
Glucose biofuel cells (GBC) using glucose and oxygen fuels are devices converting chemical energy of the fuels to electrical energy with an addition of the biocatalysts containing enzyme molecules. Since the GBC can be operated even in physiological conditions, the glucose and oxygen that are included in human body can be utilized as the fuels for the devices embedded into human body. However, critical problems to be solved still remain. In particular, its slow reaction rate should be improved. To do that, the use of mediator may be an appropriate solution. With the introduction of the proper mediator, electron transfer rate for the anodic and cathodic reactions can increase. To optimize the electron transfer rate and explain the electron transfer mechanism, a new electron transfer mechanism dubbed as a mediator embedded electron transfer (EMET) is introduced. With the mechanism, the target mediator, which can show outstanding benefits, is well immobilized on the enzyme and substrate materials. In this conference, I will explain the related electron transfer theory, the synthetic procedure of corresponding biocatalysts and the configuration and performance of GBC utilized.
To keep high energy conversion efficiency under variable electricity demand/supply is a highly important requirement for next-generation energy system which includes energy conversion processes. While SOFCs are expected to have high efficiency, they cannot avoid the drop in fuel utilization under abrupt output change.
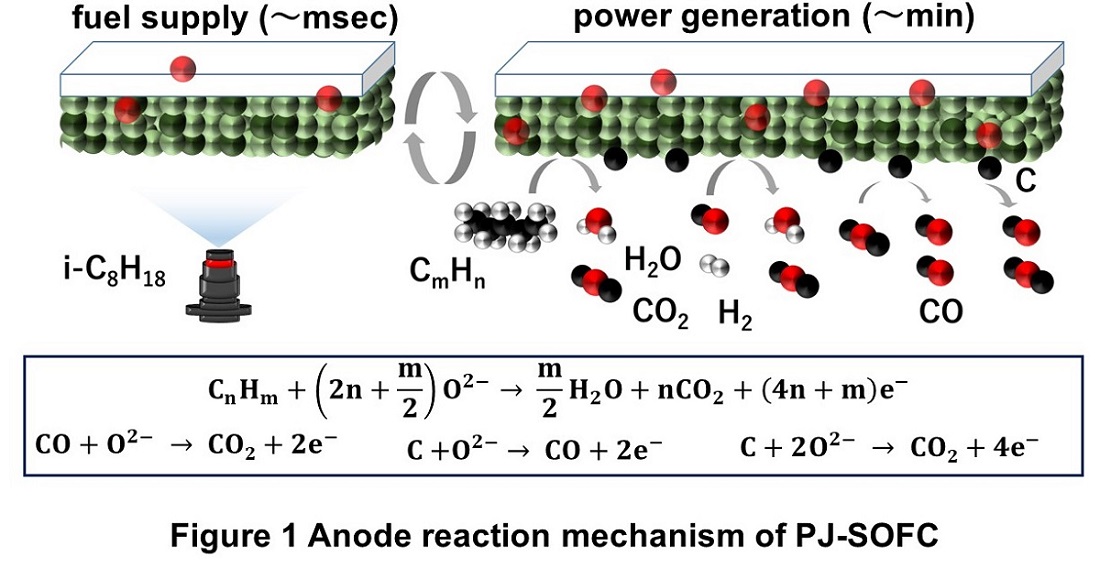
We proposed pulse jet solid oxide fuel cell (PJ-SOFC) which adopts the direct supply of liquid fuel via injector set below the anode. PJ-SOFC can keep high fuel utilization even in steep output power variation because it can change the gas atmosphere around the anode locally even with a small amount of fuel.
We have discussed the relationship between electrochemical/thermochemical reactions and the gas atmosphere around the anode, but the power generation property with steep output variation was not discussed, and the availability of PJ-SOFC as a load-followable SOFC was not evaluated.
In this research, we investigated the influence of operating condition for anode degradation quantitatively to realize stable operation under variable output with keeping high fuel utilization. Also, the advantage of PJ-SOFC for load following was discussed based on the actual data of the smart grid system “Ene-Swallow” developed at our laboratory and implemented at TokyoTech.
The power generation properties were investigated by electrolyte supported coin cells with YSZ electrolyte. Anode material was Ni/YSZ (3:2 wt%) and Ni/GDC(3:2, 1:1 wt%), and cathode material was LSM/ScSZ. Power generation was conducted at 900 °C with fixed fuel utilization as 60%.
Maximum power density was achieved 239 mWcm-2 with Ni/GDC(3:2). Better performance and stability were obtained with Ni/GDC(3:2) than Ni/YSZ(3:2). Higher stability was achieved with Ni/GDC(1:1) whereas higher power density was achieved with Ni/GDC(3:2). Step response was demonstrated and 1.3 times of output change, from 80 mWcm-2 to 105 mWcm-2, could be achieved in a moment without causing fuel utilization decrease.
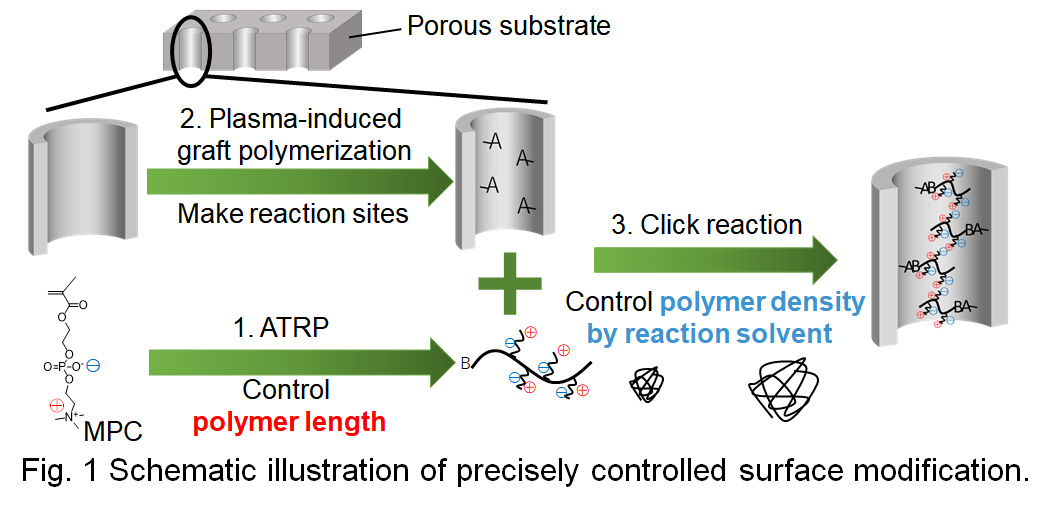
Water treatment using membranes forms a key technology for solving water shortage and pollution worldwide. Some advantages of purifying water with membrane technology include low energy and small space requirements. Fouling which causes a reduction in separation performance and shortens membrane life is a serious problem to be solved. Although surface modification with a hydrophilic polymer such as zwitterionic polymer is effective for antifouling, there is no design guideline concerning membrane surface modification due to difficulty in controlling the molecular weight and density of the modified polymer during the membrane fabrication.
Here, we propose a precise surface modification method for the water treatment membrane(Fig. 1) and evaluate the influence of graft density and length of modified polymer on fouling phenomena using the prepared membrane.
In our suggested approach, poly(2-Methacryloyloxyethyl phosphorylcholine) (polyMPC) is synthesized initially by atom transfer radical polymerization(ATRP). Then, glycidyl methacrylate is grafted to the pores of polyethylene terephthalate(PET) as the reaction point of polymer immobilization, using plasma graft polymerization, followed by the immobilization of pre-synthesized polyMPCs by Click reaction. The key advantages of this concept are that polymers with pre-determined molecular weight and narrow molecular weight distribution can be effectively introduced into the pores. Furthermore, the density of the polymers inside the pores can be controlled by considering the structure of polymer chains in each solvent during Click reaction. Surface modification was confirmed by weight change observed for the membrane before and after the Click reaction and PET membranes modified by different molecular weight polyMPCs having different densities were successfully fabricated.
Permeation test of Bovine Serum Albumin solution though the prepared PET membranes was performed using a dead end filtration system. The PET membrane modified using high molecular weight polyMPC with high density showed highest flux recovery ratio, namely such surface modification of membrane is effective for antifouling.
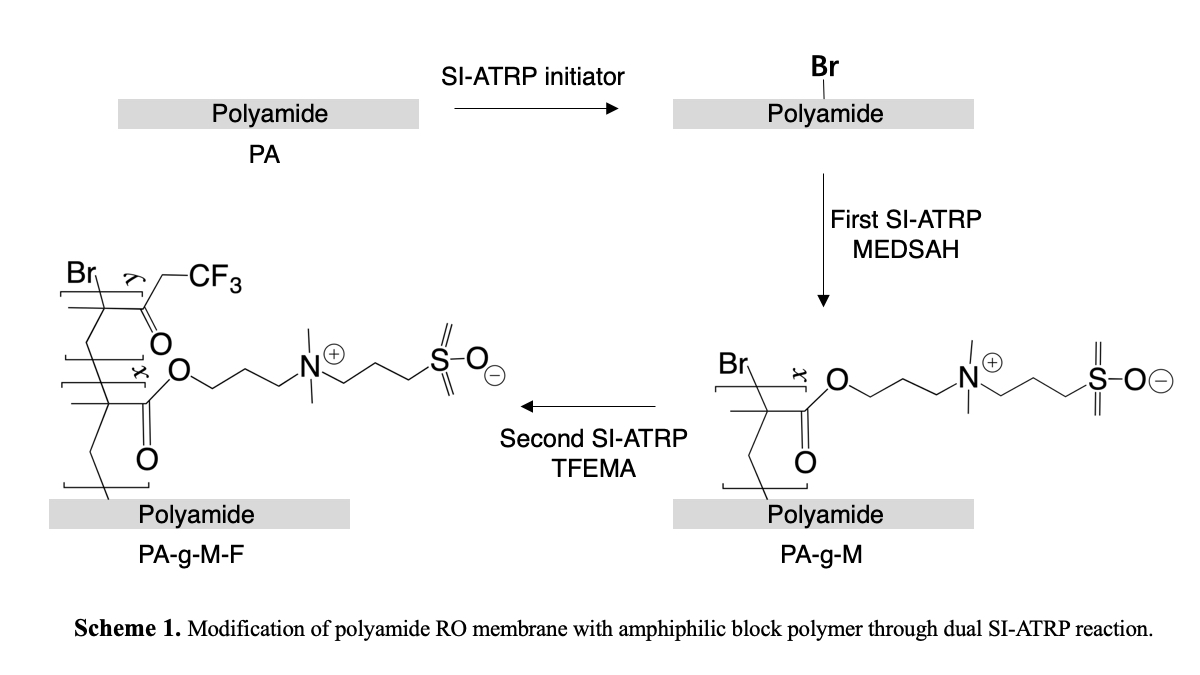
Membrane fouling is a severe hindrance to the effective application of reverse-osmosis (RO) process. For enhanced anti-fouling properties, majority efforts toward membrane surface modification have focused on rendering the surface more hydrophilic, smooth, and less charged, which prevents the foulant from contacting with membrane surface (“fouling resistant”). Probably, weakening the interfacial interaction between membrane and foulants is another strategy to tailor membrane anti-fouling characteristics, which makes adhered foulants easier to be removed (“fouling release”). Therefore, in this study, a new amphiphilic block polymer combining fouling resistance with fouling release features was synthesized by using [(2-methacryloyloxy)ethyl]dimethyl[3-sulfopropyl]ammonium hydroxide (MEDSAH) and 2,2,2-trifluoroethyl methacrylate (TFEMA) and grafted on polyamide (PA) RO membrane surface by dual surface-initiated atom transfer radical polymerization (SI-ATRP) reaction (Scheme 1). The surface composition, membrane morphology, surface hydrophilicity, and surface energy were assessed for the evaluation of the fouling resistance and fouling release potential of the membrane modified with the amphiphilic block polymer (PA-g-M-F). The long-term sodium alginate filtration experiment results indicated that compared to the pristine membrane (PA), PA-g-M-F membrane showed lower water flux decline and higher water flux recovery, remaining high stability.
Polymer composite materials are the subject of extensive studies because of their novel and excellent properties compared to their constituent materials. Dispersion stability of sub-micron sized particles in the medium is an important research theme from the point of colloidal views. And also, these particles are often used to synthesize polymer composite materials using resins and gels as matrix. Herein, dispersion of nano-materials, such as nano particles, in the matrix polymer is one of the most important problems to enhance their mechanical properties. We tackled this problem to carry out surface modification of the nano-materials, such as polymer particles or carbon nano tubes (CNTs), using amphiphilic polymers, poly-N-vinylacetamide (PNVA), synthesized thorough radical polymerization. Hydrogen bond worked between PNVA onto the modified nano-materials and hydrophilic matrix, such as polyvinyl alcohol (PVA) and nylon, to improve surface adhesions and dispersions of the nano-materials in the matrix. As a result, the mechanical properties of their composites were strengthened. When CNTs were used in PVA, the transparency of the composite was also increased owing to improvement of their dispersions. Additionally, if the CNTs formed the networks in the composites, the highly conductive and transparent composite polymer films were fabricated.
This study presents newly-developed simulation method of flow in twin screw extruder. This method based on the Hele-Shaw flow model and finite element method, and applicable wide range of screw geometry without empirical parameters. Moreover, the Hele-Shaw flow model reduced the number of unknown variables dramatically and calculation time. The simulation results of degree of fill, fiber attrition, and devolatilization phenomena were experimentally validated.
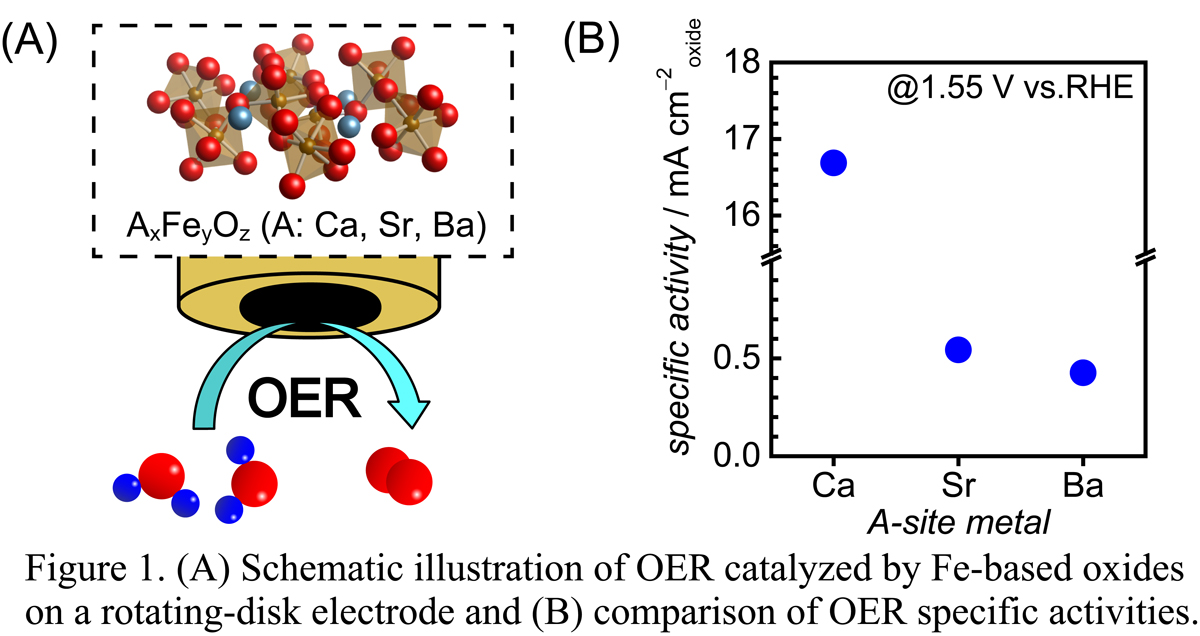
Current energy and environmental issues, alongside the depletion in fossil fuels, have increased the demand for the use of sustainable energy carriers. Electrochemical water splitting can produce hydrogen from naturally abundant water and is a clean and cost-effective energy conversion process. However, the anodic oxygen evolution reaction (OER) in the water splitting process has a large overpotential; thus, there is a need to develop highly active and inexpensive electrocatalysts for OER to overcome this barrier. Perovskite-type metal oxides with the general formula ABO3 have attracted great interest due to their excellent OER activities, facile synthesis and environmental friendliness. Several Fe-based perovskite-type OER catalysts have been previously reported, but most of these studies focused on the choice of the A-site metals. In contrast, the effects of crystalline structures and elemental compositions of catalysts on OER activity have not yet been systematically investigated, but the knowledge is crucial for the efficient design of electroactive materials. Herein, we synthesize a variety of Fe-based oxides with the general formula AxFeyOz (A: Ca, Sr, Ba) (Figure 1A) and investigate them to find promising OER electrocatalysts and to elucidate the structural and compositional effects on the OER activity. Ca, Sr and Ba were selected as A-site metals. Figure 1B shows the OER specific activities of the Fe-based oxides and reveals that a Ca-containing oxide was found to possess the highest OER activity among other synthesized oxides, exhibiting one of the best performances observed when compared to previously reported Fe-based oxides. These findings not only present a prominent electrocatalyst for OER, but also provide new guidelines for the design of metal oxides-type OER electrocatalysts.
Acknowledgement: This paper is based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO).
For large scale use of renewable energy, hydrogen society is necessary to overcome supply and demand mismatch in time and space. Polymer-electrolyte fuel cells (PEFCs) represent a superior system that exhibits high-efficiency, offering better power generation, meeting the desired levels of demand. However, in order to facilitate widespread use of fuel cells, cost and lifetime problems must be resolved. Solid alkaline fuel cells (SAFCs) are another system that holds the potential to achieve high-energy conversion efficiency without Pt catalysts. Although most of metal catalysts can be used under alkaline environment, development of durable electrolyte membranes in alkaline media is the key for this technology. We are systematically designing and developing new materials from the molecular level to the device level. In the fuel cell systems, different components such as membrane, catalysts, and catalyst layer share significant functions and work in a well-coordinated manner, and hence, the total cell system must be optimized for the best performance. The systematic design and developing approaches concerning materials for PEFCs and SAFCs are proposing. Specifically, pore-filling electrolyte membranes, carbon free Pt alloy porous capsule catalysts and durable anion-exchange membranes and direct liquid fuel cells are developed based on the approach. Future energy system and water electrolysis materials are also presented.
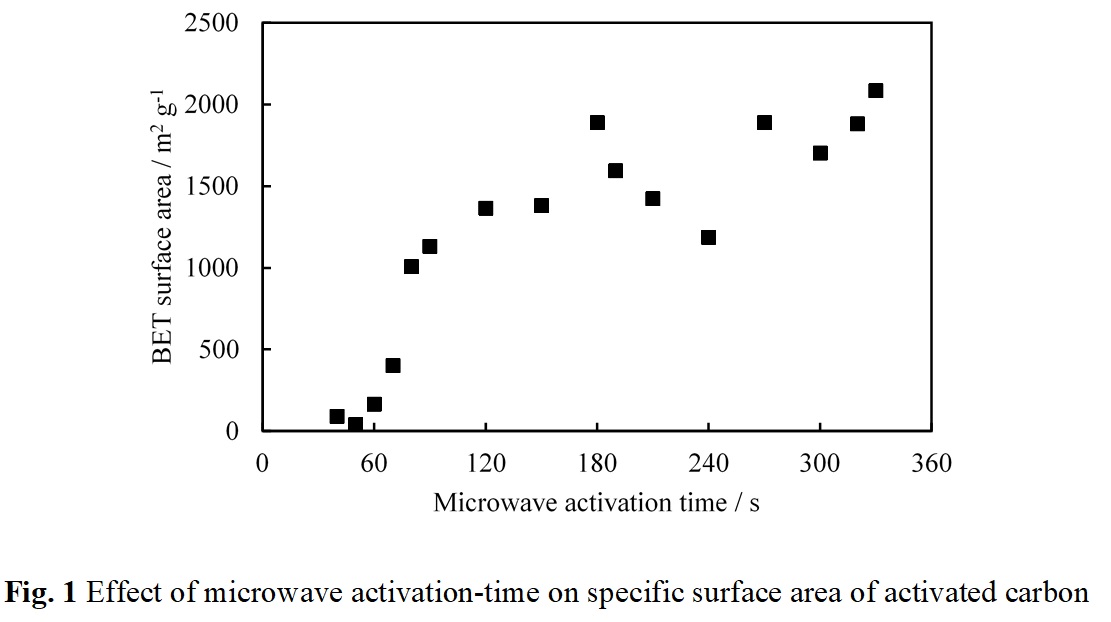
Several works reported that microwave heating can reduce production time required for activated carbon preparation. In this study, the mixture of carbonized carbon gel and KOH (mass ratio of carbon gel to KOH is 1:6) was heated by a modified microwave oven (2.45 GHz, 500 W) for 40-330 s under N2 atmosphere before being washed by distillation water. As presented in Fig. 1, BET surface area of resultant activated carbon tends to increase with an increase in microwave activation time. By using this proposed method, the large BET surface areas which are 1,007 and 2,084 m2g-1 can be achieved in the resultant activated carbon within 80 and 330 s, respectively. It has been reported that even when microwave heating is employed to reduce the activation time, the shortest record was 300 s. Such a short activation time as only 80 s to synthesize activated carbon with a large specific surface area above 1,000 m2g-1 by the proposed method is remarkable. It can be noted that bright plasma can be uniquely generated at the carbon-KOH mixture under microwave heating when the activated carbon with the large surface area is obtained. It may be considered that the carbonized carbon gel powders in contact with molten KOH may cause interfacial polarization under microwave heating, and this polarization may cause the observable plasma in the present condition, resulting in a high temperature. To date, the activation process for synthesizing activated carbon has been operated in batch system because of a long activation period. Once the mechanisms of the extremely fast activation method found in this study are clarified, a new concept of reactor design will be developed for operating in a continuous system.
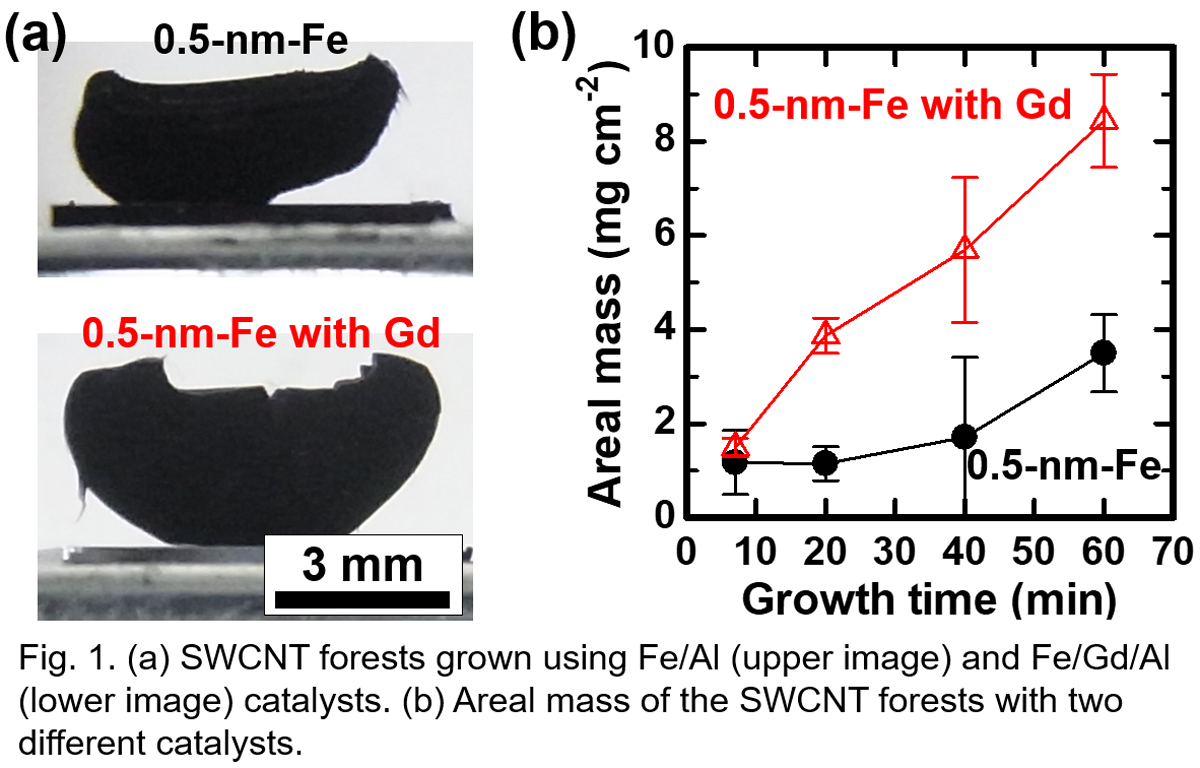
Growth of vertically-aligned single-wall carbon nanotube (VA-SWCNT) forests by the catalytic chemical vapor deposition (CVD) is an attractive method for making applications. However, the growth termination of the CNT forests is an obstacle, and the deactivation of the catalyst nanoparticles due to the structure change of the catalyst nanoparticles is thought to be one reason for the termination. In general, maintaining smaller catalyst nanoparticles which are necessary for the SWCNT growth is more difficult because the smaller nanoparticles are less stable. To realize the longer growth lifetime, engineering catalysts is crucially important. So far, Fe-Gd catalyst on Al2O3 layer was reported to realize the growth lifetime of 13 h and 22-mm-tall multi-walled CNT (MWCNT) forest at the growth temperature of 780 °C [1]. However, the growth rate is relatively low as ~0.5 μm s-1, and the possibility of using Gd for SWCNT growth was not discussed in detail.
In this work, we applied the Fe/Gd/Al catalyst to the growth of SWCNT forests, and systematically studied the mechanism behind the enhanced growth (Fig. 1a). By optimizing the catalyst condition, we achieved a high initial growth rate of ~2 μm s-1 and long catalyst lifetime of ~50 min at 800 °C. Correspondingly, the areal mass continued increasing up to ~8 mg cm-2 in 60 min (Fig. 1b). It was found that Gd layer with the thickness of less than 1 nm is effective when it is deposited between Fe and Al layers. The Raman spectra showed the radial breathing mode (RBM) peaks from the top to the bottom of the CNT forests, which suggests the continuous growth of SWCNTs.
References
[1] W. Cho et al., Carbon 72, 264 (2014).
With the increasing demand for energy supply and awareness of climate crisis, green energy generation has attracted intensive and extensive research attention during the past decades. Hydrogen, as a high energy density non-carbon energy source, has been one of the spotlights and its green production has been one of the top research topics in scientific community. Among the many existing hydrogen generation processes, electrolytic water splitting (H2O → H2 + 1/2 O2) driven by renewable energies is considered by many an everlasting process for hydrogen supply. The most concerned issue of this process is its disadvantageous production cost as compared with competing technologies, such as steam reforming of fossil fuels. Electricity accounts for more than 50 % of the production cost for electrolytic water splitting. Consequently, extensive efforts have been devoted to develop more efficient catalysts to lower the over-potential and thus electricity consumption of the process to strengthen its competiveness. To function well as an efficient catalyst for electrolytic water splitting, not only material but also nanostructure are critical. In this presentation, several recent examples from our lab are offered to illustrate the point.
Titanium dioxide (TiO2) photocatalysts have been extensively studied for water treament, air purification and antibacterial. Due to it has many properties such as chemical stability, electronic properties and especially strong photocatalysts. However, TiO2 should be irradiated with ultraviolet (UV) radiation for photocatalytic activity. In this study, Ag doped Titanium dioxide (Ag-TiO2) was synthesized by a wet ball milling sol-gel method (WBMS). Various amount Ag/TiO2 molar ratio from 0% to 10%. To study phase transition and crystallite size of Ag-TiO2 from X-ray diffraction (XRD). The particle size, surface area, morphology and electronic properties synthesized of Ag-TiO2 were determined by transmission electron microscopy (TEM), Brunauer-Emmett-Teller (BET), Scanning electron microscopy (SEM) and UV-vis diffuse reflectance spectroscopy. Photocatalytic activity and antibacterial were also investigated. The results demonstrated that when Ag/TiO2 molar ratio increased from 0% to 10%, the doping of Ag promoted the phase transformation and and inhibited crystallite size of Ag-TiO2. At 5% Ag-TiO2 showed the small particle size and, crystallite size and also presented the highest surface area. Intensity of surface plasmon absorption (SPA) peak of Ag-TiO2 increased and their peak position shifted to a longer wavelength range. Furthermore, back scatter electron microscopy (BSC) confirmed silver nanoparticle deposited on surface of TiO2 powder. The photocatalytic activity degradation of methylene blue (MB) of Ag-TiO2 increased from 1.12×10-3 to 1.62×10-3 min-1 when increasing of Ag/TiO2 molar ratio from 0% to 5%. When Ag/TiO2 molar ratio was increased more than 5% the photocatalytic activity decreased. The optimal 3–5% Ag-TiO2 for antibacterial was found high antibacterial for Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) under visible light.
Manufacture of Composite Materials for Bullet-proofing Helmets Using Multi Reinforcement Based on Epoxy Resin
Tania Natasha Dharmakusumah1*, Asep Handaya Saputra2
1tania.natasha@ui.ac.id; E-mail: taniadharmakusumah@gmail.com
2asephandaya@gmail.com
In recent years, Indonesia has faced many military challenges which are the center of attention of the military defense industry. Military Defense industry players in Indonesia such as BUMN (State-Owned Enterprises) and private companies, need new innovative material technology to make military equipment components such as bulletproof helmets that are light and practical so as not to disturb the mobility of military personnel. For this reason, this study uses composite materials consisting of multi reinforcement, such as carbon fiber and Kevlar, and epoxy resin as a matrix. The method of making is done by an open molding technique, hand lay-up. The choice of reinforcement with the right number of layers must be done to see the performance of carbon fiber and Kevlar to form a composite material that has bullet resistance, through Barcol hardness test and ballistic test. To find out how many layers are needed for one specimen by calculating the thickness. The factors that influence the thickness calculation are tensile strength values, the average resistance force of composite material specimen, cross-sectional area, and kinetic energy produced by bullets. The calculation results will be tested through ballistic tests using weapons and bullets that are in accordance with the National Institute of Justice Standards for bulletproof helmets.
Keywords: Carbon fiber, Kevlar, epoxy resin, hand lay-up, ballistic helmet
Silica aerogel is regarded as one of the most promising thermal insulating materials due to its extremely low thermal conductivity. The porosity higher than 90% and the pore size smaller than micron scale make the thermal conductivity lower than that of air. The bottleneck to the practical application of silica aerogel is its mechanical fragility. In this study, the composite aerogels comprised of individually crosslinked silica and chitosan networks were fabricated and their thermal insulating properties were investigated.
Silica sol was prepared by hydrolyzing methyltrimethoxy silane (MTMS) in an acetic acid aqueous solution containing cetyltrimethylammonium bromide (CTAB), urea and chitosan. Then glutaraldehyde (GTA) was added and the solution was heated to accelerate the crosslinking of chitosan with GTA and the gelation of silica. The obtained composite hydrogel was converted to aerogel via supercritical CO2 drying. The balance between the rates of crosslinking of chitosan and gelation of silica was the important factor of obtaining the uniform composite aerogel. The composite aerogels had the smaller average pore sizes and more uniform pore size distribution than the silica aerogel without chitosan. The thermal conductivity of the composite aerogels was nearly the same as that of the silica aerogel. The bending elastic modulus of composite aerogels was slightly higher than that of silica aerogel.
Calcium phosphate ceramics, such as hydroxyapatite (HAp: Ca10(PO4)6(OH)2) and β-tricalcium phosphate (β-TCP: β-Ca3(PO4)2) have been applied as bioactive and substituted materials for hard tissues in dental and medical fields due to excellent biocompatibility and osteoconductivity. For bioceramics, it will be very important to have absorption capability of biomaterials incorporated into bio-metabolic system because solubility absorption or giant cellular absorption may be indispensable for bioceramics harmonized with bone remodeling. The partial dissolution- precipitation with supersonic treatment is a convenient wet synthesis technique adding biomimetic characteristics for surface modification of biomaterials. Supersonic wave can bring bubble cavitation and make hot spot. So, many chemical reactions are activated by the formation of radical groups and the locally rising temperature. The purpose of this study is to investigate surface structure and nature of modified porous ceramics and to clarify the absorption characteristics in animal experiments. Commercial β-TCP products were modified to functionally graded calcium phosphate ceramics by the partial dissolution–precipitation (PDP) technique, which involved a supersonic treatment at 120 W and 38 kHz in 2% HNO3 solutions containing Ca2+ and PO43– ions. The dissolution efficiency of porous β-TCP ceramics drastically increased with time, depending on the porosity of ceramics. Enhancement of micro-pores and propagation of nano-cracks were recognized by the supersonic treatment for 7 min. After the specific PDP technique, HAp nano-crystals were precipitated all over the pore wall surfaces in macro- and micro-pores of the ceramics. The β-TCP and PDP-TCP ceramics were implanted into the subcutaneous tissues of back parts in 4 week-old Wistar rats. At 3 weeks after the implantation, the PDP-TCP ceramics showed more numbers of osteoclast like giant cells and better affinity for body fluid than original β-TCP ceramics. Based on these results, it was found that PDP-β-TCP ceramics would be one of bioactive scaffolds inducing good giant cell-absorption.
The control of the interface of micro and nano-composite materials are essential for developing environmentally benign methods for manufacturing microcapsules for Drug Delivery Systems (DDS). However, toxic organic solvents and multiple steps were often used which were unsuitable for manufacturing microcapsules for DDS in conventional methods. Here, we will talk about new methods we developed to control the interface of micro- and nano-composite particles and bubbles using a high pressure techniques. These methods used pressure-induced phase separation using super critical carbon dioxide (scCO2) solution and ultrasound irradiation under high pressure conditions. Some medicine, such as Levofloxacin were used as core materials and polymers having pH-responsive functions such as Eudragit E100 or L100 were used as coating materials. The coating materials were dissolved in scCO2 solution containing a co-solvent. As the pressure of the high pressure cell slowly decreased into atmospheric pressure, microcapsules were formed in the cell. The structure of the microcapsules was observed by SEM equipped with an electron probe microanalyzer (EPMA) devices. In order to confirm the absence of co-solvent in the capsule, we used the FT-IR. In the later part of the talk, we will introduce a novel method for producing and quanitfing micro- and nano- bubbles. These micro- and nano- bubbles were produced in a specially designed high pressure cell equipped with an ultrasonication horn located in the vessel. This technique is unique as micro phase separation between the high pressure gas and liquid interphase can be achieved through direct sonication, leading to the enhancement of micro- and nano- sized bubble production. The characterization of the mico- and nano- bubbles as well as its comparison with other conventional methods and possible future applications will be discussed.
Gold nanomaterials are of technological importance in catalysis, photovoltaic conversion, sensing, and biomedicine. The attractiveness of gold nanomaterials lies in their unique surface plasmon resonance phenomenon, which arises from the collective oscillation of surface electrons when the materials interact with incident electromagnetic waves. An important application utilizing the phenomenon is the surface-enhanced Raman scattering (SERS) technology for the detection of trace amounts of chemicals such as heavy metals, pesticides, explosives, narcotics, contaminants in food and water, etc. In particular, the inertness and non-toxicity of gold makes it favorable for personal care as well as diagnostic purposes. The surface plasmon resonant property strongly depends on the particle size, particle shape, and aggregation states of the material, hence morphological engineering is an efficient strategy for developing new plasmonic gold materials. Great efforts have been made to prepare gold nanostructures with varying morphologies. To date, spherical, rod-like, flower-like, sheet-like, raspberry-like, polyhedral, branched, hollow, and chiral nanoparticles have been synthesized. Besides, satellite-like and other sophisticated morphologies have been prepared through self-assembly or multi-step synthesis schemes.
Here we report an unconventional gold nanostructure through facile one-pot synthesis in aqueous solution from chloroauric acid precursor. The architecture is featured by pomegranate-like interior structure and raspberry-like surface morphology. The pomegranate-like gold structure achieves tunable plasmonic resonance absorption within 580-730 nm and shows SERS activity. The SERS measurements were conducted with the paper-based SERS technique using thiophenol as the probe molecule and a portable Raman spectrometer. The results showed that the pomegranate-like gold structure achieved a detection limit of 10 ng thiophenol. The performance is comparable with commercial paper-based SERS substrates, indicating the potential of pomegranate-like gold nanostructure for practical applications.
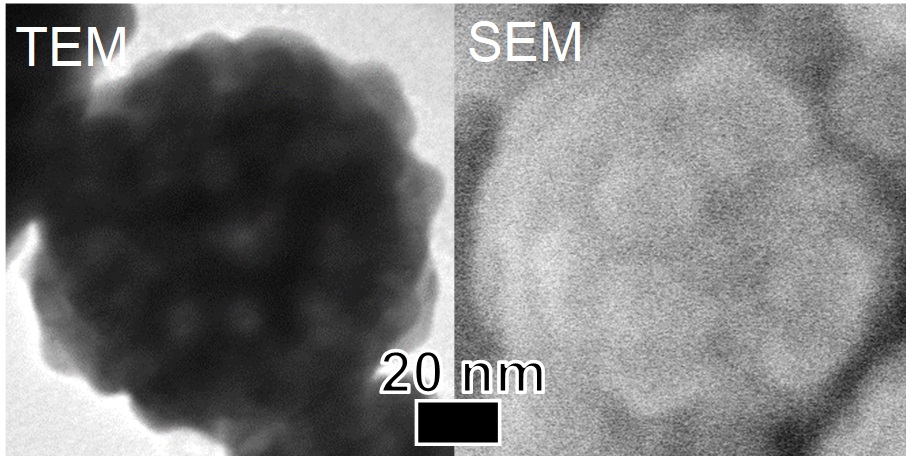
Fluorescent polymer particles immobilized with biological molecules on their surface are widely used as fluorescent probes and labels for biomedical applications. From this viewpoint, the present work proposes a simple method for fabricating such particles, which is one-pot formation in water medium. Submicron-sized monodisperse polymer particles not only surface-immobilized with sugar chain having a specific ability to be adsorbed to viruses and proteins but also incorporating fluorescent dye were fabricated with the proposed method. This method used octyl-β-D-glucopyranoside (octyl-β-D-glc), in which an octyl group is glycoside bound to a glucose skeleton as a sugar, and coumarin 7 as a fluorescent dye. During polymethylmetacrylate (PMMA) particle formation by soap-free emulsion polymerization, it is expected that the octyl group enter the PMMA particles due to its hydrophobicity and the glucose is in contact with the water medium due to its hydrophilicity. For the simplicity of the preparation procedure, both octyl-β-D-glc and coumarin 7 were added to the reaction solution at the beginning of the polymerization. The figure shows a SEM image of PMMA particles containing octyl-β-D-glc and coumarin 7. The monodisperse particles were obtained, and their average particle size was approximately 250 nm. Immobilization of octyl-β-D-glc on the particle surfaces was evaluated using concanavalin A (ConA) as a lectin, which has the selective affinity with glucose. The amount of Con A adsorbed onto the surfaces of particles fabricated with octyl-β-D-glc was twice larger than that with no octyl-β-D-glc, which confirmed successful octyl-β-D-glc-immobilization. The PMMA particles containing octyl-β-D-glc and coumarin 7 emitted fluorescence with a peak around 503 nm originated from coumarin 7. The spontaneous incorporation of coumarin 7 into the PMMA particles as well as surface-immobilization of octyl-β-D-glc on the particle surfaces took place during the particle growth in the proposed one-pot soap-free emulsion polymerization.
Flow microreactors have been widely studied for synthesizing monodispersed nanoparticles by utilizing their enhanced mixing performance owing to the shortened diffusion lengths. Mixing time is used exclusively to discuss the performance of mixing, however, more detailed parameters such as concentration profiles in the mixing space can affect the particle characters. In this study, we examined various nanoparticle synthesis processes with changing mixer structures and operating conditions. Various model materials were studied; calcium carbonate as inorganic crystal, Pt as a noble metal nanoparticle, ELM-12 as a metal-organic framework, and polystyrene as polymer latex. Two types of micromixers, tee mixers and micro-jet mixers, were employed with varying the inner diameters while keeping the Reynolds numbers. In this manner, factors involved in the nanoparticle synthesis processes with rapid mixing were separately analyzed and discussed. We found that change of the feed orientation with respect to the inlets of the micro-jet mixers can dramatically change the particle characters, implying the important role of detailed mixing behavior besides mixing time. The results showed that non-classical theory on the nucleation and growth processes should be considered for discussing the process precisely.
Microparticles of extracted compounds from seaweeds have been carried out by electrospraying method. The extracted compounds were obtained from hydrothermal extraction of Eucheuma cottonii (E. cottonii) and Gracilaria sp. The hydrothermal extraction was conducted in a semibatch extractor at temperature of 160oC, pressure of 7 MPa, and water flow rate of 1 mL/min. Subsequently, the extracted compounds were combined with polyvinylpyrrolidone (PVP) at various concentrations of 4, 6 and 8% w/v. The solution was sprayed via nozzle of 0.5 mm from positive electrode to negative collector. The distance between spinneret and collector was varied at 6, 8 and 10 cm. The applied voltage was 12, 14, and 16 kV. Electrospraying was carried out for 4 h with solution flow rate of 0.05 mL/h. The particles formed were analysed by SEM and FTIR to determine the particle morphology and their functional groups, respectively. In order to understand the change of materials by temperature, the particles formed were examined by thermal gravimetry analysis (TGA) method. Moreover, the antioxidant activity of particles was also measured by DPPH assay. Based on the experimental result, the highest mass production of particles was obtained at PVP concentration of 6% with applied voltage of 16 kV and distance of 6 cm. The highest mass production of particles from E. cottonii and Gracilaria sp were 19.97 mg and 20.02 mg, respectively. The mass production of particles increased with an increasing PVP concentration and voltage. The morphology of particles was spherical with particle size less than 3 μm. The increasing applied voltage and distance decreased the particle size. The antioxidant activity of particles was relatively high with the highest antioxidant efficiency for E. cottonii and Gracilaria sp of 0.182 min-1 and 0.106 min-1, respectively. The results indicated that electrospraying is promising method for production of pharmaceutical particles.
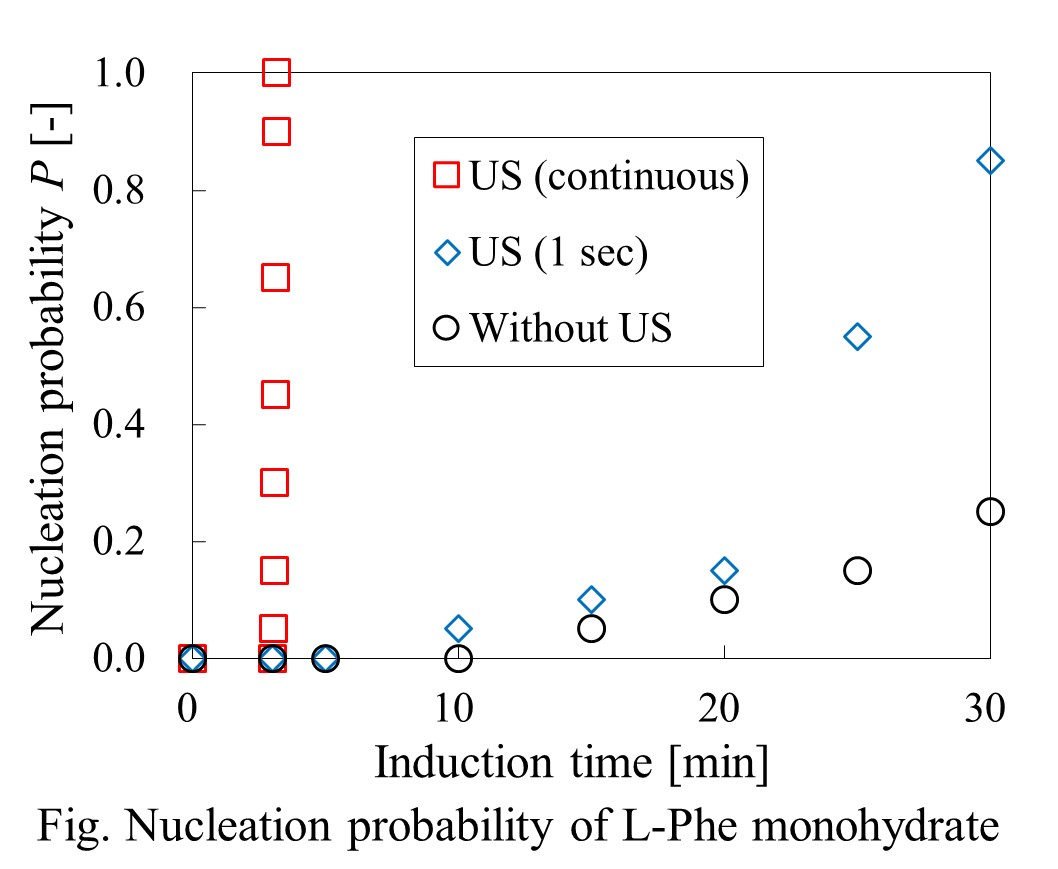
Amino acids are the components constituting proteins, and widely utilized as medical raw materials, food additives for seasonings and preservatives, and chelating agents and buffer agents of cosmetics. Most of the amino acids show polymorphism. Differences in the crystal structures affect the physical and chemical properties: solubility, stability, melting point, density, heat capacity, thermal conductivity, and optical activity. These differences influence the process acceptability, bioavailability, filtration, and tablet processes of pharmaceutical, food, and specialty materials. Therefore, an effective method for polymorph control is required in the crystallization process in order to improve the functionality of amino acid crystals and the process efficiency. As the method for polymorph control, sonocrystallization seems to have a great potential. The application of ultrasonic power causes localized mixing, heating and pressure oscillation that could contribute to control supersaturation, nucleation and crystal growth. During crystallization, sonication reduces the induction time, supersaturation, and metastable zone width. It is considerable that ultrasonic irradiation causes the nucleation directly, and sonocrystallization seems to be a simpler method to generate a desired polymorph. In this study, primary nucleation of L-Phenylalanine (L-Phe) in supersaturated solution by ultrasonic irradiation was investigated. Initially, predetermined amount of L-Phe was dissolved in purified water, and 2 mL of the L-Phe solution was added into a test tube and cooled in thermostat bath. Subsequently, in order to investigate the effects of ultrasonic irradiation, ultrasonic horn was inserted into the solution and ultrasonic wave of 20 kHz was irradiated. As a result, nucleation of L-Phe crystal was induced by ultrasonic irradiation, and nucleation probability was increased while the ultrasonic wave was irradiated. In addition, short time irradiation also caused the increase of nucleation probability. Moreover, in order to investigate the possibility of polymorph control, sonocrystallization experiment of each polymorph was performed.
