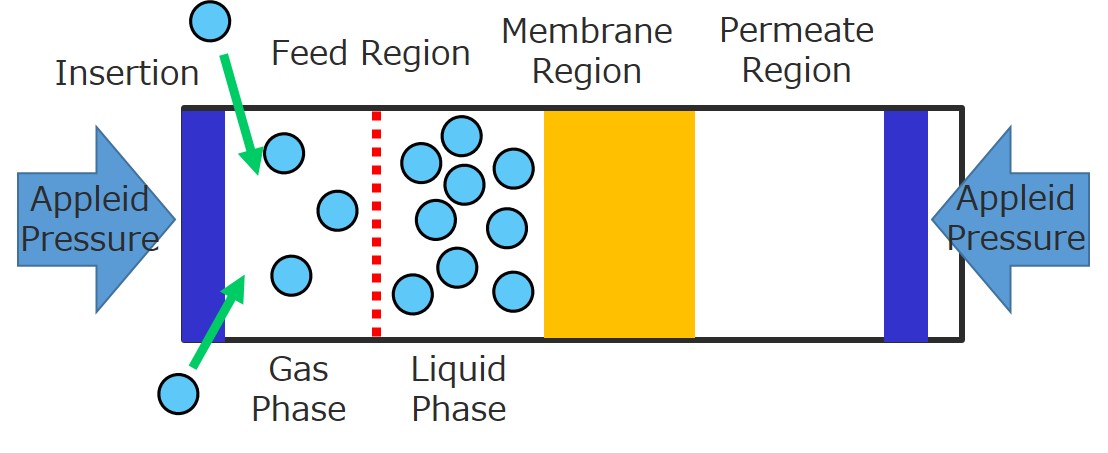
This paper offers how to realize quick refolding of high concentration protein via microchannel flow membrane dialysis. E. coli. are suitable host cells for mass production of proteins due to their fast proliferation and translation rate. On the other hand, proteins produced by E. coli. are often expressed as insoluble inclusion body, which have no activity. In that case, the “refolding” process to recover the original protein structure and activity is required. A quick refolding of high-concentration protein is important issue for industrial process but generally quite difficult. Dilution method can quickly refold protein but the concentration becomes low, while dialysis method can refold high concentration protein but requires long time. Considering the rate determining step of the dialysis is the slow permeation of denaturant through dialysis membrane, here, we propose the design to realize both of quick and high-concentration protein refolding by using microchannel flow dialysis.
Dialysis membrane was sandwiched with two microchannels; feed channel and permeation channel. Carbonic Anhydrase (CA) (as model enzyme) denatured by denaturant (guanidinium chloride (GdmCl)) was fed into feed channel. On the other hand, refolding buffer was fed into permeation channel. The denaturant was quickly removed from feed channel to permeation channel through dialysis membrane due to high specific membrane surface area in microchannel flow. As a result, CA can be refolded in only twenty minutes, which are much shorter than the time for conventional dialysis method, and just a bit longer than the time required for refolding of CA. Furthermore, the microchannel flow process realizes almost 100% protein recovery and active recovery for CA with concentration as high as conventional dialysis method within the quick process. The demerit of the microchannel flow is low flow rate but it can be easily solved by numbering up and increasing flow rate with longer channel flow.
A “Biomembrane” is a highly-organized self-assembly of biomolecules (i.e. lipid, protein etc.) and a key interface for the survival of biological cell. The “Membranome” can be defined as the properties of vesicle (or liposome), which arise from the bilayer molecular assembly of amphiphiles, focusing on “emergent properties” which are not present in the individual components, and is gradually recognized as an important research methodology to investigate the potential functions of vesicles (or liposome) and to apply them for the bioprocess design. “Self-Organizing System”, such as liposome or vesicle, possesses several benefits in the recognition of (bio)molecules, where it can recognize them with (i) electrostatic, (ii) hydrophobic interaction, and (iii) stabilization effect of hydrogen bonds at its surface. A key of next chemical engineering is the use of “Self-Organizing System”, where “enthalpy-driven” nature of chemical process would be converted to “entropy-driven” one. We call this strategy as “Bio-Inspired Chemical Engineering”. In this study, the basic and applied aspect of the self-organizing system were reviewed, especially focusing on chiral separation and chiral conversion process.
Reference:
P. Walde et al., Chem. Commun., 50, 10177-10197 (2014). T. Ishigami et al., ACS Appl. Mater. Interf., 7, 21065-21072 (2015). T. Ishigami et al., Langmuir, 32, 6011-6019 (2016). M. Hirose et al., Langmuir, 31, 12968-12974 (2015). F. Iwasaki et al., ACS Omega, 2, 91-97 (2017). F. Iwasaki et al., ACS Omega, 2, 1447-1453 (2017).
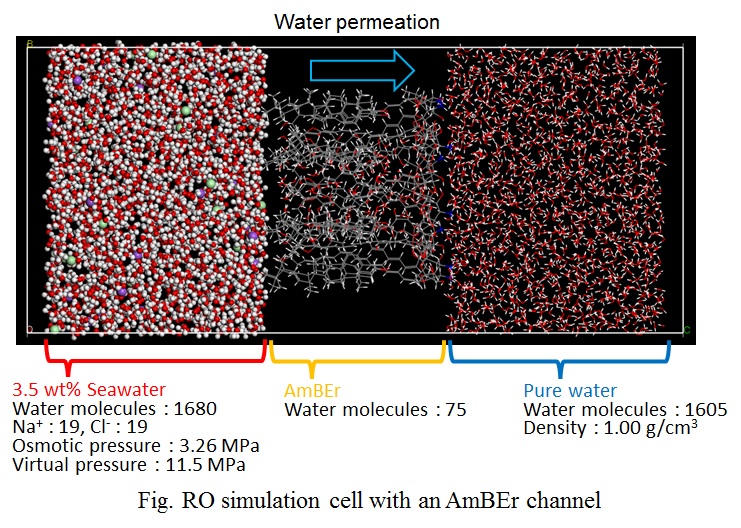
Polyamide and cellulose triacetate are known to be materials for reverse osmosis (RO) membranes, which are used in desalination of seawater. In order to deal with increasing water demands all over the world, a development of higher performance RO membrane and membrane processes are required. A drastic change in RO membrane materials would lead to one of the solutions to improve RO membrane performance. Amphotericin B-Ergosterol (AmBEr) is a biomimetic channel, and water and specific ions can be selectively transported through the channel [1]. RO membranes in which AmBEr channels can be embedded suitably are expected to be utilized high performance RO membranes. In order to design high water permeable channels, it is indispensable to understand microscopic channel structures and water transport mechanisms in a molecular scale.
In this study, a quasi-non-equilibrium MD simulation technique with applied (RO mode) or osmotic (forward osmosis, FO mode) pressure difference of several MPa was conducted to estimate water permeability through an AmBEr channel (Fig.). We had successfully carried out direct simulation of forward osmosis (FO) water permeation in artificial water channels [2]. In RO simulation, the virtual pressure was applied by increasing density of seawater side in RO simulations. Simulated FO and RO water permeability for an AmBEr channel were equal to or greater than the water permeability in Aquaporin [3] and carbon nanotube [4] water channels. No permeation of Na+ and Cl- ions were observed during the simulation time of several nanoseconds. The results suggested the excellent performance of AmBEr channel as an artificial biomimetic membrane material.
[1] H. Wu et al., J. Memb. Sci., 545, 229 (2018), [2] H. Wu et al., Desalination, 424, 85 (2017), [3] M. Kumar et al., Proc. Natl. Acad. Sci., 104, 20719 (2007), [4] B. Corry, J. Phys. Chem. B, 112, 1427 (2008)
Nanofiltration has been applied to various separation systems, in particular, for the separation of aqueous or organic solvents. For a development of more efficient membrane process, better understanding of separation mechanism of solvent including multicomponent species is of fundamental. Molecular modeling becomes a powerful tool for prediction of membrane performance and design of membrane materials for particular separation systems. Our group has reported a novel simulation scheme of molecular dynamics for the permeation of feed solution through nano porous membranes. Molecular dynamics of multi component species in the feed, however, is still difficult to be simulated because a feed concentration of multicomponent such as ions cannot keep at a constant in our simulation techniques as well as other conventional non-equilibrium molecular dynamics scheme. In this paper, to model multicomponent solutions through nanofiltration or reverse osmosis membranes, we will present a novel molecular dynamics technique that can control the feed concentration including multicomponent species at constant. This simulation technique is completely new and first methodology that could model multicomponent solution systems in membrane separation from atomistic level, as long as our knowledge. We will report that this simulation technique works well and can produce the permeation of multicomponent feed solution. The flux and ions selectivity were calculated and compared to the theoretical values to examine the validity of our proposed scheme.
We have synthesized 80-cm-long mordenite membranes on mullite supports by secondary growth method. Mordenite has a moderate Si/Al ratio of 3–10 and regular pore size of 0.65 × 0.7 nm. These advantages allow mordenite membranes to be a potential candidate for separating water from acetic acid solutions with excellent acidic-resistant property and hydrophilicity. The enhancement of flux as well as high reproducibility is critical for industrial application of mordenite membranes. To improve the flux of membrane, we rapidly prepare mordenite membranes on macroporous mullite supports in a fluoride-containing precursor gel. The molar composition of synthesis solution is 1SiO2: 0.08Al2O3: 0.25Na2O: 0.2NaF: 40H2O. The hydrothermal treatment is carried out at 170 °C for 5 h. After synthesis, the as-synthesized membranes are characterized by XRD, SEM and pervaporation (PV) test. The surface SEM image of the membrane prepared under optimal conditions shows compact and highly intergrown zeolite layers composed with ellipsoidal polycrystalline grains. The membrane thickness is approximately 8 μm. High-flux mordenite membranes exhibit a long-term acid stability for a 90 wt% HAc/H2O mixture at 75 °C, the flux and separation factor ultimately keep stable at approximately 1.03 kg m-2 h-1 and 4500 for 10 d. Furthermore, mordenite membranes are successfully scaled-up from 10 cm to an industrial scale of 80 cm with transverse crystallization. These high performance 80-cm-long mordenite membranes with good roeprducibility show a promising industrial application for dehydration of water-acetic acid mixtures.
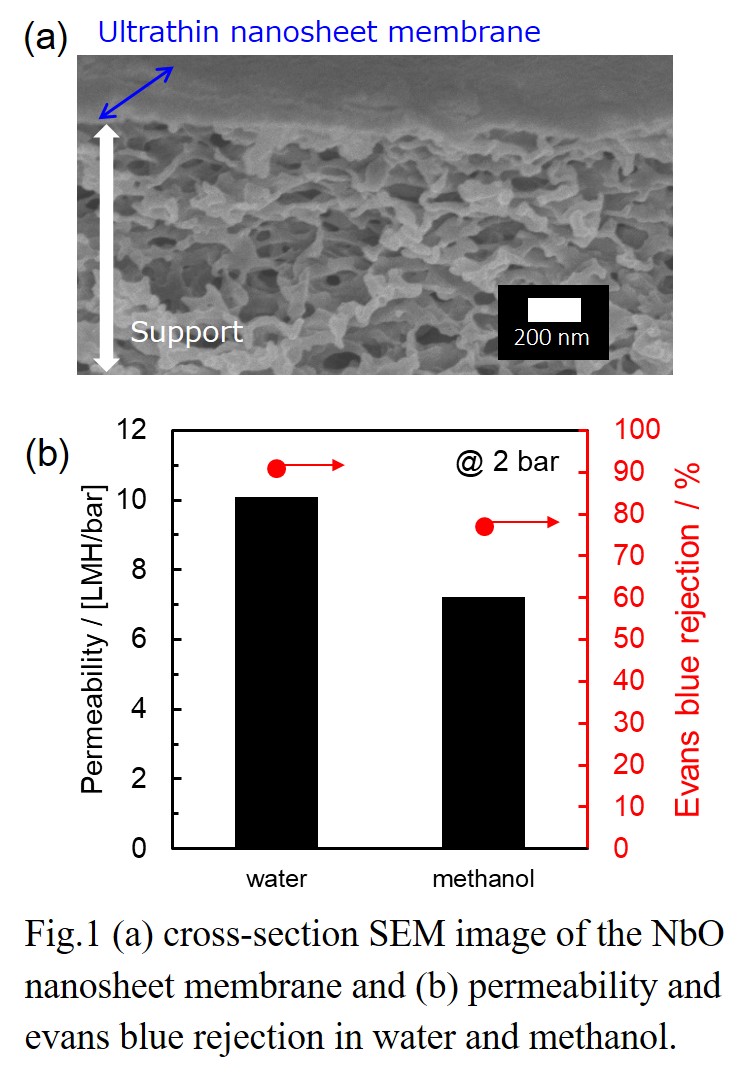
Two-dimensional (2D) nanosheet membranes are expected to function as size-selective molecular separation membranes, based on their unique atomic thickness with micrometer lateral dimensions. Stacked nanosheet membranes are formed by assembling single molecular sheets into thin membranes, and contain 2D nanochannels between the stacked sheets that allow water or solvents to pass through whilst rejecting unwanted solutes [1]. Therefore, they represent promising materials for potentially high-functional membranes for liquid separation such as water treatment and organic solvent filtration. The structural stability of nanosheet membranes during filtration is also a critical issue for their application.
In this study, we fabricated nanosheet membranes using single 2D nanosheets of niobium oxide (NbO) on a porous cellulose nitrate or anodic alumina support by a simple vacuum filtration. The thickness of NbO membranes were controlled by adjusting the volume of nanosheet colloidal solution used during the vacuum filtration. The stacked NbO nanosheet membranes had a dense structure and were highly stable during separation tests, because of the chemical cross-linking between nanosheets. The method allowed the formation of nanochannels in the NbO membranes. The NbO membranes showed high rejection performances against anionic dyes and salts in water [2,3]. Furthermore, membrane structure and separation performances in alcohols were also investigated. There was little difference in the interlayer spacing of the stacked structure between water and alcohols, suggesting the stable layered structure in alcohol solvents. The NbO membranes demonstrated relatively high rejection performances such as 85% rejection for Evans blue (EB, Mw: 960.8) in methanol. The channel structure and separation mechanism for NbO membranes in water and alcohols will be discussed.
[1] G. Liu, et al., Angew. Chem. Int. Ed.55, 2–16 (2016), [2] K. Nakagawa et al., Chem. Commun. 53, 7929-7932 (2017), [3] K. Nakagawa et al., Sep. Purif. Technol, 219, 222-229 (2019)
High purity propylene is an octane-enhancing chemical and also feedstock to industrially important chemicals. Purification of propylene from propane mixture is technologically and financially challenging because of their close boiling points. ZIF-8 membrane has the potential to separate propylene from propane effectively due to the gate opening effects, although the theoretical ZIF-8 aperture size (0.34 nm) is smaller than the kinetic diameters of propylene (0.4 nm) and propane (0.43 nm). In this work, defect free ZIF-8 membranes were successfully developed from the secondary growth seeding technique with sodium formate as deprotonating agent that facilitated continuous, well-intergrown ZIF-8 membrane on α-Al2O3 support. The defects formed by the crack formation in the membrane was steadily and effectively removed by the uniquely discovered self-healing property that the sodium formate extended. The ZIF-8 membranes demonstrated their excellent molecular sieve separation capability for equal molar propylene/propane mixture with the highest separation factor of 115 and average propylene permeance of 50.40×10-10 mol/m2 s Pa.
In this talk, I will report the development of novel omniphobic membranes and operation modes for various process applications with membrane contactors (MCs). An omniphobic membrane was fabricated for membrane distillation (MD) by effectively depositing ZnO nanoparticles on a hydrophilic glass fiber (GF) membrane to create hierarchical re-entrant structures, followed by surface fluorination and the addition of a polymer coating to lower the surface energy of the membrane. The omniphobic membranes possessed a particulate membrane morphology and an extremely high fluorine concentration on the surface. The omniphobicity of the fabricated membrane was indicated by the contact angles for water and ethanol, which were as high as 152.8±1.1 ° and 110.3±1.9 °, respectively. In particular, it will focus on the development of tuned hydrophobic, hydrophilic and asymmetric wettability membranes. Membrane contactors are membrane systems that can find application in different fields of industrial interest, covering, for example, gas-liquid operations, liquid-liquid extractions and vapor-liquid distillation. In past decade, the use of membrane absorption to capture carbon (membrane absorption, MA), to prepare emulsions (membrane emulsification, ME), to recover resources from sea (membrane crystallization, MCr) to carry out distillation processes for water scarcity issue (membrane distillation, MD) has been subject of many research activities worldwide. Focus of this talk will be placed on the applications of the developed novel aerogel membranes for MA and MD processes to resolve the carbon abundance and water scarcity issues, respectively, for achieving an ultimate goal toward a sustainable planet.
Silica membranes have been developing as hydrogen permselective membranes. A counter diffusion chemical vapor deposition (CVD) method is the preparation method for the silica membranes. One of the problems for the application of hydrogen permselective membranes is hydrothermal stability. In this study, effects of organic groups in the silica structure on hydrothermal stability was investigated.
γ-alumina (φ10 mm, L30 mm, Noritake Co..) was used for a porous substrate. γ-alumina layer or silica sol-gel layer was coated on the porous substrate. Aluminum sol 5S (Kawaken Fine Chem. Co) with polyvinylalcohol (PVA) was used for the coating of the γ-alumina layer. The parent sol of Tetraethoxysilane (TEOS):EtOH: H2O:HNO3=1:0.1:4:5 was employed for the silica coating. The coated substrates were calcined at 600 °C. CVD was carried out by using HTMOS (Hexyltrimethoxysilane) or TMOS (Tetramethoxysilane) as a silica source at 450 or 600 °C for 15 min. Single gas permeation tests were carried out by using H2 and SF6 at 270 °C.
The hydrothermal stability under steam was examined for the TMOS derived membrane deposited at 550 °C and for the HTMOS derived membrane deposited at 450 °C. The time courses of H2 permeance through the both membranes were shown the figure. The H2 permeance through the TMOS derived membrane decreased gradually, and the permeance was reduced by 37% under the steam treatment for 12h. On the other hand, the reduction rate of the H2 permeance through the HTMOS derived membrane on the silica coated substrates after 23h of the steam treatment was only 10%. In order to confirm the effects of coating of the substrates, the results through the membrane deposited on the coating of the γ-alumina layer were also shown as open plots in the same figure. The H2 permeance looks the similar indicating that there were little effects on the HTMOS derived membranes.