Controlling of crystallization is a critical issue for chemical process in food and pharmaceuticals. In particular, nucleation behaviors of crystals are dominated by a variety of factors such as supersaturation degree, interfacial energy and solute characteristics. So far, we have performed droplet-based crystallization of lysozyme using a microfluidic device and found that nucleation frequency, N/N0, in monodisperse droplets would be a promising way to estimate the nucleation rate of solute. In this study, furthermore, the droplets with different sizes gives us a useful information for nucleation behaviors. From the results of droplet-size dependency in the nucleation frequencies of several solutes, nucleation in droplets was quite different from that in bulk solution.
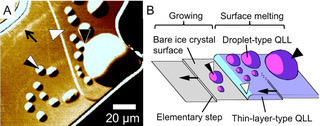
The phase transition of ice crystals governs a wide variety of phenomena on the earth. Hence, the molecular level understanding of the phase transition of ice is crucial to unlock the secrets of various phenomena. To visualize ice crystal surfaces at the molecular level, we and Olympus Corporation developed laser confocal microscopy combined with differential interference contrast microscopy (LCM-DIM) [1], which can visualize individual elementary steps (0.37 nm in height) on ice crystal surface [2]. By using LCM-DIM, we observed in-situ ice crystal surfaces under various conditions. In the conference, we will show the following two topics.
1) Thermodynamic origin of two-types of quasi-liquid layers (QLLs) with different morphologies (droplets and thin layers) [3]: with increasing water vapor pressure, the wettability between QLLs and ice crystal surfaces become significantly better, resulting in the changes in the wetting states from partial wetting (droplets) to pseudo-partial wetting (the coexistence of droplets and thin layers). The thickness of the thin layers agrees with the value predicted by the quantum theory calculation.
2) Novel uptake mechanism of HCl in ice crystals [4]: HCl gas, which triggers the depletion of the stratospheric ozone, is adsorbed on ice crystal surfaces. Then droplets of a HCl aqueous solution appear on the ice crystals surfaces. As the ice crystals grow, the HCl droplets are embedded in the ice crystals. This novel uptake mechanism allows ice crystals to store ten-times larger amount of HCl in ice crystals than the solubility of HCl in ice crystals.
[1] G. Sazaki et al., J. Cryst. Growth, 262, 536-542 (2004).
[2] G. Sazaki et al., Proc. Nat. Acad. Sci. USA., 107, 19702-19707 (2010).
[3] K. Murata et al., Proc. Nat. Acad. Sci. USA., 113, E6741-E6748 (2016).
[4] K. Nagashima et al., Cryst. Growth Des., 18, 4117-4122 (2018).
Reaction crystallization has been attracted attention because it can simultaneously perform synthesis, separation and purification process. To enhance productivity in the crystallization process, continuous flow system is used instead of conventional batch process. It is known that increasing of nucleation and growth rate is effective strategy for enhancement of the productivity in continuous flow production process. In particular, supersaturation is a main factor to achieve high productivity because nucleation and growth is promoted directly under high supersaturation condition. Unfortunately, agglomeration occurs when supersaturation increases, which affects purity of products and downstream process such as sieving or milling operation. Therefore, a novel process for enhancement of productivity without agglomeration is required.
According to the previous study, it is known that the nucleation is accelerated by shear stress. In addition, mass transfer promotes the growth rate. In this study, we focused on Taylor Vortex. Taylor Vortex is regular vortex ring and occurs continuously between rotating inner and stationary outer cylinders. Previous studies reported that Taylor Vortex shows high shear stress and fast mass transfer. This is expected that productivity can be enhanced without agglomeration by using Taylor Vortex flow. In addition, setting the various operating points is allowed because its mixing rate is extremely high compared with that of conventional batch system. Therefore, the novel crystallization process based on Taylor Vortex that is Reactallizer was proposed to obtain crystalline product with high quality in reaction crystallization.
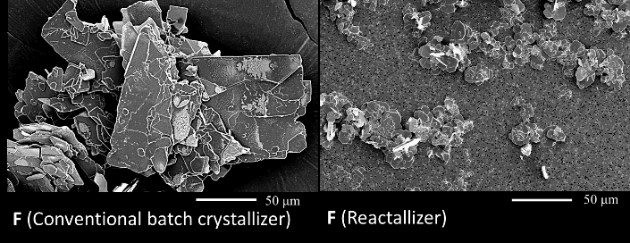
In this study, sodium L- aspartate - hydrochloric acid was used as feed. L-Aspartic acid is target product. From the result of SEM observation, it was showed that the size and agglomeration of obtained crystals using Reactallizer were improved comparing that of batch process (Figure 1). This means that our proposed crystallizer could provide fine crystals continuously without agglomeration in reaction crystallization.
Desired enantiomer crystals are obtained from a racemic solution using a preferential crystallization. However, undesired enantiomer is also crystallized from its solution, and consequently, the purity of product particles decreases. The different types of polymorphs indicate the different crystal structures although their molecular structures are the same. The solubilities differ by the types of polymorphs. If the different types of polymorphic crystals are deposited by the kinds of enantiomers, it is expected to be obtained the high purity products using a preferential crystallization. The purposes of this study are to examine the types of polymorphism crystallized from enantiomer aqueous solutions of glutamic acid (Glu) when phenylalanine (Phe) is added into their solution and to carry out a preferential crystallization of Glu.
When an L-Glu aqueous solution saturated at 333K was cooled to 323K and the a type of L-Glu seed crystals were added into its solution, the b type of L-Glu crystals was obtained. The b type of L-Glu crystallized from an L-Glu solution by addition of D-Phe. On the other hand, when L-Phe was dissolved into an L-Glu solution, the a type of L-Glu crystals was obtaned. Therefore, it is considered that L-Phe would inhibit the solvent-mediated polymorphic transition of L-Glu from an a type to an b one.
When an D-/L-Glu aqueous solution saturated at 323K was cooled to 313K and the a type of D-Glu crystals was seeded into its solution, the purity of product particles decreased with durations. On the contrary, since the L-Phe was dissoved in an D-/L-Glu solution and a preferential batch crystallization of D-Glu was carried out, the purity decreases seldom happened. Therefore, it is considered that the purity decrease for a preferential crystallization can be controlled by combination with polymorphism.
Metal-organic frameworks (MOFs), an emerging new porous materials, have been developing rapidly during the past more than two decades, due to their tailorable and diverse structures/compositions, specific properties, and various potential applications, such as in adsorption, separation, sensing, catalysis, and membrane fabrication. Some advantages of MOFs are the possibility of tuning their structures and properties at the molecular level for target application, the facility of their synthesis, and the infinitude of their selection/formation. Using pre-designed metal nodes and organic ligands has allowed the rational design and construction of these new materials for specific applications, to some extent. So far, basic research has made great progress, but applied research has been pushing forward slowly. Our recent works are focusing on the construction of chemically stable MOFs and the exploration of their applications in several fields, including adsorption and separation, catalysis, conductivity, and membrane fabrication, to promote their practical applications. Particularly, we emphasize the design at the molecular level, the new synthetic method for functionalized MOFs, the high chemical stability of MOFs, and the fabrication of MOF-based membranes, as well as their applications in separations, sensing, and catalysis.
Recently hybrid perovskite materials have attracted great attention as a promising candidate of next-generation optoelectronic devices because of their unique properties such as high absorption coefficient, long charge carrier's diffusion length, ambipolar charge transportability, convenient bandgap tunability, adjustable exciton binding energy, high quantum efficiency, and solution processability. Hence, the hybrid perovskites have been of great interest in optoelectronic devices including solar cells, image sensors, X-ray detectors, light emitting displays, thin film transistors, non-volatile resistive random access memories, hologram memories, thermoelectrics, and so on. Among them, I have focused on a development of next-generation hybrid perovskite solar cells. Here, I would like to introduce and share what I have studied on and what I am studying.
Just in time last year 2018, United Nations Environment Programme has launched major global campaign to fight against plastic wastes to eliminate the major sources of marine litters from micro size particles from cosmetics and waste generated from single-use plastics. Although the campaign is mainly to aware the consumers to reduce the usage of single-use plastic products, many times the single-use plastic products remains unavoidable in human life due to hygiene and convenient purposes. Inasmuch, many people suggest biopolymers are the replacement for non-degradable polymers, not only because derived from renewable resources, but most of the biopolymers are equalized as biodegradable polymers. The misconception about biopolymers are environmental friendly materials may lead to unresolved plastic pollution problems while consumers have to pay higher for the biopolymer products typically packaging. The fact is that biopolymers such as polycaprolactone is derived from the sources of petroleum, while biopolymers such as polylactic acid (PLA) and polyhydroxyalkanoate (PHA) are sourced from renewable agriculture (see Figure 1). Although many people think that agricultural sources should be more environmental friendly, the reality is that growing of crops like cassava and corn to produce starch as the main sources of fermentation ingredients required consumption huge quantities of water for irrigation, synthetic fertilizers, pesticides and herbicides from petroleum derivatives, harvester needs fuel to works and most important the entire polymerization process require large quantities of water and energy resources to produce the biopolymer resin. Also, the transportation of agricultural yield from the farm to factory and resin from factory to the consumers can also consume tonnes of fuel. Eventually production of biopolymers can cause higher emission compared to conventional polymers which available widely in the market. There are also issue of shorter shelf life of food packing with biopolymers resulted from poorer gas permeation resistivity. In conclusion, the consumers are urged that the most important efforts to solve the plastic pollution are through education on wisely usage of plastics while managing plastics wastes more responsible.
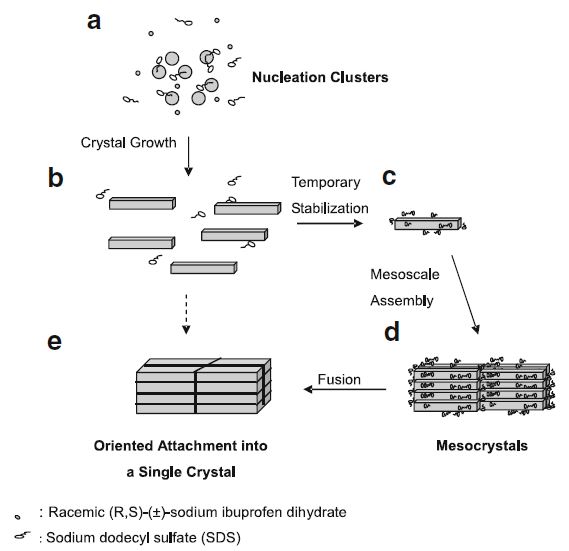
Purpose. The aim of this paper is to enhance the dissolution rate of racemic (R,S)-(±)-sodium ibuprofen dihydrate via a bio-inspired method of growing mesocrystals.
Materials and Methods. Mesocrystals of racemic (R,S)-(±)-sodium ibuprofen dihydrate were successfully prepared from a supersaturated aqueous solution of racemic (R,S)-(±)-sodium ibuprofen dihydrate having the initial degree of supersaturation, S0, of 1.326 and the initial saturated concentration, C*, of 0.986 mol/l at 25 °C with sodium dodecyl sulfate (SDS) at a concentration of 0.10 g/l. Dynamic light scattering, scanning electron microscopy, powder X-ray diffraction, differential scanning calorimetry, and optical microscopy with cross polarizers were employed to understand the formation mechanism and to characterize the superstructures of the SDS generated mesocrystals.
Results. The SDS generated mesocrystals were the assembly of the oriented attachment of racemic (R,S)-(±)-sodium ibuprofen dihydrate nano-sized platelets under the mediation of the side-to-side interaction between SDS and racemic (R,S)-(±)-sodium ibuprofen dihydrate. The SDS generated mesocrystals contained a mixture of the racemic compounds in α- and β-forms and the resolved racemic conglomerate in γ-form with no detectable amount of SDS. The dissolution rate of the SDS generated mesocrystals was more rapid than the one of its counterpart made by conventional crystallization pathway.
Conclusions. The crystallization of racemic (R,S)-(±)-sodium ibuprofen dihydrate in the presence of SDS yielded well-faceted, well-separated, but almost perfectly three-dimensionally aligned nano-sized platelets. This kind of bio-inspired mesocrystal superstructure has definitely opened a new doorway for crystal engineering and pre-formulation design in pharmaceutical industry. The future work is to study the mesocrystal formation of some other active pharmaceutical ingredients in organic solvent systems and
to develop an efficient method for screening the additives.
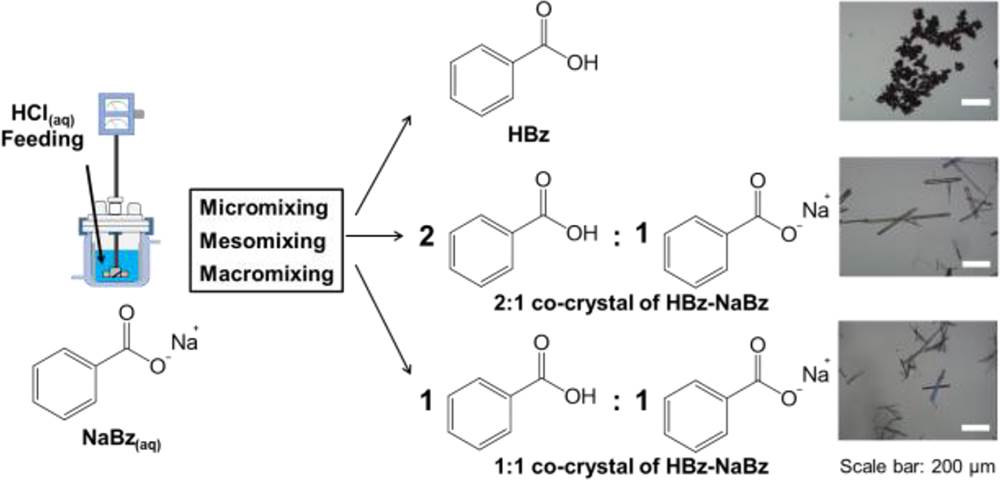
The aim of this study was to investigate the mixing effect on the stoichiometric diversity of benzoic acid–sodium benzoate (HBz–NaBz) cocrystals. The crystallization of HBz–NaBz cocrystals in a 500 mL sized glass vessel was monitored under different agitation speeds and feeding rates of HCl aqueous solution. Under good micromixing and macromixing, the HBz crystals, 2:1 and 1:1 cocrystals of HBz–NaBz were crystallized out rapidly, and all crystals were transformed to a mixture of 2:1 and 1:1 cocrystals of HBz– NaBz in a relatively short amount of time. However, the crystallization of 1:1 cocrystals of HBz–NaBz was delayed by poor micro-, meso-, and macromixing simultaneously. The compositions of the products were altered in different mixing conditions even given the identical experiment time. It was feasible to harvest pure 1:1 or pure 2:1 cocrystals of HBz–NaBz by reaction crystallization through the control of the mixing condition, concentration of reactants, and experiment time. The ternary phase diagram was also constructed for the HBz–NaBz–water system.
In this paper, authors summarized and overviewed examples of crystallization process applied to waste and wastewater treatment, in order to remove and recover substances, and clarified strategies how to apply crystallization in the environmental fields. In case of acidic and higher concentration waste, solution crystallization was applied by combining cooling and anti-solvent crystallization. Examples of the atomic field in which Uranium recovery was available by UNH cooling and anti-solvent crystallization, followed by purification process. In application to the waste water from metal surface treatment, metal and organic acid recovery based on anti-solvent-crystallization was investigated, considering solubility in rich acid solution. In case of lower concentration wastewater, reaction crystallization of sparingly soluble crystals was applied to obtain larger crystals of better quality, by adapting an original strategy of seeding policy, and also impurity control. Phosphate crystallization makes it possible to utilize recovered phosphorus effectively as fertilizer. The MAP Process in which a magnesium compound is added recovers magnesium ammonium phosphate. The MAP Process, which requires ammonium ions in the stage of crystallization, is applied to the anaerobic digested sludge or sludge filtrate, maximum phosphate recovery rate is considered by discussing the relationship between the crystal particle size. The design guidelines on both processes have been clarified. Large scale demonstration plant is in operation at Kobe Harvest Project. On the fluoride ion removal process, Organo developed the fluidized-type and agitation type process by using crystallization process. Ni2+ recovering process from used bath of electro-less plating process by reduction crystallization, in which Ni2+ is removed as a form of Ni metal, by using reducing agents with using nickel powder as seeds Crystallization will become an effective tool to remove and recover substance from waste and wastewater having much impurity.
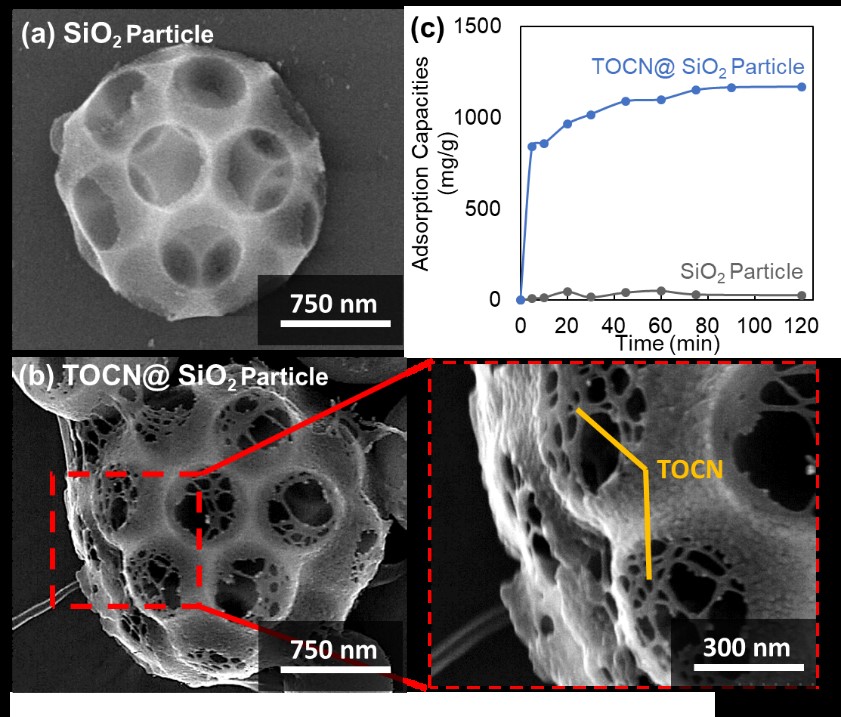
Protein adsorption is a complex process that needs to be addressed as a necessary demonstration of the successful performance of separation, drug delivery, biosensor, and other applications in biomedical. The development of adsorbent material plays a critical role to enhance protein adsorption performance. Tempo Oxidized Cellulose Nanofiber (TOCN) is a promising material owing to biocompatible, homogeneous width, highly disperse in wet-state and highly-negative charge. Even so, there is no research regarding the utilization of TOCN, especially in the particle form as a protein adsorbent material. In addition, TOCN is facing some problems such as not stable in dried-state, easily aggregated, tends to have dense morphology in dried-state that leads to the low specific area. For use as an adsorbent, stable and highly-dispersed TOCN would be desired even in the dried state. In this study, we introduced the possibility of TOCN as an effective adsorbent to specific protein considering their high content of carboxylate groups and negative charge, and a concept of TOCN decorated macroporous SiO2 (TOCN@macroporous SiO2) particle for enhanced its stability and dispersibility in dried-state. The result shows that prepared TOCN@macroporous SiO2 particle has a stable morphology with unique TOCN structure, highly negative zeta potential (-62 ± 2 mV) and surface area (30.8 m2/g) compared to pure TOCN particle. The presence of SiO2 particle allows higher stability and dispersibility which leads to a rich site of electrostatic interaction to exhibits an outstanding adsorption capacity of lysozyme which is higher than that of previous adsorbents. The material and its structure proposed in this study have a more considerable potential of application in the biomedical field.
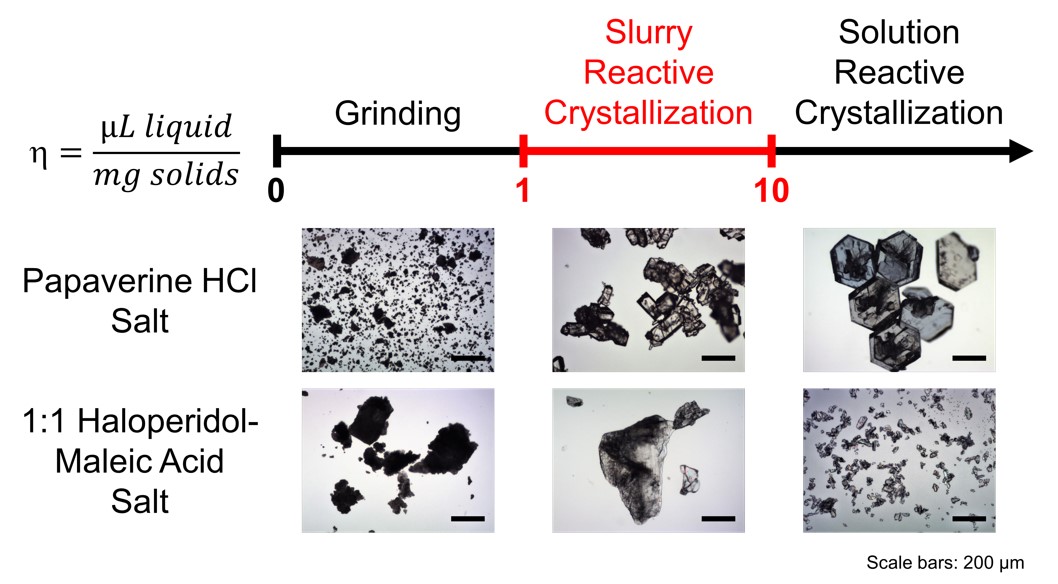
Papaverine HCl was successfully suspended by slurry reactive crystallization with the use of isopropyl alcohol (IPA) at 25oC, a solid-to-liquid ratio of 0.19 g/mL, an aging time of 8 h, a yield of 82.0 w/w %, crystal sizes of 200-400 μm, and the value for enthalpy of fusion of 154.5 J/g. The poor solubility of papaverine in IPA and better solubility of papaverine HCl in water-containing IPA had made the homogeneous nucleation of papaverine HCl dominate. Crystal size and crystallinity of papaverine HCl were time and temperature dependent. On the other hand, the 1:1 haloperidol-maleic acid salt was also successfully suspended and generated by slurry reactive crystallization with the use of water at 25oC, a solid-to-liquid ratio of 0.18 g/mL, an aging time of 8 h, a yield of 82.0 w/w %, crystal sizes of 500-1000 μm, and the value for enthalpy of fusion of 84.9 J/g. The poor solubility of haloperidol and 1:1 haloperidol-maleic acid salt in water had made the heterogeneous nucleation of 1:1 haloperidol-maleic acid salt dominate. Crystal size and crystallinity of 1:1 haloperidol-maleic acid salt became less sensitive to time and temperature. Comparing with grinding, solution reactive crystallization by cooling and solution re-crystallization by cooling, slurry reactive crystallization was a simple, robust, straightforward, low-constant-temperature, low-solvent-volume and environmentally benign process giving comparable yield, particle size distribution, and crystallinity. Moreover, the use of a poor solvent in the slurry reactive crystallization enabled the recycling of the mother liquor without any significant loss in yield and crystallinity up to three cycles.
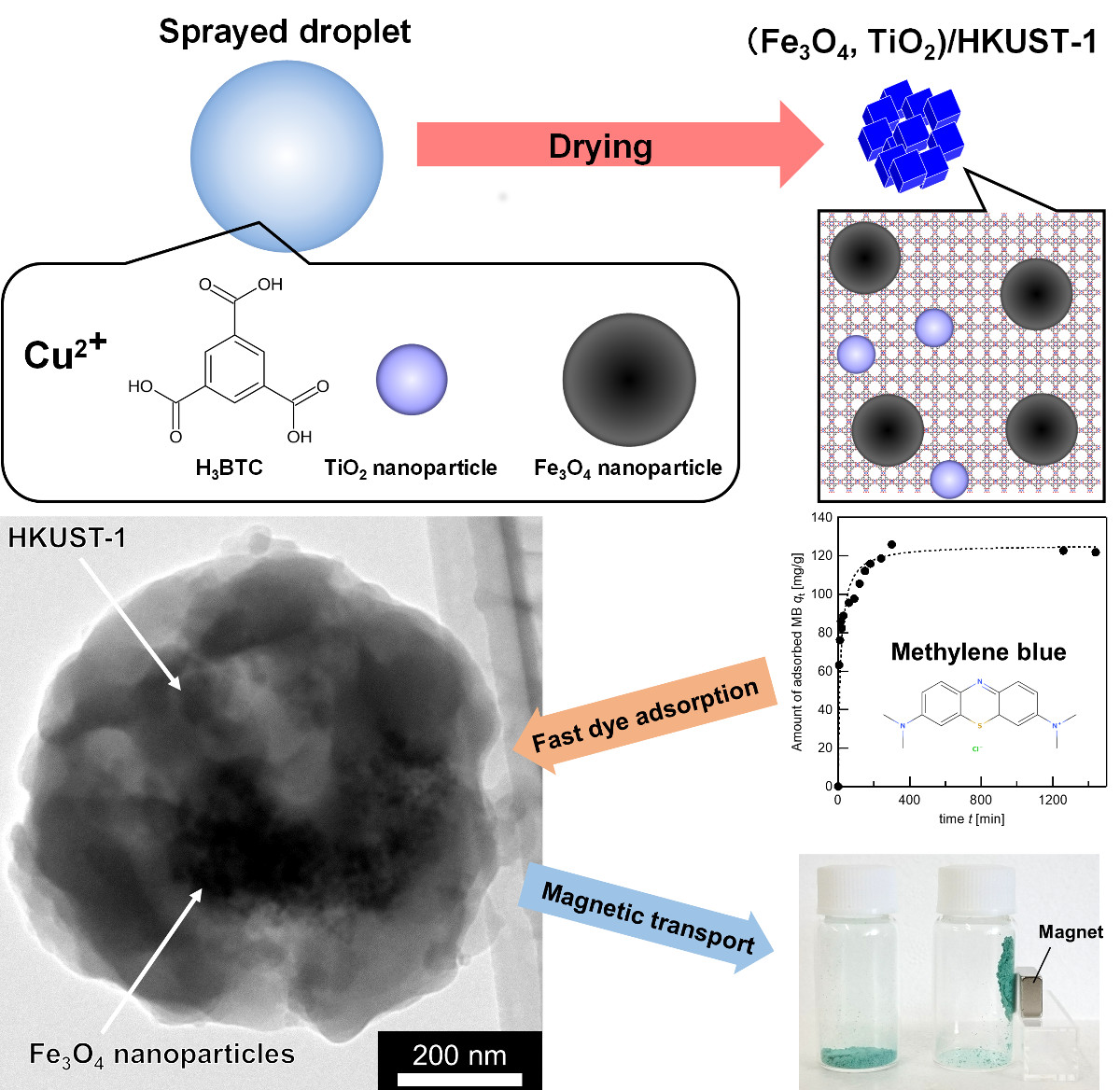
Metal–organic frameworks (MOFs) incorporating other functional nanoparticles have attracted research interest due to the synergistic functionality when combining these components. In this study, we demonstrated the fabrication of MOF composites incorporating one or two types of nanoparticles by a spray-assisted synthetic process. This process enables to synthesize MOF nanoparticle agglomerates in a short time (less than 10 ms) by self-assembly of metal ions and organic ligands during solvent evaporation from sprayed droplets of the MOF mother solution. We fabricated HKUST-1 (Cu3(BTC)2; BTC3- = 1,3,5-benzenetricarboxylate) and incorporated either Fe3O4 or TiO2 nanoparticles or both.
Nanocomposites of HKUST-1 with incorporated nanoparticles can be obtained after spray-drying of the mother solution. The amount of nanoparticles incorporated can be controlled by simply changing the concentration in the spraying solution. Further, by adding both Fe3O4 and TiO2 nanoparticles to the solution, they can both be incorporated into the same HKUST-1 crystals. HKUST-1 incorporating Fe3O4 nanoparticles have superparamagnetic properties due to the incorporated magnetic Fe3O4 nanoparticles. The nanocomposites have a high surface area (>1,200 m2/g), giving very high adsorption capacities for methylene blue (>700 mg/g). Furthermore, all HKUST-1 nanocomposites exhibit good stability for reuse as an MB adsorbent.
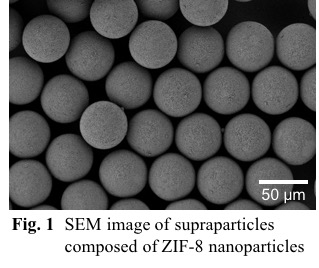
Metal-organic frameworks (MOFs), which consist of metal ions and organic ligands, possess high porosity and large surface area. An interesting feature of MOF is that particle downsizing from micron to nanoscale changes its adsorption characteristics. For application purposes, those nanosized particles have to be shaped into millimeter-sized beads or pellets. However, mass transfer is a typical problem especially when nanoparticles form aggregates with randomly-packed structures. It is thus necessary to establish a process to assemble MOF particles into ordered structures, or “supraparticles”, in which gaps between constituent particles serve as mesopores to improve mass transfer.
Here we focus on zeolitic imidazolate framework-8 (ZIF-8), which is a subclass of MOFs, as a building unit. We synthesized ZIF-8 particles with the size of ca. 200 nm using a central collision type microreactor and prepared ZIF-8 suspension droplets by mixing a suspension with an oil phase in a microfluidic device, followed by the drying of droplets to form ZIF-8 supraparticles. As shown in Fig. 1, monodisperse supraparticles successfully formed and the supraparticle size can be controlled by the ZIF-8 particle concentration and the microchannel width. Adsorption isotherms of N2 in the supraparticles showed a hysteresis between adsorption and desorption branches possibly derived from mesopores. Importantly, no hysteresis loop was observed in ZIF-8 powders, which indicates that the supraparticles have hierarchical structures with the intercrystalline mesopores. Furthermore, BET surface area of obtained supraparticles is independent of supraparticle size, which indicates the successful fabrication of ordered structures with interconnected pores via a bottom-up self-assembly. The pore size distribution and pore volume are possibly determined by the arrangement and monodispersity of ZIF-8 particles. Detailed investigation on the effects of the supraparticle morphology on mass transfer is now under progress.
Assembling of small particles onto larger particles is a useful technique toward various microstructural material design. Although many colloidal hetero-aggregation systems have been reported, most systems suffer from serious aggregation and solidification at high solid concentrations (c.a. > 15 vol%) which was induced by very strong attractive interaction between small and large particles. Herein, we successfully prepared a flowable 50 vol% silica/silica toluene suspensions comprising from micron-sized silica particles covered with submicron-sized silica particles based on surface design. The micron-sized particles stabilized with polyacrylic acid partially complexed with oleylamine (PAA-OAm) and submicron-sized particles stabilized with polyethyleneimiene partially complexed with oleic acid (PEI-OA) were mixed in toluene. The cross-sectional FE-SEM image of the in-situ solidified suspensions showed that sub-micron particles were attached on micron particles. It is expected that the hetero-assembly was driven by the acid-base interaction between free carboxyl/amino groups of PAA/PEI on two kinds of particles. It was also found that decreasing the OA content of PEI-OA fixed on submicron-sized particles resulted in gelation of concentrated suspensions under low coverage of submicron-sized particles on micron-sized particles. Contrary, when the OA content increased, the suspension showed flowable state with small apparent viscosity and little hysteresis in the flow curves. These results support that increasing oleyl chains on the hetero-assembled particles suppress aggregation in concentrated suspensions owing to steric and osmotic repulsion between the hetero-assembled particles in toluene. In summary, colloidal hetero-assembly of binary system in flowable 50 vol% suspensions were achieved by controlling the acid-base interaction between small/large particles and stabilization of resulting hetero-assembled particles by repulsive interaction.
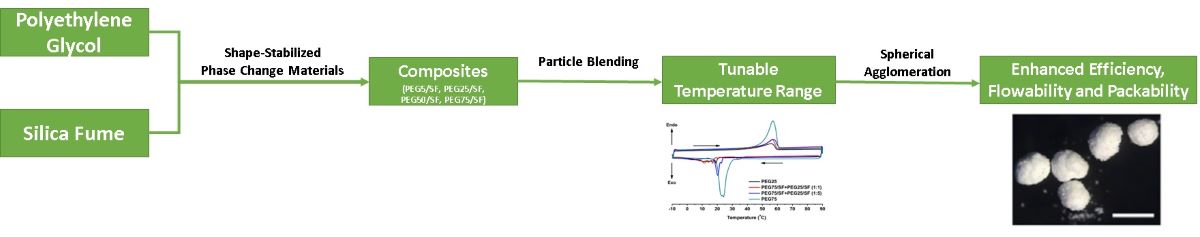
The aims of this study were to impregnate polyethylene glycol (PEG) 4000 in low-cost silica fume (SF) to form phase change material (PCM) composites with cementitious value, and to provide a quick-fix design for PCM (1) with tailor-made thermal properties and behaviors by particle blending of two types of polyethylene (PEG)/silica fume (SF) composites having different PEG wt% loading, and (2) with enhanced physical properties by turning the powdery PEG/SF composites into round granules through spherical agglomeration. The simple composite blending method was used to broaden and tune the application temperatures in response to variable conditions and environments without the need of searching for new materials to mitigate global warming. Spherical agglomerates of PEG/SF composite exhibited a good homogeneity in thermal properties and low Carr's indices indicating of excellent flowability, packability and compatibility, and offering an enhanced contact area for heat transfer and uniform mixing with other building materials. Noticeably, the agglomerates displayed higher heat capacity values of solid phase, Cps, and liquid phase, Cpl, than those of the composite determined by temperature-history method. The thermal stability of PEG75/SF composites was also attested by the small enthalpy loss, and the highly reproducible melting and solidification behaviors after more than 100 temperature cycles
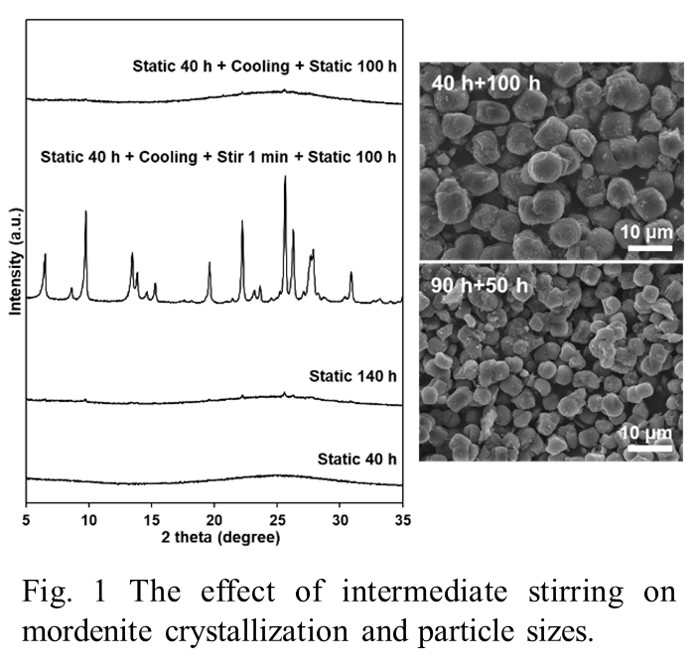
Crystallization of zeolites in hydrogel system usually requires long synthesis time, leading to products in the form of polycrystalline aggregates with broad particle size distribution. In the present study, we demonstrated that the crystallization of mordenite in an organic-free dense hydrogel system (0.275 Na2O: 0.025 Al2O3: 1 SiO2: 25 H2O) can be induced by an intermediate stirring method—quenching and opening the reactor in the middle of synthesis to stir the substances for 1 minute. Moreover, the particle size and size distribution of the crystalline products was found to vary with the intermediate stirring timing. These results suggest the crystallization in this system may follow the autocatalytic nucleation theory, and the number of nuclei changing along with synthesis time can also be estimated. Our observations on the gel particles show that worm-like particles (WLPs) formed large spherical condensed aggregates (CAs) with ca. 10 μm diameter through aggregation and coalescence. The formation of spherical CAs can take place at the very beginning of the synthesis (5 h) under static condition. Based on the results, we speculated that mordenite nuclei can form inside WLPs, and the formation of large CAs as well as the increasing packing density of the dense hydrogel phase further reduce the chance for these nuclei to contact with liquid phase and grow.
Modification of zeolites by the incorporation of divalent Zn in the silicate framework is attracting extensive attention because it results in higher anionic charge density and creates new active acid sites. However, it is a great challenge to incorporate Zn into the framework of highly siliceous MFI-type zeolites without Al. In addition, Zn tends to precipitate under basic synthetic conditions. In this study, ball milling, a mechanochemical method, was employed to prepare Si–Zn oxide composites from mixtures of fumed silica and ZnO as the starting materials for the zeolite synthesis. The dispersion of Zn on an atomic level inside the silica matrix was realized by mechanical forces. Then, the Si–Zn oxide composites were subjected to hydrothermal treatment in the presence of an additional Si source and a structure-directing agent to synthesize MFI-type zincosilicate zeolites with high Zn contents (Si/Zn=36.9–13.4) but negligible Al impurity (Si/Al>900). The successful incorporation of Zn into the zeolite framework was confirmed by several characterization techniques, including DR UV-Vis, FT-IR, XPS and solid-state 29Si MAS NMR. Co2+ ion exchange experiments showed the superior selectivity and capacity of MFI-type zincosilicate zeolites for divalent cations as compared with those of aluminosilicate analogs. The mechanochemically treated Si–Zn oxide composite plays a crucial role in the synthesis of MFI-type zincosilicate zeolites with high Zn contents and minor extra-framework Zn species.
Zeolites are crystalline microporous aluminosilicates that are widely used as adsorbents, ion-exchangers, and catalysts owing to their large micropore volumes with well-defined pore structures, ion-exchange abilities, and solid acidities. It is well known that the zeolites degrade through several mechanisms, such as interactions with metal cations and steam. High-temperature (> 800 °C) steaming conditions, to which the zeolites are exposed in regenerators for removing cokes in fluidic catalytic cracking (FCC) processes, have been a hindrance for the degradation of FCC catalysts, and over 400,000 t/yr of catalysts has been discarded. More recently, zeolites also have been utilized in ammonia selective catalytic reduction (NH3-SCR) of NOx under high temperature steaming conditions. Therefore, hydrothermal stability is one of the most important factors for the application of zeolites. Here, we provide a simple method to improve hydrothermal stability of zeolites. Several types of commercially available zeolites were treated and showed excellent stability under very high temperature (up to 1150 °C) steaming conditions. The target temperature was achieved after 75 min of ramping and was kept for 3 h for testing using a house-made durability test apparatus, followed by cooling to room temperature for over 3 h. Water was introduced through a syringe pump and mixed with dry air from a gas cylinder to achieve 10 vol.% steam at atmospheric pressure. Samples before and after hydrothermal treatment were characterized by X-ray diffraction, scanning electron microscopy, nitrogen adsorption/desorption, and so on.
Developing efficient and low-cost routes for the synthesis of zeolites is of high significance, considering the ever-emerging applications and increasing consumption of these materials. Our group has established the ultrafast synthesis of zeolites and demonstrated that the continuous flow synthesis could offers a number of advantages for zeolite production. Sharp viscosity increase taking place in the zeolite formation, however, renders a continuous flow synthesis very challenging. We herein show that the emulsion system creates a unique opportunity to address the viscosity issue, allowing the zeolite products to smoothly flow out of the reactor in forms of a suspension. The emulsion-based, continuous flow syntheses of three important zeolites – ERI, *BEA and CHA were achieved, proving this method to be a general approach.
Recently, co-authors have made it possible to densify B4C (Boron carbide) even at low pressure firing. It is expected that basic research and practical application research on B4C based ceramics will be promoted.
Evaluation on sliding characteristics was carried out mainly using B4C - SiC (Silicon carbide) ceramics prepared by low pressure sintering as test specimens. As a result, we've got some interesting findings. First, According to the observation using TEM(Transmission Electron Microscope), it was confirmed that at the interface of B4C and SiC phases voids were not observed or if any few , indicating that both were firmly bonded. Secondly, in polishing and subsequent sliding tests, it was found that a step having a height of several tens of nanometers was formed on the surface due to slight difference in wear rate between B4C and SiC. Since the step spreads over the entire sliding surface, it has a structure like a relief, we called this a relief structure.
Generally, it is said that the coefficient of friction is governed by the true contact area and the shear force at the interface. Specifically, the friction coefficient is influenced not only by control factors such as load and speed, but also by complicated phenomena such as the reaction occurring at the interface with the mating material and the lubricant, as well as the chemical and physical state of the solid surface. However, there are very few research reports that systematically examined them.
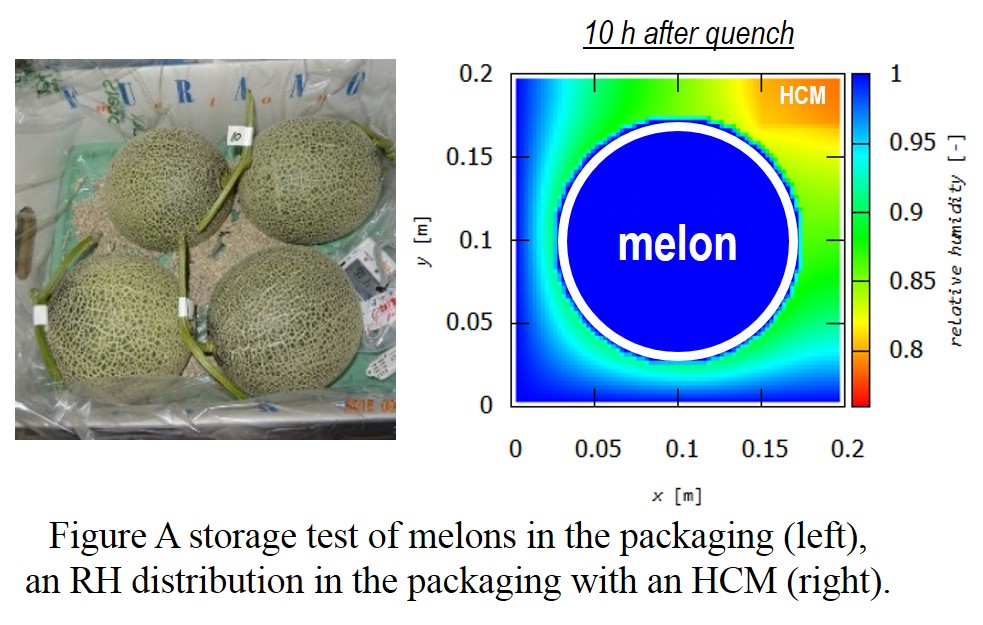
Modified-atmosphere packaging is a readily available technique to extend storage life of fruits. This is achieved just by keeping fruits in packaging bags made from high gas barrier films. Meanwhile, this is often not effective for fruits with high rates of transpiration (evaporation of water) such as melons. The relative humidity in the packaging (RH) reaches 100% due to the transpiration, resulting in condensation followed by mold growth on their surfaces. Recently we succeeded in decreasing the RH to 50% using humidity control materials (HCMs). Although condensation can be prevented, the RH is so low that melons lost too much water and were spoiled. Since the RH depends on multiple factors of HCMs (property, utilization), the RH ideal for melons (90%) is expected to be achieved through their optimization. However, this has been time-consuming because it requires multiple storage tests for data acquisition.
Simulation-assisted approaches to design HCMs have been studied in architectural engineering. Humidity distribution in rooms can be predicted through solving the modified equations for heat and mass transfer. The aforementioned factors can readily be optimized to achieve the target RH with a small number of experiments. In this work, we established a time-effective method to design HCMs for the packaging through this approach.
Three porous carbons with various water adsorption isotherms were investigated as HCMs. RH distribution of the packaging containing one of the HCMs was calculated when this was quenched (25 °C to 3 °C). The ideal environment, where melon was surrounded by atmosphere with 90RH%, was observed when the HCM with type IV isotherm (5 g) was located at the center of the packaging. This result corresponds with that of the actual experiment, where the RH was 92%, indicating reliability of our approach.