Membrane separation technology has been paid attention for one of the energy saving technologies. A silica membrane is categorized as one of the typical inorganic membranes, and the silica membranes show high hydrogen permselective performance comparing with the other inorganic membranes, such as zeolite and carbon membranes. On the other hand, it is generally known that permselective performance of silica membranes are affected strongly under hydrothermal conditions.
In this study, influence of steam on permeation performance of the DMDPS-derived silica membrane was evaluated to develop the silica membranes having relatively high hydrothermal durability. The silica membrane was prepared by counter-diffusion chemical vapor deposition method on a porous alumina support purchased from Nikkato co., Japan. After membrane preparation, permeation performance was measured using single component H2, N2 and SF6, respectively. Heat-treatment of the membrane was conducted at 573 K for 50 h. During heat-treatment, H2, N2 and SF6 permeances showed approximately constant value. To evaluate effect of heat-treatment, durability test was conducted under 0.1 ~ 1.0 mol% steam co-existed conditions at 573 K by using heat-treated and non-treated silica membranes. Comparing influence of steam on permeation performances of these membranes, permeance decreasing ratio of heat-treated membrane showed lower value than that of non-treated membrane in any steam concentrations. In addition, in-situ diffuse reflectance FT-IR spectra of heat-treated and non-treated silica powder was obtained. The heat-treated silica had less amount of adsorbed water molecules than the non-treated silica because the silanol group was decreased owing to heat treatment. From these results, it is considered that hydrothermal durability of the silica membrane is determined by the silanol group density.
In generally, permeation phenomenon in microporous membrane can be divided in three steps, adsorption, diffusion, and desorption. Though investigation of these factors in membrane is quite important to understand permeation mechanism, these factors are hardly evaluated in direct.
We previously reported non-destructive adsorption measurement for zeolite membrane.1 In this study, we estimated diffusion property of hydrocarbon in silicalite-1 membrane from gas permeation measurement.
Silicalite-1, pure silica zeolite with MFI-topology, membrane was synthesized by a seed-assisted crystallization method on an outer surface of porous tubular a-alumina support (Noritake, i.d. = 7 mm, o.d. = 10 mm, average pore size = 150 nm).
Diffusion and desorption behavior was evaluated as follows. At first, permeation flux of helium through silicalite-1 membrane was detected. After that hydrocarbon vapor were fed to a membrane with flowing helium. When micropore of zeolite membrane was saturated by adsorbed hydrocarbon, the helium flux drastically decreased. Finally, hydrocarbon feeding was stopped, and then micropore would open by desorption of hydrocarbon. Thus, we can estimate the diffusion and desorption behavior of hydrocarbon from the rate of helium flux increase by pore opening. In addition, diffusion coefficient of hydrocarbon in mocropore could be calculated by using Fick's second law.
(1) M. Seshimo, K. Matsumoto, M. Mastukata, Evaluation of micropore volume of zeolite membrane under non-destructive condition by nitrogen adsorption method, 16th International Conference of Inorganic Membrane, P2.11, July, 2016.
Acknowledgment
This work was partially supported by JST CREST (Japan Science and Technology agency, Create REvolutionary technological seeds for Science and Technology innovation program), Grant Number JPMJCR1324, Japan.
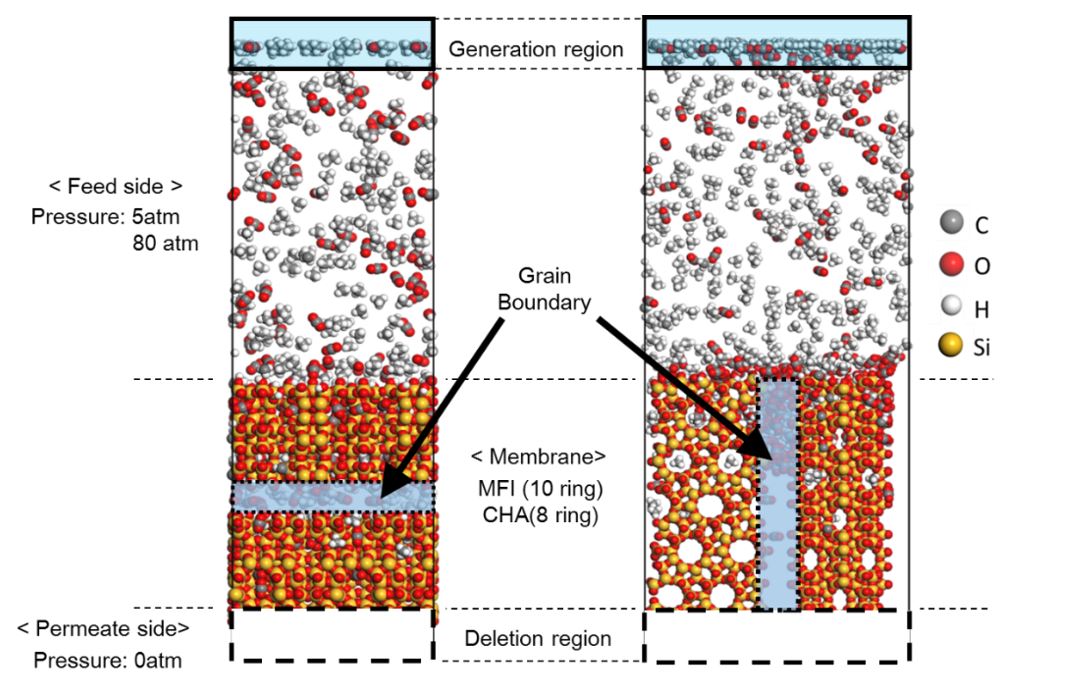
Zeolite membranes have unique properties such as molecular sieving and thermal stability. Therefore, the practical use of zeolite membranes for a purification process of natural gas has been investigated. Grain boundaries in inorganic membranes are known to degrade membrane performance, however, the detailed information of effect on selectivity still difficult to predict prior to the permeation test. Non-equilibrium molecular dynamics (NEMD) method is one of the theoretical prediction method of separation characteristics in inorganic membranes. The NEMD simulation is an atomistic-scale modeling, thus this can easily apply to the prediction of permeability and selectivity in multicomponent gas systems. The effect of trace components for membrane performance can be also investigated using NEMD.
In this study, we investigate the separation properties of CHA and MFI type zeolite membranes by NEMD for CO2/CH4 systems, and elucidate the mechanism of the effect of grain boundary on selectivity for ternary separation systems. Two different types of zeolite membranes having different pore sizes of MFI and CHA are considered to investigate the effect of pore size on selectivity. Two different models used in NEMD are shown in the figure. In the left side model in figure, grain boundary exists insides the zeolite crystal. In the right side model in figure, grain boundary runs lengthwise of zeolite crystal. In Generation region in the feed side gas molecules appear at every certain period. In Deletion region in the permeate side a permeated gas molecule is removed from the system. Our NEMD results indicates that the presence of grain boundary could increase the selectivity, which means that a control of grain boundary is a key factor to enhance the selectivity. In the conference, we will discuss the details of the effect of grain boundaries as well as pore size on this interesting phenomena regarding the selectivity.
Palladium membranes made of pure palladium, palladium-silver, palladium-copper, etc. are promising in terms of high selectivity to hydrogen. Because palladium is limited in resources and costly, various attempts have been made to reduce necessary amount of palladium preparing not only membranes supported by porous materials but also foil-shaped thin self-supported membranes. Hoverer, self-supported membranes are difficult to seal with frames of modules because welding is not easy for thin foils. To utilize self-supported membranes in practice to process large amount of hydrogen, technology to compose modules is necessary.
In this study, therefore, membrane modules for self-supported palladium membranes have been developed. A prototype module had eight sheets of palladium-silver foils 0.02 mm thick and 30 mm × 60 mm wide including sealing area. The foil-shaped membranes and spacers were alternatively accumulated and combined by thermal diffusion. The combined structure was set inside a stainless-steel container and welded between one of the spacers and the container. The module had three ports for feed, permeate and retentate gases. The module size was 34 mm × 44 mm × 64 mm excluding three ports.
Hydrogen permeation performance of the module was investigated using 1-MPa pure hydrogen as a feed gas at 623 K. As a result, 6 L(at 293 K, 1 atm)/min hydrogen was obtained from the permeate port. Durability was proved at least for 467 hours. This kind of modules are expected to enlarge the applicability of palladium membranes.
Membrane gas separation have the potential to reduce energy consumption in chemical industry. Currently polymeric membranes are widely used. However, their applications are limited because of their low selectivity. Carbon molecular sieve (CMS) membranes have higher permselectivity than commercially available polymeric membranes and lower fabrication cost than silica and zeolite membranes. The CMS membrane are expected to expand the range of application of membrane gas separation. One of problems to be considered for application is the effect of impurity. In this study, we have investigated the effect of coexisting component on gas permeation and separation through CMS membranes. Mixed gas permeation experiments have been carried out for binary mixtures of hydrogen (H2)-nitrogen(N2) and tertiary mixtures of H2, N2 and water vapor. Two CMS membranes prepared from a polyimide have been used.
Permeance to H2 at a constant partial pressure did not change with increasing N2 partial pressure for both membranes. Permeance to N2 at a constant N2 partial pressure did not changed with increasing H2 partial pressure for one membrane. However, N2 permeance increased with increasing H2 pressure for another membrane. Consequently selectivity of H2 over N2 decreased with H2 partial pressure for the latter membrane.
Coexisting water vapor decreased both H2 and N2 permeance for both membranes. However, H2/N2 selectivity decreased for one membrane and it increased for another membrane. Water vapor had a complex effect on a H2/N2 mixed gas permeation for CMS membranes.
New class of tough gel membranes containing a large amount of ionic liquids (ILs) in an inorganic/organic double-network, termed inorganic/organic double network ion gel (DN ion gel) was fabricated. The inorganic/organic double-network could be easily synthesized in the same IL pot, which allowed the preparation of freely shapeable DN ion gel, including a film shape. The DN gels showed excellent mechanical strength (more than 25 MPa of compressive fracture stress) without any leakage of ILs under compression. From tensile stress loading/unloading test, it was confirmed that the excellent mechanical strength of the DN ion gel was owing to the inorganic network which acted as a sacrificial bond to dissipate the loaded energy and the organic network which played a role of hidden length to sustain large deformation.
The gas permeation under pressurized condition was examined by using the tough ion gel membrane containing more than 80 wt% of an IL. As expected, the ion gel film was not destroyed under high trans-membrane pressure differences up to 700 kPa. Regarding the CO2 separation performance, the CO2/N2 selectivity was almost same as that of the supported ionic liquid membranes. The DN ion gel membrane with optimized network composition and 80 wt% ionic liquid sustained about 1200 barrer of CO2 permeability and 25 of CO2/N2 selectivity for more than 300 h at 50 oC under humid condition. In addition, it was confirmed that the CO2 permeability as well as CO2/N2 selectivity of the ion gel membrane containing more than 90 wt% of an IL was almost same as that of the supported ionic liquid membrane. The superior gas permeability of the ion gel membranes stem from the fact that there is almost no diffusion resistance in the ion gel owing to its low polymer content.

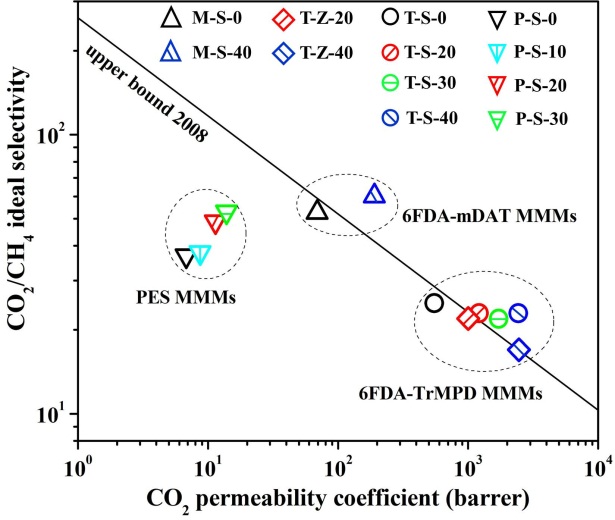
Membrane separation technology has been investigated and applied into purify natural gas, especially removing CO2 from CH4 to reduce the pipeline corrosion and increase heating value. Polymer membrane has easy processability, high reproducibility but reaches a limit in a trade-off between permeability and selectivity (Robeson upper bound line). Inorganic membrane owns excellent separation property and mechanical stability, while its high-cost membrane fabrication demands improvement. Blending inorganic particles into the polymer matrix to prepare mixed matrix membranes (MMMs) could be considered as an appropriate method due to it could combine both advantages of polymer membrane and inorganic membrane.
In this study, nanoporous SAPO-34 zeolite (~0.38 nm) and ZIF-8 particles (~0.42 nm) were severally added into polyimide (6FDA-TrMPD and 6FDA-mDAT) and polyethersulfone (PES) to prepare MMMs for CO2/CH4 separation. The filler, polymer and N-methyl pyrrolidone (solvent) were mixed to form a homogeneous solution with filler content in polymer about 0 wt.% ~ 40 wt.%. The solution was cast on a glass plate and the formed MMMs were peeled off after drying. The single gas permeation through the membranes was tested by vacuum method at 1 atm and 35 °C.
According to the figure, 6FDA-TrMPD/SAPO-34 MMMs (T-S-0~40) and 6FDA-TrMPD/ZIF-8 MMMs (T-Z-20~40) possessed the highest CO2 permeability. The 6FDA-mDAT/SAPO-34 MMMs (M-S-0~40) provided the best CO2/CH4 ideal selectivity. Addition of SAPO-34 could both increase the gas permeability and selectivity of PES/SAPO-34 MMMs (P-S-0~30) and 6FDA-mDAT MMMs. In term of 6FDA-TrMPD MMMs, all the SAPO-34 and ZIF-8 could only enhance the gas permeability at a slight loss of selectivity. Nevertheless, the separation performance of 6FDA-TrMPD MMMs containing 30%~40% SAPO-34 (T-S-30 and T-S-40) still exceeded the Robeson upper bound line (2008) of the polymer membrane, which was the same as the 6FDA-mDAT MMM with 40% SAPO-34 zeolite (M-S-40).
In recent years, microfiltration is used in wastewater and drinking water treatments, and food and pharmaceutical industries. In the process of using microorganisms, the feed solution is a complex mixture comprising bacterial flocks and microbial metabolite. Therefore, it is essential to clarify the mechanism of flux decline behaviors in microfiltration of microorganism and its metabolite. The key objective of this study is to examine the effect of polysaccharide-protein interaction on membrane fouling caused by microbial metabolite since the microbial metabolite is composed mainly of polysaccharides and proteins. Microfiltration experiments were performed in a dead-end filtration mode using various types of solutions at pH of 4.5 and 6.5 under constant pressure condition. Sodium alginate (SA) with the molecular weight (MW) of 129 kDa was used as model polysaccharide, and lysozyme with the MW of 14.3 kDa, the isoelectric point (pI) of 11.0 and bovine serum albumin (BSA) with the MW of 67 kDa, the pI of 5.1 were used as model protein in this study. The flux decline behaviors were observed in the case of mixture of polysaccharide and protein, and in particular, the filtration rate of solution containing SA and BSA was influenced by the solution pH. The BSA molecule is negatively charged at pH 6.5 and positively charged at pH 4.5, while at both pH values, the lysozyme molecule has a net positive charge, and SA molecule has a negative charge. It is obvious that the effect of the surface charge of protein on the filtration resistance should be considered.
Algal bio-fuel as alternative to fossil fuel has been attracting lots of attention due to the global warming and depleting natural fuel resources. However, the cost of bio-fuel production is relatively high. Especially, harvesting and dewatering of microalgae suspension are reported to account for 20 - 30 % of the total cost, because suspended solid (SS) concentration of microalgae culture is low with 0.02 - 0.06 wt%. Therefore, efficient dewatering process is required. This study examined the performance of dewatering of microalgae suspension by cake filtration using filter cloth.
Two hundred milliliter of microalgae suspension with 0.02 wt%-SS was filtered at constant pressure (70 kPa) using filter cloths (24.4 cm2 of effective area) made from polypropylene, which have different pore size 0.8 - 14 μm. Average permeate flux was increased from 5.9 to 2600 L/(m2 h) with the increase in pore size, while SS rejection was decreased from 94 to 53 %. The filtration behavior was analyzed by existing filtration models. As a result, when filter cloths with smaller pore size than algal cell size (about 8 μm) were used, filtration mode was finally settled into cake filtration. On the other hand, for the filtration using filter cloth with 14 μm of pore size, filtration mode was not settled into cake filtration. Then, in order to conduct cake filtration using filter cloth with 14 μm of pore size for expecting high permeate flux, pre-concentrated suspension (0.1 wt%-SS) was filtered with the intention of forming a cake layer quickly by the deposition of many algal cells on the filter surface. Consequently, microalgae suspension was successfully concentrated up to 18 wt%-SS with 360 L/(m2 h) of average permeate flux and 99 % of SS rejection. These results suggest the potential of microalgae dewatering by cake filtration with high flux and rejection.
Expression is the separation of liquid from a solid/liquid system by compression due to the movement of the retaining walls of the filter chamber. In the expression of slurry material, the application of mechanical pressure to the retaining walls causes a sudden increase of hydraulic pressure uniformly throughout the slurry. Initially, the process of expression proceeds based on the principle of filtration, where the thickness of filter cake increases over time. Filtration terminates when the whole slurry forms a layer of filter cake, and the consolidation of the cake follows. Exact identification of the time when the chamber is filled with the cake is essential for an effective expression operation. The electrokinetic response of expressed material provides us with some information on the status of the filter chamber. In this work, we have measured the time course of electric potential difference (EPD) between the retaining walls to elucidate the electrokinetic aspects of mechanical dewatering. The experimental apparatus used in this study consists essentially of a piston press with a cylinder and a piston. Filter media are placed at the cylinder bottom and the very end of the piston. EPD between the filter media was monitored during the experiment. As can be seen from the figure, the absolute value of EPD increases with the progress of the filtration period, followed by the decrease during the consolidation period. It was observed that the time when the absolute value of EPD began to decline coincides with the time when the filtration period ended. We have derived a theoretical equation of streaming potential for a flow path of the expressed material and combined it with filtration and consolidation theories to calculate the theoretical time course of EPD of expressed material. It has been found that EPD reflects liquid pressure variation throughout the solid/liquid mixture.