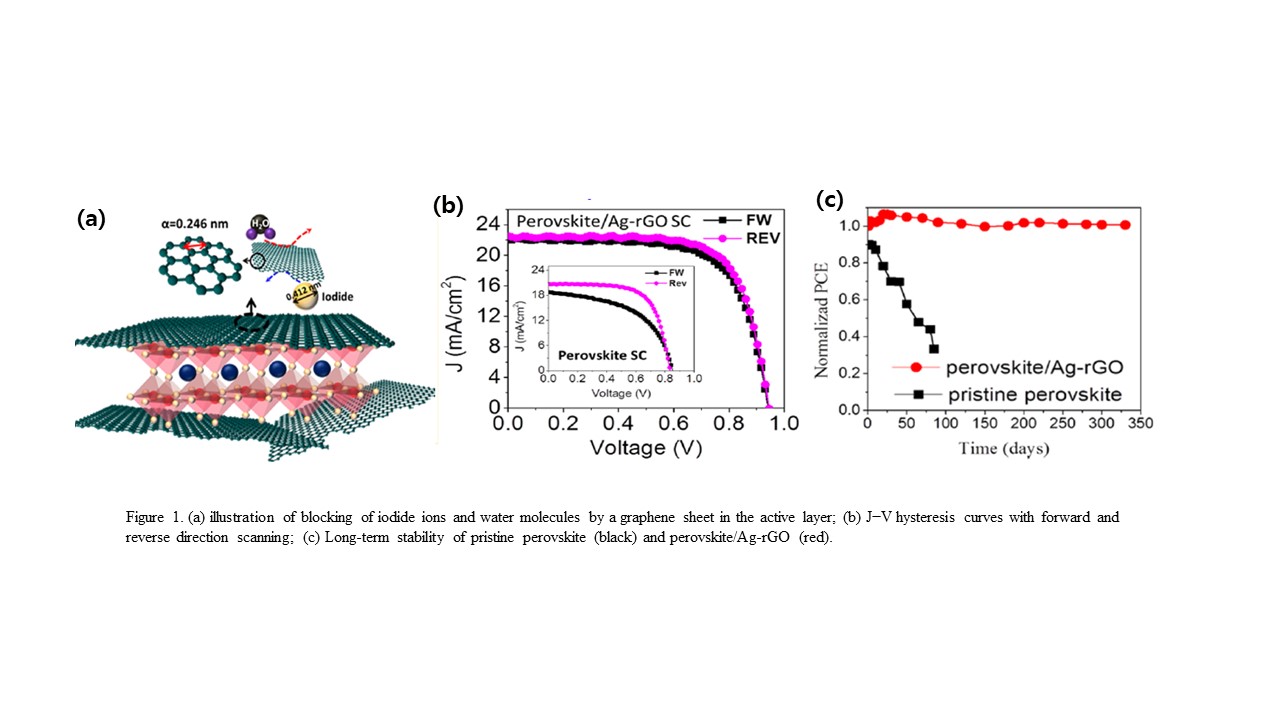
Poor stability and reproducibility of perovskite solar cells (PSCs) have prevented the devices from practical applications of industrial standard photovoltaic modules that can withstand sustained long-term operation under outdoor conditions. Furthermore, most of presented high efficiency PSCs utilize halogenated antisolvents such as toluene and chlorobenzene to assist perovskite crystal growth with large grain-size, excellent coverage and high uniformity, but they are highly toxic and detrimental to environment. To solve such issues, we developed simple methods for the production of functional nanocomposites such as Ag-rGO, perovskite-NiO, perovskite/Ag-rGO, NiO-carbon-graphite, etc and utilized them for the fabrication of ambient-air and antisolvent-free processed stable, hysteresis-free PSCs. In addition, to solve interfacial degradation whcih affects device performance Al2O3/NiO layers were utilized for interface engineering between elecrtron transport layer and active layer. By introducing the functional composites into PSCs with interface engineering, we obtained high efficiency of > 18 % and fill factor of > 78 % with excellent reproducibility. More importantly, the devices without encapsulation showed significant enhancement in long-term stability and the photovoltaic parameters sustained its stability over 330 days with retaining over 95 % of its original values under ambient condictions (see Fig. 1).
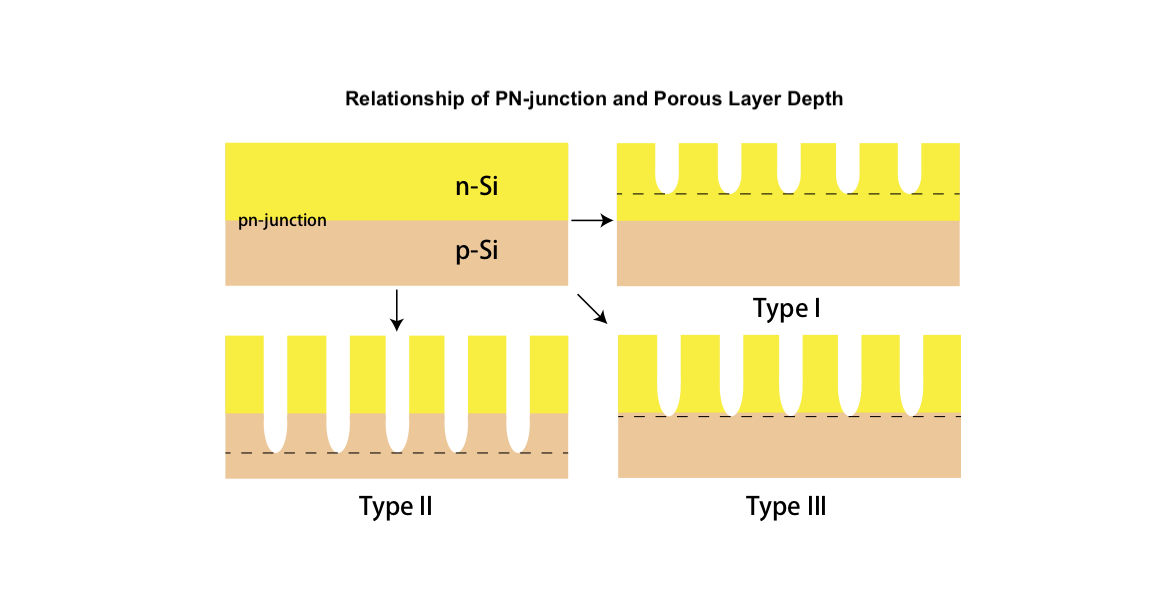
Silicon tandem solar cell is one specific type of multi-junction solar cell, which has the potential to exceed the efficiency limit of single-junction silicon solar cell and eliminates the lattice mismatch found in hetero-junction type. The porous silicon (PSi) is a potential candidate as a top cell due to its wider bandgap, which is originated from quantum size effect, for higher Voc and low reflectance leading to enhancement of light absorption.
However, the surface area and defects are highly increased compared to crystalline Si (c-Si). Therefore, suitable passivation is necessary to reduce surface recombination. The representative method to passivate silicon solar cell is atomic layer deposition (ALD). However, the cost of ALD is too high to be widely used. In this research, a low-cost electrochemical passivation technique was developed for achieving uniform passivated layer on PSi.
In this study, the PSi with porous thickness of 200-250 nm was fabricated through anodization process in HF solution. The photoluminescence (PL) was measured to observe the bandgap of P-type PSi. Then it was passivated by either electrochemical passivation in HCl or ALD (Al2O3) to compare the carrier lifetime and nanostructure. Finally, solar cell performances of electrochemical passivated samples were measured.
The result of solar cell performance shows the improvement of Jsc and efficiency of PSi solar cell compared to c-Si; the 30 seconds electrochemical passivated PSi yielded 29.8 mA/cm2 and 10.7%, and the c-Si yielded 23.4 mA/cm2 and 8.2%, while both Voc are not much different. However, the PL data shows that the bandgap is increased from 1.1 eV (c-Si) to 1.9 eV. Therefore, in order to improve Voc, the surface passivation and configuration of front electrode are key factors to reduce recombination and increase lifetime. Also, the relationship of porous structure and pn-junction depths is important.
To reduce the cost of Monocrystalline Si solar cell and the amount of Si by thinning the wafer with keeping high efficiency is promising approach. A major method to obtain monocrystalline thin film Si is the epitaxial growth by Chemical vapor deposition(CVD) and layer transfer process. Si is epitaxially deposited on a seed layer such as double layer porous Si(DLPS) with top seed layer and bottom sacrifice layer. The rate and the yield of epitaxy by CVD and the defect level caused by the roughness of the seed layer were critical issues. We developed the method to fabricate high quality film by smoothing the surface of DLPS within nanometer level with zone heating recrystallization(ZHR)[1] that selectively heat the surface by scanning of lamp heater. Also we developed rapid vapor deposition(RVD)[2] to get the high deposition rate over 10 μm/min by heating Si source over 2000 °C. By applying these methods, we obtained the monocrystalline Si in 3 min and the critical effect of lowering the roughness of DLPS in less than 0.3 nm is confirmed[3][4].
In this work, to fabricate high quality solar cell, we (1)investigated the key factors to change the roughness of DLPS and (2)evaluated the semiconductor properties of monocrystalline thin film Si. The surface smoothing of DLPS were inhibited by oxidation and by changing the atmosphere to H2/N2 promoted the decrease of the surface roughness. After modifying the method to fabricate over 35 μm thin film Si by RVD in one time, the thin film Si with passivation Al2O3 film on both sides by ALD showed the sufficient life time as monocrystalline Si film by the life time measurement.
[1]A.Lukianov et al.,Appl.Phys.Lett 108(213904)1-4(2016)
[2]Y.Yamasaki et al.,CrystEngComm 18-3404-3410(2016)
[3]C.Takazawa et al.,ECS.Transaction 75(31)-11-23(2016)
[4]K.Hasegawa et al.,CrystEngComm.in press20(2018)1774-1778
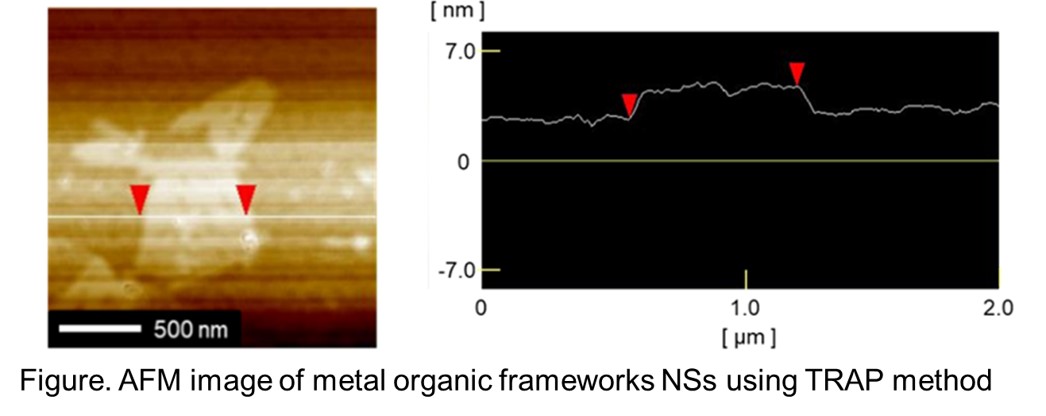
Recently, nanosheets (NSs) have been attracted attention owing to their various unique properties. The synthetic methods of NSs are divided into two categories: top-down exfoliation methods and bottom-up methods. However, both methods have some problems. As one of the former methods, NSs can be obtained from the exfoliation of layered materials; the thickness of the obtained NSs are a few nm, but this method can be applied only to layered materials. As one of the latter methods, NSs can be obtained from the chemical vapor deposition on a smooth substrate; this method can synthesize NSs of various chemical compounds, but the NSs are not so thin. It is required to develop a versatile method to synthesize thin NSs.
We focused on the two-dimensionally confined space in a bilayer of hyperswollen lyotropic lamellar (HL) phases of amphiphile solutions as a sandwich-like reaction fields (SRFs) for the synthesis of thin NSs; HL phases are classified as one of lyotropic liquid crystalline phases. The SRFs with several nm thickness are separated from each other by about several hundred nm. In fact, NSs were successfully synthesized in the thin hydrophobic SRFs inside the bilayers of aqueous amphiphilic solutions. The thickness and horizontal width of the NSs are a few nm and several hundred nm, respectively. The thickness of the obtained NSs is almost constant. We have developed such a synthesis method and named it "two-dimensional reactors in amphiphilic phases" (TRAP) method.
Here, we present the NSs synthesis using TRAP method. We confirmed that TRAP method can be apply to the hydrophobic materials made from both hydrophilic and hydrophobic ingredients such as metal organic frameworks (Figure). Furthermore, we found that it is possible to synthesize NSs in the thin hydrophilic part of the SRFs.
Surface modification plays an important role in determining interfacial adhesion between substrate and electroless-deposited (ELD) metal layer. In this presentation, we will share our experience regarding to control of surface modification on either organic or inorganic substrate and its relation to the adhesion strength of subsequent ELD metal layers. In particular, case studies of adhesive ELD on silicon wafer, glass sheet, Ajinomoto build-up film and photoimageable dielectric will be reviewed. It is concluded that with sufficient control of surface modification, the adhesion strength of ELD layer can be largely enhanced due to formation of either covalent bond or donor-acceptor-like interaction.
To meet the increasing demands in portable microelectronics, thin film pattern formation on plastic substrate surfaces has recently received great interests. Metal conductive interconnects, as the essential components in any electronic devices, built on flexible or even stretchable plastic substrates are necessary for the realization of portable or wearable devices. However, due to the incompatibility between metal and plastic molecules, it is challenging to resolve the surface bonding at the metal/plastic interfaces. Moreover, in order to manufacture conductive interconnects with fast speed and low costs, printing procedures with high reliability are also needed. To address these problems, we developed several ink formulation and pre-treatment methods to improve the adhesion between metal coatings and the plastic substrates. In addition, related process parameter adjustments in printing procedures are also provided in this talk to give general guidelines for reliable pattern formation on either 2D or 3D microstructures. Same approach can also extend to fabricate various sensors with great performance. Several examples, such as temperature and humidity sensors, will be given in this talk to show the potential aspects of printing technology in microelectronics.
Coating layers of nanoparticles exhibit a mechanically fragile property upon semidrying. In contrast to fluidic inks where precise shaping is difficult owning to surface tension, viscosity and wetting, semidried layers can selectively be transferred onto a printing plate and thereby enabling patterning with micrometer-scale features. Reverse offset printing and adhesion contrast planography use this patterning principle and allow to generate high-resolution patterns of various functional nanomaterials such as gold, silver, copper, ITO and others. Here, we used a simple discrete element method to simulate the patterning step so as to reveal its patterning mechanism at the constitutive particle level. The modelling clarified the rule of cohesive force between particles and their interfacial adhesion against a substrate in the pattern formation. In the presentation, the criteria for successful patterning is discussed together with the comparison with experimental results.
Irradiation of UV light onto a photo-reactive solvent/monomer/initiator ternary solution film triggers polymerization and a spontaneous phase separation between polymer-rich and solvent-rich phases. Subsequently, the volume occupied by the solvent was replaced by air when the solvent-rich phases were dried, resulting in a certain film porosity. This process does not require multi-step rinsing procedures to remove either phase in a selective solvent, providing a promising method for roll-to-roll high-speed fabrications of porous polymer films. Despite extensive previous studies on solvent-free photo-reactive systems [1], no quantitative data is currently available on the evolutions of local composition distributions in solvent-based reactive coatings. In this study, we developed a confocal Raman spectroscopy technique to directly determine local polymer concentrations across the film. We used polyester acrylate (M9050, Toagosei Mw = 1000~1500) as the photo-reactive monomer, and 1, 3 α-alkyl amino phenone (Irugacure 379 EG, BASF) as the photo-initiator. Methyl isobutyl ketone (MIBK, Wako) was chosen as the solvent, which is immiscible with the reactant of the polymerization reaction. The solution was dropped on a glass plate and dried at 30 °C for 1 min prior to the irradiation of light of wavelength 365±5 nm. To avoid beam scattering at phase-separating interface in the Rama spectroscopy, the solvent was replaced by a reactive monomer to match the local reflective indices in the cured film [2]. The results indicated that the polymer concentration profiles near the top surface were tunable by solvent drying condition, whereas those near the bottom were almost independent on the condition.
References
[1] H. Nakanishi, T Norisuke, Q.T.Miyata, J. Phys Chem Lett. 4 (2013) 3978-3982.
[2] H.Yoshihara, M.Yamamura, Journal of Coating Technology Research, in press
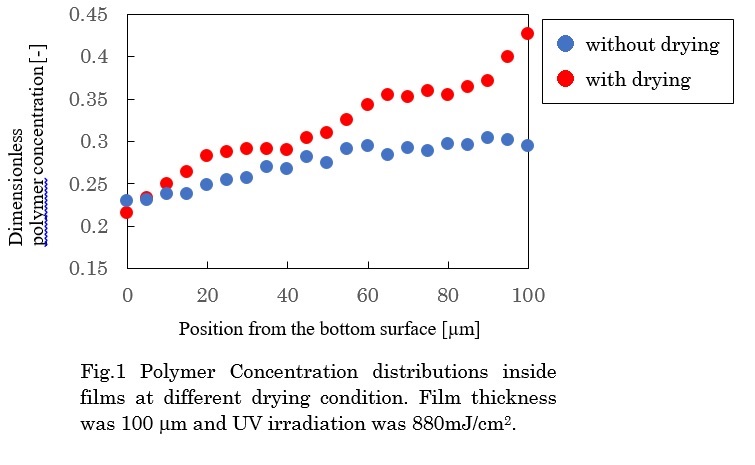
The term “back diffusion” is sometimes used to describe local diffusion of solvent in the opposite direction with respect to its evaporation. Such a diffusion emerges when we stratify a solvent-rich liquid layer on top of a solvent-lean layer and simultaneously dry them. The back diffusion across the two-layer interface alters not only drying rates and residual amounts of the solvent but also microstructure developments in drying coatings. Despite practical utilities to create stratified thin liquid layers in printable electronics and many other functional coatings, the role of back diffusion in drying kinetics of two-layer coating is still poorly understood. In this study, we present drying kinetics of solvent-base two-layer polymeric solution films coated simultaneously or in tandem. The layers contain tetrahydrofuran (THF) as solvent, and polystyrene (PS) or cellulose acetate butyrate (CAB) as a polymeric binder. The blade coating method was used to stratify two solutions layers of different concentrations. The decrease in mass of the wet-on-wet coating was measured by with an electronic balance and recorded to calculate the solvent drying rate. The results showed that, in the case of stratified PS/THF binary coatings with the same solvent concentration of the top layer, the solvent drying rate monotonically decreased with decreasing the bottom-to-top solvent mass ratio, indicating a retarded drying due to a back diffusion of the solvent. However, the opposite trend was observed in drying of a PS/THF layer on top of a CAB/THF layer; the drying rate increased with increasing the bottom-to-top solvent mass ratio even when the solvent concentrations in the top layers were the same. This puzzling drying behavior was qualitatively explained by considering spontaneous phase separation between immiscible PS and CAB solutions and development of interconnected domain interfaces that act as a solvent diffusion pathway across the thickness.
Continuous liquid coating is used to produce various films or sheet-like products, such as adhesive tapes, optical films, display panels, etc. It is also a strong candidate for nanoparticle assembly film production. Slot coating is a popular high-precision coating methods, because the film thickness is directly controlled by the flow rate and substrate speed rather than coating liquid properties. However, many high-performance multi-functional films for electronics and optical devices require nano- or micro-sized particles inside a coating liquid. As the environment requirements push to use less solvent, the coating liquid becomes dense suspension. Consequently, the liquid shows complex non-Newtonian behavior including viscoplasticity, i.e. exhibiting little or no deformation up to a certain level of stress. This study presents fundamental aspects of film formation flows used in coating processes and the impact of rheological properties on the operating limits of a coating method in both experiments and computations.
Slot coating is a pre-metered coating method widely used to produce a variety of thin films such as magnetic tapes, battery electrodes, optical films, and so on. In industrial applications, a high-speed coating is desired in order to lower the manufacturing cost. However, it often requires the usage of diluted low-viscosity liquids to ensure the stability of a coating flow. Such dilution of a coating liquid could result in an increase of the drying load more than the dryer's capacity, the cost increase for solvents treatment, or the insufficient quality of a coated product caused by the severe drying condition. The most cost-effective way is to achieve a high-speed coating with a concentrated high-viscosity liquid.
For this purpose, we studied the simultaneous dual-layer slot coating, having a high-viscosity top layer and a thin low-viscosity bottom layer. We found that the coating window could be greatly expanded compared to the single layer coating of the top layer. Visualization of a coating bead through a transparent glass backing roll revealed that the location and the shape of the layer-interface was the key factor to determine the stability of dual-layer coating. Furthermore, we found three flow regimes regarding the interface shape, and examined the effect of coating parameters such as film thickness ratio and viscosity ratio to those flow regimes. Finally, we will discuss the mechanisms to explain each of the flow regimes.
Polymer electrolyte fuel cells (PEFCs) are one of the most promising power sources for vehicles. However, there are several hurdles preventing the spread of PEFCs, including reducing cost, improving performance and durability. Key measures to help resolve these issues include controlling the structure of the electrode catalyst layers. Catalyst layers are porous layers prepared from the catalyst ink consisting of ionomer solutions, carbon black supporting Pt nanoparticles, and dispersing solvents. Drying of a thin film from the catalyst ink on a substrate often causes the formation of cracks. Since cracks in catalyst layers of a fuel cell affect performance and durability, it is important to obtain the factors that control the crack formation.
Herein, this study aimed to identify the factors affecting crack behavior in terms of the dispersion state in catalyst ink. Critical crack thickness (CCT) is examined as a parameter for cracking characteristics by changing the formation of Pt/carbon by adding two types of solvents with different hydrophobicity.
The CCT was found to be four times greater for catalyst layers obtained from catalyst inks that are well dispersed by ionomer adsorption than for catalyst layers obtained from catalyst inks with a network of structured agglomerates. This suggests that the CCT decreases due to the poor affinity between the ionomer and Pt/carbon.
These results suggest that well-dispersed catalyst inks produce homogeneous distribution of Pt/carbon and ionomer in catalyst layers with high fracture toughness, while catalyst inks with network of structured agglomerates produce dense aggregates with small primary pores that generate high drying stress, and self-organizing free ionomer that cause stress concentration in catalyst layers with a high risk of cracking.
In conclusion, the present study has demonstrated that crack behavior can be controlled by ionomer adsorption on the Pt/carbon in catalyst ink and the resulting dispersion state.
Self-healing polymer coatings is applied on the surface of metal to prevent corrosion. The addition of cellulose nanofibers as pathway of healing agents into polymer coating could be improved the self-healing properties in our previous research. In the present research, polymer coatings blended with nanofibers and two types of inorganic healing agents, which are zinc sulfate and zinc phosphate, was applied on the surface of carbon steel. Release behavior of two types of inorganic healing agents from polymer was measured using mass change. The electrochemical impedance of scratched specimen was measured in corrosive solution. As a result, release rate of two types of inorganic healing agents from polymer was different. The release of zinc sulfate was fast, and the release of zinc phosphate was slow but long. The release amount of healing agents was increased by addition of cellulose nanofibers. The polymer coating added cellulose nanofibers and the two types of healing agents showed excellent self-healing performance for corrosion inhibition of carbon steel. This was caused by the fast and long release due to two types of inorganic healing agents.
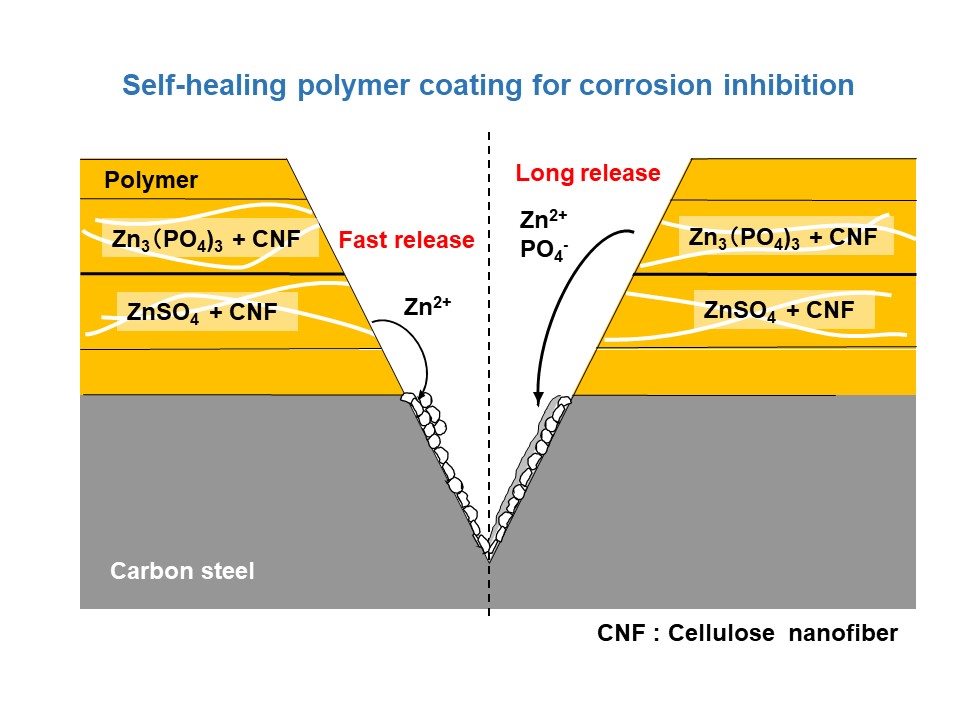
An aqueous dispersion of latex particles produces uniform resin layer. The addition of silicate and coupling agent can improve the toughness. In this study, the effects of those additives on the film formation process was studied. A sample coated glass substrate was placed in a container heated to 100 °C. A confocal displacement sensor placed above was used to measure film thickness change. Additionally, an expanded laser beam was irradiated on the surface. The reflected lights were interfered to produce speckle pattern containing bright and dark spots. The variation of the speckle pattern was recorder using an objective lens and CMOS camera. From the inspection region (20 x 100 pix) of the speckle pattern, 100 lines containing 20 pixels were vertically aligned to produce long lines of 2000 pixels. The long pixel lines of more than 9000 frames, being corresponding to 100sec, were then horizontally aligned to produce area time history speckle pattern (A-THSP). Various types of stripes were found in A-THSP. An oblique striped pattern frequently observed indicates the translation of speckle pattern in the longitudinal direction of the inspection region. This pattern suggests the formation of convective flow. After that, highly active random speckle pattern was followed by vertical or horizontal stripe pattern. Vertical stripe suggests uniform and periodic change of speckle pattern. When film formation was terminated, static speckle pattern attributes to constant brightness and horizontal stripes. No significant change in film thickness was observed in the convective flow regime, while thickness was drastically decreased in the random pattern regime. The transition from vertical to horizontal stripes can be detected only by A-THSP. In this stage, an interval between the bands became larger with time, suggesting the inhibition of activity of film and the termination of film formation process.
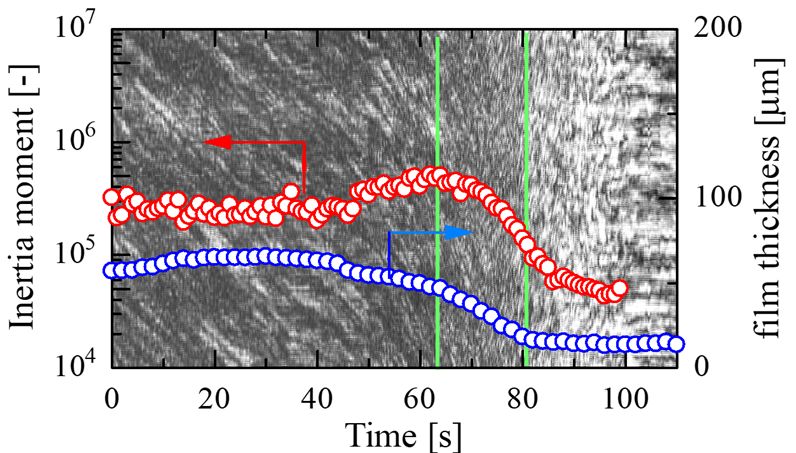
Colloidal suspensions are coated on substrates and upon drying produce various functional materials, whose quality is determined by the microstructure composed of colloidal particles. The structure formation of the particles during drying is governed by the competition between the Brownian motion of the particles and the recession of the free surface according to liquid evaporation. The Brownian motion tends to distribute the particles uniformly, while the receding free surface drives the formation of concentrated particle layers. The drying rate falls with a decrease in the liquid permeability of the growing concentrated layers. The structure formation of the particles is reflected in the drying characteristics, of which the control is required for the improvement of material quality as well as the reduction of drying time. We then construct a model to investigate how drying conditions and suspension properties affect the drying characteristics.
We have developed a simulator of colloidal suspensions: Structure of NAno Particles (SNAP). In the present study, we extend a simple mathematical model adopted in SNAP-L, where the Brownian motion of particles is described by a stochastic differential equation called the Langevin equation. We additionally construct a model to consider drying rate falling by estimating the permeability of formed concentrated layers. The drying characteristics calculated by our model is shown in Figure. Using the present model, we propose how to control the drying characteristics by initial drying rates and interactions between particles.
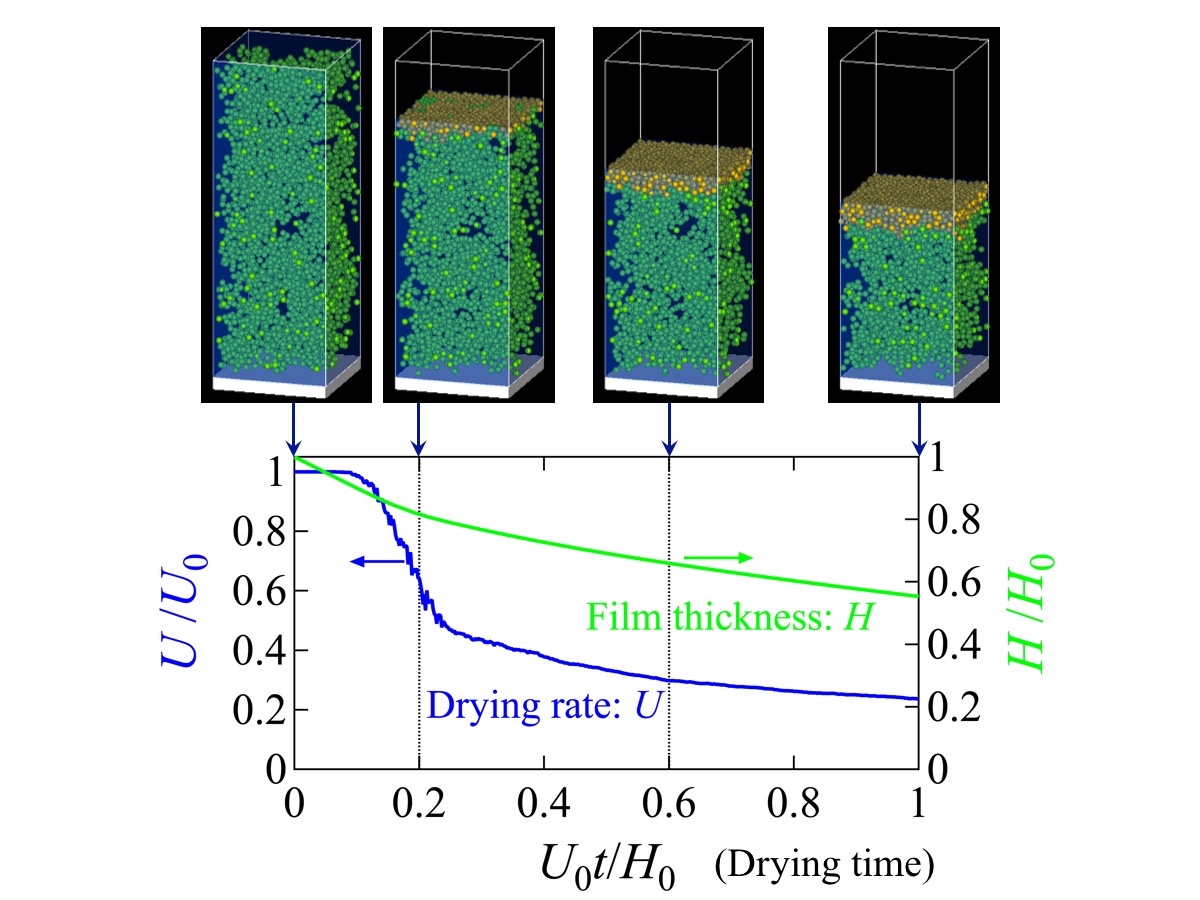
Self-assembly is an attractive technique to fabricate colloidal crystals, which are promising for various applications. However, few works focused on the formation process of colloidal crystals based on interaction forces acting on particles, which play a critical role in self-assembly. Here we produced colloidal crystals and controlled their size by adjusting the balance between the attractive force induced by the depletion effect and the repulsive force due to overlapping of the electric double layers of particles.
We used polyethylene oxide (PEO) as a depletant and sodium chloride (NaCl) as a salt and systematically investigated the effect of their concentrations. Figure 1a shows a typical image of depletion-induced colloidal crystals composed of 3 μm polystyrene particles under the condition of 0.4 g/L PEO and 10 mM NaCl. The decrease in the depletants resulted in a dispersion state (Fig. 1b), while the increase produced aggregates (Fig. 1c). Under a lower salt concentration condition, a larger amount of the depletants (i.e., the stronger attractive force) was necessary for the crystallization because of a longer Debye length.
Further, the depletants of 0.7 g/L without salts resulted in a formation of large crystals with an average size of 33 μm while the addition of 1 mM NaCl decreased the size to 15 μm. In addition, the crystal formation without salts was much slower than that with 1mM NaCl because of a higher energy barrier caused by a longer Debye length. These results suggest that a higher energy barrier produces a fewer number of "nuclei" of colloidal crystals, resulting in larger domains during assembly. Hence, we demonstrated that the formation process of crystals and their size can be controlled by adjusting the balance between repulsive and attractive force.
Figure 1. Images of (a) depletion-induced colloidal crystals, (b) dispersion state, and (c) aggregates
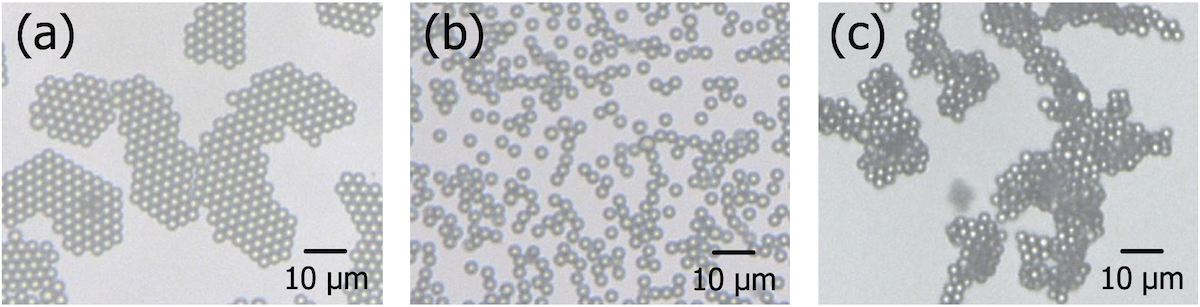
Drying of colloidal suspension is a common industrial process and widely used for manufacturing products, such as paints, ceramics and electrodes. During drying of a colloidal suspension, particles move towards the drying interface, and they form particulate films. Many papers have reported drying kinetics of colloidal suspensions and directional drying is frequently used in those studies. Suspension is introduced into a narrow space between two glass plates. Evaporation occurs from one open side, which induces flow to the drying interface. Because flow is confined in a narrow space, it has been widely accepted that only horizontal flow is induced by drying. However we have found that there is a complex circulation flow. We used a unidirectional drying cell (Hele-Shaw cell) to dry silica colloidal suspensions. Fluorescent particles were added to trace flow inside of the drying cells. A circulation flow appeared around the interface between the packed layer of particles and the suspension although the suspension was confined in a narrow space with a height of 100 μm. The width of circulation flow region was strongly dependent on the growth rate of packed film and the gap height of cells. The origin for the circulation flow and possible impacts of the flow on film formation processes are discussed.
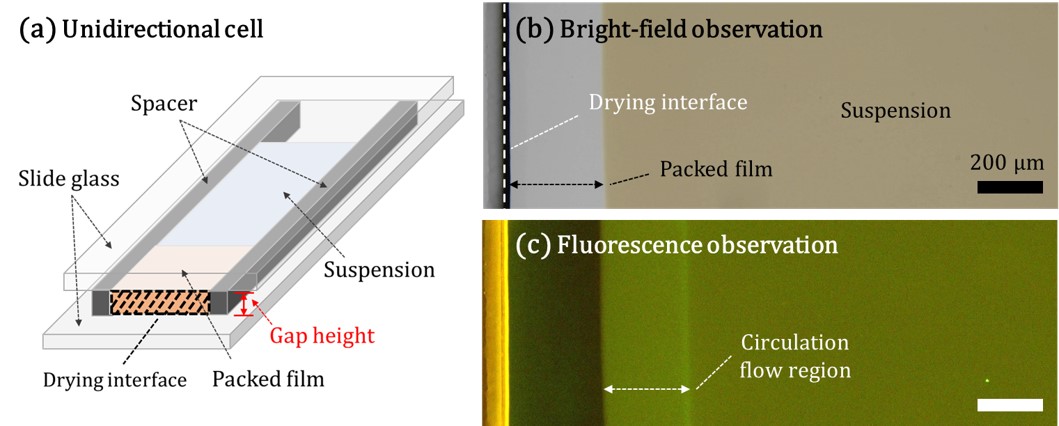
Some solid particles stabilize water-oil interface and particle-stabilized emulsion forms. We prepared Water-in-Oil (W/O) emulsions using hydrophobic silica particles as a stabilizer. Drying of droplets was observed. Due to evaporation of water, droplets shrunk. We found that the ways to droplets shrunk varied depending on the storage time (aging time) of droplets as shown in the Figure. Immediately after preparation, droplets shrunk isotropically during drying (Mode 1). After several-days aging, droplets shrunk but wrinkles formed on surface of the droplets (Mode 2). Further aging induced buckling of drying droplets (Mode 3). We revealed that those changes closely related to particles affinity to water. Particles were hydrophobic and they did not have good wettability to water in praparation process. However wettability of water increased during aging. Formation of wrinkles and buckling of droplets suggested that the droplet interface became solid owing to packing of particles. Because particles at the interface were compressed due to drying-induced shrinkage of droplets, particles packing occurred at some point. We consider that wettability of particles is a key issue in packing. Simple drying experiment of particle-stabilized water droplets would provide us with important information about how packing of particles occurs on two-dimensional interface.
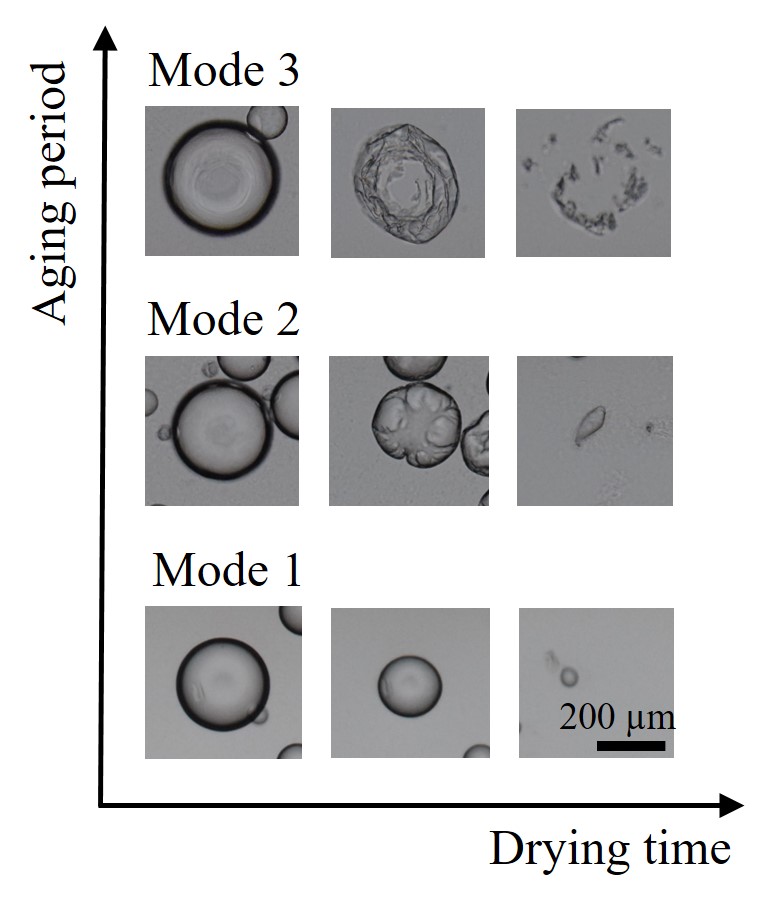
Performance of emulsion significantly depends on interface control. This study focused on interfacial layer formation of particles for oil-water emulsion system and its emulsion stabilization effect. In order to investigate interfacial localization, adsorption, and desorption of clay particles in the presence of surfactant, interfacial modulus of oil-water interface was measured under the control of surfactant loading. Depending on the balance of wetting property of particles between oil and water, localized particles responded to surfactant differently, especially depending on ionic characteristics of surfactant and the oil phase. Natural particles responded rapidly to surfactants and enhanced to desorb to the other phase as the concentration of surfactant increases. Organically surface-modified clays localized at the interface with the presence of surfactant and slowly formed viscoelastic complex interface of particles. (this work was supported by the National Research Foundation of Korea(No. NRF-2018K2A9A1A06086766))
The primary distinction between gel and non-gel is ascribed to whether it macroscopically flows or not. This visible criterion for discriminating the abovementioned two states which clearly differ from each other is based on the kinematics of a gelling solution, where we need to make a decision on whether it still exhibits flowable appearance or not. In most cases, gelation is observed as irreversible arrest in the motion of the gelling solution. Therefore, the direct observation of such a transient and transitive process is regarded as the only viable approach to detecting the actually occurring gelation. Generally in chemical studies, gelation tends to be discussed in the light of the formation of a percolated network-like structure of chain-like molecules or some alternative microstructures with elongated geometry. Thus, it is almost our habitual to attempt to obtain the clues for the gelation by means of some effective physicochemical measurements which have been established in the piles of abundant theories and experiences. Nevertheless, in the present work, we attended to the direct observation of the moment at which the arrest of the flow in the gelling solution occurs. For the suitable condition for the direct observation of the very moment of the gelation, we employed high-speed motion picture which captured the gelation occurring in only a few seconds. In advance, we had found an appropriate system which undergoes such a prompt gelation, where silicate oligomers stabilized in weak acid polycondense triggered on adding ethanol solution of imidazole which worked as a highly effective basic catalyst. In the high-speed observation at approximately 104 fps, the enforced flow in the gelling solution was seen arrested at 1 to 10 ms, whereas the overall gelation of the whole solution gave the observers the visual impression that it occurred and terminated in 0.1 to 0.4 s.
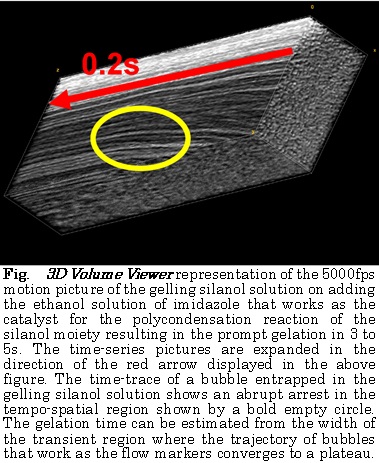
Groundwater contamination of arsenic is a global concern. The challenges in removing arsenic are low adsorption rate, lack of selectivity, regeneration and performance at natural conditions. Two forms of arsenic are predominantly found in nature, Arsenate and Arsenite. To remove Arsenate, As(V), from ground water by adsorption using a composite of cationic polymer gel impregnated with iron hydroxide. The cationic polymer gel that we used is N,N-dimethylamino propylacrylamide, methyl chloride quaternary (DMAPAAQ).
We prepared the gel using a unique method where Sodium Hydroxide and Iron (III) Chloride wasSVA added to the monomer and initiator solutions, respectively, to maximize the FeOOH contents in the polymer structure of the gel.
Results suggest that the maximum As(V) adsorption capacity of our gel, DMAPAAQ + FeOOH, was 123.4 mg/g. At pH7, the maximum As(V) adsorption capacity of our gel was higher than that of other adsorbents currently being used. Selectivity analysis suggests that in the presence of Sulphate and Chlorine, the gel can selectively adsorb As(V) effectively (see Fig. 1).
Regeneration experiments were successful for eight continuous adsorption-desorption cycles with the same gel pieces with 87.6% efficiency at neutral pH levels. Conventional desorption processes use NaOH for desorption. Since NAOH is harmful to human health, we used NaCl instead.
Our newly developed gel composite, DMAPAAQ + FeOOH, adsorbed As(V) effectively and the adsorption amount was higher than the other adsorbents at neutral pH levels. The gel selectively adsorbed As(V) and was regenerated with 87.6% efficiency. The novelty in our research is in the unique preparation method of the gel and the high adsorption performances at neutral pH levels, selective adsorption of arsenic and its reusability. In future, we will examine the performance of the gel in removing As(III) and the applicability in the natural groundwater.
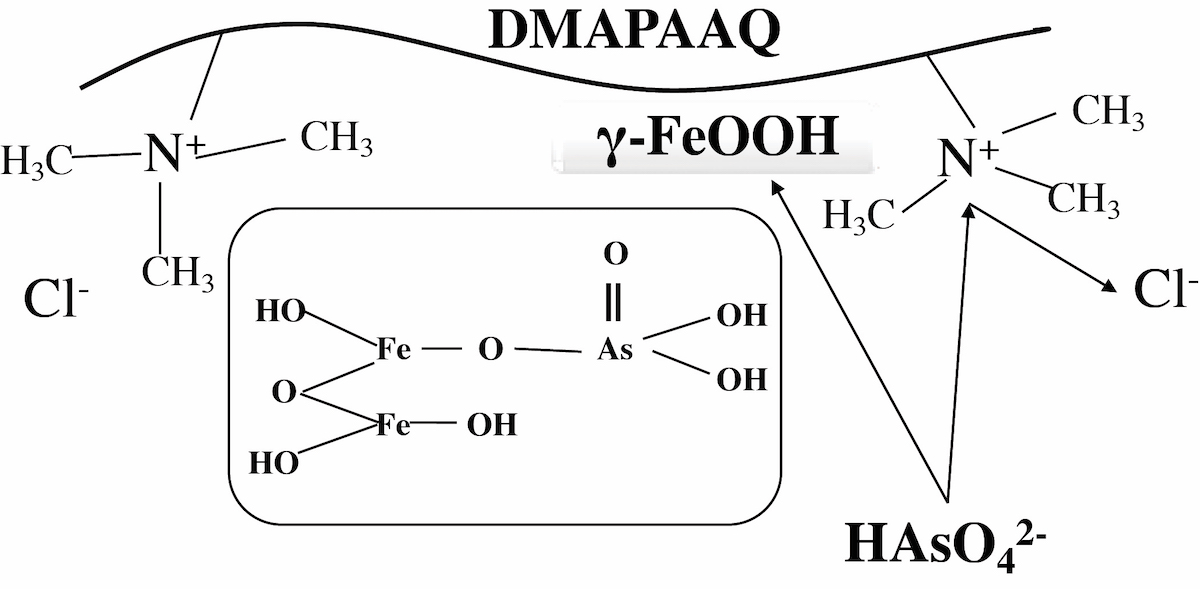
Self-assembly behaviors of fatty acid molecules are sensitive to surrounding pH conditions. Here, we developed a novel methodology to create hydrogel materials using fatty acid and its derivatives. Phase behaviors of oleic acid (OA)/monoolein (MO) mixtures were controlled by pH titration, wherein vesicle, oil-in-water emulsion, and cubic phase (cubosome) were formed at pH 9.0, 7.0, and 5.0, respectively. Rheological properties of cubosomal gel were characterized, and the mechanical properties of cubosomal gel could be improved by modifying with polymerizable fatty acid molecules. In addition, the mesoscopic membrane properties of OA/MO assemblies were systematically characterized by Raman spectroscopy and fluorescent probe Laurdan. It is concluded that the rigidity of the membrane could be key to maintain the mechanical properties of cubosomal gel materials.
Reference: K. Suga et al., Langmuir, 34 (5), 2081-2088 (2018). K. Suga et al., J. Chem. Eng. Japan, 52 (3), 311-316 (2019). K. Suga et al., Langmuir, 32 (30), 7606-7612 (2016). K. Suga et al., Langmuir, 30 (43), 12721-12728 (2014).
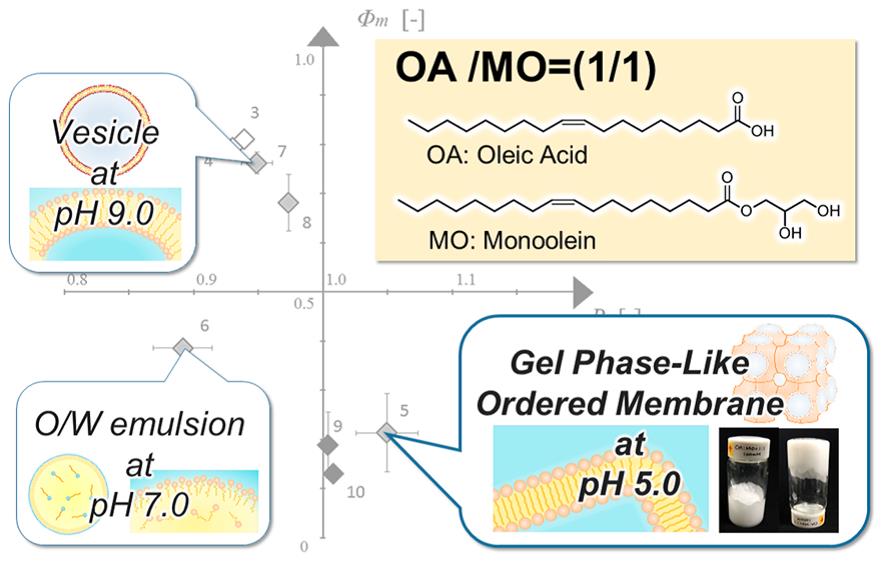
In recent rising environmental problem, competition and interest for CO2 gas capture materials have been increased. Solid CO2 adsorbents such as activated carbon and zeolite have been used for CO2 capture, however, those CO2 capacity or adsorbed kinetics were dramatically decreased in high humid condition. We have been investigated hydrogel film for CO2 capture to show relatively high CO2 capacity in R.H > 95%, however, CO2 absorption kinetic of this hydrogel film was too slow to spend over 1 hour. In this study, we developed an aeroporous hydrogel film (as shown in Fig.1) which consist of phase separation structure for hydrophilic hydrogel phase and hydrophobic porous gas diffusion phase. In this concept, CO2 gas can smoothly penetrate through gas diffusion phase inside film and finally absorb to hydrogel film phase for short time. We prepared aeroporous hydrogel film by mixing between amine containing hydrogel particle (GPs) and acetylene black (AB) using planetary beads milling in dry state, forming film by uniaxial pressing, and humidifying under R.H > 95%. After mixing GPs and AB, obtained powder showed core-shell structure, especially primary AB particle with 30 nm coated on the surface of GPs with approximately 5 μm from its characteristics using SEM, EDS, and particle size distribution laser diffraction type of particle size distribution measurements. After preparation the film, prepared self-standing film shows submicron ordered phase separation of GPs and AB, and a lot of voids was further observed in AB phase from TEM observation. CO2 absorption and desorption kinetics of this film showed higher than that of only GPs film evaluated by pressure swing adsorption method using humid CO2 and N2 gases. We supposed that this result revealed that gas transfer micro-channels in the aeroporous hydrogel film could be maintained under high humid condition and attributed to accelerating CO2 absorption and desorption rate.

Clean water is a rare commodity to get in several areas, take Batam in Indonesia for example. In 2018 approximately 70 percent of the waterways in the area is polluted by heavy metal contaminant. In this research an efficient and economical metal separation technique is proposed for the adsorption of heavy metal ions in solutions containing heavy metal ions. This technique adsorbs undesired cations and anions at the same time by deploying both cationic and anionic gel to adsorb heavy metals in target solution. The model system consisted of metal ion (PbCl2 or ZnCl2), cationic copolymer gel, and anionic copolymer gel, metal adsorption was successfully conducted. Copolymer gel consisted of N-isopropylacrylamide (NIPAM) as a thermosensitive agent, Acrylic Acid (AAc) / Acrylamidomethylpropane sulfonic acid (AMPS) as cation adsorbent and N,N-Dimethylamino propylacrylamide (DMAPAA) as anion adsorbent. With the existence of NIPAM as one of the major constituent of the gel, gel also exhibits thermosensitive properties, hence gel can desorb and adsorb ions at different temperatures. Figure 1 shows us that adsorption of both cation and anion does occurred in every type of gel. However gels that are able to adsorb cation and anion simultaneously (Dimethyl(acrylamidopropyl)ammonium propane sulfonate (DMAAPS), NIPAM-DMAPAA+NIPAM-AAc, NIPAM-DMAPAA+NIPAM-AMPS) recover more ions compared to other gels. Simultaneous usage of cation and anion gel also increases the reusability of gels. Unlike systems that uses sulfobetaine gel such as DMAAPS gel, this system enables gels to adsorb both cation and anion without any inhibition from inter-chain, intra-chain, and intra group bonding, thus reducing the probability of the system reaching its equilibrium due to the different charges in the target solution. The tested system, where anionic and cationic gels deployed at the same instant, has the potential to efficiently recover heavy metal ion from target solution.
Keyword(s): Thermosensitive, Simultaneous, Adsorption, Heavy Metal.
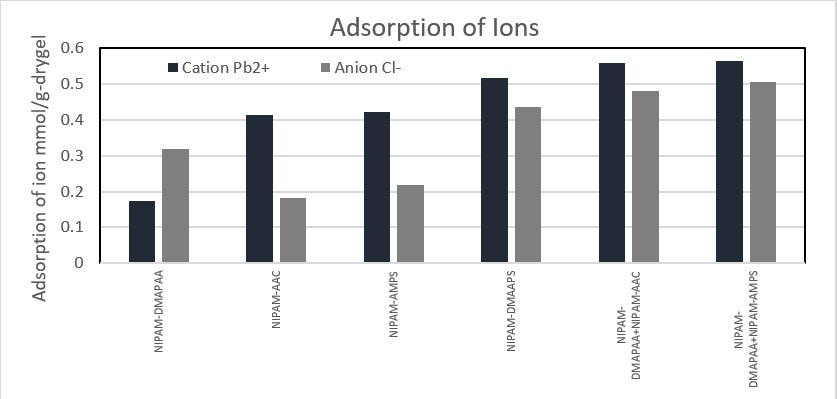
Perfluorocarbons (PFCs) have long been investigated for use in many fields including contrast agent, cancer therapy, and oxygen carriers (OCs) for a long time. In the case of OCs, PFC-based materials aim to provide adequate amount of oxygen to treat ischemic diseases or to facilitate tissue growth in 3D cell culture system for tissue engineering. Nano-sized PFC-based OCs have been studied for several decades due to the high oxygen carrying capacity and low side effects of PFC. In addition, micro-sized OCs are also attractive since they can avoid cellular uptake via endocytosis. Size control has been proposed as a requirement for developing OCs and uniform size distribution is crucial for their function of oxygen supply, durability, and biological interactions. However, it is hard to be accessed by traditional methods, such as mechanical agitations, that produce wide size distribution.
Shirasu porous glass (SPG) membrane emulsification technique is capable to produce monodisperse emulsions with precisely-controlled size. We have, for the first time, prepared PFC-based OCs using SPG membrane emulsification (Fig. 1a).1 By changing the membrane pore size, we were able to precisely control the size of emulsions and fabricate emulsions similar in size to human red blood cells (Fig. 1b). We also fabricated PFC emulsions using different types and concentrations of Pluronics (F127, F68, P85 and P103) as surfactants. The F127- and F68-stabilized micro-sized PFC emulsions were highly stable even during autoclave sterilization. The obtained emulsions were further loaded with oxygen-sensitive probe on their surfaces to indicate oxygen concentration. Their oxygen delivery to cultured cells was demonstrated using genetically-engineered HeLa cells that express EGFP in response to hypoxia.
Reference
1) Fu, X., Ohta, S., Kamihira, M., Sakai, Y., Ito, T. Langmuir 2019, 35, 4094-4100
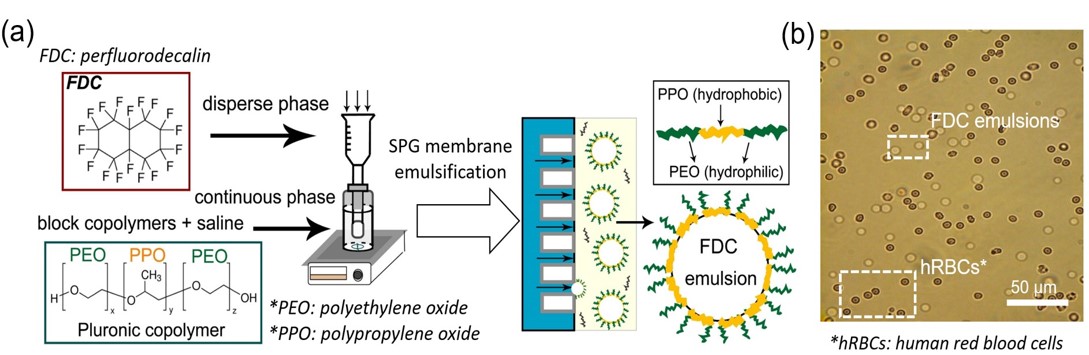
In recent years, many studies have been made in finding alternative additive to enhance the mechanical and thermal properties of polyvinyl alcohol (PVA). In this study, PVA-clay and PVA-grog composites were fabricated by solution casting method with varying additives composition. Clay and grog particles have been pre-treated by milled and sieved into micron size. The properties of composites were investigated with tensile test, scanning electron microscope, X-ray diffraction, fourier transformed infrared spectroscopy and thermogravimetric analysis. The results showed that PVA molecules could not intercalate into clay and grog particles during solution casting. The structure of composites were suspected to be phase separated instead of exfoliation or intercalation structure. The addition of clay or grog had slightly improved the mechanical strength of composites but FTIR result suggested that the improvement was not caused by hydrogen bonding between filler and PVA. The surface morphology from SEM showed that high loading of clay or grog in PVA has resulted in rougher surface and agglomeration of fillers. Based on XRD result, the average crystallite size of PVA-clay composites decreases with increasing clay loading while the overall crystallinity of PVA-grog composites increases with increasing grog loading. For thermal stability, increasing clay or grog loading have caused the thermal stability of the composites decreased slightly. Overall characterization results of the composites suggested that micron sized clay or grog particles are not suitable to enhance PVA mechanical and thermal properties.
Poly(N-isopropylacrylamide) (PNIPAM) nanogels were synthesized by emulsion polymerization using sodium dodecyl sulfate (SDS). After removal of SDS by dialysis, the surface tensions of the PNIPAM nanogel aqueous dispersions were measured by the pendant-drop method, and it was found that the surface tensions of the nanogel dispersion below the lower critical solution temperature (LCST) of PNIPAM were much smaller than those of water and comparable to those of the SDS aqueous solution. The stability of the aqueous foams generated by nitrogen bubbling thorough the PNIPAM nanogel dispersion was investigated below and above the LCST of PNIPAM. The foam prepared below the LCST was stable in some degree, whereas almost no foam was formed above the LCST. Moreover, the foam prepared below the LCST was quickly collapsed by changing the temperature above the LCST. This rapid defoaming represents that the surface activity of the PNIPAM nanogel can be switched off by the temperature increase across the LCST. In addition, we succeeded in controling the temperature dependence of the Pickering foam stability by copolymerizing the hydrophilic monomer, N,N'-dimethylacrylamide (DMAM) with NIPAM.
We report a microfluidic preparation of poly(ethylene glocol)-based hydrogel microparticles with tunable structures using phase separation in aqueous droplets, followed by cross-linking reaction. The process consists of water-in-oil (W/O) emulsion droplet formation through microfluidic emulsification, subsequent transition from the W/O emulsion droplets to water-in-water-in-oil (W/W/O) double emulsion droplets by internal phase separation, and gelation of the reactive monomer phase in the W/W/O emulsion droplets. We discuss the effect of the rate of gelation on the resulting structures of the hydrogel microparticles.
Advanced materials with low density and high strength have transformative impacts in construction, aerospace and automobile industries. These materials are realized with integrating well-designed modular building units into interconnected structures. Here, we present a hierarchical design strategy to demonstrate a new class of carbon-based closed-cellular structures (CCS). The building units are prepared by a multi-scale approach starting from functionalized graphene oxide nanosheets, leading to the microfluidic synthesis of solid-shelled bubbles with shape diversity at the micro-scale, followed by the assembly into meso-scale 3D structures. Subsequently, these are transformed into self-interconnected and structurally-reinforced CCS, resulting in graphene lattices with rhombic dodecahedral honeycomb structures at the centimeter-scale. The 3D suprastructure simultaneously exhibits the Young's modulus above 300 kPa while retaining a light density of 7.7 mg/cm3 and the elasticity against up to 80% of the compressive strain. The fabricated 3D CCS opens a new pathway for designing lightweight, strong, and superelastic materials.
Biological functions are maintained by various molecular motors, where the driving force is obtained from a chemical potential difference within the microscale. Here, we show in detail artificial vesicles that generate mechanical work from a local pH gradient. The vesicles are composed of oleate and oleic acid and exhibit rhythmic shape change[1] shown in Figure 1. This cyclic motion involves both rotation of the entire vesicle and reversal motion that reverses the vesicle structure of inside and outside, which constitute relaxation and excitation processes against a pH gradient, respectively. A cycle is constituted of these two elementary processes. In our previous work, vesicle motion was observed under an unsteady pH gradient formed by a diffusion of a base solution. As a result, the pH and pH gradient around the vesicle varied throughout the observation period. Therefore, quantitative aspects such as the driving force strength were unclear.
In this study, we observed periodic shape change under a quasi-steady state pH gradient, meaning that the pH gradient can be regarded as constant throughout the observation period. For this purpose, a device maintaining a quasi-steady state pH gradient over a sufficient period of time was made. With this setup, the pH and pH gradient around a vesicle exhibiting a shape change could be estimated. From the motion analysis of the vesicle, the driving force for vesicle motion was evaluated as a function of the pH and pH gradient. As a result, a quantitative discussion of the self-excitation and relaxation processes is provided. The present results demonstrate that this vesicle can be regarded as a molecular assembly machine working under a pH gradient.
Acknowledgements
E.N. gratefully acknowledge the financial support from JSPS KAKENHI Grant Number 19K15425.
Reference
[1] E. Nawa, Y. Nishigaki, D. Yamamoto, A. Shioi, Soft Matter, 2013, 9, 7832.
In recent years, poorly water-soluble drugs are increasing, and improvement of water solubility is considered as an important issue. Several methods have been proposed for improving water solubility, and one of them is a method of dispersing a drug by utilizing hydrophobic interaction between a water-soluble polymer having a hydrophobic moiety and a hydrophobic drug. In this method, there is a problem that only a polymer having a hydrophobic part can be used. However, it has been found that the water-soluble polymer itself inhibits the crystallization of the drug even in the absence of the hydrophobic moiety, and that it is possible to disperse the poorly water-soluble drug. This has resulted in more selectable polymers and the ability to use biocompatible polymers. In this study, we tried to use ionic polymers to disperse poorly water soluble drugs via ion-ion interact of water soluble polymers and hydrophobic drugs.
It was confirmed that with Glycol Chitosan as a cationic polymer, Indomethacin, an anionic poorly water-soluble drug, could be dispersed in water. In addition, with anionic polymer Chondroitin sulfate C sodium salt, dispersion of Lidocaine, a cationic poorly water-soluble drug, was also confirmed. Confirmation of the dispersion was conducted by UV measurement and the presence of the ion-ion interaction was conducted by IR measurement. Compared with pure water, the dispersibility of Indomethacin and Lidocaine were improved about 1,600 times and about 50 times, respectively.
In the process of manufacturing crystal products, the crystal size distribution (CSD) of product crystals is required to be monodispersed and unimodal for improving productivity. Therefore, unintentional nucleation needs to be avoided in batch cooling crystallization because it leads to broad or bimodal CSD. Full seeding and partial seeding are well known as seeding policies for inhibiting or controlling nucleation. In full seeding, a large amount of seed crystals are added to inhibit secondary nucleation. On the other hand, in partial seeding, a small amount of seed crystals are added to trigger secondary nucleation and inhibit primary nucleation. In this study, partial seeding was mainly investigated. In the previous study, the optimum operating condition for partial seeding was successfully estimated based on coefficient of variation (CV) by utilizing computation results on inorganic model substance. Product CV was assumed to be small when seeding was effectively performed. Also in this study, the optimum operating condition and the resulting product properties were estimated for various cooling and seeding policies by utilizing computation results on inorganic model substance. Cooling policy was changed not only in the total batch time but also in the cooling mode, as seen in Figure. Here, linear cooling, programmed one, and natural one were performed. The order of programmed cooling np was set to 4 for small supersaturation in an early stage of crystallization. For improving symmetry of condition, the time constant of natural cooling was set to the same value as that of inverse programmed cooling the order of which was 1/np. Seeding policy was changed not only in the mean mass size but also in the CV. The population density of seed crystal was given by the convex quadratic function. Potassium sulfate was utilized as the inorganic model substance.
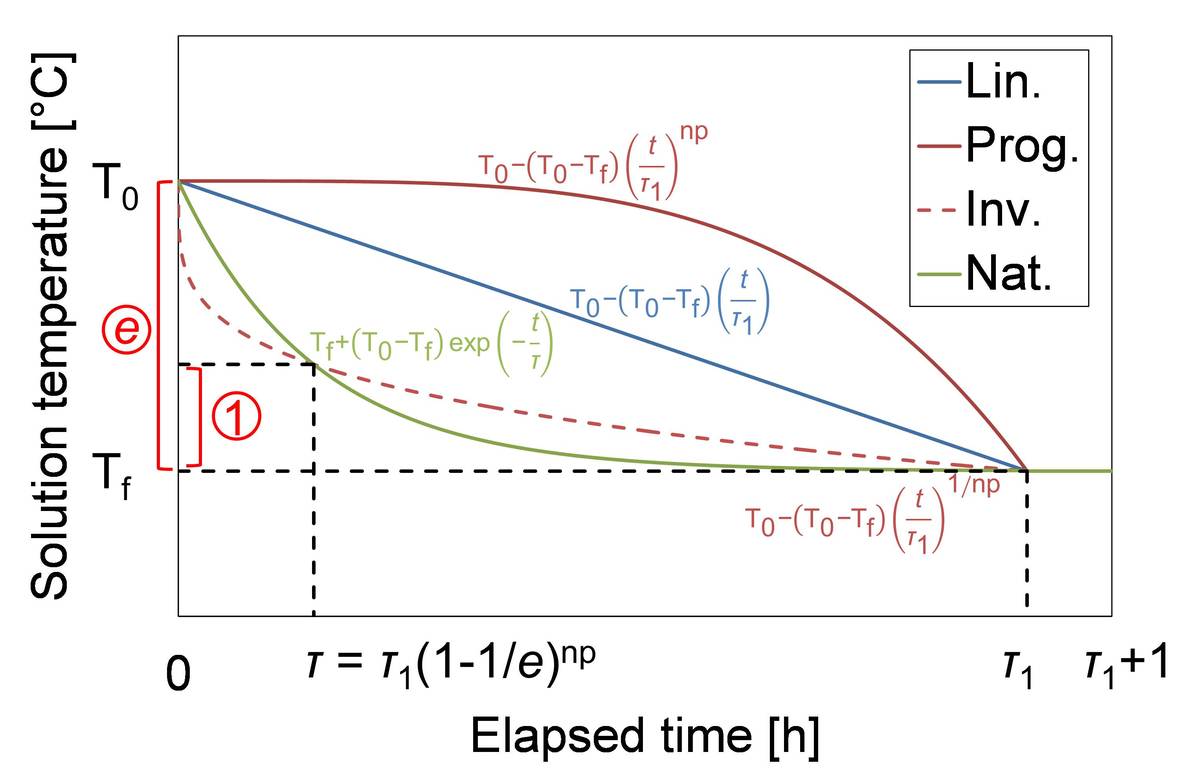
Transparent conductors are widely used for smart-phones, organic light emitting diodes and organic photovoltaics. Indium tin oxide (ITO) is the most common because of its high transmittance and conductivity. However, ITO have some defects. ITO is produced by a vapor phase synthesis and the rate is low, which makes ITO expensive. Second, indium is scarce. Finally, ITO is brittle. Therefore, the alternative transparent conductors have been studied eagerly. Among the studied transparent conductors, copper nanowires (Cu NWs) are promising. It is because copper is more inexpensive and abundant than indium. Moreover, Cu NWs are produced by a solution method, which is economical. Moreover, Cu NWs are flexible and this is advantageous for OLEDs and OPVs. Cu NWs are synthesized by a copper salt, a reducing agent and a capping agent, which determines the property, especially habit of crystals. The widely used capping agents for Cu NWs are amines. In this research, double-jet reactive reaction is applied for process of Cu NWs synthesis. In our previous study, it was difficult to synthesize Cu NWs of desired aspect ratio. On the other hand, double-jet process could control aspect ratio of Cu NWs by controlling reaction time, and to find out that concentration of reducing agent affects aspect ratio of Cu NWs.
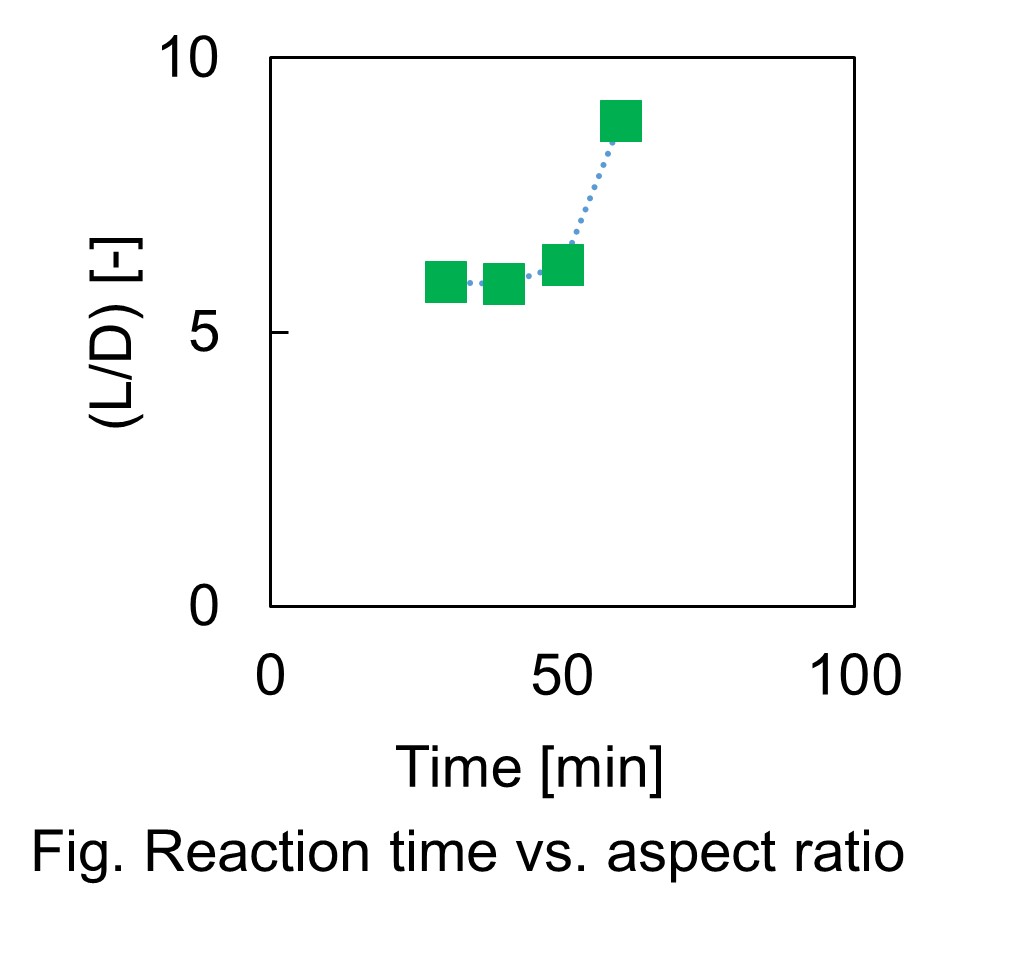
Many of the pharmaceutical compounds have asymmetric carbon. There is a need for techniques to reliably obtain one enantiomer. One of the methods of optical resolution is a preferential crystallization. In the preferential crystallization, crystals with high purity can be obtained by a simple operation. However, if chiral molecules do not racemize in solution, the maximum yield is 50%. By racemizing the chiral molecule in solution, other enantiomers can be converted to opposite enantiomer and crystallized as the desired product. So, the maximum yield would be 100%. Furthermore, repeated operations will be eliminated. In this study, the enzymatic reaction by racemase was examined as a method for racemization. We aimed to improve yield by performing preferential crystallization of DL-alanine combining it with racemization by alanine racemase. The alanine racemases gene from Streptococcus pneumoniae was artificially synthesized and expressed in E. coli shuffle T7. After several steps of purification, the enzyme of 35 kDa with electrophoretic homogeneity was obtained. The enzyme activity of alanine racemase was measured by monitoring the concentration of L-alanine with a circular dichroism dispersion meter. Specific activity for the conversion of L-alanine to D-alanine was 18.4 U/mg. Since alanine is crystallized as a racemic compound, it cannot be resolved by preferential crystallization. Thus, in order to crystallize as a racemic conglomerate, co-crystallization of alanine and p-chlorobenzene sulfonic acid (CBS) was performed. Optical resolution by preferential crystallization was performed. The alanine was dissolved at 40 °C and slowly cooled to 25 °C. When the solution reached to saturation temperature, seed crystals of D-Ala/CBS were added into the solution. After stirring for 1 hour at 25 °C, the crystals were recovered by filtration. The yield of crystals and the enantiomeric excess were 74% and 72%ee, respectively.
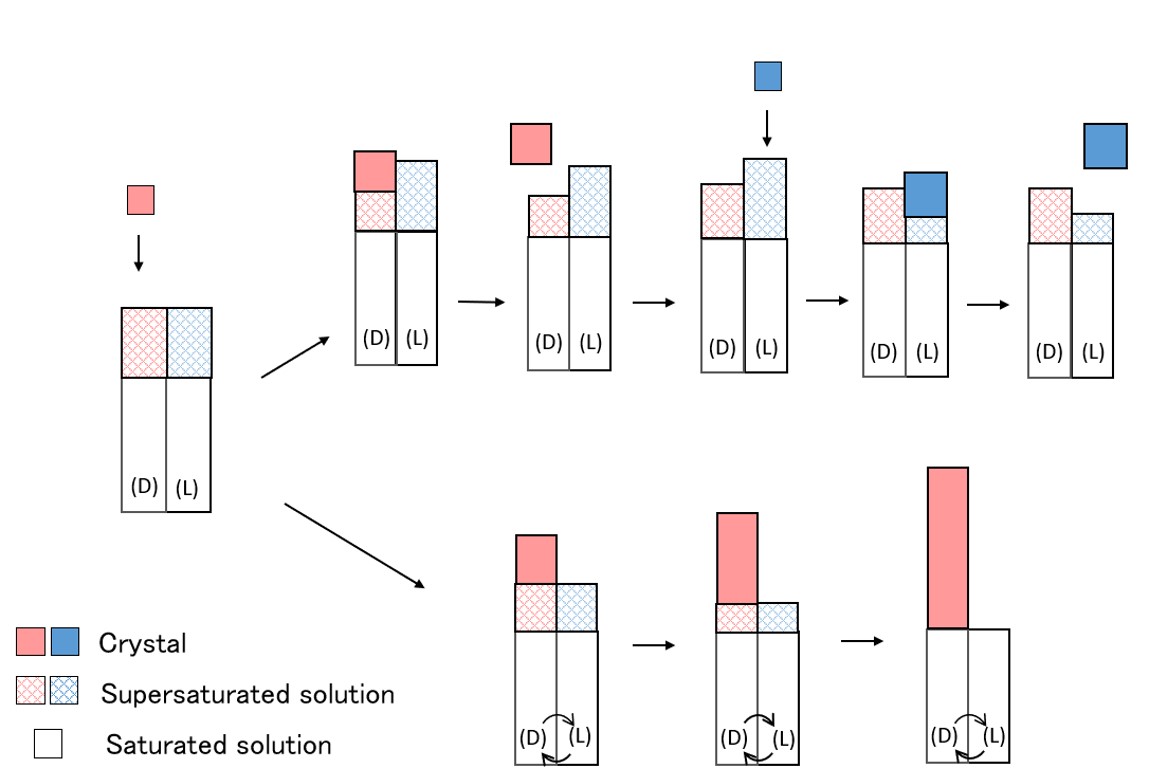
Since crystal nucleation directly affects the characteristics of the crystal, control of crystal nucleation is crucial in industrial crystallization. Previously, we reported that active diffusion of solute molecules in solution could be a trigger of nucleation. In this study, we investigated a new strategy to induce crystal nucleation. When immersing plastic pieces in solution, intermolecular interactions occur between the plastic surface and the solute molecules. When the plastic piece is moved vertically in this state, solute molecules which interact with the plastic also move together. The movement of the solute molecule reproduces unidirectional active diffusion of the solute molecule, and it is thought that crystal nucleation could be induced. In order to confirm this, we investigated the effect of the movement of plastic pieces on the crystal nucleation of L-alanine. Special experimental equipment and image processing software were developed. The plastic piece (polypropylene (PP)) was moved up and down vertically in alanine solution at a constant speed of 0.5, 2.0, and 5.0 mm/s. After that, the time when the first crystal was observed was recorded for at least 27 samples. Results were analyzed by cumulative distribution functions, and nucleation rate and average induction time were estimated. At all speeds, the nucleation rates were much higher than when the plastic was not in contact. In addition, it was also found that no crystals were generated when the plastic was immersed in the solution and left to stand. Therefore, it was suggested that the movement of plastic in solution induces nucleation of L-alanine. Comparing the nucleation rates at each speed, the nucleation rate was fastest at 2.0 mm/s. From these results, it was concluded that nucleation induction by the movement of plastic is caused through two processes, that is, the formation of intermolecular interaction and unidirectional movement of solute molecules.
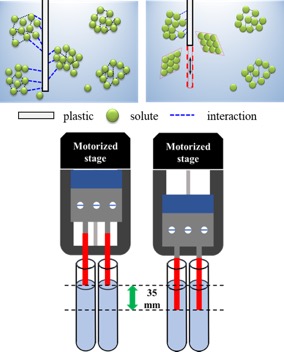
Novel small scale continuous crystallizer was developed to produce small uniform crystals. In the field of pharmaceutical crystallization, research on continuous crystallization is actively conducted. In particular, there is a need for a technique for continuously producing microcrystals without milling process. However, since the conventional continuous crystallizer has a large working volume, the residence time distribution becomes large, and it is difficult to control the crystal characteristics. Thus, we have developed a small continuous crystallizer to realize short residence time. The crystallizer is a 9 mL stainless steel device equipped with a high-speed agitator with a maximum stirring speed of 24000 rpm. Comparing with the large scale crystallizer, the average residence time is short, the distribution of residence times is narrow, and the concentration and temperature in the crystallizer are homogeneous. Therefore, small uniform crystals can be obtained. Anti-solvent crystallization of glycine was performed. Ethanol was used as a poor solvent. Almost 100% yield was attained even very short residence time (0.3-33 s). The shape and particle size of the obtained crystals were observed with a scanning electron microscope. More uniform crystals were observed compared to that obtained by the conventional crystallizer. Furthermore, it was also advantageous to polymorphic control. Due to the short residence time, the desired metastable crystals were recovered avoiding the solvent-mediated transition to the stable form.
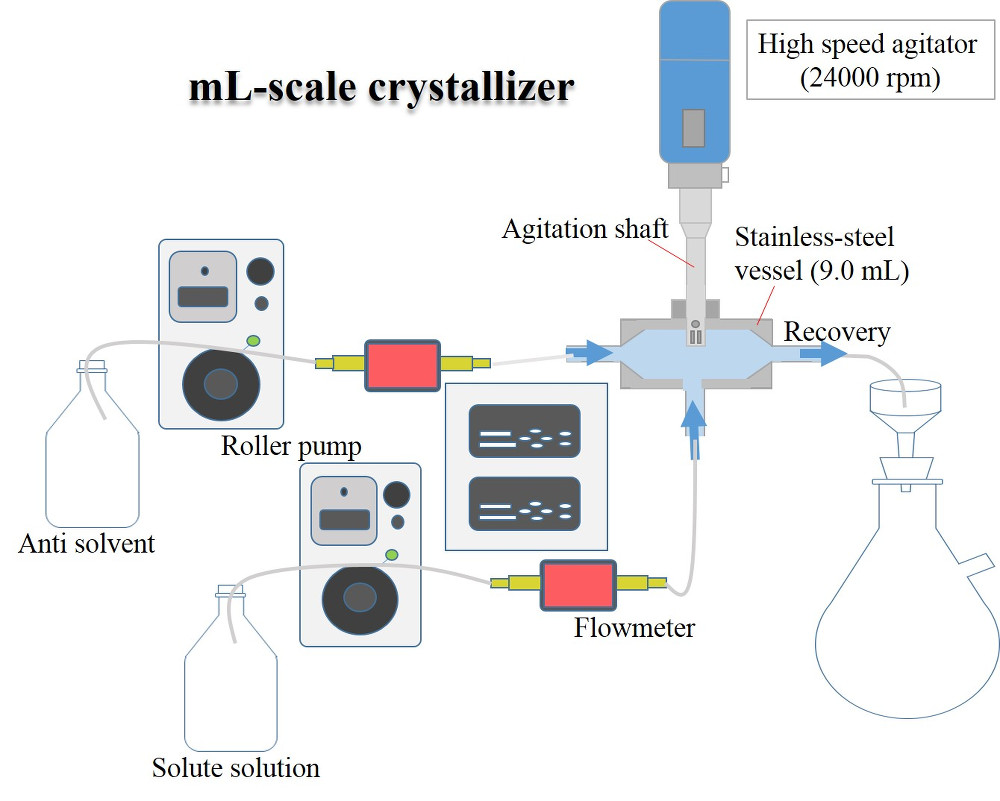
Unseeded semi-batch cooling crystallization of potassium alum (AlK(SO4)12H2O, K-alum) was carried out to obtain uniform crystals with narrow size distribution. In this paper, influence of feeding condition on crystal size distribution is mainly discussed. Seeding policy studied by the research group of Iwate University is a powerful idea for the successful inhibition of secondary nucleation leading to monodisperse crystals. However, seeding methodology is often undesired particularly in the pharmaceutical and/or food industries because the possibility of containing impurity in the seed crystals is not always ignored. The internal seeding method (unseeded method) was reported by the above research group, however, the theoretical aspect of operation design has not been clarified. This study continues the internal seeding method to develop theoretical feed condition as unseeded crystallization policy. One of the strategy to obtain narrow size distribution is the division of cooling stage (feeding stage) for the separation of nucleation and growth period.
Polymorphism occurs when a compound can exist in more than one crystalline form. Because of the different properties of polymorphs, it is advantageous to choose the proper polymorph for the desired application. In the crystallization processes involving polymorphs, the formation of the desired polymorph has to be controlled and such a process should be robust and reproducible. In the polymorphic crystallization, relative nucleation rates, relative growth rates, and the transformation of crystallizing polymorphs should be controlled individually. Therefore, the determination of the crystallization (nucleation and growth) and dissolution kinetics, and thermodynamics are important for characterization of the crystallization behavior and transformation of the polymorphs. Thermodynamics determines the polymorphic forms and their properties such as solubility. The nucleation, growth and dissolution kinetics determine the kinetics of the crystallization and transformation of the polymorphs. In this work L-histidine (L-his) is the model substance; it is one of the essential amino acids and is significant in the food and feed industries. There are two known polymorphic forms, forms A and B. Form A is the stable form and form B is the metastable form. In the present study the crystal growth kinetics of the metastable polymorph B of L-his were performed and studied. The crystal growth rates were measured in aqueous solution of L-his. The crystal growth rates increased with increasing temperature, concentration of solute, and seed mass. The growth rates were correlated to the supersaturation with a power-low equation. The results indicated that the crystal growth process was controlled by surface integration mechanism at temperature of 10 and 25 degreeC and diffusion mechanism at 40 degreeC. Growth kinetics of the polymorphs of L-his will be used to begin characterization of the polymorphic transformations and the overall crystallization rate of L-histidine.
Crystallization is used widely in industrial processes for products such as chemicals and pharmaceuticals. Stirred-type crystallizers can produce various complex phenomena such as crystal growth, abrasion, and aggregation, which affect the total number of crystal particles in a vessel and their particle size distribution. Population balance analysis of the number of crystals coupled with mass balance of the solute material provides an important means of predicting and controlling the number of crystals in the crystallizer. In this study, particularly addressing the aggregation phenomenon, methods for measuring the degree of supersaturation in solution, the particle size distribution, and the amount of aggregation were developed. Relations among them were investigated for various seeding and cooling rate conditions.
Batch-type cooling crystallization of potash alum started with seeding was examined as a model case. The solution contains crystal particles in the vessel aspirated through the quartz observation cell every 10 min. A high-speed movie camera recorded the motion of crystals through the cell. The particle size distribution was calculated from the obtained image using the original MATLAB program. Moreover, the time evolution of solution concentration was estimated from measurements of the solution electrical conductivity and temperature. The solution conductivity is affected by the suspended crystal particles. Therefore, the effects of particle size and suspension concentration on conductivity were calibrated using unimodal glass particles.
Results show that the time evolution of the solution supersaturation degree and crystal aggregation were estimated successfully based on the combination of the solution conductivity, temperature, and particle size distribution measured during the crystallization operation. When the seed crystals were few, the crystal surface area was small during the initial stage of crystallization; then the mode diameter of crystals in the final stage became large.
Low aqueous solubility of crystalline drugs is the major issue in the pharmaceutical field. Normally, two-component amorphous solid dispersion is used as conventional strategy for improvement of their solubility. Unfortunately, it is known that amorphous phase shows poor stability. Therefore, development of novel strategy for improvement of solubility is strongly desired. On the other hand, our recent study using eutectic solid dispersion found that nucleation rate enhancement of second component encourages refining eutectic phase including target component. Normally, refining eutectic phase including target component is favorable structure for enhancement of dissolution rate. Therefore, it is suggested that enhancement of nucleation rate is probably effective strategy for improvement their solubility. In addition, it is expected that formation of eutectic having a refined microstructure occur with refining the eutectic phase, which affects their solubility. Therefore, the objective of this present study is development of newly strategy for improvement of dissolution rate by nucleation rate enhancement of second component based on eutectic melt system. Sorbitol (target component)-Xylitol (second component) was used in the experiment system. In this study, we focused on two nucleation factors that are shear force and supersaturation. Therefore, we investigated the influence of seed crystal mass and cooling rate on the nucleation of the second component and dissolution rate of target component. As a result, it is showed that dissolution rate of the crystal phase was influenced by the seed crystal mass and the cooling rate. Furthermore, it was found that the dissolution rate increased with increasing of seed crystal mass and cooling rate. This means that solubility could be improved by promoting the nucleation rate of the second component. Therefore, our novel concept based on nucleation rate enhancement of second component is probably effective strategy to improve the solubility of target component.
Crystallization operation is used as the final process in chemical products manufacturing. The production of crystals with high quality is required because the final product quality strongly depends on the crystal quality. Therefore, quality control in the crystallization operation is essential.
The crystal quality is determined by desired many kinds of characteristics. Generally, the average value of each characteristic of individual crystals is used as standard whether the desired quality is achieved or not. On the other hand, it is also important to evaluate the variation of their qualities constituting the crystalline particles. In particular, their qualities tend to vary when the agglomeration phenomenon occurs. Therefore, achievement of the desired quality and improvement of the variations in individual crystal quality is strongly needed to control in crystallization process. Our present work reported that homogeneity is a useful index to evaluate variations of crystalline particles. Thereby, the purpose of this research is to develop the newly method of crystallization operation for improvement of homogeneity for agglomerated crystalline particles in reaction crystallization. In this study, L-Aspartate sodium (L-AspNa) and hydrochloric acid (HCl) were used as reaction system. L-Aspartic acid is objective crystalline material.
As a result, we found that homogeneity could be improved by supersaturation modulation operation. Unfortunately, it was also showed that using only supersaturation as an operation variable is not enough to product crystals with various qualities because it directly influenced on their characteristics such as size and shape. For this reason, pH of the solution was used as additional operation variable for quality control of crystals because solubility of L-Asparatic acid depends on pH. Consequently, production of crystals having various qualities was succeeded with keeping high homogeneity. Therefore, it was suggested that crystalline particles with high homogeneity could be obtained by using the multiple operation variable.
Quality control in the crystallization operation is required to obtain desired products because its quality depends on the properties of crystalline particles. It is known that their properties such as morphology and shape are determined by nucleation and growth of crystal. Thereby, controlling the nucleation and growth process individually is important. Normally, nucleation and growth occur simultaneously in conventional crystallizers, which means that conventional crystallizer are not suitable to obtain uniform product. For this reason, development of novel crystallizer for controlling nucleation and growth individually is needed. On the other hand, designing a newly production process based on conventional crystallizer has some advantages because of low investment cost and standard operation. Therefore, it is suggested that a crystallization process specializing nucleation should be integrated to conventional process in serial to separate nucleation and growth. We focused on the Taylor Vortex. Previous study reported that Taylor Vortex shows high shear stress due to its specific vortex ring, which is favorable flow state for enhancement of secondary nucleation. However, the driving force of nucleation and growth is determined by a supersatutration of feed solution. This indicates that the balance of each processes is limited even if nucleation is separated from growth using two continuous crystallizers. On the contrary, we designed the novel cascade type crystallizer based on Taylor Vortex. The purpose of this study is investigation of operation strategy for designed cascade type crystallizer to produce crystalline particles with uniform quality. The crystal shapes obtained by conventional and novel cascade process were evaluated using SEM. As a result, the dispersion of crystal size became narrow, which means that quality of crystal could be improved by using our designed crystallizer. We expect that our proposed crystallizer is favorable continuous process to produce crystalline products with desired quality.
The adsorption experiments were first carried out to remove the impure acetic acid from the mixed acids (phosphoric acid + acetic acid) as raw model waste solutions. The adsorbent used in this study was very effective to adsorb acetic acid selectively. The adsorption capacity of adsorbent was about 5% for acetic acid. Moreover, new adsorption-assisted crystallization was performed for purification of phosphoric acid crystals from the mixed acids. The crystallization temperature and the additional ratio of the adsorbent was changed as the experimental conditions. It was found that crystals of purity more than 99% phosphoric acid were easily obtained. The yield of phosphoric acid crystals increased as the crystallization temperature was lowered. The purity of phosphoric acid crystals increased slightly when the additional ratio of adsorbent was increased in the mixed acids. It was clarified that high purity phosphoric acid crystals can be purified efficiently by the synergetic effect of the adsorbent.