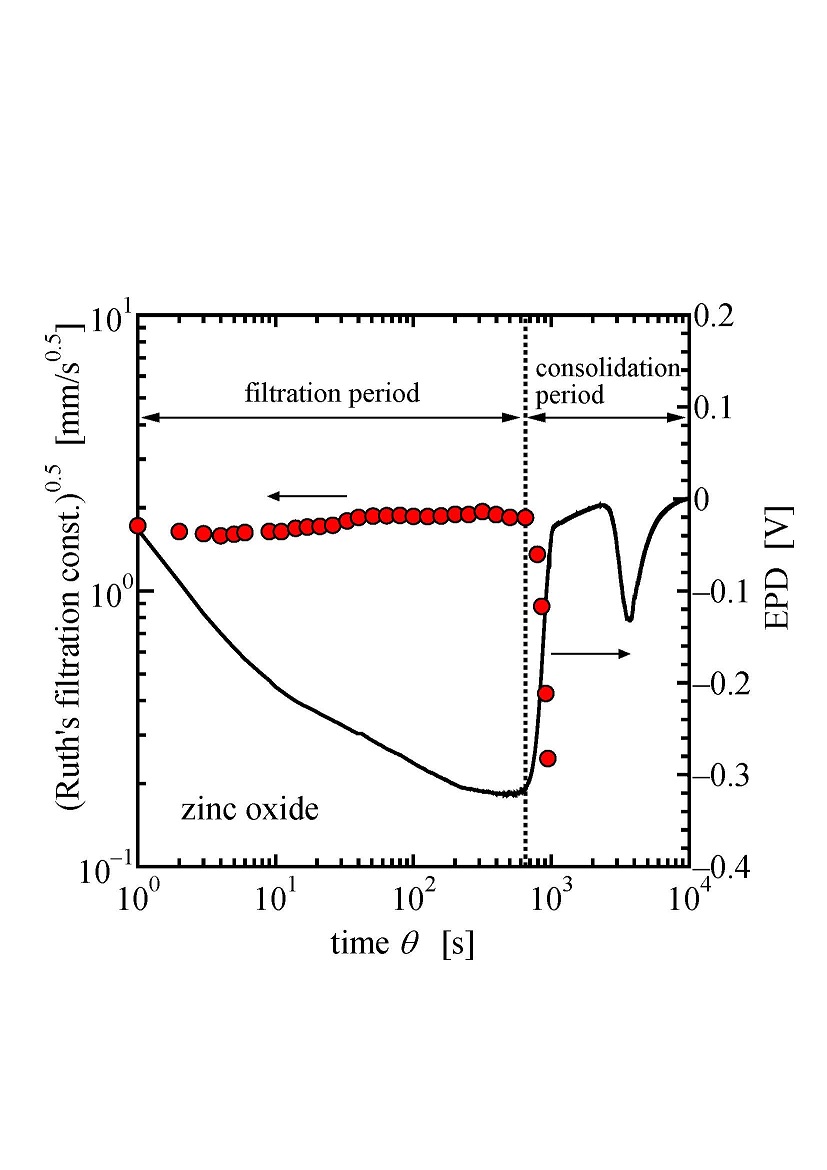
The application of membrane filtration processes has been increased because of global shortage of water. Membrane fouling is considered as a major factor limiting the use of the membrane filtration process and is the irreversible alternation in the membrane caused by specific physical and chemical interactions between membrane and various components present in the process stream. The fouling configuration can be classified into three simple mechanisms: pore narrowing by adsorption, pore blocking/plugging and cake/gel layer formation. In many actual processes the species causing the dominant fouling effects is not always known because of the complexity of the feed stream. Identifying the foulant and understanding fouling phenomena are required for adopting countermeasures for the fouling. Electrostatic and hydrophobic interactions are recognized as the major interactions causing fouling. The zeta potential of the foulant or the membrane, that is, the electrical potential at the surface of shear between the solid and the liquid, can be useful for the prediction of the electrostatic interaction. Adsorption is a major cause of membrane fouling in microfiltration of macromolecule containing solutions. The change ratio of zeta potential by protein adsorption is directly proportional to the surface coverage regardless of pH. In pore blocking or cake formation caused by fine particles the location of pressure drop in the system depends on the fouling configuration. The zeta potential reflected the local pressure drop part, where the gap between particle and pore in pore blocking filtration and the gap between particles in cake filtration. These observations shows the zeta potential will useful for monitoring the fouling configuration during the filtration operation.
Traditional pharmaceuticals have been made from chemical processes. Today, a significant fraction of valuable pharmaceuticals, such as hormones, insulin, growth factors, interferon, enzymes, drug intermediates, etc. are replaced by biotechnology processes. One of the building blocks is to make protein out of recombinant processes where extracellular or intracellular protein can be “engineered” and secreted from host biological cells which have been inserted with vectors carrying human genes. The host cells, e.g. the mammalian cells, bacteria and yeast can secret high amounts of recombinant protein in a bioreactor or fermenter. For extracellular protein, the first step in harvesting protein is to separate the protein from the cells. On the other hand for intracellular protein, the bacteria cells have to be lysed first before releasing the protein in form of inclusion body, for which separation follows. Disk stack and tubular centrifuges operating between 4,000–15,000 times gravity are popular choices to make separation of cells, cell debris, and proteins from the broth. We will discuss the various scenarios of centrifugal separation of protein in clarification, separation, classification and purification by washing during protein harvest. This is an extremely important step that affects yield and downstream processes, e.g., purification by chromatography. We will also discuss testing, modelling and prediction of protein separation in protein harvesting. Further, new technologies on separation of flocculated bio-solids and other popular biotech separation processes by centrifugation will also be discussed.

A deformable gel-packed chromatographic column was used to separate as-synthesized graphite oxide with different sizes. The synthesized gel (56 micrometer) was deformed by pressure of the fluid flow and the gaps in the gels showed a range of sizes. A suspension of graphene oxide (0.1 g/L, 10 mL) was injected, and graphene oxide in the elution had a size at 0.56 micrometer and 0.14 micrometer, whereas in half upper and bottom domain of the gel layer graphene oxide had a size at 33 micrometer and 2.9 micrometer, respectively, demonstrating that graphene oxide suspension was separated by size through gel layer. On behalf of graphene oxide, suspension of silica particle produced by dry process was also injected to the elastic gel -layer to separate silica particles due to their size and morphology.
To elute the filtered colloidal particle among the gel, the elastic gel layer was compacted and extended by the change of the applied pressure of water. At that time due to the dynamic change of the gel layer, the filtered particles was gradually eluted by the expansion of the gel's gaps as well as the convection of the fluid flow. The recovery percentage of the silica particles filtered was increased with increasing the repeated time of compaction and extension of the elastic gel layer.
The capture of solute into freezing part is difficult by the vigorous agitation of the freezing interface during the fast freezing of solution. We have been studying the applicability of ultrasonic irradiation (frequency: 20 kHz) to the agitating method, and found that the freeze concentration efficiency of solutes is improved greatly by this irradiation. In this paper, the effect of the frequency of ultrasonic irradiation on the freeze concentration characteristics for multiple solutes is examined.
Using three kind of solutions containing only one solute (Histidine, Vitamin C, and saccharose (these can widely be found in the food materials), 0.03 mol/L), we examined the freeze concentration characteristics with ultrasonic irradiation. The frequencies of ultrasonic irradiation were 20 kHz and 200 kHz, which were adjusted to the same output (11.8 W). From the experimental results, decreasing dissolved oxygen concentration (DO) can increase the concentration efficiency of all solutes in the case of 20 kHz ultrasonic frequency. On the other hand, in the case of 200 kHz ultrasonic frequency, increasing DO may increase the concentration efficiency. The DO dependence of the concentration efficiency differs depending on the frequency of ultrasonic irradiation and solute type.
Ultrasonic wave is applied in many situations, for example cleaning glasses, sensors, dispersion. In cleaning glasses, the frequency of ultrasonic is about 100 kHz. When the frequency of sonication increases to about 2 MHz and vibrates water, water becomes mist and flies away. This phenomenon is ultrasonic atomization. In this research, we applied this atomization to drying particles that is useful in chemical processes. This drying method is called ultrasonic drying.
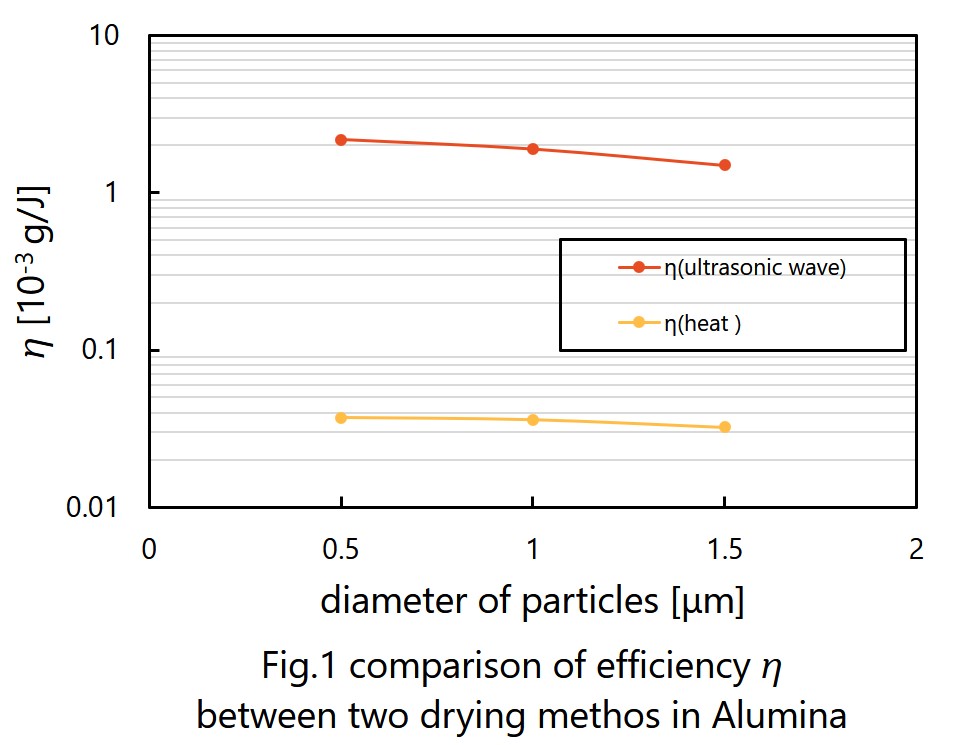
It is expected that ultrasonic drying will be faster and consume less energy than drying by heat. We had three experiments to prove them. First was testing whether ultrasonic can dry wet particles. I tried to dry fly ash by ultrasonic and observed how humidity of fly ash changed. Next is measuring the rate of ultrasonic drying. We prepared three kinds of particles, alumina, silica and fly ash. I moreover prepared three different diameter of alumina and two different diameter of silica to see whether the rate of ultrasonic drying depends on the diameter of particles. Last is comparing efficiencies of ultrasonic drying and traditional drying. We measured the loss of weight of materials by drying and the consumption of electronic energy. Efficiency of drying, as shown in Fig. 1, is defined by the weight loss divided by the electric energy consumption.
The result of first experiment was that mist appeared from the surface of particles, so we were sure that ultrasonic can dry materials. Second experiment suggests that the rate of ultrasonic drying has positive dependence on diameter of particles. This experiment, however, cannot show difference in dying rate among different particles. Efficiency of ultrasonic drying gotten in last experiment is approximately 100 times better than one of drying by heat. Considering by those outcomes, ultrasonic drying will replace some current drying methods in a few decades.
Consumer products largely originate from fossil resources which will be depleted sooner or later and contribute to CO2 emissions and climate change. Alternatives are sought with low carbon emissions and these are inexhaustible resources like plant derived biomass. Our motivation is to optimize the extraction process of lignin. The only water pre-treatment process and combination with organic solvent Soxhletation was carried out in the first stage to reduce impurities. Soda process and diluted acid process were chosen for extraction methods and microwave-assisted as the main instrument for heating up solution.
The results showed that pre-treatment process by using water following with isopropanol-hexane was given the best result. Water helped to extract sugar, alcohol, and some water soluble impurities. When isopropanol extracted the rest of polar impurities that water could not be done, Hexane was extracted non-polar impurities such as lipid. It could have extracted about 5.3 % weight loss of impurities without changed the lignin structure significantly due to TGA and FTIR results. This study aims to extract lignin in moderate temperature, pressure, and power in as little concentration and time as we could achieve. The process was done with soda process for 2 wt% NaOH concentration at 130 oC with 30-min extraction process as the best result rather than diluted acid process. The amount of lignin obtained about from this process was relatively higher both in 99.7% yield and 94.6% purity compared to other studies result. This type of lignin has similar characteristic to commercial dealkaline lignin.
This study aimed the treatment of the aqueous solution contaminated by antibiotics with duckweed, Lemna minor, which was reported to uptake organic compounds in the aqueous solution, and the removal of the antibiotics in the aqueous solution with duckweed under various conditions was measured to study the mechanism of antibiotics removal from contaminated solution. Firstly, it was confirmed that the molar concentrations of ciprofloxacin and sulfamethoxazole, which were selected as model antibiotics to be treated because of most popular antibiotics causing aquatic environmental pollution, could be reduced due to the mechanisms of hydrolysis, photo-degradation and uptake by duckweed. Ciprofloxacin was more degradable due to hydrolysis and photo-degradation in the aqueous solution than sulfamethoxazole, and the degrees of the molar concentration reduction due to uptake by duckweed were comparable for both antibiotics. In the cases of the treatment of ciprofloxacin of the initial molar concentration at 1×10-5mol/L, the reduction of the molar concentration due to the hydrolysis and photo-degradation shared 0.6 relative to the total reduction of the molar concentration, and the ratio of reduction due to uptake relative to the total reduction was 0.4. On the other hand, the molar concentration reduction of sulfamethoxazole due to the uptake relative to the total concentration reduction was 0.95, and the contribution of the hydrolysis and photo-degradation to the total reduction of ciprofloxacin concentration was so small. When the initial molar concentrations of both antibiotics increased up to 5×10-5mol/L, the contributions of these three factors to the antibiotics reduction were similar to the cases with the initial concentration as 1×10-5mol/L. Accordingly the uptakes of both antibiotics by duckweed were so influential to reduce the molar concentrations in the aqueous solution, especially for sulfamethoxazole, and this treatment method might have a potential to remediate the aquatic environment polluted by antibiotics.
The response surface methodology was applied to study and to optimize the surfactant-enhanced extraction of tea tree oil (TTO), i.e. the essential oil of Melaleuca alternifolia, by hydrodistillation method. Both Tween 20 and Tween 80 were used as surfactants added in the hydrodistillation vat with an aim to enhance the extraction yield of TTO. In principle, this study evaluated the relevance of several independent parameters, including the concentration of surfactant, extraction time, and liquid/solid ratio, against TTO yield with a design of experiment (DOE) based on response surface methodology. Central composite design (CCD) was used to optimize the processing condition of TTO extraction. The chemical compositions of tea tree oil were analyzed and quantized by GC-FID and were referred with the international standard "ISO 4730". Additionally, TTO obtained from optimal condition was examined on the stability of its microemulsion formulations as well as the antibacterial property, compared with the commercial TTO. The microemulsion stability was mainly determined by the particle size measurement with dynamic light scattering (DLS), whereas the antibacterial assay was carried out by agar disk diffusion method with Escherichia coli and Staphylococus aureus.
Keywords: Melaleuca alternifolia; Extraction; Design of experiment (DOE); Optimization
Solvent extraction (SX) is generally used for separation and purification of platinum group metal (PGM) in hydrometallurgical processes. The development of a successful SX process depends on the choice of appropriate extractants. Therefore, we have been studying the extraction behavior of PGM with newly synthesized extractants in addition to the structural properties of PGM complex anions extracted in the organic phase.
We have developed a new palladium extractant, thiodiglycolamide (TDGA), which can rapidly extract Pd(II) from HCl solution with a good selectivity and has a high oxidation resistance, compared with an industrial palladium extractant, di-n-hexyl sulfide (DHS). Currently, TDGA has been commercially available and already put to practical use in a PGM separation plant.
To date, there have been no effective practical extractants for rhodium in acidic chloride media, because the dominant rhodium species in relatively concentrated HCl solutions ([RhClx(H2O)6–x]3–x (x ≥ 4)) are poorly extracted into an organic phase. We have found that amide-containing tertiary amine (ACTA) compounds show a higher efficiency for the Rh(III) extraction from HCl solution than the conventional tertiary amine extractant, tri-n-octyl amine. The protonated ACTA molecules extract the mono-aquated dianion [RhCl5(H2O)]2– through the formation of an outer-sphere assembly, which was characterized by slope analysis, FT-IR, EXAFS, SANS, computational modeling, etc.
Prices of strategic metals and rare earth elements (REEs) have been rising over the past decade due to the global shortages in supply and increasing demands. These metals are essential components of advanced and emerging technologies associated with transport, environment, energy, defense, electronics, information and aerospace. With the utility of REEs projected to increase in the next decade, it is important to find alternative sources to conventional mining to cope with the demand. There are a few studies conducted on the potential of coal fly ashes as secondary resource of REE. Coal fly ash are waste products from coal burning in a coal power generation plants.
This research is aimed at extracting rare earth elements from coal fly ash by hydrochloric acid leaching. The purpose of this study is to explore the amenability of coal fly ash to metallurgical processing for the extraction of rare earth elements. Three process parameters were investigated in this study, namely, hydrochloric acid concentration, leaching time, and the leaching method. Results of the experiment showed that the recovery of rare earth elements increased with increasing hydrochloric acid concentration. Direct leaching method achieved higher recovery values for REEs compared to sequential leaching. Maximum recovery value for REEs was obtained at 1 h leaching time.