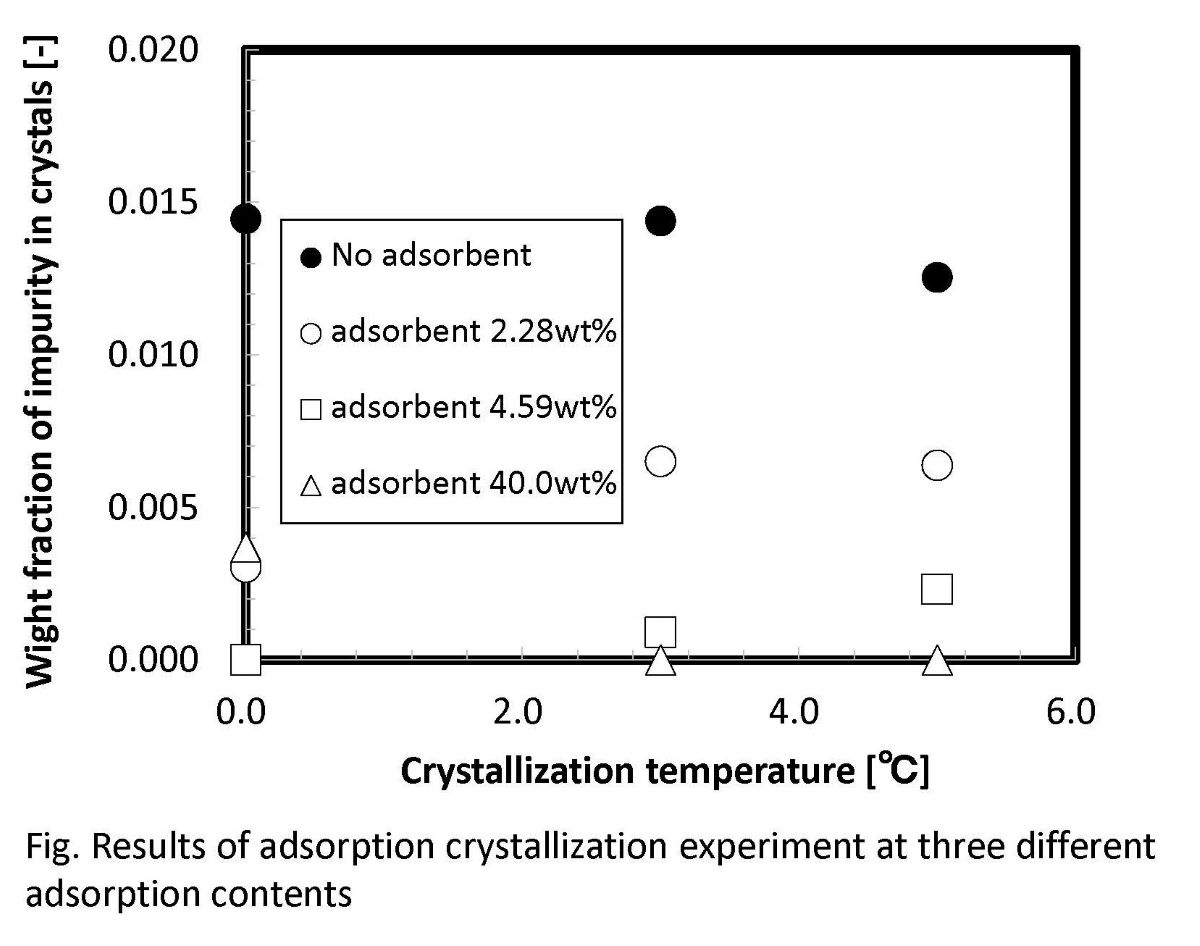
Recovery of valuable resources from waste plating solution has been of great interest from the viewpoints of both environmental protection and resource management. In this study, we attempt reactive crystallization combined with ozone oxidation to recover phosphorus species, which are contained in a waste plating solution as the reducing agent for electroless nickel-phosphorus plating, as calcium phosphate. The effects of the experimental conditions such as pH, temperature, the amount of calcium chloride added to the solution on the recovery yield are examined.
Core-shell mesoporous silica particles (MSPs) composed of a nonporous core and porous shell have attracted attentions due to higher monodispersity in particle sizes and more highly mechanical strength than mesoporous particles without a core. The core-shell MSPs can be used as packing materials to separate chemical substances in high performance liquid chromatography (HPLC) [1]. The performance of separation strongly depends on physical properties of MSPs, including particle sizes, pore sizes and shell thickness. The physical properties should be tuned to separate a variety of chemical substances.
Our group has been working on the synthesis of MSPs in sol-gel reaction where hydrolysis and condensation of silicon alkoxide are conducted in the presence of submicron-sized cores and cationic surfactants under basic conditions [2]. In conventional sol-gel reaction without cationic surfactants, sizes of silica particles formed are increased by electrolyte addition to the reaction system [3]. The increase in particle sizes by electrolyte addition has a potential of thickening mesoporous shell on the cores in the presence of cationic surfactants.
In this study, synthesis of core-shell MSPs was performed in the presence of electrolytes to increase the mesoporous shell thickness.
References
[1] Hayes R., Ahmed A., Edge T. and Zhang H., “Core–shell particles: Preparation, fundamentals and applications in high performance liquid chromatography”, J. Chromatogr. A, 1357, 36–52 (2014)
[2] Ishii H., Nara M., Hashimoto Y., Kanno A., Ishikawa S., Nagao D. and Konno M., “Uniform formation of mesoporous silica shell on micron-sized cores in the presence of hydrocarbon used as a swelling agent”, J. Sol-Gel Sci. Tech., 85, 539-545 (2018)
[3] Nagao D., Kon Y., Satoh T. and Konno M. “Electrostatic interactions in Formation of Particles from Tetraethyl Orthosilicate”, J. Chem. Eng. Japan. 33, 468-473 (2000)
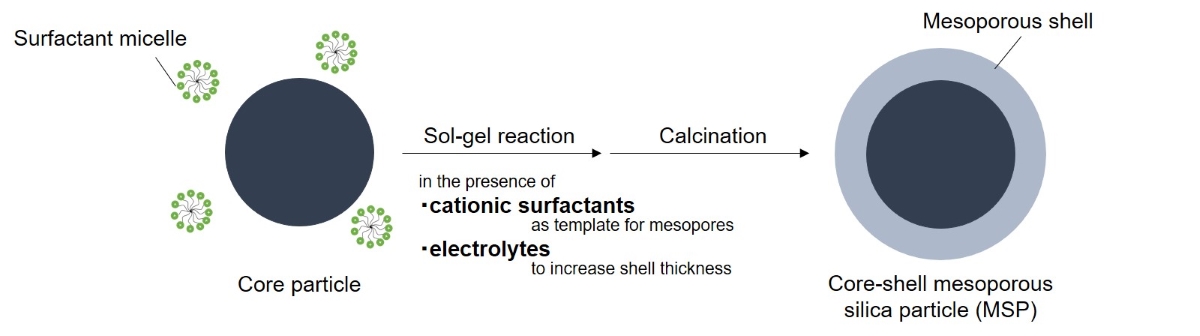
Mesoporous silica are functional materials with high specific surface area caused by regularly ordered mesopores. Mesoporous silica have been applied to absorbents, catalyst supports and drug delivery carrier. In order to apply the mesoporous silica to various fields, particulate mesoporous silica is attracting a lot of attention. One of the important factor to be controlled is pore size distributions in the synthesis of mesoporous silica particles (MSPs). Micelles of surfactants can be a template for mesopores of silica particles formed in the sol-gel reaction [1, 2]. Addition of hydrophobic molecules as a swelling agent to the synthetic system could enlarge pores in the silica matrix [1].
Our group has been working on the synthesis of MSPs in sol-gel reactions in mixed solvents of water and ethanol in the presence of cationic surfactant used as template micelles [3]. The addition of swelling agents increased sizes of mesopores in MSPs [4], although mechanism on enlargement of mesopores in the formation of MSPs is not sufficiently clarified.
The present study examined the effects of solvent composition on spherical particle formation and pore size distribution. The affinity of swelling agents to the mixed solvent was examined using different hydrophobic molecules. Analysis by Hansen solubility parameter (HSP) could correlate swelling agents with pore size distribution.
[1] J. S. Beck et al., J. Am. Chem. Soc., 114, 10834–10843 (1992), [2] T. Yanagisawa et al., Bull. Chem.Soc.Jpn, 63, 988–992 (1990), [3] H. Ishii et al., Adv. Powder Technol., 27, 448–453 (2016), [4] H. Ishii et al., J. Sol-Gel Sci. Technol., 85, 539–545 (2018)
Characteristics of particles strongly depend on the particle shape. Synthesis of anisotropic particles has been extensively studied to functionalize particles in recent years. Location and orientation of the anisotropic particles can diversify colloidal assemblies of the anisotropic particles.
To fabricate the colloidal assemblies, several methods such as suspension drying and using chemical interaction between particles have been developed. Depletion interaction is one of the powerful approaches to assemble colloids into desired structures in solution. The attractive depletion interaction between particles is induced by osmotic pressure in the presence of depletants (e.g. non-absorbing polymer, surfactants and nanoparticles). The attractive interaction is proportional to both depletant concentration and overlapping between depletant layers to which depletants cannot access.
Our group previously reported depletion-interaction-driven assembly of golf ball-like particles for development of colloidal macromolecules [1]. Golf ball-like particles have a large number of dimples on their surface.
In the present work, we tried to synthesize golf ball-like particles having hemi-rod-shaped dimples and demonstrated an assembling of the golf ball-like particles and rod-shaped particles via depletion interaction. Anisotropic particles with rod-shapes are expected to be captured by the hemi-rod-shaped dimples due to depletion interaction. The capture of particles has a potential of new purification process in which targeting particles can be intentionally collected depending on their shapes and sizes.
[1] K. Watanabe, Y. Tajima, T. Shimura, H. Ishii, D. Nagao, Journal of Colloid and Interface Science, 534, 81-87 (2019).
Recently, MoS2 is considered to be the most prospective candidate of anode materials for lithium ion batteries becuase of its high theoretical capacity (670 mAh g-1) and layered structure. However, it has usually suffered from structural deterioration, due to a large volume change on charge/discharge and low intrinsic electrical conductivity, which lead to a poor cycling performance. In this study, we have prepared porous MoS2 microspheres by spray pyrolysis with (NH4)2CO3 additive and investigated their pysical and electrochemical properties.
The precursor solutions were prepared by dissolving the required amount of (NH4)2MoS4 and (NH4)2CO3 in distilled water. Spray pyrolysis synthesis was conducted at different synthesis temperatures and concentrations of precursor solutions at a gas (3%H2+97%Ar) flow rate of 2 L min-1. The resulting samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS). N2 adsorption–desorption measurements were performed at a liquid N2 temperature of 77K. Pore volume and size distribution were estimated from the desorption branches of the isotherms by the Barrett–Joyner–Halenda model. Their electrochemical properties have been investigated using CR2032 coin-type cells.
The XRD peaks of the sample prepared at 800°C from the precursor solution of 0.05 mol L-1 (NH4)2MoS4 and 0.50 mol L-1 (NH4)2CO3 could be indexed well to the hexagonal phase of MoS2 (ICSD No. 31067) without any other phases. The desired Mo-related bond features of the sample were confirmed by XPS analysis. The pore structure analysis based on N2 adsorption–desorption isotherm led that the MoS2 microspheres with pore size less than 200 nm could be prepared and the (NH4)2CO3 additive in precursor solution could enlarge the porosity in MoS2 microspheres, especially the pore size between 20 to 100 nm. As a result, the porous MoS2 microspheres improved its cycle performance, as shown in Fig. 1.
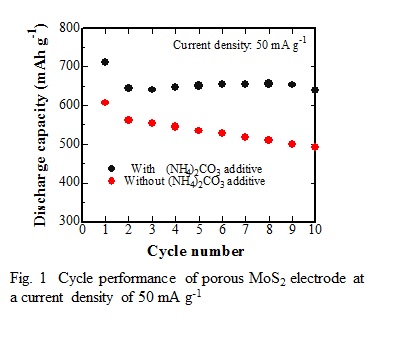
Porous carbon microspheres impregnated with transition metal-based nanoparticles have been considered as next generation materials for energy and environmental applications. In this study, we have prepared cupper (Cu) nanoparticles impregnated porous carbon microspheres by spray pyrolysis with heat treatment from the precursor solutions which appropriate amount of sucrose (C12H22O11) and cupper chloride dihydrate( CuCl2 2H2O) with citric acid (C6H8O7) additive were dissolved in distilled water, and investigated their pysical properties.
The precursor solutions were prepared by dissolving the required amount of C12H22O11,CuCl2 2H2O and C6H8O7 in distilled water. Spray pyrolysis synthesis was conducted for different synthesis temperatures and concentrations of precursor solutions at a gas (N2) flow rate of 2 L min-1. The as-prepared samples were further annealed at 800 °C for 2 h in a 3%H2/97%N2 mixture gas. The resulting samples were characterized by X-ray diffraction (XRD), field emission-scanning electron microscopy (FE-SEM) with an energy dispersive spectroscopy (EDS). Nitrogen (N2) adsorption–desorption measurements were performed at a liquid N2 temperature of 77 K The carbon content of the samples was estimated using an element analyzer (CHNS, Elementar, Vario Micro Cube).
Fig. 1a shows the XRD patterns of the sample prepared at 800 °C from the precursor solution by spray pyrolysis and then annealed at 800 °C for 2 h in a 3%H2/97%N2 mixture gas. It can be clearly seen from the XRD pattern of as-annealed sample that crystallized Cu could be obtained from the present preparation route. The N2 adsorption-disorption isotherm of the as-annealed sample indicated that it had mesopore structure, and the content of carbon was 28 wt% by CHNS analysis. Furthermore, it could be verified from the SEM-EDS analysis that the porous carbon microspheres impregnated with cupper nanoparticles could be sythesised by the present preparation route (Fig. 1b)
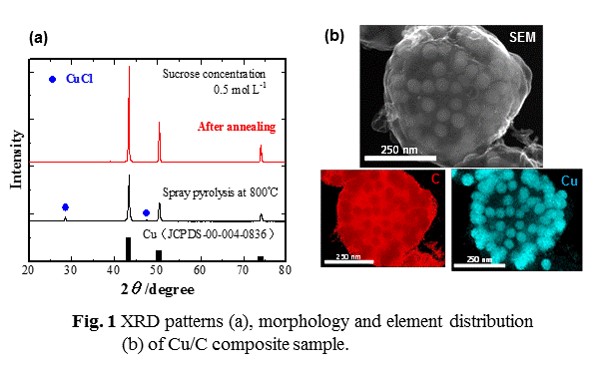
LiNixCoyMnzO2 (x≥0.33, x+y+z=1) (NCM) have a great potential as cathode materials for Lithium-ion batteries due to their abundant sources, high specific capacities and outstanding thermal stability. With the increasing content of nickel, NCM shows higher reversible capacity together with lower cost and toxicity. In this study, spherical nanostructured NCM particles were synthesized directly from homogeneous precursor solutions by spray pyrolysis followed by annealing in air atmosphere. The effect of process parameters on the physical and electrochemical properties of NCM was investigated.
LiNO3, Ni(NO3)2·6H2O, Mn(NO3)3·6H2O and Co(NO3)2·6H2O were used as the starting materials. These materials were dissolved in distilled water with the stoichiometric ratios of NMC. The starting solutions were atomized by an ultrasonic nebulizer at a frequency of 1.7 MHz. Then the generated droplets were carried to a spray pyrolysis reactor heated at different temperatures (400 -600 °C ) by the air gas with a flow rate of 2 L min-1. The as-prepared samples were further annealed at different temperatures in air atmosphere.
Crystal structures of the synthesized materials were determined by X-ray diffraction (XRD) analysis using Cu-Kα radiation. Fig. 1 shows the XRD patterns of the LiNi1/3Co1/3Mn1/3O2 (NCM-111), LiNi0.5Co0.2Mn0.3O2 (NCM-523) and LiNi0.8Co0.1Mn0.1O2 (NCM-811) samples synthesized at 500 °C by spray pyrolysis followed by annealing at 800 °C for 6 h. It can be seen that the well-crystallized NCM samples are formed with α-NaFeO2 hexagonal structure.
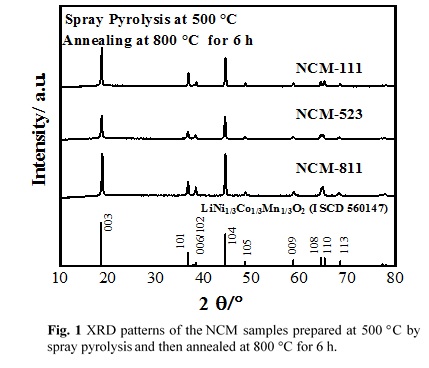
Integration of renewable energy sources with energy storage systems appears to be an encouraging approach to reach sustainability in green energy supply. It is expected that iron particles dispersed carbon microspheres (Fe@C) will find a broad application in many future generation energy storage systems and environmental issues. In this work, we have synthesized Fe@C using ultrasonic spray pyrolysis (USP) followed by annealing and studied synthesis parameters.
The precursor solution was prepared by dissolving an appropriate amount of sucrose, ammonium nitrate and iron nitrate in distilled water. Spray pyrolysis synthesis of Fe@C was carried out at 700 °C in an inert atmosphere (pure N2). Post-annealing of the samples was conducted at 700 °C for 4 hours in a reducing atmosphere (3%H2/97%N2). The obtained samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectra, and Brunauer-Emmett-Teller (BET).
Fig.1 shows the XRD pattern of the Fe@C prepared at 700 °C spray pyrolysis temperature followed by annealing at 700 °C for 4 hours. Three sharp peaks at approximately 45 °, 65 ° and 82 ° correspond to zero valent Fe while a peak at about 27 ° indicates the formation of graphitic carbon. SEM observation of the obtained sample shows that zero valent Fe particles evenly dispersed in the carbon microspheres (Fig.1 inset).
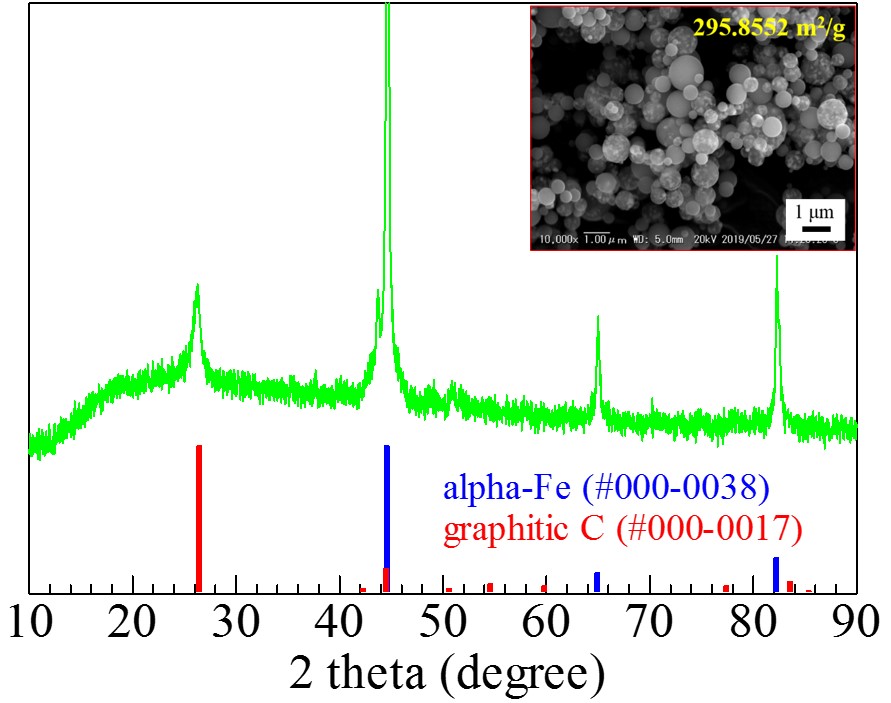
Gallium oxide (Ga2O3) is known to have five polymorphs, that are, α-, β-, γ-, δ-, and ε- Ga2O3. Among them, β-Ga2O3 is the most stable crystal structure and attracted recent attention as the material for power electronics, solar cells, and sensors. The conventional synthetic method of β-Ga2O3 nanoparticles is the so-called two-step method where hydrothermally synthesized GaOOH is calcinated at temperatures around 700 °C or above. In this presentation, we report an alternative one-step method to synthesize of β-Ga2O3 nanoparticles under solvothermal condition. In this method, gallium nitrate was dissolved in alcohol and then heated at 300 °C or above in a high-pressure reactor. The solid products were purified and analyzed by an X-ray diffractometer and a scanning electron microscope. Results showed that the crystal structure of the products gradually changed from γ-Ga2O3 to β-Ga2O3 as the reaction time increased. The average size of the produced nanoparticles was about 50 to 100 nm, which is much smaller than the products from the two-step method. This might be because our one-step method does not require a calcination process that allows the aggregation of particles during annealing.
Metal nanoparticles play an important role in catalysis, sensing, and imaging applications. Bimetallic systems are attractive for improving the physical and chemical properties by varying the elemental combination, composition ratio, and architectures of nanoparticles. However, designing the bimetallic nanoparticles are difficult because of many factors influences the nucleation and growth processes. In this study, we focused on the reduction rate of the metal precursors during the polyol synthesis of Ag-Rh nanoparticles. A microflow reactor was employed for obtaining reliable kinetic information by enhanced heat transfer performance. Reaction at elevated temperature in ethylene glycol was successfully examined by rapid heating and cooling with a microflow reactor. Ag precursor and Rh precursor showed strong interaction on the reduction rate. Reduction of Ag+ in ethylene glycol got slower with the higher reaction temperature, which contradicts to the Arrhenius law. The concentration profile of rhodium acetate would cause complex behavior due to its catalytic effect on the reduction. Elemental mapping of synthesized particles implied that the solid-state diffusion played a crucial role in the formation of alloyed Ag-Rh nanostructures.
When a small amount of water is added to a suspension in which particles are dispersed in oil, the number water between particles is formed. Such a substance is called a capillary suspension, it having fluidity change to flow characteristics like gel. However, detailed mechanisms and characteristics of capillary suspension have not been studied. In this study, the particle / oil / water capillary suspension was prepared using various particles and evaluated the rheological properties.
Firstly the particles and the oil were mixed with a dissolver stirrer to prepare a suspension. Next, capillary suspension was prepared by adding small amount of water to the suspension and stirring for 10min. Viscosity and rheology of the product was measured with a rotary rheometer. Fig.1 shows the effect of water volume fraction on the viscosity of capillary suspensions prepared by using three types of particles (Sillica(2.5μm),sillica(1.0μm),Cacao powder(2.5μm). The viscosity profile shows the maximum value at the certein water volume fraction, which we called the optimum crosslinked water content (OCWC) . From the results, the samples using particles of the same material showed the same OCWC. However, samples using different particle with same particle size showed different OCWC. When the three-phase contact angle of cocoa powder is lower than that of sillica. It is considered that the OCWC became high because more hydrophilic characteritics. It is thought that the rheological property value of the capillary suspension can be estimated by the three-phase contact angle, interfacial tension, particle size and particle concentration. By measuring the yield stress, it is possible to calculate the capillary force acting between particles of each sample. It was found that the three-phase contact angle, which varies depending on the material of the particles, is closely related to the rheological property.
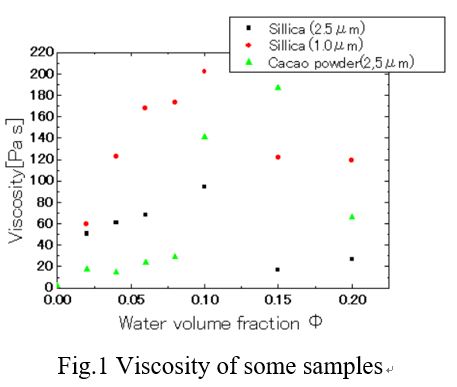
We focused on the interface between the polypropylene (PP) resin and the polyamide 6(PA6) resin, which was generated when the PA6 resin was located at the center of the carbon fiber reinforced polypropylene (CFRPP) to enhance the strengthen of the CFRPP. By adsorbing the polymer particles on the surface of the carbon fiber through the electrodeposition, the particles played a important roles to improve the interfacial adhesion between the PP and the PA6 resins. And also, the interface between CFRPP and metal was investigated to incorporate CFRPP to multi-material structures. polymer particles with the same component as the resin were adsorbed on the metal surface, and the particles were impregnated to fine defects on the metal surface, thereby improving the interfacial adhesion between CFRPP and the metal. Therefore, we examined the application of polymer particles to fabricate CFRPP for practical use.
A microlens array is the optical element which has many lenses with a diameter of several to hundreds micrometers on its surface. At present, the microlens arrays are prepared by the top-down processes such as photolithography and etching, where the productivity of the arrays is not very high. Moreover, it is difficult to obtain the arrays with smaller lenses through the top-down processes. To prepare the smaller microlens arrays efficiently, the bottom-up processes such as self-assembly are required. In the present study, we have tried to prepare the mesolens array with a diameter of hundreds nanometers from the non-close-packed (NCP) colloidal monolayers, which are prepared by the convective self-assembly (CSA) using a glass substrate and the silica particles grafted with cationic polyelectrolyte, poly(vinylbenzyl trimethylammonium chloride) (PVBTA). When the NCP colloidal monolayers are annealed, PVBTA would be removed and a part of the silica particles are thought to be melted. It is therefore expected that the silica particles and the glass substrate could fuse together to form the mesolens array, because they are mainly composed of silicon dioxide.
PVBTA were grafted by free radical polymerization on the surface of the monodisperse silica particles, and then the PVBTA-grafted silica particles and the glass substrate were applied to the CSA process to prepare the NCP monolayers. The obtained monolayers were annealed at 973 K in a muffle furnace for several hours. The annealed particle monolayers were observed by the atomic force microscope (AFM) and the scanning electron microscope (SEM). As a result, the height of the silica particles were found to decrease with an increase in annealing time, which probably indicates that partial fusion of the silica particle and the grass substrate proceeded as the annealing time increases.
Polymeric microcapsules are micron-sized particles with a liquid core and a polymer shell. The shell of the microcapsules can isolate the encapsulated substance from the external environment. Microcapsules exhibit various functions by combining different core substances with shell materials. In particular, microcapsules containing a phase change material have advantages such as an increase in heat exchange efficiency due to an increase in specific surface area and prevention of the encapsulated phase change material from leakage, which can be served as efficient heat storage materials for energy saving applications. Most of the microcapsules used as heat storage materials are made of melamine-formaldehyde materials (MFM). MFM have excellent mechanical strength and heat resistance. However, it has been reported that the resultant microcapsules contain unreacted formaldehyde which has high carcinogenic to human. There is therefore an increasing demand for reducing or removing formaldehyde from the products. In this study, we developed a process to prepare formaldehyde-free melamine microcapsules by using melamine, urea, glyoxal and 2,2-dimethoxyacetaldehyde (DME) [1] as the shell materials, based on the previous study [1] and optimized the preparation conditions for controlling the shell thickness. Specifically, we prepared a prepolymer solution and mixed the solution to oil-in-water (O/W) emulsion composed of Isopar G and aqueous solution containing poly(E-MA), as the oil and water phases, respectively [2]. We discuss the effect of glyoxal/DME molar ratio and NH2/CHO molar ratio on the formation of the microcapsules as well as the thickness of the microcapsules.
[1] G. Leon et al., RSC Adv., 7, 18962-18975 (2017)
[2] Eiji Kamio et al., Langmuir., 24, 23, 12387-13298(2008)
The behavior of drawing process was investigated by focusing on fiber stretching speed (spinning acceleration) to improve fibers' luster quality. Wet spinning method often has a problem of low luster level caused in higher spinning speed. We found that, (1) luster quality could be evaluated by fibers' arithmetic average (RΔa), which indicated fibers' roughness and (2) fibers' roughness was related to spinning acceleration by analyzing RΔa. Spinning acceleration was measured by chasing markers which was used to tie fibers. Regardless of length of spinning bath, fibers were stretched mostly during the first stretching stage. So we tried to stepwise drawing method. In case drawing ratio is 220 percent by one step, RΔa was 10.1 degree but by stepwise drawing (1st drawing ratio was 148.3 percent and 2nd drawing ratio was 148.3 percent) RΔa was decreased to 8.3 degree. We have cleared that stepwise drawing method has enabled to decrease fibers' roughness by preventing sudden change of fiber stress. In addition, we have cleared that higher temperature also improved fibers' roughness. In higher temperature, roughness was decreased in spite of higher acceleration because that fiber was easier to stretch than in lower temperature.
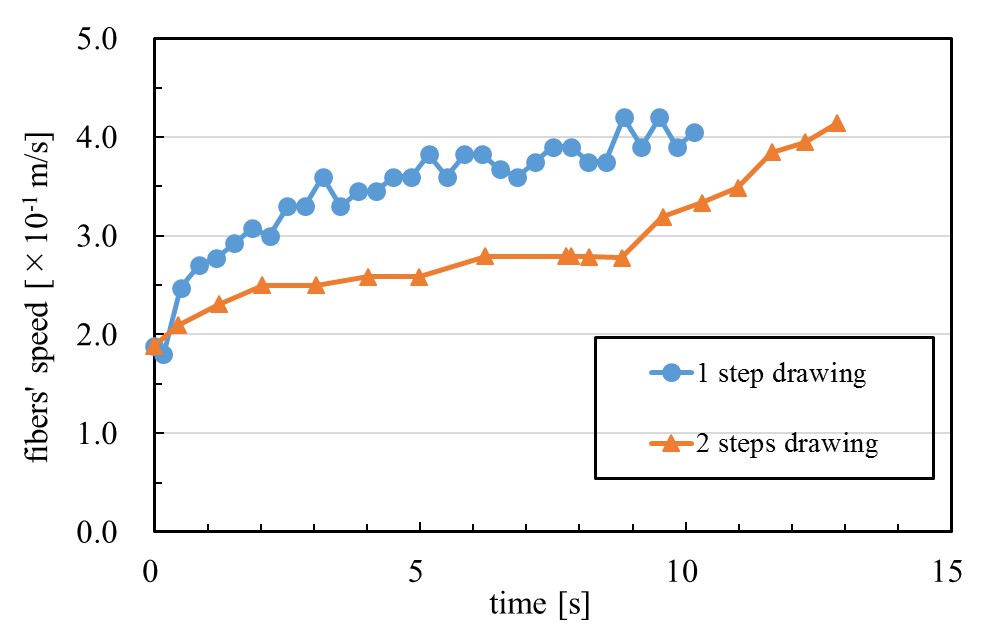
Paper-based devices have attracted a lot of attention as simple analytical platforms for bioassays. However, paper-based biosensors have still many problems, especially nonspecific protein adsorption. Detection sensitivity and selectivity of such sensors are impaired by nonspecific protein adsorption because of the drifting and denaturing of the detection ligands, the loss of target analyte during transport to the detection zone, and background noise from nonspecific protein adsorption. Typically, this problem in a sensor device is addressed by surface modification with hydrophilic polymers. For example, poly(ethylene glycol) (PEG) provides protein-repellent properties on wide variety of surfaces.
Herein we demonstrated surface modification of cellulose paper with a functional PEGylated polymer via simple dip-coating. This functional PEGylated polymer has biotin as a reactive site on the end PEG chains. In general, dip-coating with a PEGylated polymer followed by drying generates a hydrophobic surface because the hydrophobic moieties of the polymer are preferentially segregated at the air-polymer interface to minimize the surface energy. To display the hydrophilic moieties (functional parts) of a polymer on an outermost surface in dip-coating, we previously proposed that the surface segregation in the dip-coating of a PEGylated polymer can be controlled using a fluorine-containing surfactant. Based on the previous study, we here attempted to impart antifouling property and reactivity with other molecules such as antibody, DNA to the paper surface.
We synthesized PEGylated copolymers (Scheme 1) and a fluorine-containing surfactant (Rf-PEG). Cellulose paper was dip-coated with these copolymers in the presence of Rf-PEG. This coted paper had water absorbency in comparison with PMMA-coated paper and a paper coated PEGylated copolymers in the absence of Rf-PEG. Moreover, this coated paper had less nonspecific protein adsorption than a raw paper and a paper coated PEGylated copolymers in the absence of Rf-PEG. We expect that this coted paper will be applied to platforms for bioassays.
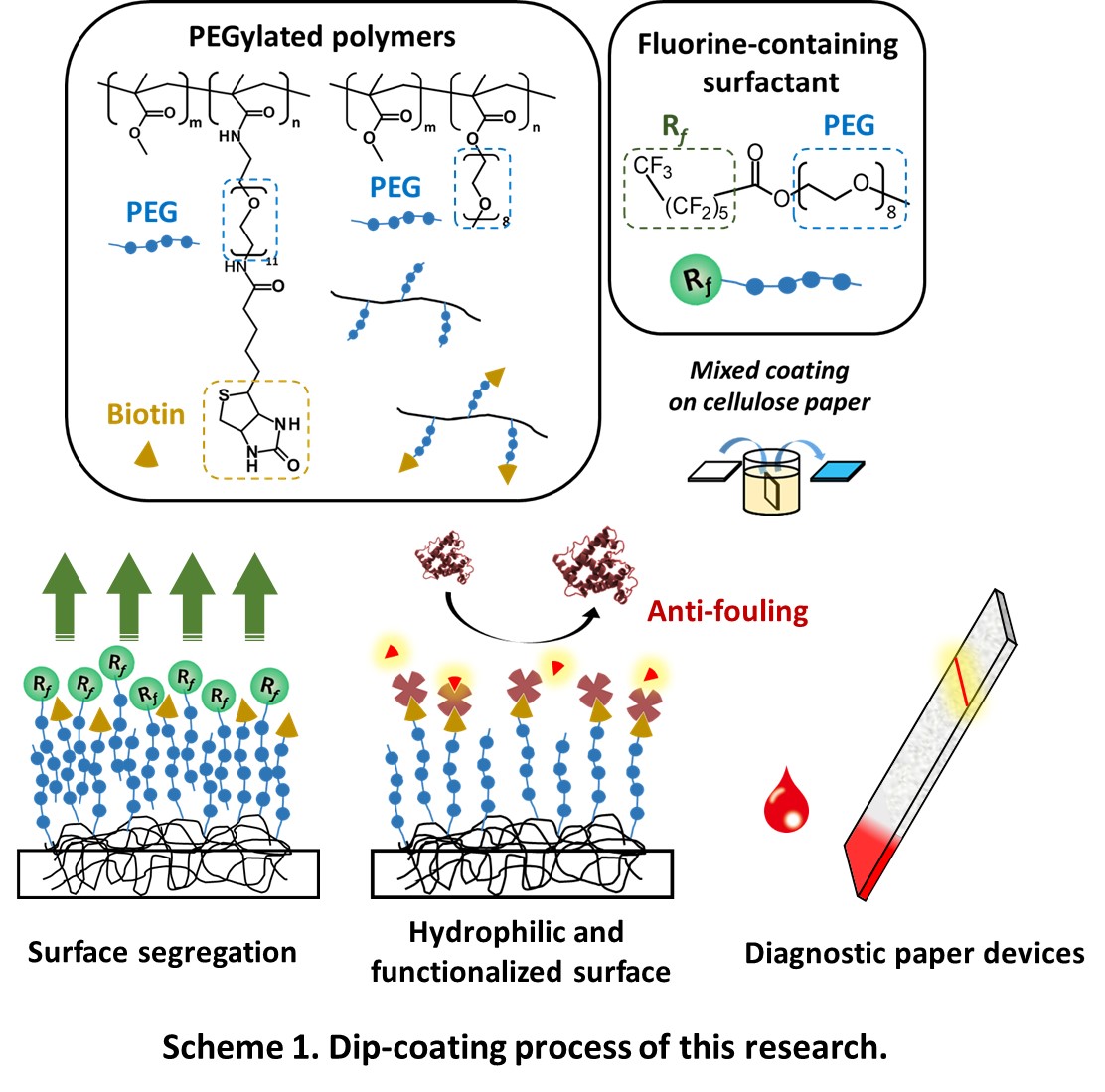
The excited electrons and holes generated upon by irradiating UV ray to TiO2 have powerful redox power. They produce reactive oxygen species (ROS) under aerobic conditions at the surface. These species rapidly decompose organic compounds to converts them into CO2 and H2O via carbon-carbon bond cleavage. TiO2 has been used as a powder to increase the catalytic effect. However, the use of particles involves the need of incorporating extra stages in the purification process, such as separation and catalyst recycling. We focused on a composite of TiO2 nanoparticles and polymer nanofibers. Polymer nanofibers have a large surface area. Polymer nanofibers are fabricated by electrospinning as follows. An electric field is used to create a charged jet of polymer solution. As this jet travels in air, the solvent evaporates leaving behind a charged fiber that can be collected on a metal plate. The purpose of this study is to develop novel composite polymer nanofibers with TiO2 nanoparticles and clarify its applicability as photocatalyst for degradation of organic compounds. N-isoprorylacrylamide-co-N-methylol acrylamide (NIPA-co-NMA) polymer was synthesized by free radical polymerization. NIPA polymer is a thermosensitive polymer, and NMA can be used as a crosslinking agent. The composite nanofibers were fabricated by electrospraying methanol containing NIPA-co-NMA polymer and TiO2 nanoparticles onto stainless-steel plate through a nozzle with application of high voltage. The composite nanofibers catalyzed decomposition reaction of methylene blue (MB) successfully.
Development of novel materials which mimic the excellent molecular recognition function of biomembranes has recently got attention. Copolymer of N-isopropylacrylamide (NIPAM) bearing β-cyclodextrin (CD) (poly(NIPAM-co-CD)) exhibits molecular recognition function, which controls its volume phase transition behavior by the inclusion of guest molecules[1]. However, the mechanism of the molecular recognition of poly(NIPAM-co-CD) has not been fully explained. N,N-dimethylaminosulfonyl-2,3,3-benzoxadiazole (DBD) is a fluorophore and changes its fluorescence intensity in response to hydrophilicity around the molecule[2]. If DBD is introduced into the poly(NIPAM) chain, changes in polarity around PNIPAM accompanying phase transition can be detected.Herein, DBD is copolymerized with poly(NIPAM-co-CD) to directly analyze the change in hydrophilicity of the poly(NIPAM-co-CD) chains by inclusion of guest molecules (Fig. 1A). We discuss the correlation between the phase transition behavior and the hydration state around NIPAM, which is controlled by molecular recognition of CD in polymer chains. The change in phase transition and hydrophilicity around the polymer chains caused by inclusion of a guest molecule, sodium 2-naphthalenesulfonate (2-NS), was investigated by cloud point measurement and fluorescence intensity measurement. Fig. 1B. shows the results of the cloud point measurement. When 2-NS were added, aggregation of the polymer was suppressed. The fluorescence intensity measurement exhibited that there was no difference in the change in fluorescence intensity by addition of 2-NS, suggesting that inclusion of 2-NS in CD did not affect the hydrophilicity around NIPAM. Further, the cloud point measurement in NaCl solution did not display suppression of aggregation even the presence of 2-NS, resulting from electrostatic shielding by Na+ around SO3– group of 2-NS which was included in CD. It implies that the suppression of aggregation in poly(NIPAM-co-EDA-CD-co-DBD) by addition of 2-NS was caused by the electrostatic repulsion of 2-NS included in CD.
[1] Macromolecules, 45, 9742-9750, (2012)
[2] Anal. Chem.,, 75, 5926-5935, (2003)
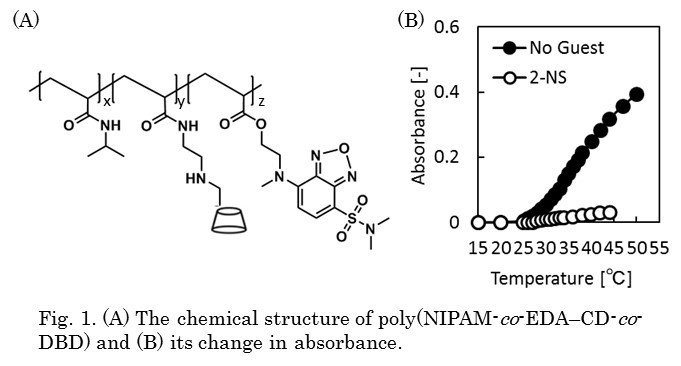
To investigate the effect of the surface properties and polymer properties on surface modification via electrostatic adsorption, the electrostatic adsorption behavior of zwitterionic copolymer on negatively charged surface was studied. A series of positively charged zwitterionic copolymer and a series of negatively charged surfaces including porous substrates and dense films were respectively fabricated. The electrostatic adsorption behavior of zwitterionic copolymer on negatively charged porous substrates were confirmed by contact angles and fluorescently labelled protein adsorption experiment. And the adsorption behavior of zwitterionic copolymer on negatively charged dense films were confirmed by quartz crystal microbalance (QCM) determination and fluorescently labelled protein adsorption experiment. The results indicate that the lower charge density on the zwitterionic copolymer bring to the higher adsorption on the charged surface, while the extremely low charge density results in the lower adsorption on the charged surface, due to the low interaction. The high density of film surface charge is beneficial for surface adsorption, while the extremely high density of the film surface charge leads to the lower surface adsorption, due to the steric hindrance of the negatively charged sites. This work provide the insight into the best strategy for surface modification via electrostatic adsorption.
Gating membrane is a porous membrane that opens and closes pores responding to heat[1] and specific ions[2]. Recently sensor system using photo-responsive material attracts attention because of several advantages such as simple operation and uninfluential nature to the substance. In this research, we aim to develop a photo-responsive gating membrane that opens up the possibility of conventional membranes.
Photo-responsive polymer composed of poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) actuator and photo-sensing spiropyran (SP) was synthesized by free radical polymerization (molecular weight : Mn =1.49 × 104 , Mw / Mn = 2.07 and SP content: 0.81 mol% ). Lower critical solution temperature (LCST) of 0.1 wt% aqueous solution is 42 °C before visible light (Vis) irradiation and 40 °C after Vis-irradiation while no LCST was observed after ultraviolet light (UV) irradiation.
The copolymer was immobilized to the polyethylene (PE) porous membrane by plasma-induced graft polymerization (Graft ratio: 5.6% and SP content: 0.96 mol% ). Permeation coefficient (Lp) of the prepared membrane increases as water temperature rises before light irradiation (Fig 2). This is because the copolymer shrinks above LCST and opens the pore of the membrane. Just after UV-irradiation, Lp of the membrane decreases as we expected, but increases then. The latter behavior may be due to elimination of the copolymer from PE substrate by UV-irradiation, as indicated by FT-IR spectral change. After Vis-irradiation, Lp of the membrane did not increase even though linear polymer shows similar LCST behavior before and after Vis-irradiation (Fig 1). This is probably because LCST behavior of grafted polymer in the pore and linear polymer in diluted solution is different.
In conclusion, we have successfully fabricated visible light-responsive gating membrane.
1) H. Kuroki et al., J. Membr. Sci. 2010, 352, 22-31.
2) T. Yamaguchi et al., JACS, 1999, 121, 4078-4079
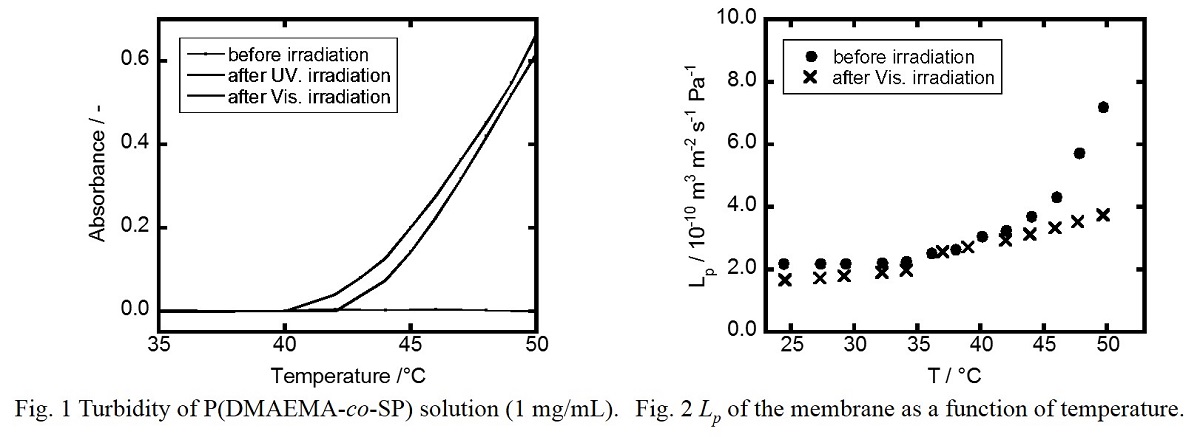
Enzymes play vital roles in biological transformations due to incomparable selectivity. Enzymatic membrane reactors (EMRs) combine enzymes with membranes, and many researchers have studied the synergistic effect of EMRs exerting on enzyme performance. Before the utility of EMRs can expand from natural aqueous media to organic solvents, robust membranes must be developed to promote enzyme protection from hostile forms of media. For this study, laccase was immobilized on an organic-solvent-resistant hydroxylated polyketone (PK-OH) membrane via covalent bonds and served as a model enzyme. Ketone groups facilitated the immobilization via hydrogen bonds, leading to a high immobilization density of 462 μg/cm2. In homogeneous aqueous-organic solvents, the activity of immobilized laccase was up to 3.5 times greater than that of free laccase towards 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid). In addition, the results also showed improved activity towards highly concentrated 2,4,6-trichlorophenol and bisphenol A (1.4-g/L). Furthermore, the activity in filtration mode showed a 240% increase over that in batch mode. The immobilized laccase maintained its activity after 40-days of storage, 10 reuse cycles, and 50 h of continuous reaction. These results show that robust polyketone based membrane support will create opportunities for the application of EMRs in aqueous-organic solvents.