Scandium (Sc) is rare and expensive metal in high demand, providing excellent characteristics for various industrial applications. Since Sc is commonly found in crustal together with Y owing to its small ionic radius, the separation of Sc from yttrium (Y) and other rare earths is required. In the present work, separation and recovery of Sc in an aqueous chloride media was investigated by solvent extraction with carboxylic acid, Versatic acid 10.
The aqueous chloride solutions of rare earths were prepared by dissolving their oxides in 1 or 2 mol/L HCl solution. Organic solution was prepared by diluting Versatic acid 10 in IP Solvent 2835. Extraction of Sc and Y was carried out by shaking the organic and aqueous solutions at volume ratio of 1 : 1 at 25oC for more than 6 h. Concentrations of the metals in the aqueous solutions were determined by ICP-AES and those in the organic solutions were calculated based on material balance.
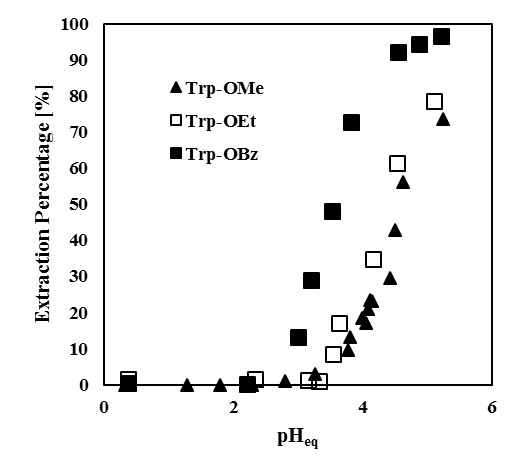
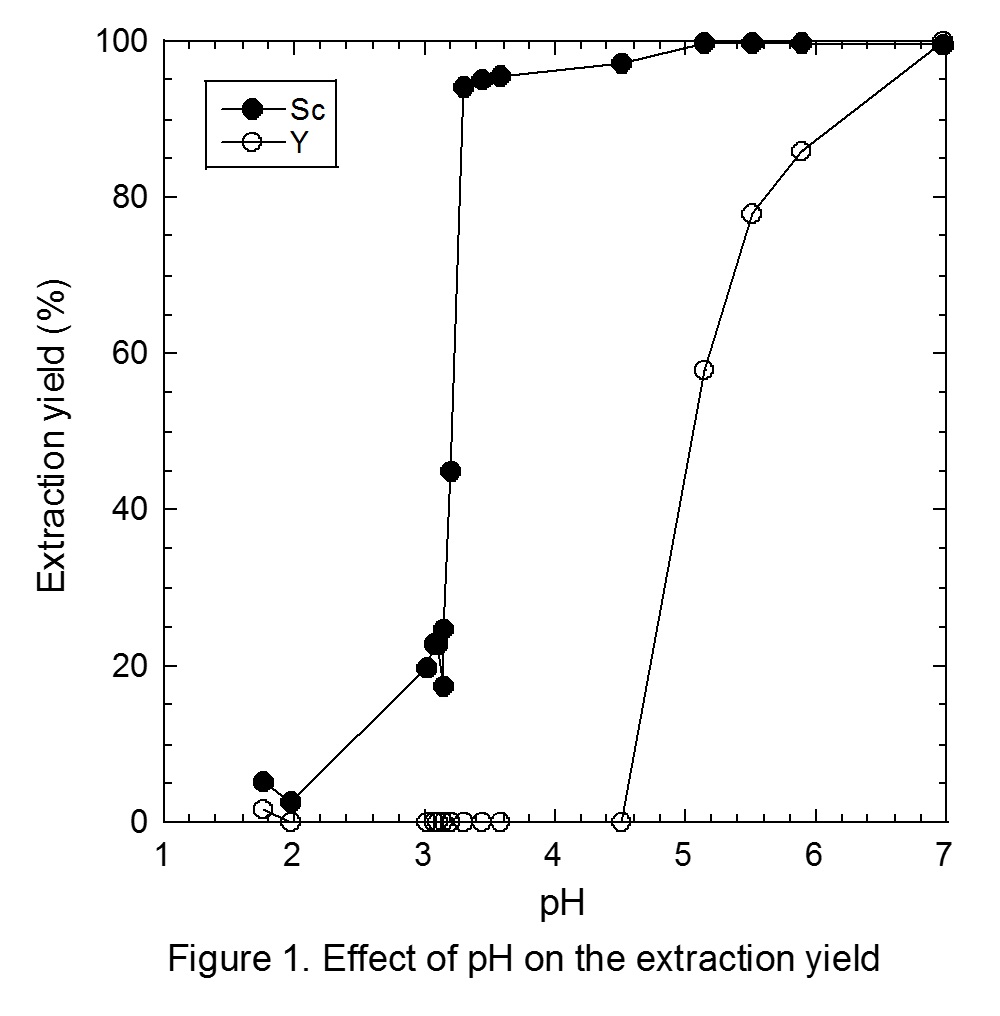
Figure 1 shows the effect of pH on the extraction yields of Sc and Y from the binary solution. Extraction of Sc proceeds from pH 3, and is increased with pH, while the extraction of Y proceeds from pH 4.5. Separation of Sc and Y is therefore easily achieved with Versatic acid 10. The conventional slope analysis of Sc with Versatic acid 10 revealed that the extraction is based on the cation exchange mechanism and the stoichiometry of Sc and the dimeric extractant of Versatic Acid 10 was 1 : 3.
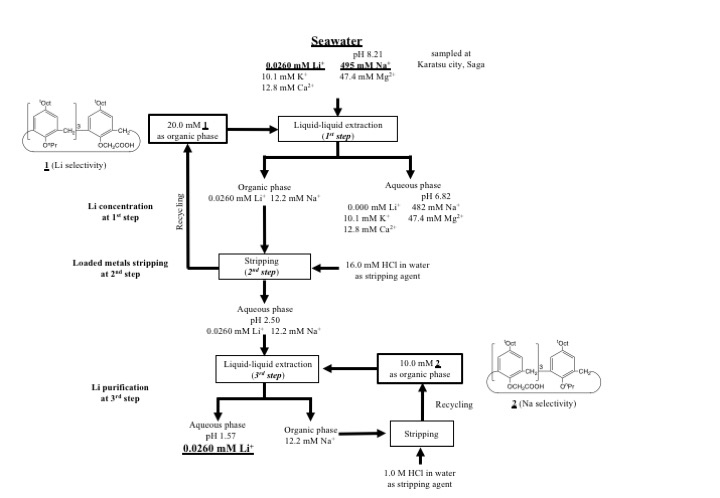
Two derivatives of p-t-octylcalix[4]arene were prepared to investigate extraction behavior of alkali metal ions. Tripropyl-monoacetic acid derivative (1) exhibited lithium selectivity among alkali metal ions, while Triacetic acid-monopropyl one (2) showed sodium selectivity. Then, they were employed for individual and stepwise recovery of alkali metal ions using microreactor system. Finally, they were employed for Li recovery from the seawater. Compound 1 was applied for Li recovery to concentrate Li ion at the 1st step and to strip the loaded metal ions, and compound 2 was employed to remove sodium ion for Li purification. Lithium ion was successfully completely recovered form seawater with two derivatives using microreactor system. Flow sheet for Li recovery with two derivatives using microreactor system is shown in Figure.
The polymer inclusion membranes (PIMs) composed by polymer and carrier without plasticizer have been used in this study. The extractant used in this study is containing in long alkyl chains and double bond. The great benefits of PIMs are to be used a small amount of extractant and less organic solvent and also are less leak of the extractant out of the membranes.
In the near future, it is expected that a large amount of wastes of used solar panels containing In(III), Ga(III) and Zn(II) is discharged. In this research, we were interested in alkyl-derivatives of sarcosine as an extractant to selectively separate In(III) and Ga(III) from Zn(II). Accordingly, a comparison of the metals(In(III), Ga(III) and Zn(II)) extraction was performed using four kind of extractants (two types of carboxylic acid and two types of sarcosine) and three polymers. Among them, We found the membrane containing poly(vinylidenefluoride-cohexafluoropropylene) (PVDF-HFP) as a polymer skeleton and N-oleoylsarcosine as a carrier. This membrane exhibited highly selective to In(III) and Ga(III) in hydrochloric acid solution. Finally we found that the PIM containing N-oleoyl sarcosine could perform the selectively separate In(III) and Ga(III) from Zn(II) from hydrochloric acid. In addition, we succeed in containing 60 % w/w N-oleoylsarcosine in the membrane.