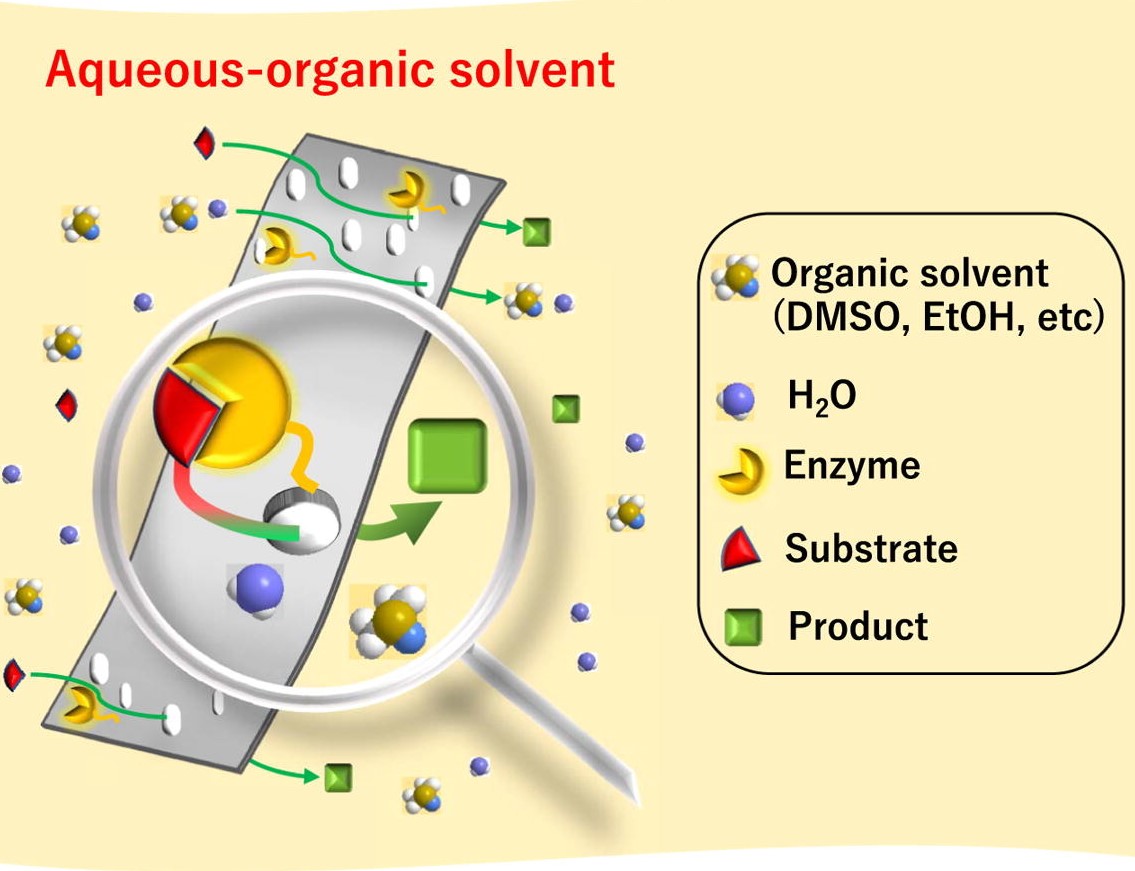
Cellulose nanofiber (CNF) is a micro-refined nano-level fibrous material that can be produced from chemical and mechanical treatment of variety of woody biomass materials. CNF has attractive characteristics, it's density is 5 times lesser than that of steel while being 5 times stronger. Moreover, it is produced from natural biomass resources therefore it is entirely environmentally friendly material1). Recently, its compounds with resins have received increased attention as alternative to pure metals owing to their light-weight and noncorrosive characteristics. In this use, defibrating and surface hydrophobization have main roles in obtaining high quality CNF. CNF is hydrophilic, while phenolic resins are hydrophobic; therefore, mixing them could result agglomeration and consequently lower the strength of the composite. In addition, the form of CNF is likely to influence the properties of the composites. Application of thermoplastic resins, such as polyethylene and polypropylene, in the formation of CNF composite materials have been widely reported. However, to the best of our knowledge, few studies have been conducted using thermosetting resins in the synthesis of CNF-reinforced composite materials. In this study, reinforcing of phenolic resin with CNF as a model for thermosetting resins was investigated. Various tests were conducted to evaluate the performance of the composites produced with CNF (produced at different physico-chemical conditions) in different proportions with the resin. Various techniques, such as Fourier-transform infrared spectroscopy, mechanical testing, and scanning electron microscopy were utilized to understand their properties. Fig. 1 shows mechanical strength of typical CNF composite. As shown, bending strength as well as tensile strength increased approximately 20% when 3 wt% CNF was used. This study suggested that reinforcement of the resin using CNF resulted in stronger and more promising composites.
References: 1) Akihiro Sato et al., Composites Part A: Applied Science and Manufacturing, 83, 72-79 (2016)
This study is to investigate the characteristics of polyvinyl alcohol (PVOH) added with different loading level of calcined fish bone (HAp). HAp was extracted from Chirocentrus nudus skeletal via thermal calcination; followed by solution casting method to produce PVOH composite films, consisting 0 phr to 5 phr calcined fish bone. In term of tensile strength, it decreases upon added with 1 phr calcined fish bone then increases at 2 phr loading level. The addition of 3 phr calcined fish bone was found to be the optimum loading level to exhibit reinforcement function; whereas for elongation at break, a decreasing trend with an increase in calcined fish bone loading level was found due to the continuity of chain mobility in PVOH being interrupted and the inducement of internal friction within PVOH matrix. For scanning electron microscopy, the wave-liked shrinkage, fibrils and agglomerates were reduced with increasing calcined fish bone loading level, indicating a high tensile strength and low elongation when increasing the loading level. In x-ray diffraction analysis, the peak broadening effect indicates a decrease in HAp crystallite size which has ruptured and well dispersed within PVOH matrix. For differential scanning calorimetry, the enthalpy of melting and crystallinity (in terms of qualitative and quantitative) possessed similar trend as the finding in tensile strength. In fourier transform infrared spectroscopy analysis, the unique peaks occurred approximately at 3263 cm-1 and 2923 cm-1, indicating the presence of O–H group bonding and C–H bonding respectively. Lastly, this study concluded that the 3 phr calcined fish bone was the optimum loading level to be employed in biomedical application such as bone scaffolding due to the high strength and compatibility.
Polymer/magnetic particle composites have high magnetic sensitivity, and thus have wide applications in biomedical fields. B. Tural et al. reported recently that if polymer containing a carboxyl group such as poly(methacrylic acid) was added to the coprecipitation solution during magnetite nanoparticle (MNP) synthesis, the polymer was immobilized on the magnetite surface immediately following particle generation. The method is one-step process that does not require complex and time-consuming manipulation.
However, so far, there are no reports on what kind of polymer (or functional group) can be used for this type of one step synthesis of polymer/MNP composite. Therefore, in this study, we tried one-step synthesis of polymer/MNP composite by using different polymers which contains different functional group to clarify 1) what kind of functional groups can be applicable for one step polymer/MNP synthesis, and 2) the effect of the polymer difference on the immobilized polymer amount.
In the experiment, the synthesis of polyacrylic acid (PAAc) / MNP was carried out using the following procedure; first, PAAc, iron (II) sulfate heptahydrate and iron (III) chloride hexahydrate were dissolved in deionized water. Then, sodium hydroxide was dropped into the solution at room temperature to proceed MNP synthesis reaction. Polyacrylamide (PAAm), polyethylene glycol (PEG), polyethylene imine (PEI) and polyvinyl alcohol (PVA) immobilized on MNP were also synthesized using the same procedure just by changing PAAc to the corresponding polymers. The results of XRD analysis showed that for all the polymers used in this study, formation of MNP was confirmed, and the fact indicated that the presence of the polymers did not affect on the synthesis of MNP. Thermogravimetric analysis (TGA) indicated that for all the polymers, polymer immobilized on MNP was successfully synthesized, and the amounts of the immobilized polymers were in the order of PEG < PEI < PAAm < PAAc.
To prevent aggregation and oxidation of magnetite nanoparticle (MNP), silica coating on MNP was proposed. Recently, Setyawan et al. successfully achieved simultaneous magnetite nanoparticle formation and the modification of its surface with silica layer by using the electrooxidation method. Advantage of the method is that the process is very simple and easy since it's a one-step process.
The purposes of this study are following; 1) to synthesize MNP with silica coating under various conditions by electrooxidation, and to examine the effect of the synthesis conditions on the characteristics of the resulting sample, 2) to immobilize thermoresponsive copolymer, poly(NIPAM-co-AA) on the MNP with silica coating, and to evaluate their immobilized copolymer amount and Cu adsorption property.
As a result of X-ray diffractometer (XRD) analysis and Fourier transform infrared spectroscopy (FT-IR) measurement, it was confirmed that MNP with silica coating was successfully synthesized using this method. When the current value was changed during electrooxidation synthesis, it was found that the minimum particle diameter(maximum specific surface area) was obtained at the current value of 0.60 A. The results of thermogravimetric analysis (TGA) showed that the maximum immobilized amount of the copolymer of 0.255 g/g-MNP was obtained when the sample synthesized at a current value of 0.60 A was used. The immobilized amount was 3 times larger than that of the sample prepared using MNP without silica coating. This is considered to be due to less aggregation of MNP during polymer immobilization. The results of Cu (II) adsorption experiment showed that although Cu (II) recovery amount through temperature swing was similar to those of conventional thermoresponsive “gel” type heavy metal adsorbents, time required for Cu(II) recycling process could be decreased to 1/10. These results showed the effectiveness of this method.
Graphene-based materials have been studied for prospective applications in many fields, including catalysts and electrode materials. Graphene-based materials can be made by two kinds of approaches. The first one is the bottom-up approach represented by a chemical vapor deposition method. The second one is the top-down approach via graphene oxides. Although the latter approach is suited for mass production, it is difficult to control the crystallinity and the amount of defects by this approach. Therefore, tailoring some properties depending on applications has remained a significant challenge. We reported that it is possible to control the crystallinity and the concentration of defects by microwave-assisted (MW-assisted) annealing for carbon materials by adjusting distances between carbon particles through changing the bulk density. However, it can be difficult to change the bulk density depending on some types of carbon materials. This study is aimed at controlling the crystallinity and the concentration of defects regardless of the kind of carbon materials through development of a new up-flow reactor for MW-assisted annealing. Crystallinity as well as the concentration of defects were investigated by multiple techniques including Raman spectroscopy and thermogravimetric analysis. During MW irradiation, we observed moderate fluidization of sample particles in an up-flow reactor. Therefore, distances between carbon particles were adjusted, through which the concentration of defects was controlled. Our results indicate that extend of changing of the concentration of defects depends on the sample particle size and the gas flow rate during MW irradiation. Moreover, samples annealed by the up-flow reactor exhibited high oxidation resistance in air in comparison with ones treated by a down-flow reactor.
Magnesium oxide (MgO)-templated mesoporous carbons were modified with hydrated zirconium oxide (ZrO2·xH2O) synthesized through hydrolysis of zirconium chloride oxide (ZrOCl2) to prepare adsorbents for fluoride ion (F-) removal from water. Powder X-ray diffractometry (XRD) revealed that ZrO2·xH2O was successfully formed on the surface of mesoporous carbons. Adsorbed amount of F– was observed as a function of time by using pristine mesoporous carbons, modified mesoporous carbons, and ZrO2·xH2O prepared without carbon. The figure shows the F– concentration of solution (CF) as a function of contact time. The pristine carbon was found to adsorb little amount of F–. In contrast, the modified carbon and ZrO2·xH2O were capable of removing F– from water. The amount of F– removed with the modified carbons was larger than that observed using ZrO2·xH2O. This result indicates that the capability of ZrO2·xH2O to remove F– is improved by attaching it to the mesoporous carbon surface. The authors presume that this positive effect is probably provided by formation of ZrO2·xH2O on the carbon surface giving crystals that are smaller than crystals fomed without carbon. The F– removal was described by conventional adsorption isotherms. The other results such as effect of mesopore size and pH on the F– removal will be shown at the conference.
Porous carbon materials have attracted much attention because of their large surface area, large pore volume, chemical inertness, and electrical conducting properties. Mesoporous carbons with well-tailored pore system are essential for a number of applications, such as adsorbents, catalyst supports, and energy storage devices. Recently, a great deal of effort has been made to develop a method of synthesizing mesoporous carbons, including hard- and soft-templating techniques. Hard-templating techniques require preparation of inorganic template such as mesoporous silicas, impregnation of pores with appropriate carbon precursors, carbonization, and subsequent selective removal of inorganic template using HF
or NaOH. Another recently developed approach is based on the application of soft-template such as triblock copolymers access for simpler and facile synthesis of mesoporous carbons because of the avoidance of multiple step to prepare and remove the hard-template. However, the soft-templating techniques still suffer from long preparation durations and the excessive use of solvents for the formation of mesostructure in solutions through the self-assembly process, which causes low efficiency, high production cost, and environmental burden.
Herein, we investigate a highly efficient and simple approach to synthesize mesoporous carbons using solid raw materials under solvent-free conditions. The solid–solid approach has potential advantages for large-scale production, low cost, environmental-friendly process. Mesoporous carbons were obtained by the solvent-free technique using various combinations of solid raw materials with adjustments of the molar ratio.
The demand for high-performance thermal interface materials (TIMs), which enhances releasing heat from the electronic devices, is increasing for further performance improvement and miniaturization of electronic devices. The performance of TIM is characterized by the total thermal resistance RTotal which is the sum of the bulk thermal resistance and the contact thermal resistance between the TIM and the opposing substrate. Therefore, it is required for the TIM to have high thermal conductivity for vertical direction κ⊥ and softness, thus conventional TIMs consist of filler particles having high thermal conductivity and soft polymer matrix. By using fillers with high aspect ratio and aligning them vertically, κ⊥ can be improved [1]. We previously fabricated TIM using carbon fiber (CF) having high thermal conductivity and high aspect ratio. By a simple process using shear force, CFs were filled in the thermoplastic elastomer (TPE) matrix with vertical alignment and high mass ratio of over 50 mass% (Fig. 1a) [2]. However, the total thermal resistance is as high as 100 mm2 K W-1 possibly because of the poor connections between the CFs and between the CF and the opposing substrate surface. This time, expanded graphite (EG) having high thermal conductivity and flexible sheet-like structure is added as a sub-filler (Fig. 1b). Thermal resistance was measured by the steady-state method and plotted against the TIM thickness (Fig. 1c). The thermal resistance decreased with the EG content, resulting in the significant reduction of the thermal resistance to RTotal = 19 mm2 K W-1 with 19 mass% EG. This value is similarly small as the high-performance TIM of an indium sheet. The improvement comes from both the improvement of the thermal conductivity (slope) and the reduction of contact thermal resistance (intercept).
[1] K. Uetani, et al., Adv. Mater., 26, 5857 (2014).
[2] R. Yamada et al., The 51st FNTG symposium, 2P-38 (2016).
Electrochemical sensor based on redox cycle of repeated oxidation and reduction has advantages of compactness and high sensitivity. To promote the redox cycle, two working electrodes for oxidation and reduction should have large surface area, be placed closely, be insulated electronically while be conductive ionically each other. The typical example is the interdigitated electrode (IDE) (Fig. 1a), but the fabrication of the microstructure requires complex processes using lithography. In this work, we propose a new structure similar to electrochemical capacitor in which a separator made of cellulose nanofiber (CeNF) paper holds two working electrodes (WEs) made of carbon nanotube (CNT) paper (Fig. 1b). Cyclic voltammetry (CV) using K4[Fe(CN)6] aqueous solution shows the effectivity of this structure as a sensor (Fig. 1c).
Larger current was obtained with 3D electrodes of 10 μm-thick CNT papers and separator of 3 μm-thin CeNF paper, enabling detection of K4[Fe(CN)6] for concentrations down to 10 μM.
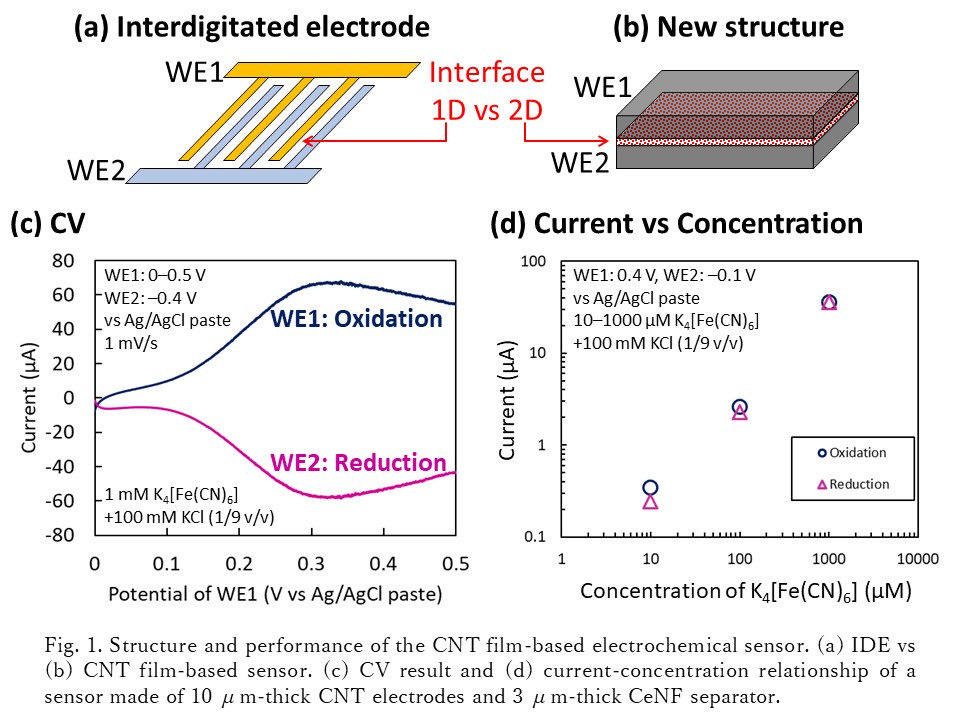
The conventional hydrogen storage materials such as metallic alloys are heavy and difficult to transport, and there is a problem that their costs are high. Therefore, in this research, we aim to develop hydrogen storage materials using carbon nanohorn (CNH), which is a lightweight and inexpensive carbon material.
CNH dispersed with metal nanoparticles was prepared using arc-in-water method. After that, the amount of absorbed hydrogen was measured under the condition of 0.95 MPa and 25 °C, and the influence of the metal composition and the content on the amount of absorbed hydrogen was clarified. It can be seen in Fig. 1 that there is the optimum metal content to maximize the hydrogen storage capacity. The increase in the amount of hydrogen storage with the increase in the metal content in relatively low metal content range is considered to be due to the increase of hydrogen spillover effect (dissociative adorption of H2 on carbon surface close to metal nanoparticles). Further, the decrease in the storage amount with metal content in high metal content range is considered to be caused by the decrease in carbon surface per material. Also, it can be observed that the amount of hydrogen storage becomes the highest when using Ti to disperse in CNH among Fe, Ni, Zn and Ti.
In addition, semi-empirical molecular orbital calculation was conducted in order to theoretically verify the hydrogen spillover phenomenon on the metal dispersed CNH. As a result, the hydrogen spillover effect is likely to occur at high pressure. It was shown that the hydrogen spillover effect can easily occur when H2 molecules present at the interface between Fe cluster and C atoms.
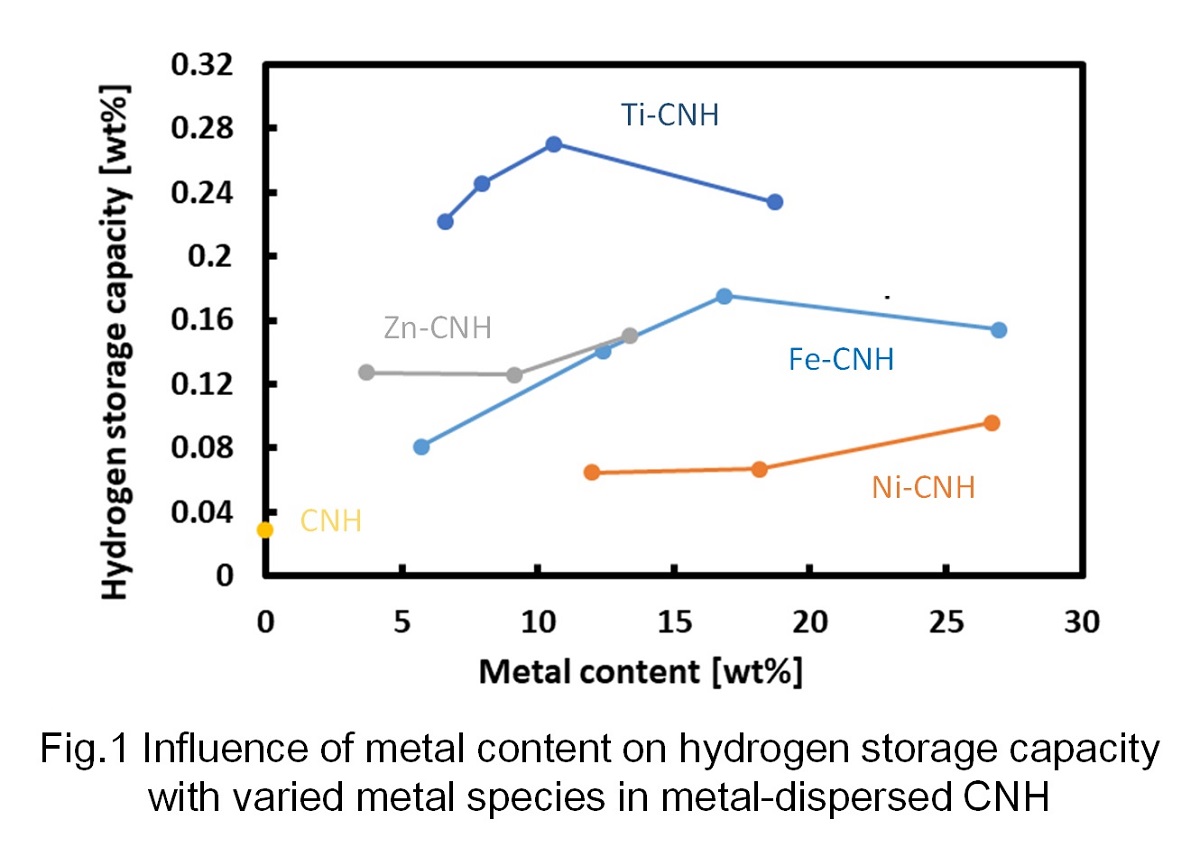
Metal-air battery and fuel cell are widely considered a promising candidate for next-generation renewable energy. One of the main problems of these technology is the catalyst for oxygen reduction reaction (ORR) that mainly depending on Pt, a very expensive material. The oxides of several transition metals have also been proposed for the purpose. Here, we propose nitrogen-doped carbon aerogel from coir fibers as an alternative metal-free electrocatalyst for the ORR. The nitrogen-doped carbon aerogel was prepared from coir fibers via simple ammonia–urea method followed by freeze drying and pyrolysis.Nitrogen molecules also have been well doped into the carbon structure, as confirmed by the existence of C=N functional group in FTIR spectra. Ammonia treatment could exfoliate the carbon structure causing defects and disorders, enhance the specific surface area, and maintain the morphology of carbon aerogel during carbonization. Kouteckỳ-Levich plots presented that the obtained nitrogen-doped carbon aerogel involved n ≈ 2 electrons towards ORR. The results suggest that the prepared nitrogen-doped carbon aerogel is a promising metal-free electrocatalyst toward ORR through 2e- pathways.
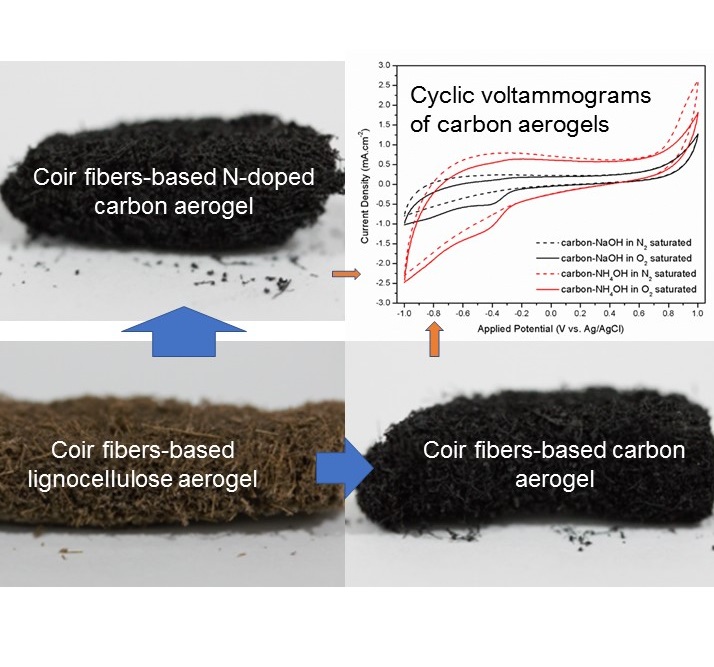
Proton-exchange membrane fuel cells, which can provide clean and efficient electricity, contain precious Pt electrocatalysts due to the sluggish kinetics of the oxygen reduction reaction (ORR). One of the major strategies to enhance the ORR catalytic activity is to control the morphology of the catalytic particles. Herein, a facile shape-directing mechanism was developed, and highly active Pt-based electrocatalysts were synthesized. Moreover, ammonium hexachroloplatinate(IV), which is known as recycling intermediate, was used as metal precursor of the synthesis experiments. It was revealed that NH4+ ions can behave as shape-directing agents in the presence of Cl– ions and O2. Based on the mechanism, Pt nanoparticles were synthesized in aqueous NH4Cl solution, forming a plate-like morphology that selectively exposes the {111} facets. Then, ammonium hexachroloplatinate(IV) and Cu salts were reduced together under the same synthesis condition, leading to Cu-doped Pt nanoplates. The Cu-doped Pt naonplates exhibited 3.7-times higher ORR catalytic activity than the commercial Pt catalysts. The present study, in which recycling intermediate has been used as metal precursor of highly active electrocatalysts, suggests a possibility to interconnect between material synthesis and recycling process.
Polymer electrolyte fuel cells (PEFCs) have received a great deal of attention for its utility in various applications, owing to their high energy conversion efficiency and clean emissions. However, a sluggish oxygen reduction reaction (ORR) rate and the low durability of conventional catalysts in PEFCs limits the wider commercialization prospects. To address these issues, our group had developed a carbon-free, connected platinum–iron (Pt–Fe) nanoparticle catalyst having porous hollow capsule structure. This catalyst exhibited an enhanced ORR activity as well as excellent durability against start-stop operations, due to the carbon-free structure. [1] Thus, the connected nanoparticle catalysts are identified promising, and further improvement in the ORR activity and durability can cater to the growing demands for PEFCs.
In this work, we developed a new connected catalyst, platinum–cobalt (Pt–Co) with a chemically ordered structure (FIG. 1A) having different catalyst atomic structures to that of connected Pt–Fe. Herein, we employed a new synthesis method for the catalyst processing, having the combination of silica coating and high temperature annealing as shown in Fig.1B. The SEM images (FIG. 1C) of the obtained Pt–Co catalysts showed a hollow capsule structure, indicating the formation of a connected Pt–Co network. The XRD patterns of the catalysts showed the peaks corresponding to an L12 type chemically ordered structure, when the annealing temperature was higher than 500 °C. Additionally, chemically ordered degree in the connected Pt–Co catalysts was successfully controlled by annealing temperature and time. Thus, we succeeded in the development and structural control of a connected Pt–Co catalyst composition for the first time. Investigating structural effects of connected Pt–Co catalysts on the ORR activity and durability is expected to provide useful guidelines to design an advanced connected nanoparticle catalyst for application in PEFCs.
References
[1] T. Tamaki et al., Energy Environ. Sci., 8, 2015, 3545–3549.
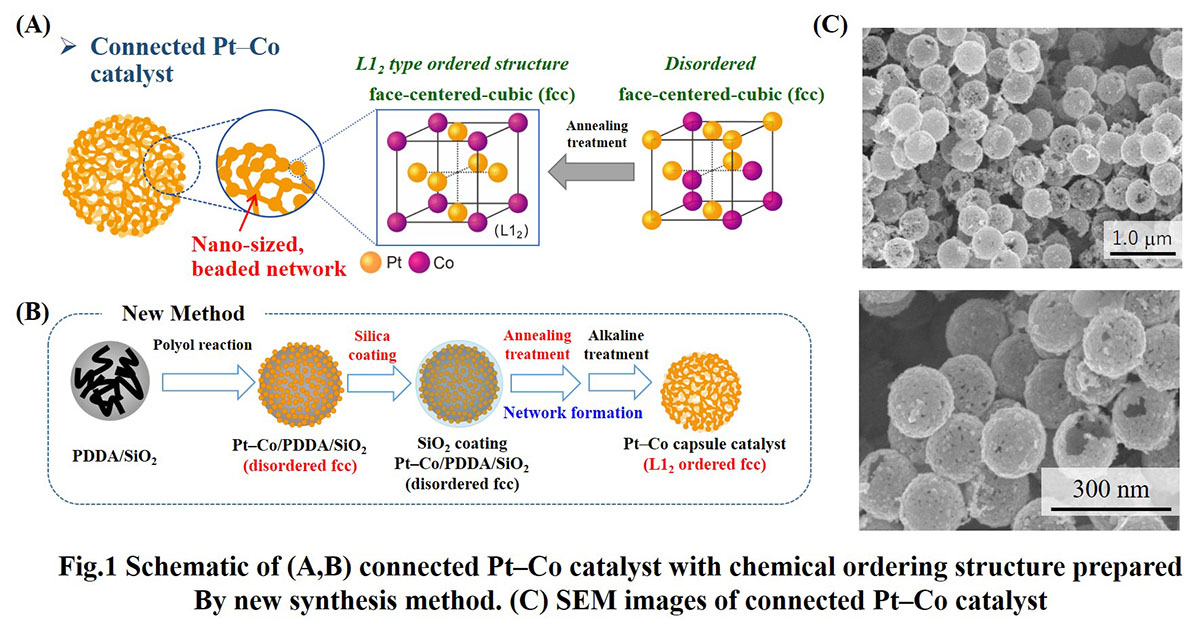
Polymer electrolyte fuel cells (PEFCs) have attracted much attention as promising energy conversion electrochemical devices. However, a conventional cathode catalyst of platinum nanoparticles supported on carbon black (Pt/C) exhibits a sluggish oxygen reduction reaction (ORR) rate and a low durability against start–stop and load cycle operations. In our group, a carbon-free, connected platinum–iron (Pt–Fe) nanoparticle catalyst with porous hollow capsule structure (FIG. 1A) has been successfully developed for an ORR. [1] This catalyst provides enhanced ORR activity and excellent durability during start-stop operations. However, there is a dissolution of alloyed metal occurring during load cycles, resulting in reduced ORR activity for the connected Pt–Fe catalyst.
In this study, we focused on a chemically ordered face-centered-tetragonal (fct) structure (FIG. 1A) to improve the load cycle durability of the Pt–Fe catalyst. The conventional synthesis method of connected Pt–Fe catalyst using a supercritical fluid treatment produces low fct degree (40–50%). Here, we propose a new and simple synthesis method using the combination of silica coating and high temperature annealing as shown in FIG. 1B. Using this method, we succeeded in preparing connected Pt–Fe catalysts having nano-sized network and a high fct degree (70–80%). The obtained catalyst exhibited a ten-times higher ORR specific activity, compared with that of the Pt/C. Furthermore, as showed in FIG. 1C, the connected Pt–Fe catalyst with the high fct degree (74%) exhibited a higher retention of ORR activity after 10,000 load cycles, compared to the low fct catalyst (44%). Thus, this study demonstrated that a highly ordered fct structure greatly contributes to the suppression of dissolution of metal, resulting in an improved load cycle durability of the connected Pt–Fe catalyst.
Reference
[1] T. Tamaki et al., Energy Environ. Sci., 8, 2015, 3545–3549.

We present an electrochemical reaction system that enables the generation of electricity, employing waste plastics as raw materials. These plastics can include polyvinyl alcohol, polyurethane, nylon, vinylon and polyethylene terephthalate. In the proposed process, the polymers are partially or completely dissolved in phosphoric acid (H3PO4) at temperatures of 100 °C or higher. When a physical mixture of plastic solid and H3PO4 solution was supplied to the anode in a temperature-controlled fuel cell, the in situ dissolved plastic component was oxidized to CO2, protons and electrons as a result of anodic polarization. This oxidation reaction at the anode was optimized by designing a catalyst support with an enlarged, ordered pore structure. An active oxygen species, believed to be the OH· radical, also plays a key role in the plastic oxidation reaction. The fuel cell demonstrated in this work functioned continuously to generate power densities on the order of mW cm-2(Fig.1). Conventional plastic disposal systems based on combustion emit toxic pollutants. The present study demonstrates a process that overcomes this issue by allowing the treatment temperature to be significantly decreased.
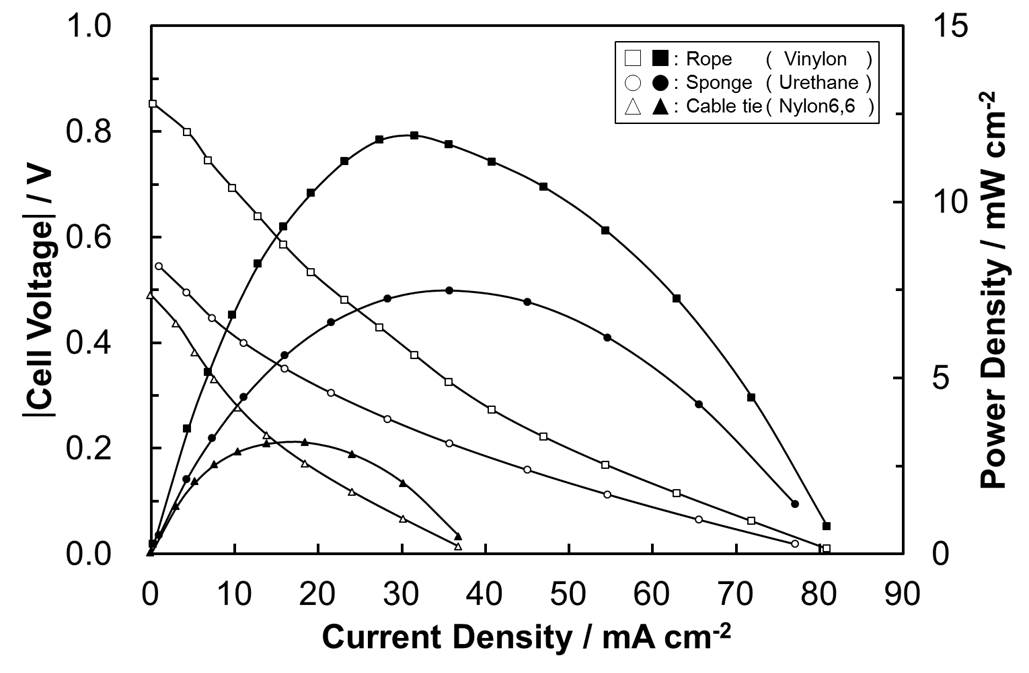
Transparent conductive oxides (TCO) with high visible transmittance and low resistance have been used in applications of optoelectronics. Dielectric/Ag/dielectric (DAD) multilayer was proposed to meet the requirements of low resistance and enough transparency [1]. In this work, a Cu-Ag bi-layer was proposed to serve as the mid-layer in oxides, including TiOx and WO3. All the films were prepared through E-beam evaporation at room temperature. The optical and structure properties of the D/Ag/Cu/D were studied by X-ray diffraction, UV-Vis, SEM, TEM and XPS. The surface morphology of Ag will be flattened by the Cu buffer layer, which can reduce the critical thickness of Ag layer. The thickness effects of Ag and Cu film on the optical properties of the D1/Ag/Cu/D2 were also investigated. According to the UV/VIS transmission spectra, the TiOx (35 nm)/Ag (12 nm)/Cu (3 nm)/TiOx (35 nm) exhibited the highest average visible light transmittance of 88%. The figure of merit (FOM) of TACT stacking is ~ 126 mΩ-1 at the wavelength of 550 nm. Using WO3 with an thickness of 40 nm as the capping layer above Ag/Cu/WO3 (WACW) shows a FOM of 73 mΩ-1. Use of Ag/Cu bilayer can widen the transmittance of the D1/Ag/Cu/D2 with an appropriate Ag and/or Cu thickness. The experimental results were also compared with the optical simulation to figure out the role of the ultra-thin Cu buried layer.
As chemical industries have grown gradually, strong alkaline chemicals are commonly used in almost all industries worldwide. Despite their usefulness, alkaline chemicals can be very dangerous as they can cause severe intoxication. In particular, gaseous alkaline chemicals require more precautions because they spread very quickly without being noticed in case of leakage. Therefore, it is important to detect strong alkaline chemical leaks promptly and easily. In this study, to detect gaseous alkalis immediately with naked eyes, we fabricated colorimetric textile sensors based on halochromic dyes. Three halochromic dyes with superior pH sensitivity were synthesized and applied to textile surface through screen printing method. The sensitivity to gaseous alkaline chemicals of the fabricated textile sensors was investigated. Ammonia was used as a gaseous alkaline chemical and the test was conducted at a concentration of 1 to 100 ppm. All of the fabricated sensors showed a high reaction rate and distinctive color change under alkaline condition due to the high pH sensitivity of the dyes. These results indicate that the fabricated textile sensors can be a promising candidate for practical sensor applications.
Recently, nanosheets (NSs) have attracted attention because of their various fascinating properties. We have developed the synthesis method of NSs inside bilayers of hyperswollen lyotropic lamellar phases of amphiphile solutions; the distance of the bilayers with several nm thickness is kept several hundred nm. We named the synthesis methods "Two-dimensional Reactors in Amphiphilic Phases" (TRAP) method. We have already successfully synthesized NSs of polystyrene and metal organic frameworks (MOF) in the thin hydrophobic sites inside the bilayers of aqueous amphiphilic solutions. To extend the range of the application of the TRAP method, we focused on aluminosilicate that is commonly synthesized in the hydrophilic reaction fields.
Aluminosilicate can be a precursor of zeolites with molecular-sized pores (0.3-1.2 nm-sized pores), which lead to the unique functions of zeolites: catalysts, adsorbents, ion-exchangers and membranes. CHA-type zeolites (CZs) are preferable catalysts for methanol to olefins (MTO) processes, which are important for the production of ethylene and propylene requied for polymer industry. CZs, however, have some problems; in particular, the pores are quickly clogged with by-products, and the resulting restriction of the reaction site and the diffusion induces the catalyst deactivation. One of the most promising solutions of the problem is the downsizing of the catalysts. Because the downsizing effect is based on the decrease of diffusion path length, CZ-NSs should have a potential as catalysts with one of the ideal shapes for the MTO processes.
Here, we report the preparation of the suspension of aluminosilicate NSs as a precursor to synthesize CZ-NSs using the TRAP method. Atomic force microscopy (AFM) and transmission electron microscopy (TEM) of the obtained CZ-NSs indicate that their thickness and horizontal width are a few nm and several hundreds of nm, respectively (Figure). This is the first example of CZ-NSs with a few nm thickness.
Seawater is currently used for desalination and salt production, and then the bittern is discharged by the operation. The bittern contains various resources, but most of them are unutilized. It is important to consider the resource recovery process from the bittern in Japan. Therefore, we focused on synthesis of layered double hydroxides (LDH) from the components of the bittern. The LDH is a clay mineral and has anion exchange ability. In this study, the anion exchange property of LDH from the bittern was investigated in comparison with that from ingredients of LDH stoichiometric concentration without components of Na, K, etc which include in the bittern but not in the basic LDH.
The simulated bittern was adjusted based on the actual composition of the bittern and was mixed with Al solution. The precipitate of LDH obtained by dropping into sodium hydroxide solution with coprecipitation method at a high pH region and the powder samples were obtained by filtration from the slurry. The structure, size, shape and ion concentration and anion exchange property of the LDH were analyzed by XRD, SEM and ICP.
The precipitated LDH from the bittern was Mg-type hydrotalcite as results of the XRD measurements. Then, the synthesis of Ca-type hydrocalumite can be also synthesized at another higher pH region. In the syntheses, all LDHs had Chloride ions as charged intercalants for keeping charge neutrality in the layer structures. The anion exchange property was investigated between Cl- and PO43-. The exchange structures were checked by interlayer distance variation before and after the exchange operation. The anion exchange capacity of the hydrotalcite form the bittern showed lower than that from the artificial ingredients.
Zeolites are crystalline microporous aluminosilicates that are widely used as adsorbents, ion-exchangers, and catalysts owing to their large micropore volumes with well-defined pore structures, ion-exchange abilities, and solid acidities. However, the pore sizes of zeolites are usually smaller than 0.7 nm, and the diffusion of molecules in the micropores is often a rate-determining step. Therefore, nano-sized zeolites attract much attention. Proper supports should be used for practical use of nanosized zeolites to prevent their aggregation, and porous inorganic submicron fibers are desirable supports for nano-sized zeolites due to strength, high formability and high surface areas. We prepared zeolite-containing submicron-fibers by centrifugal spinning. The centrifugal spinning is a method using a centrifugal force as a driving force and was developed recently for mass production of various fibers. After careful tuning of preparation conditions and fiber compositions, obtained fiber showed high BET surface areas even after repeated water adsorption test.
To meet increasingly strict regulations on emission control, there has been a high demand on effective catalysts for ammonia selective catalytic reduction (NH3-SCR) of NOx. Although the CHA zeolite dominates the current NH3-SCR market, other small pore zeolites have been studied as equivalent or more efficient catalysts. AFX zeolite is considered to be a superior catalyst for this reaction, which has not yet been applied for industrial use. This situation is in part due to partly because of its long synthesis time (typically, one week or even longer). A more efficient synthesis can help greatly save cost and energy which will pave the way for its industrialization towards emission control. In the work, we demonstrate the ultrafast synthesis of AFX zeolite from the hydrothermal conversion of FAU zeolite, which resulted in fully crystalline product in as short as 12 min.
We carried out the synthesis in the autoclave with a typical recipe, which took 7 d to yield AFX zeolite. The ultrafast synthesis was carried out in a tubular reactor made of stainless steel tube (1/4 inch) which features fast heating and cooling. To prompt the crystallization, seeding method was applied where the autoclave product was used as a seed; the composition of the synthesis precursor was optimized where FAU zeolite was used as a raw material; and, the synthesis temperature was elevated to 210 °C. Although the crystallization time was shortened, the synthesis resulted in the co-crystallization of ANA zeolite as a byproduct. To overcome this issue, the AFX seed was found to play an important role. With a seed synthesized by an improved method, the pure AFX zeolite was obtained after a synthesis of 12 min. This ultrafast synthesis method would facilitate the mass production of AFX zeolite boost its application as a de NOx catalyst.
The synthesis of zeolites, typically carried out in batch reactors like autoclaves, takes a time so long (typically, on the order of days) that the crystallization of zeolites had been believed to be very slow in nature. Long periods of hydrothermal treatment also cause a burden on both energy efficiency and operational costs. Recently, we have reported the ultrafast syntheses of a class of industrially important zeolites within several minutes. We present herein a continuous flow method for the synthesis of ZSM-5 using pressurized hot water with extremely high temperature (370 °C) as the heating medium. With a carefully tuned gel, the crystallization could be completed in tens of or even several seconds.
Direct mixing of synthesis precursor and the pressurized hot water in a millimeter-sized continuous flow reactor could result in immediate heating up to high temperatures; consequently, the crystallization of ZSM-5, from amorphous state to full crystallinity, proceeded to completion at a remarkably fast rate in a system without the addition of any seed. The well-tuned synthesis precursor, obtained by aging the initial aluminosilicate gel at 90 °C for a certain period, triggered the nucleation and ensured the formation of ZSM-5 without any byproduct at extremely high temperatures. The crystallization rate surpassed the decomposition rate of OSDA because of the creation of a favorable environment for crystallization. When a gel aged for 16 h was employed, fully crystalline ZSM-5 was obtained after a synthesis for 6 s. SEM image shows that the ZSM-5 synthesized after 6 s exhibited well crystallized facets. These unexpectedly fast crystallization rates in a continuous flow apparatus helped us to approach the limit of the zeolite crystallization.
Graphene oxide (GO) contains abundant oxygen-containing functional groups, which is an effective adsorbent to remove organic and inorganic pollutants. SBA-15 possesses large surface area, large pore volume, adjustable pore size, and thick silica wall. In the current study, we attempted to synthesize GO/SBA-15 nanocomposite. The large surface area of SBA-15 can provide abundant adsorption sites and plays a crucial role in increasing the adsorption efficiency and reuse of GO. This investigation characterized GO/SBA-15 materials through transmission electron microscopy (TEM), field–emission scanning electron microscopy (FESEM), X-ray diffractometry (XRD), surface area analysis, and Raman spectrometry. Various experimental parameters including the initial concentration of dye, calcination temperature, and pH of the solution were investigated. Immersion of GO into mesoporous SBA-15 increased methylene blue (MB) adsorption capacity. Increasing the initial concentration and solution pH enhanced the adsorption efficiency of GO/SBA-15. Optimal MB adsorption was observed at a calcination temperature of 550 °C. N2 adsorption-desorption measurement indicated that the GO/SBA-15 samples were mesoporous materials with uniform pore distribution. The addition of GO did not affect the mesostructure of the SBA-15 framework. The GO/SBA-15 possessed a large surface area of 891 m2/g, large pore volume of 1.035 cm3/g, and wide pore diameter of 6.50 nm. TEM of the synthesized GO/SBA-15 revealed that GO was homogeneously coated on the surface of mesoporous SBA-15. The adsorption isotherm and kinetics were more closely represented by the Langmuir model and pseudo-second order model, respectively, indicating that the adsorption type was monolayer adsorption. Reutilization experiments were carried out to investigate the stability and regeneration ability of the GO/SBA-15 materials. The adsorption capacity still remained above 95 % after five-time reused, demonstrating the excellent regeneration of the GO/SBA-15 materials. This method of preparing GO/SBA-15 composite is potentially useful for removing organic pollutants from wastewater.
Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. Nowadays, the flame retardancy of polymers are the major concern. In this study, Polycarbonate (PC) was selected to develop an efficient flame retardant (FR) of high temperature polymer. A series of organo phosphorus flame retardants (FRs) based on aromatic phosphate and cyclic phosphate, mainly Biphenyl bis(cyclic 1,3-propanediol phosphate)(BP-CPP) and Biphenol bis(cyclic 2,2-dimethyl-1,3-propanediol phosphate)(BP-DMPP), were synthesized for polycarbonate. Their thermal stability and flame retarding efficiency as a single-component additive were investigated and compared with resorcinol bis(diphenyl phosphate) (RDP). Flame retarding efficiency was evaluated by the UL-94 test method. The V-0 rating was achieved at RDP/ BP-CPP loading of 6/2 wt% for polycarbonate, which is far better than that of resorcinol bis(diphenyl phosphate) and cyclic phosphate-based FRs. And its flame retarding performance was studied by the UL-94 and thermogravimetric analysis (TGA).
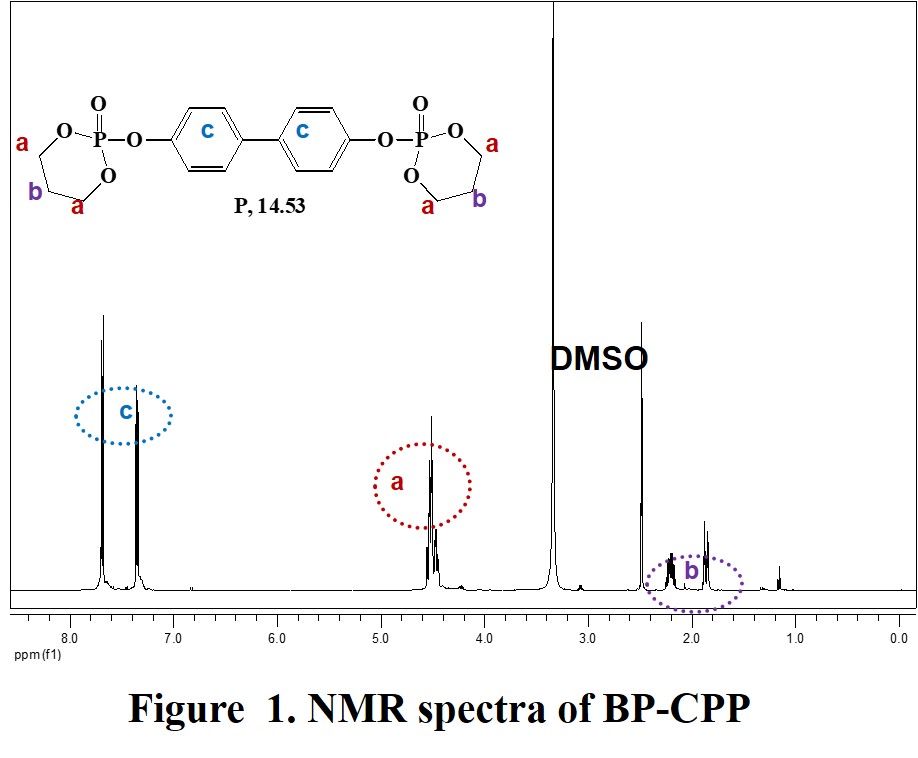
Porous materials have been widely applied to the different fields, such as adsorbents, heterogeneous catalysts, drug loading and delivery. In the current studies, the porous materials are mostly constructed by gas foaming, photolithography, templating, etc. Here, we report an innovative manufacturing process for the fabrication of three-dimension porous structures containing other ingredients including the organic and inorganic via the chemical vapor deposition (CVD). The porous materials were made by poly-para-xylenes (PPXs) replacing the origin sublimating templates via the sublimating/deposition process, and the ingredient that cannot be sublimated would be encapsulated within the PPXs porous materials. The mechanism of the porous structures is that the pores are formed by the gas vapor occupying the remaining vacancies lifted by sublimated template. By adjusting the sublimation rate of the template and the rate of PPXs deposition, the porosity can be controlled. In this report, we present three examples including the magnetic porous particles, cell-containing scaffolds and the protein-encapsulated porous particles. This novel approach overturns the general concept that vapor deposition forms uniform thin film and also provides another way to manufacture multifunctional porous materials.
In this study, a oriented organometallic frame/polyvinyl alcohol (MOF/PVA) nanofiber material was prepared by electrospinning. PVA was uniformly mixed with a MOF to form a solution. In this experiment, zinc nitrate Zn(NO3)2) and 2-methylimidazole were used to synthesize ZIF-8 powder material by in-situ process, and then added to 40mg, 120mg and 200mg respectively. The 10wt% PVA polymer solution was thoroughly stirred, the precursor was disposed, and the electrospinning process was performed. The crystal structure and scanning electron microscope (SEM) were detected by X-ray Diffaction (XRD). Observing the trend of fiber arrangement and the surface morphology of ZIF-8 grown on the fiber, the weight change of the sample detected by Thermogravimetric Analysis (TGA), and the Fourier Transform Infrared Spectroscopy (FTIR) test sample The change of properties before and after mixing of different contents.
As a results, it was found by SEM observation that PVA/ZIF-8 10 wt% was formed by bead-like fibers after electrospinning. From this phenomenon, ZIF-8 was uniformly dispersed in a polymer solution to reunite ZIF-8 by electrospinning technology. ZIF-8 can be evenly distributed in the fiber. XRD analysis showed that there were indeed various characteristic peaks of ZIF-8 in the composite fiber, and it was further confirmed that ZIF-8 was contained in the fiber.
Metal-organic frameworks (MOFs) are porous material composed of metal ions and organic ligands, which have ordered crystalline structure with micro pores and high surface area. MOFs are recently focused as materials for various applications. In the development of the MOF-based mixed-matrix membranes for membrane separation processes, the size and the shape of particles are the important factor for membrane performances. Zeolitic imidazolate framework-L (ZIF-L) is one of the two-dimensional MOF which is focused as materials for mixed matrix membrane; however, the control of the shape and the particle size has not been sufficiently reported yet.
In this study, we report the effect of surfactants on the synthesis of ZIF-L. Surfactants can adsorb on the specific surface of the MOF crystal in synthesis, and can control the size and the shape of the resultant MOF particles. In the synthesis of ZIF-L, we used cetyltrimethylammonium bromide (CTAB) as cationic surfactant, sodium dodecyl sulfate (SDS) and sodium dodecylbenzene sulfonate (SDBS) as anionic surfactants, TWEEN(R) 20, 40, 80 as nonionic surfactants. We synthesized ZIF-L in the presence of various surfactants with various concentrations and characterized the crystal structures using X-ray powder diffraction, the size and the shape of the particles using scanning electron microscope, porous structure using nitrogen adsorption measurements. The characteristics of obtained ZIF-L particles are highly influenced by the species of surfactants, and the details will be reported in the presentation.
Metal-organic frameworks (MOFs) are novel porous materials composed of metal ions and organic ligands. MOFs have ordered porous structures and show adsorption and molecular sieve properties. As one of the applications, MOF-based mixed-matrix membranes (MMMs) have been reported for gas separation. The morphology of MOF particles is the important factor for membrane performances. It is reported previously that MMMs prepared by stacking two-dimensional (2D) MOFs horizontally in the membrane plane can exhibit high gas selectivity by reducing the permeation of non-permeable component. Zeolitic imidazolate framework-7-III (ZIF-7-III) is one of the 2D MOFs which is composed of zinc ion and benzimidazole and forms layered crystal structure with micropores which diameter is approximately 0.29 nm. In the previous report, composite membrane was prepared using ZIF-7-III nanosheets which was made by exfoliation of layered ZIF-7-III particles. The initial ZIF-7-III particle size should be influential on membrane performances; however, there has been no report on the control of ZIF-7-III particle size.
In this study, we report the control of ZIF-7-III particle size using water-in-oil microemulsion. Microemulsion was prepared using heptane as continuous phase, water as dispersed phase, cetyltrimethylammonium bromide (CTAB) as surfactant and 1-hexanol as co-surfactant. Synthesis of ZIF-7-III was conducted in microemulsion, and obtained white powder was characterized using powder X-ray diffraction and scanning electron microscope. Reaction conditions such as reaction temperature, concentration of CTAB were highly influential on the morphology of resultant ZIF-7-III particles. The details will be reported in the presentation.
Metal-organic frameworks (MOFs) are an emerging class of nanoporous materials. Owing to their highly ordered nanopores, MOF membranes are promising for higher selectivity and permeability than polymer membranes which dominate industrial membrane separation. However, high cost of support materials and lack of robust membrane fabrication methods are problematic. Here, to establish a versatile fabrication method, we investigated the formation process of MOF membranes on hollow fibers, originally used for water purifier filter, which are cheap and easily available.
Here we focused on zeolitic imidazolate framework-67 (ZIF-67) composed of Co2+ ions and 2-methylimidazole (MIM) linker, which is a family of MOFs, and applied a secondary growth method. In this method, seed crystals were deposited on the fiber by immersing the fiber in MIM and Co(NO3)2 aqueous solutions alternately, followed by the seed crystals growth by immersing seeded fibers in a mixed aqueous solution of MIM and Co(NO3)2 (growth solution). We first investigated effects of concentration ratio of growth solution (R = [MIM]growth / [Co2+]growth). A small R resulted in the deposition composed of needle-like crystals with many gaps (Fig. 1a), while a large R produced a thin film densely packed by rhombic dodecahedral crystals (Fig. 1b). However, resultant films showed no separation selectivity.
To improve the membrane performance, we modified the growth process. Immersing seeded fibers in a growth solution with a small R was followed by subsequent growth under a large R so that ZIF crystals first grow vertically and then merged with each other by filling gaps in the second growth. This two-step process successfully produced a thick and dense membrane (Fig.1c) with separation factor of 1.15 for air and propane, which suggests that the two-step growth reduces cracks. Hence, we demonstrated that the stepwise control in the crystal growth direction enables the membrane densification.
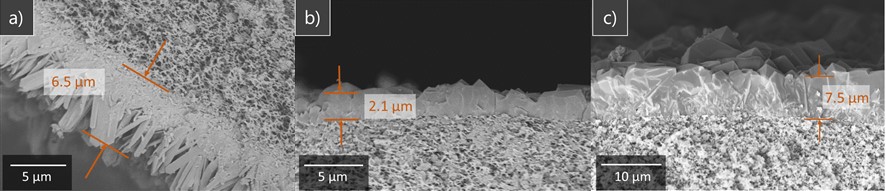
Metal-organic frameworks (MOFs) are new porous materials discovered in the 20th century. MOFs are synthesized through self-assembly of metal ions and organic ligands, and they have regular arrays of micropores and a large surface area. In addition, by selecting components, MOFs exhibit various crystal structures with tunable pore size. From these characteristics, MOFs are expected to be applied to the medical field, especially drug delivery systems (DDSs). In DDSs, the drug with necessary amount are transported to the right place at the right time by spatial and temporal control. Generally, a carrier for containing or binding the drug is required for DDSs. Although it is common to use a liposome and a polymeric micelle as the carrier, the high inclusion method of drugs has not been established. In recent studies, therefore , MOFs have attracted much attention as a new DDS carrier with high drug inclusion.
In this study, the drug-release behavior from MOFs by a pH change was investigated. As model MOFs, we selected zeolite imidazolate framework-8 (ZIF-8) and Universitetet i Osolo-66-NH2 (UiO-66-NH2). It is reported that the crystal structures of ZIF-8 and UiO-66-NH2 collapse under acid and base conditions, respectively. As model drugs, indomethacin and ibuprofen were selected. We found that ZIF-8 included indomethacin and its dissolution rate from ZIF-8 was the fastest at pH 5.0. On the other hand, UiO-66-NH2 included ibuprofen and its dissolution rate from UiO-66-NH2 was the best at pH 8.5. These results will lead to the development of the new pH responsive DDS with high drug capacity.
Hydrogels are used in applications such as separation, reaction, and drug delivery systems. The diffusivity of solute in hydrogels is an important parameter. For example, the concentration and the diffusivity of solutes, such as oxygen and NH4+, in the hydrogel can affect the activity of ammonia-oxidizing bacteria (AOB) immobilized within the hydrogel for an ammonia wastewater treatment system. The goal of this study is to develop a novel method to measure the diffusivity of solute in spherical hydrogel using a microsensor. A microsensor measures the chemical environments at the scale of the micrometer-sized sensor tip, as well as the concentration of solute in hydrogel. Millimeter-sized spherical hydrogel is suitable for the experiment using the microsensor and the analysis of diffusion kinetics. The spherical polymeric hydrogel was prepared using a production method combining sedimentation polymerization and two-fluid atomization, developed in our previous study. Oxygen, NH4+, and N2O were used as model solute for AOB-catalyzed reactions. The experiment on oxygen diffusion was carried out as follows. Firstly, a microsensor was inserted in the center of the spherical hydrogel and the hydrogel was placed in anaerobic water purged with N2 gas. Then, water was purged with air and the dissolved oxygen (DO) concentration in the hydrogel was measured continuously. The DO concentration was approximately 0 during initial period, and increased with time up to approximately 230 mmol/m3 in air-saturated water. Diffusivity of solute in hydrogels was successfully analyzed using the solution of the Fickian diffusion equation within a sphere.
Pd catalyzes various kinds of reactions such as automobile exhaust emission treatment and organic synthesis. In this study, we focus on polymer gel as a heterogeneous catalyst carrier. The gel is a polymer of a three-dimensional network structure swollen with a solvent and can be handled like a solid, but the inside of the gel is also like a liquid. The aim of this study is to develop a polymer gel supported Pd catalyst using various polymer gels, to clarify the influence of the absorption and adsorption ability of the gel and the holding state of Pd(II) ion on the catalytic activity to construct a design guideline for a high performance gel catalyst. The model polymer gel was copolymer gel of allyl mercaptan (AM) having a thiol group interacting with Pd(II) ion and multicomponent, and gel having amino group and ammonium group in side chain. The various kinds of gels were synthesized by free radical polymerization. Pd(II) ion-adsorbed gels were prepared through the Pd(II) ion adsorption experiment, and subsequently Pd nanoparticle-loaded gels were prepared by the reduction of Pd(II) ions using NaBH4. The model organic synthesis was Suzuki-Miyaura coupling (synthesis of biphenyl with phenylboronic acid and iodobenzene as reactants) using both catalysts Pd(II) ion-adsorbed gels and Pd nanoparticle-loaded gels. The adsorption properties of Pd(II) ions of various gels, characteristic properties of gel supported Pd, and Suzuki-Miyaura coupling reaction characteristics using gel supported Pd were investigated.
The excited electrons and holes generated upon by irradiating UV ray to TiO2 have powerful redox power. They produce reactive oxygen species (ROS) under aerobic conditions at the surface. These species rapidly decompose organic compounds to convert them into CO2 and H2O via carbon-carbon bond cleavage. On the other hand, they can also catalyze organic synthesis reactions such as carbon-carbon bond formation. The reactions must be carried out in dry anaerobic conditions in order not to produce ROS that can damage organic compounds. Usually, TiO2 has been used as a powder to increase the catalytic effect. However, the use of particles involves the need of incorporating extra stages in the purification process, such as separation and catalyst recycling. We focused on a composite of TiO2 nanoparticles and a polymer gel in which TiO2 nanoparticles are dispersed and immobilized in the polymer gel as a heterogeneous catalyst. The purpose of this study is to develop a novel TiO2 nanoparticle-loaded polymer gel and clarify its applicability as a catalyst for organic synthesis reaction. As polymer gels, N,N-diethylacrylamide gel, N,N-dimethylacrylamide gel, and N-isopropylacrylamide gel were used. Commercial TiO2 nanoparticle (AEROXIDE® TiO2 P-25) with 21 nm in diameter was used. The composite gels were synthesized by free radical polymerization. The 4-iodetoluene dehalogenation reaction as a model organic synthesis reaction was performed by a batch operation using 4-iodetoluene, diisopropylethylamine, dried composite gel, and acetonitrile as a solvent. The reaction was irradiated with UV light and stirred at room temperature under a nitrogen atmosphere. The composite gels catalyzed the reaction successfully.
Sodium alginate aq. is known to form hydrogel on mixing with CaCl2 aq., where Ca2+ works as the crosslinker. Megascopically, the above process is observed as instantaneous gelation. The authors have been interested in capturing the quick gelation in terms of how fast it occurs on actual mixing of these two starting aqueous solutions. First, 1wt% sodium alginate aq. was added dropwise into 10M CaCl2 aq. and the time evolution of the morphology of the droplet after the impingement onto the surface until the stabilization of its outer surface was recorded in a high-speed motion picture. The difficulty in fulfilling our observational purpose relying on the above method was revealed that the gradual morphological alteration toward the spherical geometry is temporarily too moderate to be detected by the high-speed motion picture method. In order to capture the arrest in the deformation of the gelling solution more effectively, we needed to choose a situation where the tiny surficial undulation of the gelling solution is incessantly induced by being in contact with 10M CaCl2 aq. In the intensively discharged gelling solution out of an injection syringe, it was found that the straight linearity of the gelling solution in the translational motion was observed while it kept in the state of a flowable solution. The beginning of the deviation from the as-injected linear flow implied the vanishing fluidity. Thereafter, the gelling solution was seen to behave like rod-like objects forming an entangled coil. From the discharging velocity of the gelling sodium alginate aq. at the exit of the injection syringe, the timescale for the gelation was estimated to be 10-3s in the order of magnitude.
In many countries, arsenic level in groundwater exceeds the acceptable limit. Although we removed Arsenate in our previous research, removal of Arsenite is harder. The purpose of the study is to adsorb Arsenite, As(III), from ground water by adsorption using a composite of cationic polymer gel impregnated with iron hydroxide. The cationic polymer gel that we used is N,N-dimethylamino propylacrylamide, methyl chloride quaternary (DMAPAAQ).
The gel was prepared in a unique method where Sodium Hydroxide and Iron (III) Chloride was added to the monomer and initiator solutions, respectively, in order to maximize the FeOOH contents in the polymer structure of the gel.
Transmission Electron Microscope (TEM) images show that our gel contains visible FeOOH particles in its structure. The Thermogravimetric Analysis (TG) indicates that the maximum weight percentage of FeOOH particles in the gel is 53.7%. Generally, there are three types of FeOOH. We performed Mössbauer spectroscopy to determine the type of FeOOH contained in the gel. The results match with that of γ-FeOOH. Previous arsenic removal experiments found that γ-FeOOH helps to remove both As(III) and As(V). Our studies suggest that in the structure of DMAPAAQ + FeOOH gel, the polymer structure of DMAPAAQ adsorbs 35.6%, whereas γ-FeOOH components adsorb 64.4% of arsenic. Hence, DMAPAAQ + FeOOH can adsorb arsenic successfully. FTIR analysis suggests that when As(III) and As(V) were adsorbed by the DMAPAAQ + FeOOH gel, changes such as shifts, appearances and disappearances in several surface functional groups occurred. These results further prove the adsorption of arsenite to the gel.
Our study successfully showed that the newly developed gel composite, DMAPAAQ and FeOOH, adsorbed As(III) effectively because of having γ-FeOOH components in the gel structure. The novelty in our research is in the unique preparation method of the gel, and It's characterization.
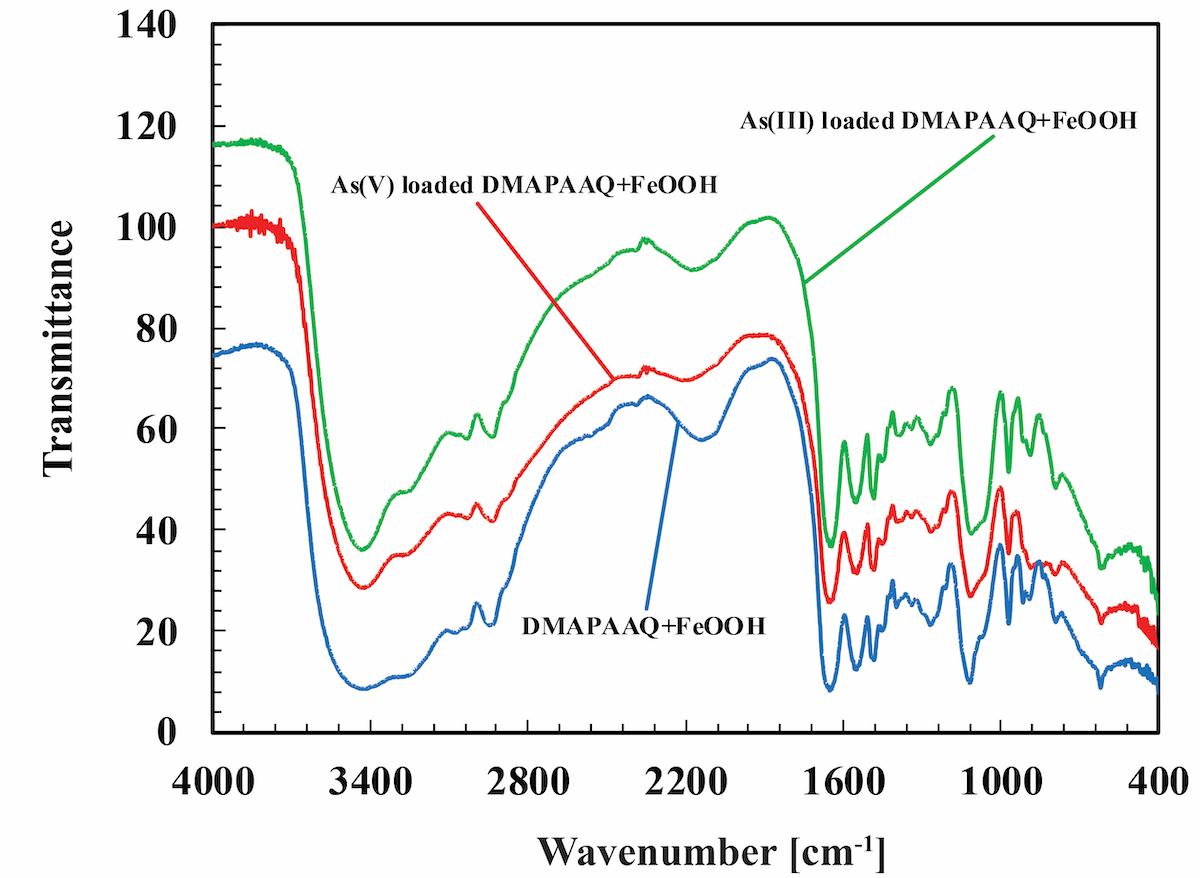
Catalysis by Heteropoly acids (HPAs) and polyoxometalates (POMs) having a higher demand worldwide, as it can be designed to accelerate complex reactions and be more environmentally friendly. However, recycling of water-soluble solid catalysts remains a problem. The synthesis of a recyclable composite with catalytic properties is the key to better use of HPAs and POMs. Many researches have mentioned the method of synthesis by immersing a porous carrier in a supported solution. However, the catalytic abilities of the previously studied composites after multiple uses have rarely been mentioned. In this research, a novel idea is proposed to synthesize a heteropoly acid supported composite. A complex hydrogel with catalytic properties was synthesized by mixing an anionic monomer with a heteropoly acid. The heteropoly acid particles were inserted inside the hydrogel by the interaction forces between the anions. Thus, preventing the water-soluble heteropoly acid from being lost during the catalytic reaction. The complex hydrogel is consisted of the anionic monomer 2-acrylamide-2-methylpropanesulfonic acid (AMPS) as a carrier, N,N′-Methylenebisacrylamide (MBAA) as a crosslinker and the typical keggin-type HPA: H3PW12O40, which was synthesized by one-pot method. Then the composite is divided into five groups. Following this, the five groups were washed by deionized water for 24h, 48h, 72h, 96h and 120h respectively. The existence of H3PW12O40 crystalline nanoparticles in the composite was confirmed by X‐ray diffraction (XRD). After comparing the samples from different groups by Scanning Electron Microscope (SEM), all the samples have similar surface structure and nanoparticle aggregation state. Nanoparticles were observed from the samples of each group by Transmission Electron Microscope (TEM). From the thermogravimetric analysis (TGA), the particle content rate of samples from each group was kept at about 17% as the washing time increased. It indicates a higher potential of this synthetic method to a wide range of applications.
Removal of heavy metals from ground water and soil has been a common problem both in industry and agriculture, since high concentration of heavy metals are harmful to environment and human health through water and food. In this study, a simple and effective removal method of various harmful anionic ions such as arsenic acid, selenium acid, and chromium acid from soil and water was proposed. Generally, these anions were removed by cationic adsorbent or cationic ion exchange resign. However, various adsorbents have low ability to remove heavy metals from low concentration solutions, and it sometimes desorb those ions when interfering ion exist. We present new ion removal method by using hydrogel. Anionic hydrogel of 2-Acrylamido-2-methylpropanesulfonic Acid Calcium salt (AMPS-Ca) and cationic hydrogel of N,N-Dimethylamino Propyl Acrylamide (DMAPAA gels) were used to remove anionic heavy metal ions. Each heavy metal ion could be partially removed by both hydrogels. It was found that removal of arsenic and selenium by AMPS-Ca hydrogel as attained by the formation of heavy metal calcium salts in the hydrogel. On the other hand, the crystallinity of the heavy metal salt was not found in the DMAPAA gel. It is found that removal of heavy metals by each hydrogel was made by adsorption or formation of heavy metal salt in the hydrogel. In addition, the various hydrogels used in this study could absorb water as well as sodium acrylate. Therefore, it is suggested that they also provide a method of solidify the heavy metal contaminated sludge discharged in tunnel excavation work. The contaminated sludge will be recycled after the treatment of the hydrogel.
Introduction
The demand for lithium-ion batteries has rapidly increased in recent years, and the supply will be insufficient in near future. Therefore, the establishment of lithium recycling process is required to solve the problem of the increase of the waste lithium batteries. However, conventional lithium recovery processes are complicated and costly. We have presented new simple lithium recovery process1) using hydrogel with a quaternary amine ((3-Acrylamidopropyl) trimethyl ammonium chloride)(APTAC). In this research, the reaction mechanism of lithium and carbonate ion are investigated.
Result and discussion
A schematic diagram of the process is shown in Figure 1. At first, APTAC gel was immersed in the solution containing anion which exchanged Cl-. The gel was then immersed in LiCl aqueous solution. The Li+ reacted with the anion in the gel to produce lithium salt. The Li+ was recovered as the lithium salt within the gel.
We investigated which anion was suitable for Li+ recovery and found that the HCO3- recovered the lithium ion best among PO43-, HCO3- and CO32-. HCO3- reacted with Li+ more than their stoichiometric ratio, 1.0, when the initial concentration of Li+ was more than 1000 ppm. It is considered the Li+ initially reacted with HCO3- to form metastable LiHCO3 when its initial concentration was below 1000 ppm. Then H+ in a part of the LiHCO3 exchanged with Li+ in the solution to form stable Li2CO3 when it was higher than 1000 ppm. Therefore, Li+ was recovered more than double of the stoichiometric ratio.
The elemental composition of the gel after the Li+ recovery was analyzed by XPS. The gel had the C-O single bond that was not exist in the original APTAC gel. We found that Li+ reacted with HCO3- and was recovered as LiHCO3 or Li2CO3 in the gel.
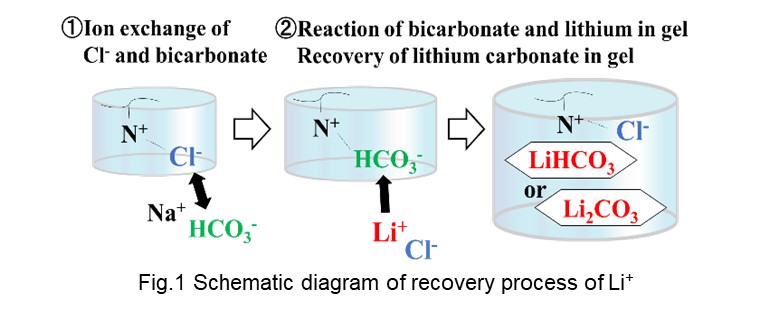
Gel bead can be used as a carrier for drugs and fertilizers and its diffusion characteristics has been studied for controlled release of chemical substances. In our study, the effect of the addition of the dispersed phase on the diffusion rate of substances in hydrogel was investigated. The oxygen diffusion rate in hydrogel containing microbeads was measured to study the effect of the size and amount of microbeads on the diffusion inhibition.
Figure 1 shows the effect of microbead addition on apparent diffusion coefficient of oxygen in hydrogel. The apparent oxygen diffusion coefficient was calculated by solving one-dimensional unsteady diffusion equation with measured data of dissolved oxygen concentration profile in the gel. First, the apparent oxygen diffusion coefficient in the gel containing the microbeads was smaller than that without the microbeads. It was confirmed that the addition of the microbeads inhibited oxygen diffusion.
Next, the apparent diffusion coefficient decreased with an increase in the microbeads holdup. On the other hand, the diffusion rate did not depend on the microbead size. To develop the estimation method of apparent diffusion coefficient, diffusion in hydrogel containing microbeads was considered by analogy with the diffusion theory in porous medias (Ohashi et al., 1993) where diffusion path is lengthened by solid obstacles. The experimental values agreed well with the model. This suggests that the apparent diffusion coefficient can be estimated from the volume fraction of the added dispersed phase.
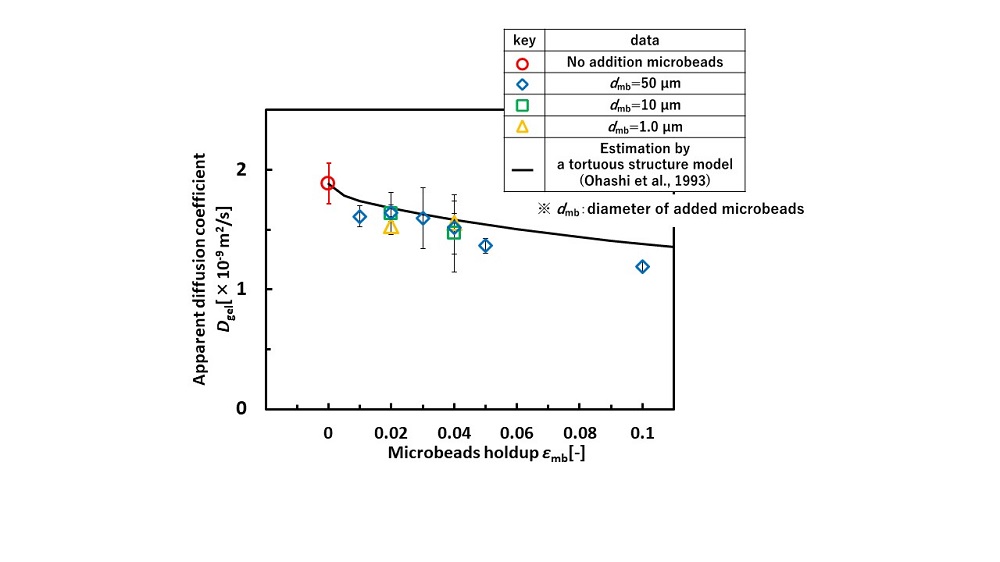
Heat dissipation is one of the most important issue for designing high performance devices. Thermal interface materials (TIMs) are used to promote heat transfer at the solid-solid interface between IC chip and heat radiator in electronic devices. Presently, composite materials of thermally conductive fillers and polymers are widely used, however polymers have problems of low thermal stability and low thermal conductivity. Here, we propose and report Ag aerogel films composed of Ag foils sandwiched with Ag porous layers (Fig. 1a,b). The film was fabricated by simultaneously forming Ag particles by gas-evaporation and depositing Ag particles on an Ag foil. The boat-foil distance was carefully optimized to make the Ag porous layer soft and uniform. The steady-state measurement showed a thermal resistance as small as <20 mm2K/W (Fig. 1c), which is similarly small as the high performance TIM of an indium sheet. Our aerogel TIM, made of Ag having a melting point (962 °C) much higher than indium (157 °C), can expectedly be used for thermal management under high-temperature conditions.

Hydrogel have good flexibility and biocompatibility, so they cause little damage by implantation in the body. Furthermore, since hydrogels can release drugs, they are expected to be applied to a local drug delivery system. The amount of drug used can be reduced and side effects can be alleviated by the local drug delivery system. However, the hydrophilicity of hydrogel interfere result in suppression the controlled release of hydrophobic drugs such as anticancer agents or antibiotics. We have developed hydrophobically-modified gelatin (HMG) and could prepare a hydrogel by only hydrophobic interaction. HMG hydrogel do not require the use of chemical crosslinkers, which are often toxic to the body. In addition, HMG hydrogel have hydrophobic segments in its structure, which can absorb hydrophobic drugs and expect to control their release. As a factor to control the amount of adsorbed hydrophobic drug in the hydrogel, we focused on the strength of hydrophobicity of hydrophobic group. HMG with different alkyl chain length (C4~C12) of modified hydrophobic group were synthesis. The swelling ratio of these hydrogels were decreased in increasing the chain length of alkyl group modified to HMG. This result means that the increase of alkyl chain length intensifies the hydrophobic interaction. Then, HMG hydrogel was adsorbed with uranine used as a model for hydrophobic drugs. The adsorption test exhibited that the amount of hydrophobic drug adsorbed in HMG hydrogels could be controlled by varying hydrophobic alkyl chain length. The controlled release of the drug was confirmed by drug release experiments in vitro. In conclusions, HMG hydrogel could control drug adsorption and delivery.
Novel organic-inorganic hybrid material was developed as a LED sealant.
Polysilsesquioxane with -SH group (PSQ-SH) and acrylic monomer were selected as inorganic and organic components, respectively. Trimethylolpropane Trimethacrylate (TRIM) which is a trifunctional monomer, has been selected as an acrylic monomer, and curing of the material was performed by the Thiol-ene reaction.The LED light emitting substrate was sealed using the prepared PSQ-SH/TRIM hybrid material, and a light emission test was performed. However, there was a problem that cracks occurred in the hybrid material during lighting. Therefore, Ditrimethylol propane tetraacrylate (DTMPA), which is a tetrafunctional monomer, was newly selected to prepare a PSQ-SH/DTMPA hybrid material in order to enhance the strength and heat resistance of the material. The organic component can be reduced by the increasing the number of the functional group in the monomer, and a more inorganic rich material can be prepared. The heat resistance can be improved by increasing the inorganic components in the hybrid material. The mechanical strength of the hybrid material can also be improved by increasing the polymerization density. Therefore, the characteristics of novel PSQ-SH/DTMPA hybrid material were evaluated and compared with PSQ-SH/TRIM hybrid material. As the contents of the evaluation, the heat resistance was evaluated by confirming the weight reduction start temperature using the Thermalgravimetry-differential thermal analyzer(TG-DTA) analysis, the mechanical strength was also evaluated by bonding the glass substrates with a hybrid material and measuring the sealing strength. The obtained results did not improve with respect to the heat resistance even if the monomer was changed.However, the mechanical strength was greatly increased by changing the monomer.
In conclusions, it was possible to suppress the generation of cracks at the time of lighting by using the PSQ-SH/DTMPA hybrid material compared to PSQ-SH/TRIM hybrid materials in the light emission test.
Rod-shaped nitroxide radical (NR) compounds with a five-membered ring NR moiety in the mesogen core (LC-NRs) show unique intermolecular magnetic interactions in their liquid crystalline (LC) and isotropic liquid (Iso) phases above room temperature; their magnetic susceptibilities increase abruptly at the phase transitions from the crystalline (Cr) to LC phases. This phenomenon is called magneto-LC effects. This effect is considered to originate from inhomogeneous intermolecular magnetic interactions. In fact, we confirmed that the effect was enhanced by adding a small amount of (2S,5S)-2 to (2S,5S)-1 (Figure). We also synthesized a new LC-NR with an azobenzene moiety (2S,5S)-3 whose trans and cis isomers have similar shapes to (2S,5S)-1 and (2S,5S)-2, respectively. Since an azobenzene moiety shows the photoisomerization, we expected to change reversibly in inhomogeneity of intermolecular magnetic interactions of (2S,5S)-3 due to the change of the molecular shapes during the photoisomerization. Here, we report the synthesis of a new LC-NR with an azobenzene moiety in the mesogen core and the effect of the photoinduced phase transitions on magnetic properties.
We observed the phase transition behaviors under ultraviolet (UV) or visible (Vis) light irradiation by polarized optical microscopy and measured UV-Vis light absorption spectra. We also measured electron paramagnetic resonance (EPR) spectra under continuous irradiation of UV or Vis light.
Photoinduced phase transitions occur only between LC and Iso phases under UV or Vis light irradiation for (2S,5S)-3. This is likely to arise from the increase of the ratio of cis-isomer by the UV light irradiations, which could reduce the orientational order parameter. EPR spectra indicate that magnetic susceptibility increases under UV light irradiation and decreases under Vis light irradiation. We confirmed that bidirectional switching of the magnetic properties of (2S,5S)-3 occurs under alternating irradiations of UV and Vis light.
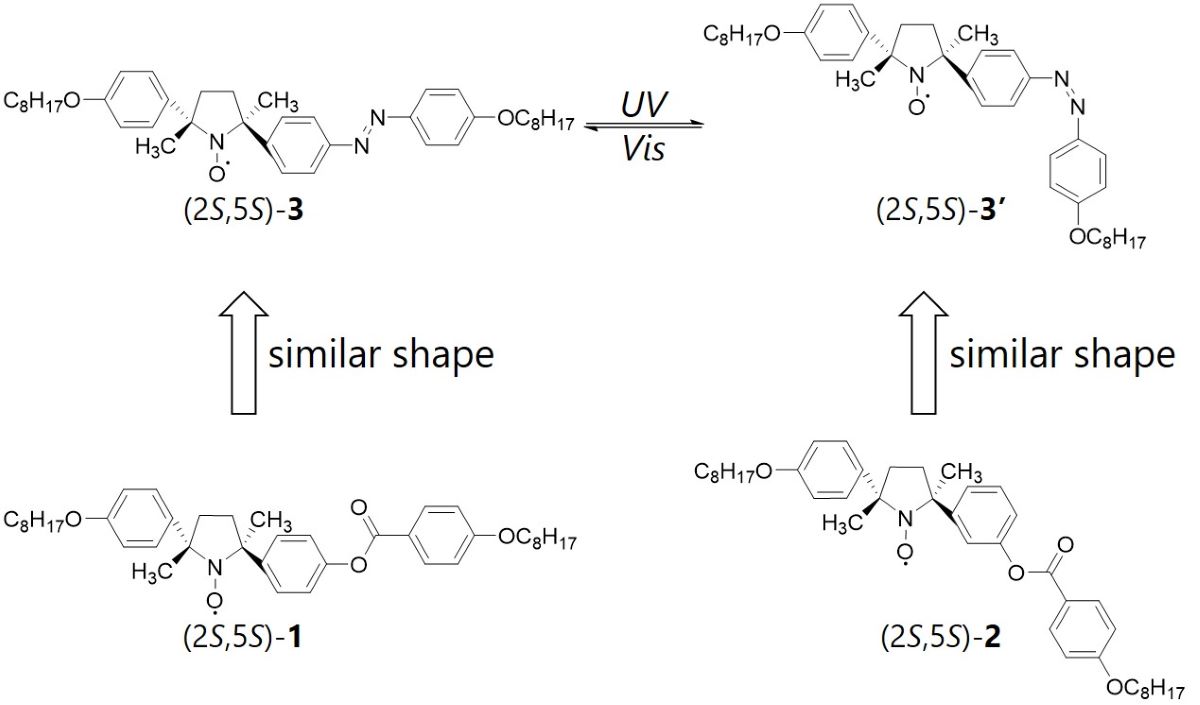
With the increasing demands on wearable devices, the stretchablity of various electrical devices have drawn much attention recently. The most commonly used method to enhance mechanical performance of stretchable polymers is introducing nanofillers to form a composite system. Polydimethylsiloxane (PDMS) is the most widely used silicon-based elastomer, and has been widely used in many medical, biological, and microfluidic applications. In the literature, various nanofillers, such as carbon nanotubes, graphene sheets, and silver nanowires, have been well dispersed in PDMS to demonstrate the flexibility and versatility of PDMS composites.
On the other hand, because of the chemical inertness, it is difficult to include ionic fillers in PDMS. PDMS composites with ionic filler usually have problems like filler aggregation, delamination, phase separation, or fractures, under stretching conditions. To address this problem, in this work, a water-in-oil type emulsion is used as a carrier for water-based ionic materials. The relationship between applied sheer rates and droplet size will be examined to elucidate the emulsion process. Moreover, emulsion stability will also be carefully characterized to investigate the effectiveness of various emulsifying agents. In summary, this research provides a new method for PDMS composite synthesis and opens a new avenue to stretchable polymer applications.
In the last two decades, many reports revealed the novel functional properties of natural and synthetic peptide lipids. Previously, we also reported the high self-assembling ability of various peptide lipids and demonstrated high cytotoxicity of some peptide lipids to animal cells. Peptides can be substrates for many enzymes in a living system. For example, tyrosine kinase adds a phosphate group to a tyrosine residue in a target protein, which plays a key role in signal transduction on cell growth and differentiation. Gene mutation in tyrosine kinase leads to uncontrollable signal transduction and causes many cancers. In other words, tyrosine kinase activity of some cancer cells is different from other cells.
Here, we prepared novel peptide lipids that underwent phosphorylation of its peptides by tyrosine kinase in animal cells and evaluated their cytotoxicity. First, we synthesized different types of peptide lipids by solid-phase peptide synthesis, which were composed of fatty acids (C8 – C16) and peptides containing tyrosine (Fig. 1). The peptide sequences were designed according to tyrosine kinase substrates. The synthesized peptide lipids were purified by HPLC and confirmed by MALDI-TOF MS. When a phosphate group is added to tyrosine in a peptide lipid by tyrosine kinase, the hydrophilicity of the peptide lipid increases. We conjectured that a peptide lipid is transformed to anti-cancer drugs in cancer cells. We expected that phosphorylated peptide lipids should have cytotoxicity different from the original peptide lipids (Fig. 1). The present study proposes the novel effective treatment of drug-resistant cancer cells based on kinase activity.
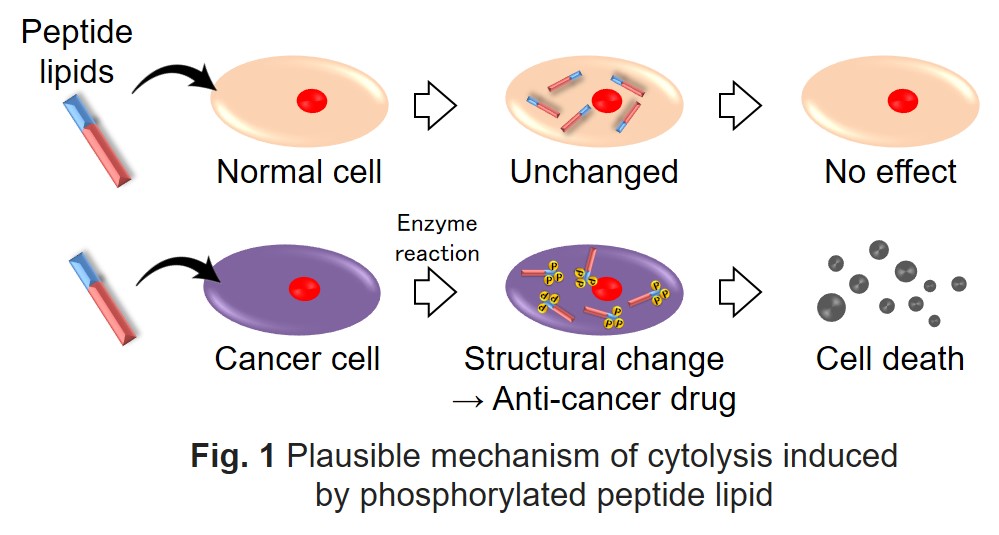
Lipid vesicles are closed bilayer membrane(s) consisting of amphiphilic lipid molecules such as phospholipids formed in aqueous media. Lipid vesicles can encapsulate various water-soluble biochemical molecules into their internal water phase, therefore lipid vesicles have been studied as biochemical microreactors surrounded by lipid bilayer membranes, those are structurally similar to biological cells. However, the utilization of lipid vesicles as microreactors is limited by their preparation method because it is generally difficult to obtain vesicles containing biochemical molecules such as enzymes with high encapsulation efficiency and controlled diameter.
Recently, we developed a novel vesicle preparation method by the combination of multiple emulsification and solvent evaporation techniques (Kuroiwa et al.: J. Am. Oil Chem. Soc., 93, 421-430, 2016). In this method, at first, water-in-oil (W/O) emulsion containing water-soluble enzymes was prepared by membrane emulsification. Next, water-in-oil-in-water (W/O/W) multiple emulsion containing above W/O emulsion as a dispersion phase was formulated by microchannel emulsification with water-soluble polymeric emulsifiers. After removal of organic solvent in the oil phase of W/O/W emulsion by solvent evaporation under the ambient condition, finally we obtained enzyme-containing lipid vesicles. The diameter of lipid vesicles reflected that of the starting W/O emulsion droplets. For porcine pancreas lipase, the activity-based encapsulation yield was determined to be ca. 30%. These value was quite high compared with values for conventional vesicle preparation methods. Lipase-catalyzed reaction inside lipid vesicles was carried out by supplying a substrate (fluorescein diacetate) from the external water phase to internal water phase of lipid vesicles. The hydrolysis of the substrate was proceeded as comfirmed by fluorescence of hydrolytic product, fluorescein, from internal water phase of vesicles. This result suggests that the substrate permeated through lipid bilayers of vesicles and then it was hydrolyzed by lipase inside vesicles. We also evaluate the encapsulation characteristics of a proteolytic enzyme.
Structural color is color produced from materials having microscopic structures that can interact to visible lights via interference and/or scattering phenomena. Artificial structured materials have been widely developed by fabricating various nano-sized structures using synthetic organic or inorganic materials. In contrast, development of structural colored materials based on natural biomolecules have not studied enough although very vivid structural colors are widely observed in nature, for example, in butterfly wings, beetle shells, and bird feathers.
In this study, we developed a novel structural colored material using millimeter-sized molecular aggregates consisting of natural amphiphilic lipids including phospholipids. A lipid mixture consisting of phosphatidylcholine, cholesterol, and additives (various monoalkyl compounds) was dissolved in n-hexane and placed onto aqueous phases with various pH values. After evaporative removal of n-hexane, molecular aggregates with visible size (larger than 1 mm) were formed in the aqueous phase. The shape of molecular aggregates was film-like or spherical, depending on lipid composition and pH value in the aqueous phase. Small/wide angle X-ray scattering measurements revealed that the molecular aggregates exhibited the liquid crystalline polymorphism and their phase structure was related to their macroscopic shape. Among them, spherical aggregates with cubic liquid crystalline phase could be converted to aggregates exhibiting colorization by controlling the salt concentration of their external aqueous phase. The reflection spectra of visible lights from the colored molecular aggregates suggested that their colorization was attributed to the internal structure of the molecular aggregates. Electron microscopic analysis demonstrated the existence of periodic structures with the characteristic distance corresponding to wavelength of visible lights. We believe that our finding would contribute to development of novel lipid-based colorization materials that are potentially applicable for foods, cosmetics, and display materials.
Amphiphilic molecules form self-assembled aggregates, such as micelles and vesicles. These aggregates are expected to be applied as a drug carrier and a microreactor. Polarity of the hydrophobic region is an important parameter to enbed hydrophobic drugs and substances. In this study, the relative permittivity was evaluated by using fluorescent probes. These probes were synthesized by conjugation of 1-pyrenemethanol and dicarboxylic acids, succinic acid (P-C3-COOH), suberic acid (P-C7-COOH) and dodecanedioic acid (P-C11-COOH). Aggregates were prepared by non-ionic sorbitan surfactants, Span 40 and Tween 40. The mixture of these surfactants forms various kinds of aggregates, Tween 40-rich and Span 40-rich mixtures tend to form micelles and vesicles, respectively. Figure 1 shows the relative permittivity of aggregates using P-C3-COOH. Relative permittivity of vesicles at low temperature was apparently higher than that of vesicles at high temperature, it is due to the localization of P-C3-COOH. Rigid structure of vesicles prevented P-C3-COOH to insert into the deep hydrophobic region. Increase of temperature change vesicles more fluid, resulting in decrease of relative permittivity. The measurement using P-C7-COOH shows a similar result as that using P-C3-COOH, while it seems that behavior of P-C11-COOH was different from that of P-C3-COOH and P-C7-COOH. The relative permittivity of micelles did not change regardless temperature. On the other hand, relative permittivity of vesicles was increased from 10 to 30 °C. It was related to phase transition temperature of vesicles. These results help to assume localization of drugs or substances in the aggregates, such as the considering about to apply as a drug carrier or a microreactor.
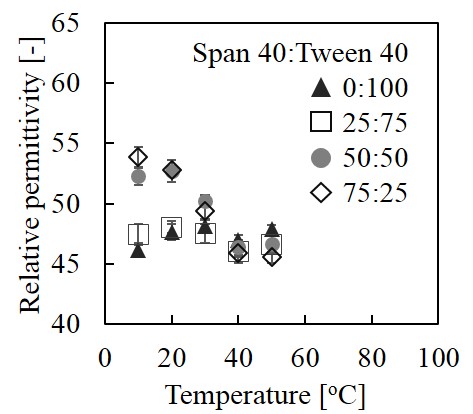
Bicelle is a disk-shaped self-assembly with bilayer membranes like vesicle, and its size is small as well as micelle. In addition, bicelle with a large disc diameter aligns to the magnetic field. Because of these unique properties, bicelle has been attracting tremendous attention as NMR measurement medium. In the formation and application of bicelle, the ordered structure of bicelle bilayer membranes is particularly important. In this study, bicelles were prepared by DMPC and DHPC, then the ordered properties of the bicelle bilayer membranes were characterized by combining the fluorescent probe method and induced circular dichroism spectroscopy (ICD). ICD is a phenomenon that CD activity occurs when achiral molecule DPH is ordered highly within a chirality lipid membrane. The ICD intensities at 360 nm decreased with increasing temperature, suggesting that the motion of the DMPC molecule was limited at low temperature, and the orderly orientation of DPH in the bilayer membrane was lost. The ICD intensity of bicelle was stronger than that of conventional vesicle. Furthermore, the aggregated bicelles, such as stacked bicelle, can be designed by modifying the membrane surface properties of bilayer region.
References: P. Walde et al., Langmuir, 13, 1668 (1997). S. Taguchi et al., Colloids and Interfaces, 2 (4), 73 (15 pages) (2018).
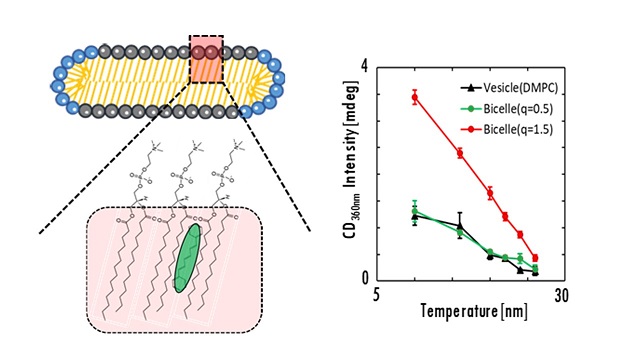
The "gas-liquid two-phase mixed flow" mechanism that fine bubble generation uses rapidly swirling water which shear gas and making small bubbles in water. Based on this mechanism, sending liquid such as oil into the swirling water instead of gas and it able to be emulsified. At present, evaluation methods for emulsions can be considered as particle size distribution and dispersion stability. However, in systems where the separation behavior is not good, it is a problem to catch only some dispersed oil particles and use them as a whole evaluation. In order to express numerical evaluation including the oil particles which can not be dispersed, we use chemical oxygen demand (COD) as numerical evaluation. The figure shows the temporal change of the amount of volatilizing oil calculated from the COD of emulsions sample (1)-(3). The dispersed phase used was of three types: sample (1) only one oil, sample (2) an anionic and a nonionic emulsifier were mixed and added to the oil, sample (3) an anionic and an another kind of the nonionic emulsifier were mixed and added to the oil. It was confirmed that COD decreased and sample (1) has a large amount of volatilized oil because there were many oil particles that can not be dispersed. The performance of the emulsion could be expressed numerically, including the oil particles which could not be dispersed. We also directly analyzed volatile components and compared them with COD results.
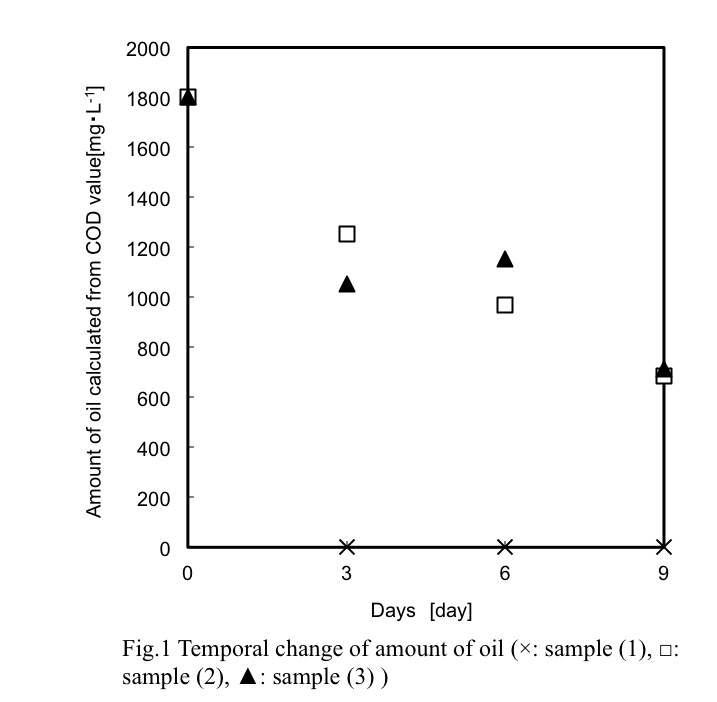
In this study, a plant-derived alkyl polyglucoside (APG) surfactant with alkyl chain of C8- C10 was formulated with the essential oil of Melaleuca alternifolia (tea tree) in the presence of propylene glycol as co-surfactant. This nonionic surfactant with a tradename as Triton CG 110 is reported to possess a CMC at 1748 ppm at 25oC. Typically, tea tree oil (TTO), commonly used in variety of cosmetic and health care products, was extracted from tea tree leaves by Triton CG110-assisted hydrodistillation. The extraction yield was 6.68% under the conditions with (1) 645.4 ppm Triton CG110, (2) at a liquid/solid ratio of 24.5, and (3) for 128 mins as the extraction time. The pseudo-ternary phase diagrams were investigated at the different weight ratios of surfactant mixtures (Smix = CG/PPG) as 1:1, 3:1, 6:1 with hydrodistilled TTO and commercial TTO by water titration method at room temperature. One-phase region consisting of micelles and microemulsion (μ_phi) was painted in gray in Figure 1. The greater the amount of Triton CG110, the wider phase compositions of microemulsion formed. For example, the largest microemulsion region was occurred at a surfactant mixture of 6:1. No liquid crystalline phase was observed at all ratios of Smix under the cross-polarized microscope. Particularly, the effect of storage temperature on selected microemulsion made of 1% TTO with various concentrations of Triton CG100/PPG (3:1 w/w) were recorded. Shelf stability was examined on microemulsions right after preparation and in one month with dynamic light scattering. These results also showed that microemulsion made of 1%TTO, 9% Triton CG100/PPG (3:1 w/w) was insensitive with time and temperature during long time. Antimicrobial activity and antioxidant activity were evaluated with microemulsion made of 1% TTO.
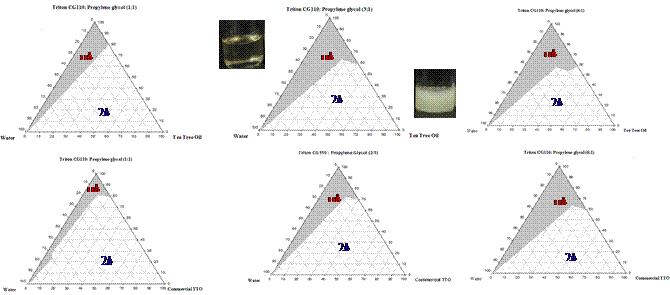
Recently, Pluronic surfactants (PEO–PPO–PEO triblock copolymers, where PEO: poly(ethylene oxide)x and PPO: poly(propylene oxide)y) were found to show CO2-responsive aggregation behavior in water. In earlier paper, mesoporous silica prepared in a hexagonal liquid crystal/water system was swollen by CO2 and the mesopore size was tuned by pressure of CO2 atmosphere. It suggests various potential applications of CO2-responsive surfactant. To clarify mechanism of CO2-responsive behavior of Pluronic surfactant assemblies in water, this study examined effects of temperature, pressure, PEO and PPO lengths on growth of Pluronic surfactant micelles in water under high-pressure CO2 atmosphere.
Aqueous micelles of Pluronic surfactants F108 (x = 150, y = 55) and F127 (x = 100, y = 65) showed no significant change in hydrodynamic diameter of micelles even at high CO2 pressures of 100–300 bar. In contrast with those Pluronic surfactants, F68 (x = 80, y = 30) and F88 (x = 100, y = 20) displayed clear micellar growth with increasing CO2 pressure. Figure 1 shows relationship between the diameter of aqueous micelles and density of CO2 atmosphere at 75 °C and the surfactant concentration 1 wt% for F68, a nonionic EMULGEN 4085 having the C14H29-tail and the PEO (x = 85) close to that of F68, and a nonionic BrijS100 (x = 100) having the C18H37-tail and the PEO chain (x = ~100) similar to that of F88. The tendency that the higher the CO2 pressure (or CO2 density) the larger the micellar diameter was considerably small for nonionic surfactants without a hydrophobic PPO chain, compared with Pluronic surfactants having corresponding PEO units. Especially the Pluronic surfactants with PEO lengths x = 80–85 were found to exhibit significant micellar growth with increasing CO2 density.
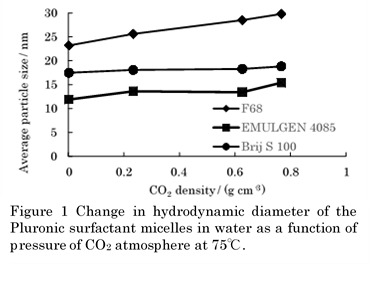
Real-time monitoring of small molecules, such as reactants, dissolved gas molecules or metabolic products, during biological conversion is of paramount importance for understanding the metabolic pathway, optimizing conversion efficiency and enhancing the reproducibility of bioprocesses. Generally, detection of such small molecules during biological conversion are largely dependent on chromatography-based techniques, such as high performance liquid chromatography and gas chromatography. However, these techniques are time-consuming, requiring more than 10 minutes for the separation of sample in the column. Moreover, off-line sampling process is inevitable for the analysis which has a risk of causing contamination. For these reasons, development of a novel method that is suitable for real-time monitoring of biological conversion is required. Here, we propose the plasmonic detection of small molecules in biological conversion by using plasmonic nanostructures produced using capillarity-mediated inverse transfer. Nanoparticles assembled at air/water interface are transferred onto solid substrates through the reconstruction of the assembly interface along the direction of gravity by a capillary tube, enabling the transfer of nanoparticles on solid substrate via direct contact. Small molecules are preconcentrated on plasmonic nanostructure through electrostatic attraction and chemisorption. Preconcentration of small molecules increases the number of molecules affected by strong near-field enhancement at the proximity of the metallic surfaces; thereby, allowing the detection of trace amount of small molecules in the solution.
A living matter moves as an efficient energy conversion system under a non-equilibrium state. The study of this motion may be associated with creation of innovative energy conversion system. Fundamental study with a model system involving similar characteristics to living matter is one of the powerful approaches for understanding and application of such types of motion. In this study, we have studied the motion of aluminum float driven by the spontaneous flow at oil/water interface: The oil and water phases contain di(2-ethylhexyl) phosphate (DEHPA) and Fe2+, respectively, whereby spontaneous instability develops at the interface. Spontaneous flow is generated by a local compression of the adsorbed DEHPA layer by an interfacial flow, and the generated flow compresses the surrounding DEHPA monolayer to cause the subsequent flow. This suggests that spatiotemporal control of compression points rectifies the flow pattern, resulting in a regulated motion of a float.
As an example, a rectangular aluminum foil is placed in an arc-shaped triangular space as shown in Fig.1(a). The aluminum foil exhibits a reciprocal motion. This motion repeats itself many times. Such a motion was not obtained without the confined space. The motion of the aluminum foil results from the compression of DEHPA layer between the aluminum foil the wall, because the compression induces the next flow. As the other example, a ratchet gear made of aluminum foil is placed at the interface where powder are dispersed in the oil/water interface(Fig.1(b)). The ratchet rotated in one direction, that lasted for more than 10 minutes. This rotation may be considered to be caused by the compression of DEHPA layer around the aluminum foil assisted by the powder acting as a wall. These motion may lead to the creation of an innovative energy conversion system.
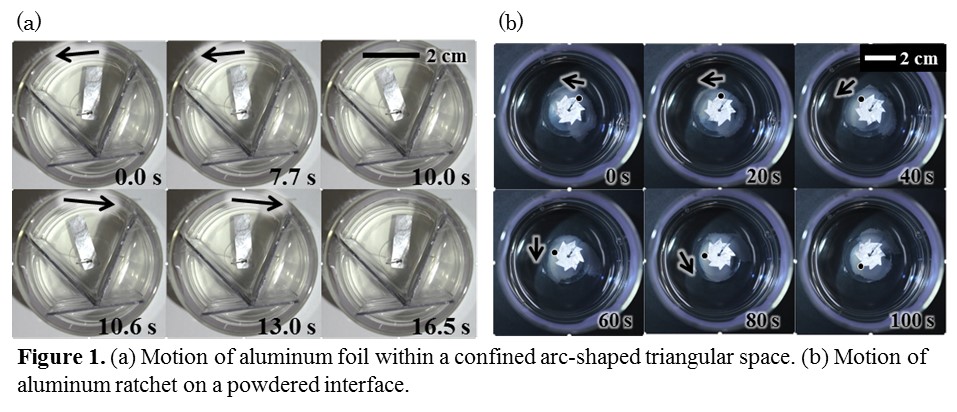
Graphite consists of many carbon sheets. A single layer of the carbon sheets is called graphene. Graphene is a material having good electrical conductivity and high mechanical strength. Industrial applications of graphene are expected in the wide range of fields. However, graphene is usually aggregated in solvents because of strong hydrophobic interaction. This causes the difficulty of the manipulation and the loss of electrical conductivity of graphene.
We previously succeeded in dispersing graphene using poly(3-hexylthiophene-2,5-diyl) (P3HT), which is the conductive organic polymer, in an organic solvent. However, P3HT dispersed graphene only in an organic solvent, which leads to large environmental concerns. Here, we aimed at the preparation of graphene aqueous dispersion using poly(3-(4-sulfophenyl)thiophene-2,5-diyl) (P3ST), which is a water-soluble polythiophene derivative.
P3ST is obtained by deprotecting neopentyl groups of poly(3-(4-neopentylsulfophen-1-yl) thiophen-2,5-diyl) (P3NST). We mixed graphite flakes with P3NST in chloroform and conducted ultrasonication for exfoliation and dispersion of graphene. After the removal of chloroform, the graphene-P3NST complex was heated at 220~230 °C for deprotecting neopentyl groups of P3NST. Then, we added water and redispersed the graphene-P3ST complex in water by ultrasonication. After the redispersion was left for 20 h, the supernatant was collected. The supernatant was diluted 2 times with water and its absorbance at 800 nm was measured to evaluate the dispersion of graphene.
Fig. 1 shows the absorbance at 800 nm of the dilute solution of the supernatant. The x axis shows heating time for deprotecting neopentyl groups of P3NST. The results showed that dispersion of graphene in water was improved by heating the graphene-P3NST complex. The deprotection of neopentyl groups produced sulfo groups in the polythiophene derivative and the sulfo groups would generate electrostatic repulsion between the graphene nanosheets in water to stabilize the dispersion.
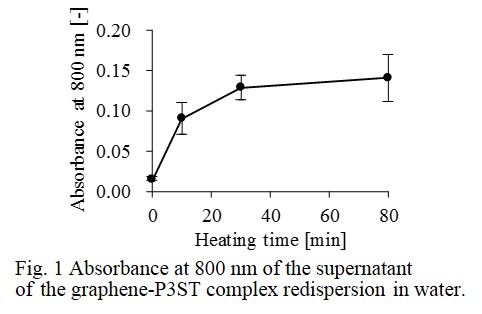
Cu-free click reaction is one of the most famous reactions for conjugating DNA, proteins and cells. Recently, a Cu-free clickable surface is attracting as a platform of immobilizing biosubstances for preparing biosensors. In previous studies, inorganic substrates (e.g., silicone and glass) were used, and they needed surface activation by plasma and chemicals. In this study, we synthesized a Cu-free clickable functional polymer, and a Cu-free clickable surface was prepared on a plastic substrate by polymer coating without pre-treatment.
We synthesized a methyl methacrylate (MMA) and hydroxyethyl methacrylate (HEMA)-based backbone copolymer. Then, dibenzocyclooctyne (DBCO), a Cu-free clickable functional group, was conjugated to the hydroxy group of PMH to get a Cu-free clickable polymer, poly(MMA-r-HEMA-DBCO) (termed PMHD). PMHD was dissolved in ethyl acetate, and a plastic substrate was coated with the polymer solution and dried to prepare a Cu-free clickable surface. AFM and SEM observations revealed that dip-coated surfaces were flat compared with bare substrates.
We also synthesized an azide-conjugated fluorescent substance and estimated the amount of clickable moieties on the polymer-coated surface using Cu-free click reaction between the surface and the fluorophore. The surface density of Cu-free clickable moieties was proved to be 63 pmol/cm2 on PMHD-coated PMMA substrates. This meant that a Cu-free clickable surface was easily prepared on a plastic substrate by polymer coating. To investigate the potential of the functional surface as a platform of a biosensor, azide-conjugated oligo DNA was immobilized on the clickable surface. In consequence, 5.4 pmol/cm2 azide-conjugate oligo DNA was immobilized and hybridized with the complement DNA on the PMHD-coated functional surfaces.
In summary, we synthesized a functional polymer (PMHD), and succeeded in the preparation of a Cu-free clickable surface by simple polymer coating. The investigation of DNA immobilization revealed that the functional surface had high potential as a scaffold of biomatters.
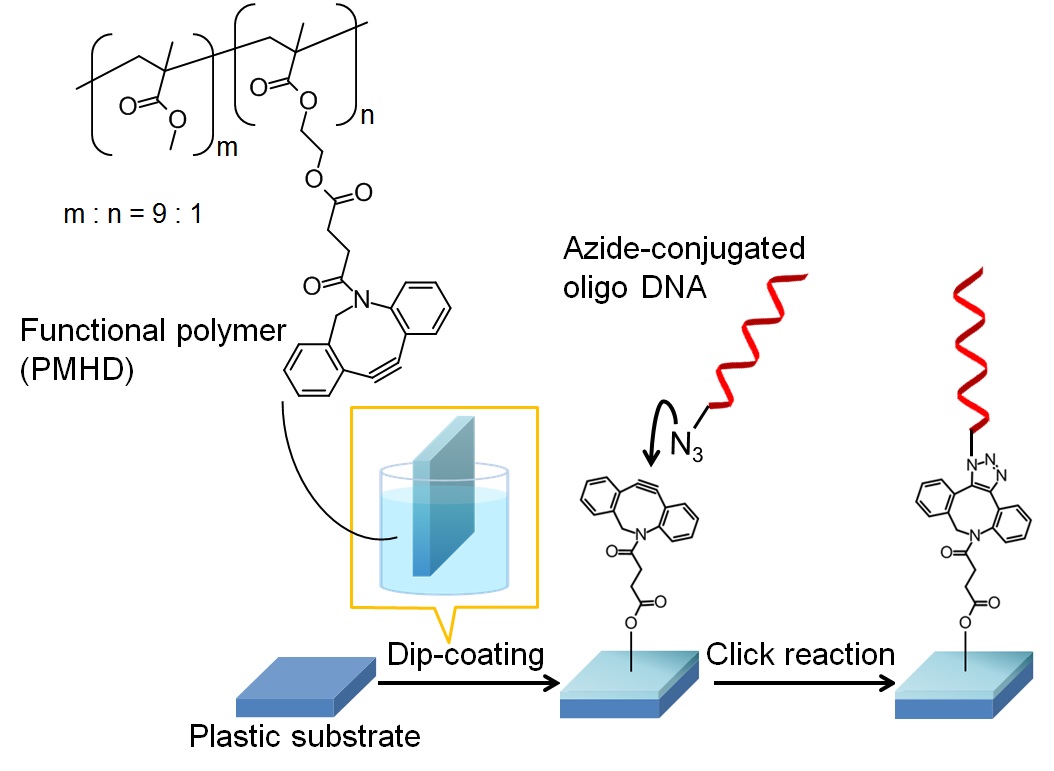
In recent years, separation membranes have attracted a lot of attention: that is widely applied to seawater desalination treatment, separation/recovery of exhaust gas, etc. The materials of the separation membrane are classified into inorganic or organic one, and the latter has generally advantages of low cost, low density and easy handling. In the widespread use, it is indispensable in our lives.
At present, a new preparation process using phase separation in liquid phase has been developed. The outline of this process is 1) selection of a polymer, its solvent and anti-solvent, 2) mixing of them, 3) emergence of phase-separation at specific compositions and 4) drying and formation of porous separation membranes. However, the formation mechanism of the membrane is not clear in the drying process and should be clarified for design of the membrane structure. In this study, poly(vinyl alcohol) (PVA), water and γ-buthyrolactone(GBL) were selected as ingredients for the membranes. Then, the concentration changes of mixed samples have been investigated in the formation of the porous membranes of PVA by the drying process.
As a first approach, preparation of the phase diagram for the three components system was carried out.When the concentration of the liquid sample changed by adding a component slightly, the turbidity was measured. The binodal curve was found by connecting of the turbidity points in PVA-GBL-water systems. The trace of concentration change in drying processes were drawn in the obtained phase diagram. The structures of PVA on the trace line was measured by SEM and DSC measurements were carried out. The relationship of drying rate and PVA structure was elucidated with the structural and thermal data.
Plastics have some practical characteristics; in recent years, the modification of plastic surfaces has received considerable attention for new application. The display of reactive functional groups on plastic surfaces is one approach of the modification of plastic surfaces and it is very important for adhesion and immobilization of biomolecules and polymers. In this study, we displayed amino groups and immobilized gold nanoparticles (AuNPs) on plastic surfaces. Preparation of AuNPs-modified surfaces will realize new applications such as fabrication of conductive plastic surfaces, immobilization of thiol compounds on a surface of a plastic substrate.
We synthesized a methacrylate-based copolymer containing amino groups in the side chains, in which the amino groups were protected with tert-butoxycarbonyl (Boc) groups. The protection groups of amino groups in this copolymer were hydrophobic, leading to the display of the amino groups on the outermost surface of a PMMA substrate after dip-coating. We deprotected the Boc groups and displayed amino groups on the surface of the substrate. Quantitation of the amino groups was carried out using a cleavable fluorescent compound. The amount of amino groups displayed on the surfaces was 10.3 pmol/cm2.
AuNPs, which were 13 nm in diameter, were immobilized on the plastic surfaces by simply immersing PMMA substrates in Au-colloidal solution for 30 minutes. The acrylic substrate displaying amino groups turned red after immersion (Fig. 1), comparing to the bare substrate and the substrate displaying Boc groups. We measured absorbance of these substrates after immersion at 520 nm, which was maximal absorption wavelength of Au-colloidal solution. Absorbance of the substrate displaying amino groups was the highest among the three different kinds of substrates. We conjectured that electrostatic interaction between amino groups and negatively-charged AuNPs led to the immobilization of AuNPs on the surface.
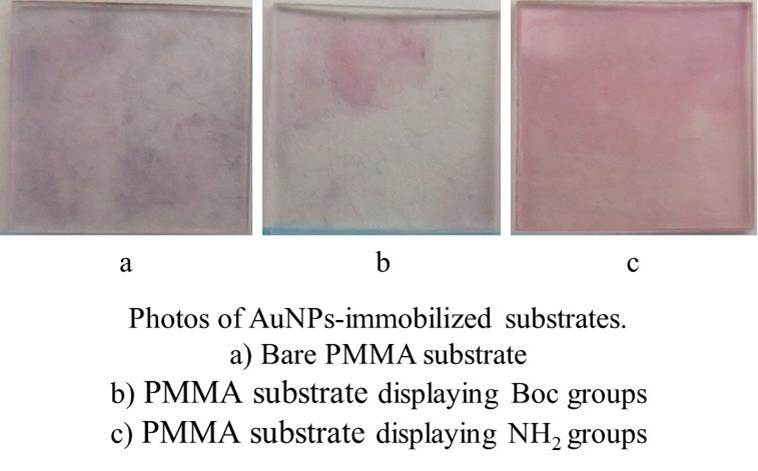
In drying of particle dispersion films, drying defects such as cracking or the like are caused by capillary forces acting between the particles. Previous studies have shown that cracks develop when the dry film thickness exceeds a critical thickness, referred to as critical cracking thickness (CCT)[1]. Polymeric additives can increase CCTs as they (i) adsorb on particle surfaces to induce steric repulsive forces between the neighboring particles, (ii) adsorb at air-liquid interface to reduce the surface tension, and thus the capillary forces, or (iii) show physical crosslinking between particles. However, few quantitative data are currently available to clarify how polymers suppress the propagation of cracks. In this study, we aim at investigating the effects of polymer adsorption on critical cracking thicknesses of drying particle dispersions.
We used Carboxymethyl cellulose (molecular weight: 1850000 g/mol, Dai-ichi Kogyo Seiyaku) as polymer. The sample dispersion containing TiO2 (diameter: 150 nm, concentration:15 wt%) and polymer was dried on a tilted hot stage. The dried film surface was observed by an optical microscope to find a position where cracks developed with a length of 30 μm or longer. The film thickness at that position was measured using a laser focus displacement meter to determine CCTs.
The results showed that CCT was nearly constant at polymer concentrations lower than the critical concentration of c* ~ 0.05 wt%, and agreed with that in polymer-free particle dispersions. At polymer concentrations above the critical value, on the other hand, the CCT monotonically increased with increasing the polymer concentrations. The adsorption measurements indicated that the onset of polymer adsorption on the particle surface emerged at the same polymer concentration, c*, indicating that crack propagation was suppressed by polymer adsorption on the particle surface.
References
[1] K.B.Singh and M.S.Tirumkudulu,Phys.Rev.Lett.98(21)(2007)218302
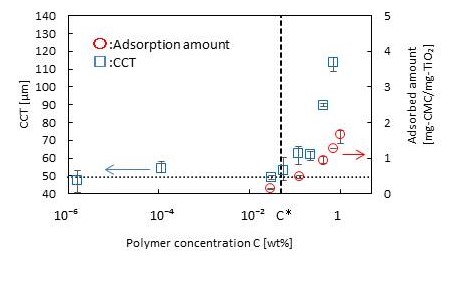
Shape of particles in dense suspension influences on the rheological properties. We focused on wrinkles generated by buckling as the periodic structure on the surface of elastomer particles, and evaluated the suitable method to control the obtained particle size and the shape of wrinkles.
Polydimethylsiloxane (PDMS) was used as the base material for wrinkled particles. The monomer and curing agent were mixed (10:1 (w/w)) to obtain the uncuring PDMS sol, and then the sol was mixed in a 15wt% polyvinylalcohol/H2O solution by the following methods, magnetic stirrer (ST), homodisper (HD), and homogenizer (HG). The PDMS droplets were filtrated with the porous membrane to fractionate the droplet size. In order to cure PDMS, the droplet suspension was heated at 80 °C for 3 hours. Finally, the surface of the PDMS particles were oxidized with a mixed acid solution to form wrinkle structure. The size of PDMS were measured from the image of optical microscope and/or dynamic light scattering.
The order of PDMS droplet size was roughly ST > HD > HG, and the particle size distribution was very broad from several 10 nm to above 100 μm. To separate out the particles with desired size, the HD pre-mixed PDMS suspension (1000 rpm, 5 min) was filtrated by using a shirasu porous glass membrane (pore size: 1 μm). The filtrated PDMS suspension shown a more narrow size distribution (d50 ~2.5 μm (Figure)). However, after surface oxidation of mixed acid, surface wrinkling on the filtrated PDMS particles was not observed by a SEM. Only PDMS particles of 15 μm or more in the diameter obtained from HD pre-mixed suspension were buckled into wrinkling. The wavelength of wrinkle was about 450 nm (d~15 μm), which tends to increase slightly with the particle diameter.
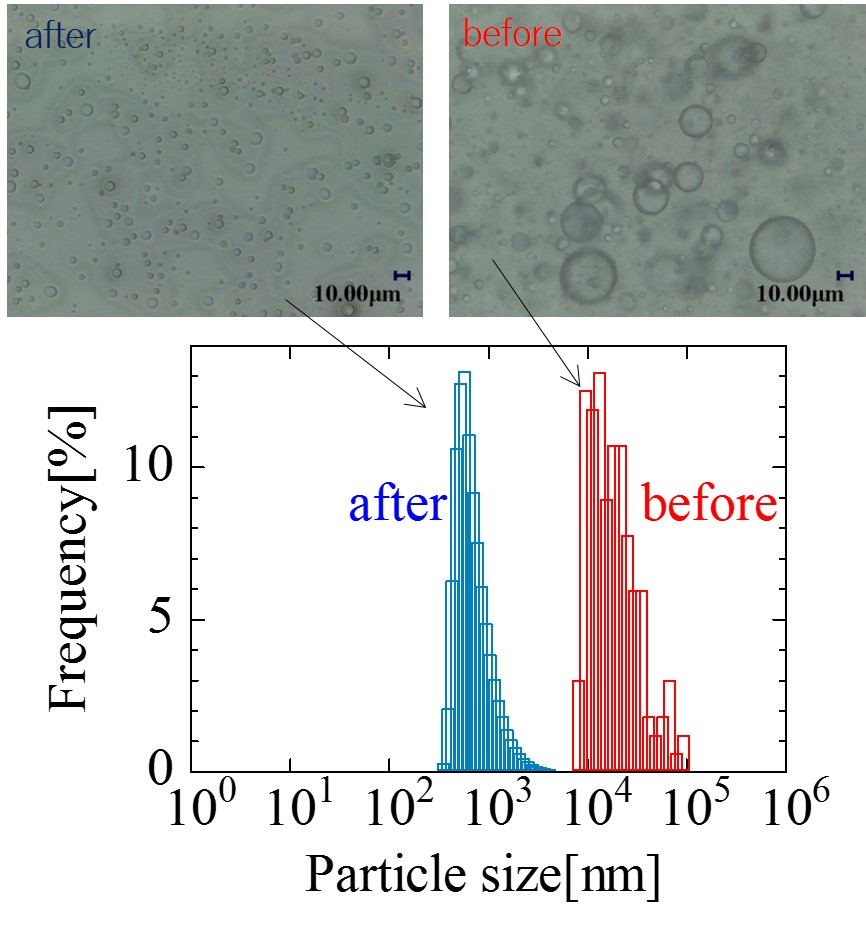
The organic luminescent material easily loses its luminescence once it is exposed to moisture, and the highly reactive cathode with low work function will be easily corroded by moisture and oxygen. To solve this problem, We demonstrate flexible moisture barrier film encapsulation for organic electronics using low temperatures process method. To realize the flexible barrier, the best practice is to make the barrier as multilayer structure. Low temperatures process applied the atomic layer deposition(ALD) method and sputtering method to deposit barrier films. Inorganic layers work as moisture barrier and very thin thickness provide the flexibility and planarization. The moisture permeation characteristics of the optimized Al2O3/SiNX multilayer films were finally measured using MOCON's WVTR measuring instrument and WVTR values lower than the detection limit of the measuring device (less than 5.0 x 10-5 g/m .day) were obtained.
This study is to investigate the optimal time of applying current assisting during employing hot-pressing Bi-Sb-Te films to improve the thermoelectric properties. First, an average 5 um of grinded P-type of Bi-Sb-Te powers was used to formulate a slurry paste to coat on a polyimide substrate for the current assisted hot pressing. It shows that the resistivity decreases with increasing the current density, and then increases with further raising the current density. The main reason is speculated that increasing the electric driving force at low current density can eliminate defects by reducing the obstacles of carrier transmission and formatting Sb-rich phase to form a bridge between grains. However, the excessive current density will generate stronger electric driving force causing a large amount of joule heat to increase the resistivity. Also, the case of “hot pressing and current assisting simultaneously” or the case of "current assisted hot pressing after completion of hot pressing" cannot achieve the best improvement of the thermoelectric properties. Analyzed by surface reaction method, the best thermoelectric efficiency can be obtained at 1000 A/cm2 with 3 min hot pressing followed by 7 min current-assisted hot pressing. Applying hot pressing ahead can increase contact area between particles to make current density distribution more uniform, which can generate less extra joule heat and larger electric driving force, leading to further reducing resistivity to 1.04 mΩ·m and increasing power factor to 2880 μW/K2m. Compared with the case of hot pressing and current assisting simultaneously for 10 min and the case of current-assisted hot pressing for 7 min after hot-pressing for 10 min, the power factor increases 34% and 20%, respectively.
An innovative bio-functional lenses can control multiple surface properties independently for bio-medical applications. The bio-functional lenses was fabricated based on a chemical vapor deposition encapsulation process using functionalized poly-p-xylylenes. Our device exhibits excellent biocompatibility, high transparency, heat resistance and stable in vacuum. The bio-functional lenses applied in many area, such as artificial crystal or cell incubator. Here we reported a method for the fabrication of bio-functional lenses which constructed via vapor deposition of poly-p-xylylenes on template and shape is controlled by oil. Because the lenses constructed by liquid, it is softer than solid that apply in artificial crystal. On the other hand, the lenses have some characteristic such as stable in vacuum, biocompatibility, and perfect curvature. Another research, we try to put Human Adipose Stromal Cells(ASC) in the lenses which is encapsulated by oil and observed in Transmission electron microscope (TEM). The advantage of lenses is high transparency that easily observed in TEM and durable in the environment of vacuum. We build an excellent cell incubator so that we can observe life phenomenon in TEM instead of killing cells.
Perovskite solar cells have been intensively studied because they have high efficiency over 20%1 and are fabricated by a low-cost wet process. The ideal cell structure is a simple planar type with a multi-layer of substrate/electrode/electron transport layer/perovskite power generation layer/hole transport layer/electrode. Titanium dioxide (TiO2) is used as a compact blocking layer as well as an electron transport layer. The underlying TiO2 layer would also play a role in determining the structure of a perovskite layer. To improve the efficiency of the solar cell, it is important to control the structure and physical properties of the TiO2 layer. The ultrasonic spray method is one of the wet coating processes. It enables to control film structure by nuclear growth on the substrate and to deposit large-area and continuous film, having advantages of applicability of the Roll to Roll process upon scale-up to mass-production. The aim of this study is to analyze the relationship between coating process, film structure and physical properties of TiO2.
Titanium bis acetylacetonate diisopropoxide (Ti(acac)2(OiPr)2) and titanium tetrachloride (TiCl4) were used as soluble precursors in ethanol. The ultrasonic spray method was used to deposit TiO2 films on two different types of SnO2 films. The structure of the TiO2 films was investigated by X-ray diffraction and X-ray absorption spectroscopy. In the case of Ti(acac)2(OiPr)2 solutions, TiO2 films had anatase and rutile structures with (101) orientation, while the films deposited with TiCl4 solutions showed only a rutile structure with (101) orientation. The local epitaxial growth of rutile TiO2 on rutile SnO2 structures would be the origin of the observed rutile (101) orientation. We will discuss the effect of the structure of the TiO2 films on the physical properties of perovskite solar cells.
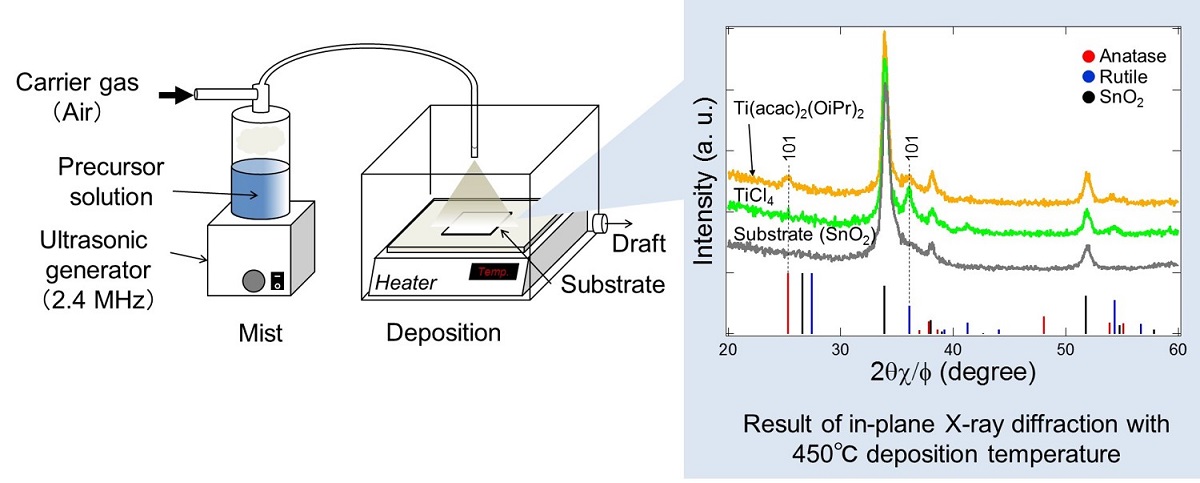
Fluorinated compounds often exhibit a very low surface energy, and these can be used as antifouling and antifogging agents. However, high F-content compounds are generally expensive and bioaccumulative. Our previous research found that non-fluorinated surfactants having trimethylsilyl groups decreased aqueous surface tensions as low as a short or middle fluorocarbon-tail surfactant can (i.e. ~22 mN/m). To develop fluorine-free water-repellent materials comparable to fluorinated one, this study synthesized 3,4-propylenedioxythiophene monomer having 3-trimethylsilylpropyl group (ProDOTSiMe3, Fig. 1) and obtained poly(ProDOTSiMe3) films on gold surfaces by electrodeposition of the monomer.
Electrodeposition was carried out by placing a gold plate (working electrode), a glassy carbon electrode (counter electrode), and SCE in a glass cell containing 10 mL dehydrated acetonitrile solution with 0.1 M tetrabutylammonium perchlorate and 0.01 M ProDOTSiMe3. Water repellency and morphology of the surfaces were also studied. SEM observation clarified that the gold surfaces after electrodeposition were covered with dense poly(ProDOTSiMe3) nanofibers with a large roughness. Water contact angle on the gold surface was 141.6 °± 4.7 °, similar to the values 136.8 °and ≥ 150 °for 3,4-propylenedioxythiophene monomers having two isopropyl-terminated alkyl chains[1]. Interestingly, the poly(ProDOTSiMe3) surfaces exhibited parahydrophobicity due to the wenzel-state.
References
[1] Thierry Darmanin, Claudio Mortier, Julian Eastoe, Masanobu Sagisaka, Frederic Guittard., RSC Adv., 2014,4, 35708–35716
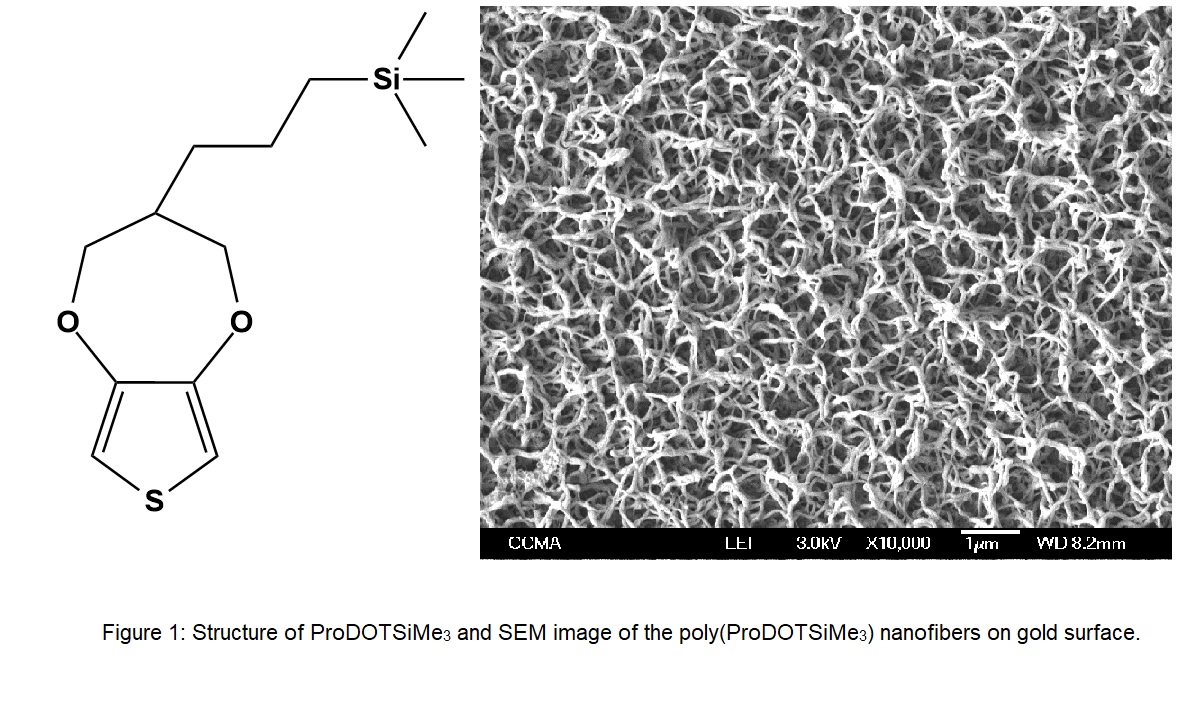
A probe needle for a probe card which is employed in a current energization inspection of an integrated circuit chip in a semiconductor wafer manufacturing process has been made of tungsten, rhenium tungsten, beryllium copper, etc. Particularly, tungsten is a material which is excellent in wear resistance and the excellent toughness is popularly used. However, the contact resistance is raised and the liability of probe card deteriorates on the inspection because tungsten is easily oxidized due to the frictional heat which is generated by the frictional wear or the generation of heat. In order to solve this problem, it is desirable to use gold or platinum material, which is strong in corrosion resistance, as a probe needle. However, in case of gold, it cannot be used in contact with the pad due to its ductility. In addition, there is no economical efficiency because the process cost is increased.
In this study, we platted on the surface of tungsten needle with 1 ~ 5 um of gold, platinum, and nickel to prevent the oxidation of tungsten needle by frictional heat which can be used sufficiently as a probe from being corroded by heat. The undercoat was conducted with nickel, and then, gold, platinum, or nickel was plated. The change of electric resistance after plating was examined. Because the needle thickness is very thin, the over-plating occurred at the tip of tungsten needle. To reduce over-plating, new zig for needle was invented and plating conditions were optimized.
Figure1. The microscope cross-sectional images of bare tungsten needle, the plated tungsten needles with nickel, gold, platinum.
High-chromium cast iron, which is a wear resistant material, is a ternary white cast iron generally containing 7% or more of Cr. It forms many carbides of high hardness, for example M23C6 and M3C, M7C3 (M = Fe, Cr)(1).Therefore, it is used as a material for rolling rolls used in rolling processing and slurry pumps for slurry liquid transfer. However, the rolling conditions are becoming more severe in terms of energy saving and quality improvement in rolling processing, and the slurry pump has problems of wear and corrosion due to high concentration slurry liquid. In order to cope with the current situation, it is important to change the solidified structure by adjusting the Cr and C content, in order to improve the low toughness, corrosion resistance, and wear resistance. Changes in the composition and cooling rate that affect the solidification structure are factors related to the mechanical properties of cast iron. Direct observation of the solidification process in which carbides and base structures appear is thought to be important because it leads to the elucidation of the growth mechanism and can be expected to control the size, amount and formation position of the crystal. Direct observation of metals under high temperature is carried out to clarify the growth mechanism of the metal structure. For this purpose, it is necessary to use a laser microscope which is not affected by the radiation emitted from the molten metal. In this research, our purpose to establish the observation condition of the growth process of base structure and carbide using laser microscope and to elucidation the formation of structure
(1)Tomoo Sato, Taiji Nishizawa, Joo Ishihara, hardness of carbides in iron and steel, Journal of the Japan Institute of Metals, Vol. 23, No. 7, pp. 404-405, 1958