We successfully developed a novel nanofiltration membrane that enables the separation of Mg2+ and Ca2+ from effluents in the electrodialysis process in salt productions. For that purpose, we first fabricated the polyamide layers using piperazine and trimesoyl chloride onto microfiltration membranes as supports, and then immobilized poly(2-methacryloyloxyethyl)trimethylaminium chloride) (polyMTMA) covalently on the surfaces and the pore walls of the polyamide layers by the plasma graft polymerization method. The polyMTMA grafted on the substrates made the membranes surface positively-charged, and at the same time reduced the pore sizes. Throughout the systematic nanofiltration experiments using various single salts with concentrations of 1.9 x 103 ppm, a membrane with a grafting amount of 0.24 mg cm-2 showed the following rejection performances that positively-charged nanofiltration membranes typically exhibit; the observed rejections (Robs) for NaCl, CaCl2, MgCl2, Na2SO4, CaSO4, MgSO4 were 0.20, 0.64, 0.65, 0.21, 0.35, 0.44, respectively. We also carried out Mg2+ separation tests from Na+-rich solutions by using aqueous solutions containing NaCl and MgCl2 with the ratios of 9:1, 14:1, and 19:1 as feed. The results indicated that around half of Mg2+ ions were retained with the permeation of more than 80% of Na+ ions.
Acknowledgement: Part of this study was financially supported by a grant from the Salt Science Research Foundation, Japan.
Oil/water separation has attracted more attention in both of academia and industry due to the increased amount of oily wastewater from the industrial process and frequent oil spill accidents, causing fatal damage to ecosystem and people's health. How to treat the oily wastewater has become a big challenge all over the world. In this work, an innovative aliphatic polyketone (PK) membrane surrounded by hydrophilic silica nanoparticles with hierarchical micro/nanoscale structure was developed by a APTES-assistance deposition process. With the APTES help to make the PK membrane positive charged, the negative charged silica was uniformed deposited on the membrane surface. The resultant silica-d-PK membrane displays superhydrophilicity, ultralow oil-adhesion underwater superoleophobicity, and distinctive self-cleaning ability, as well as excellent solvent resistance. More important, the membrane shows ultrafast and anti-oil-fouling separation performances for different oil-in-water emulsions. Therefore, it is very promising for large-scale applications.
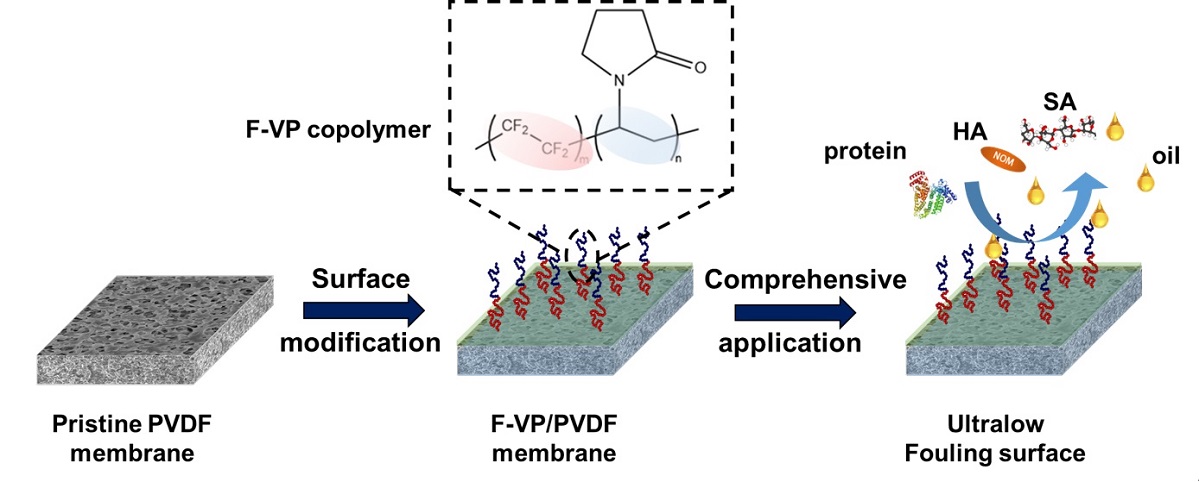
Fouling problem caused by oil and other pollutants is one of the most severe challenges for membranes used for the purification of comprehensive oil-in-water emulsions. Poly(tetraflouride-r-vinylpyrrolidone) (F-VP) with low-adhesive superleophobicity is a class of novel material for fouling-repellent membrane modification to achieve highly-efficient separation of complex oily wastewater. In this work, the construction an ultrathin F-VP layer with a controllable thickness of 2 μm supported by polyvinylidene difluoride (PVDF) substrate was achieved, via an extremely simple, scalable and one-step surface modification process. Benefiting from the ultrathin fouling-resistant skin layer, the resultant F-VP/PVDF membrane exhibited a superior comprehensive fouling-resistant and fouling releasing property, while maintained a highly mass-transfer efficiency without significant flux sacrificing. The intrinsically non-fouling nature of F-VP modification layer endowed the F-VP/PVDF membrane with superoleophobic property to various oils, superhydrophilic/under oil superhydrophilic properties, and excellent antifouling property for comprehensive oil-in-water emulsions. It is capable of efficiently separating the oil-in-water emulsions with a high water permeability of 4612 L m-1 h-1 bar-1, high emulsion permeability (10000 ppm soybean oil-in-water emulsion) of 461 L m-1 h-1 bar-1 with high rejection ratio of >99.9%, and an outstanding stable fouling-resistant and fouling-releasing property of ~93% flux recovery, over 5-time continuous cyclic tests. Overall, this work provides an insight into a facile preparation of advanced composite membrane with ultralow fouling-propensity property, which shows a great potential in treating practically challenging emulsified wastewater.
Membrane bioreactor (MBR) technology has many advantages from the views of high product water quality, small footprint and high possibility of treated water reuse. The critical challenges of MBR are membrane fouling and high energy consumption. Membrane air scouring is the main cause of high energy consumption in MBR. In fact, overall energy consumption can be reduced by operating at high flux or low air flow, on the other hand, serious membrane fouling will occur under high suction pressure. We investigated the fouling mechanism and main foulant type in high flux operation (42 LMH) comparing with low flux (25 LMH). The total amount of protein and polysaccharide clogged in the membrane at high flux operation was 2 times higher than that at low flux operation. Those clogging foulants in membrane pores were considered to be responsible for serious fouling at high flux operation. In order to mitigate those foulants, low concentration chemical cleaning, so-called maintenance cleaning (MC) was applied.
Additionally, we focused scouring efficiency to remove foulant from the membrane at low air flow operation. In order to increase scouring efficiency, new MBR structure that has 3 times higher scouring efficiency than the current MBR structure was developed. Scouring efficiency was improved by increase of the probability of contact of air and membrane in the new MBR.
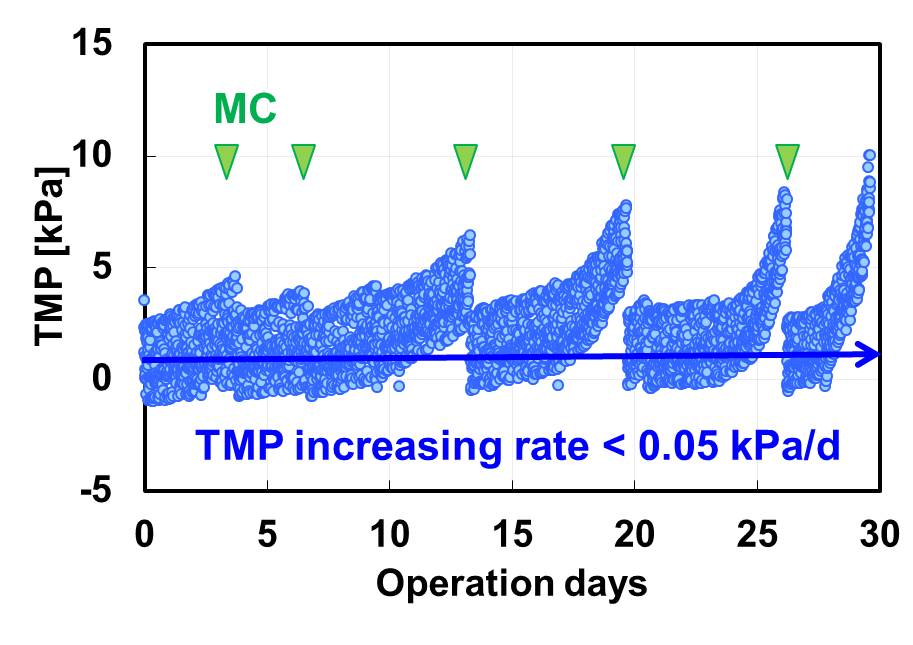
A verification test using new membrane module at high flux and low air flow was conducted for a month with periodic MC every 3-7 days (Figure). The base membrane TMP (Trans Membrane Pressure) increasing rate was maintained at less than 0.05 kPa/d. The aeration energy consumption per product water was reduced 80% in high flux and low air flow operation.
Aromatics have numerous applications in the chemical and petrochemical industries (Odedairo and Al-Khattaf 2011). Transalkylation which includes transfer of alkyl group from one molecule to another is one of the prominent catalytic processes for the conversion of aromatics, especially for the production of industrially important products like xylenes, ethylbenzene, cumene, and cymene (Brown and Nelson 1953). The products of transalkylation reactions have commercial importance as they are derived from low-valued by-products like diethyl or diisopropyl benzenes, toluene, and benzene. An attempt was made to transalkylate low-valued toluene to industrially important product of great commercial value. A series of zeolite (LaB, CeB, and PrB) containing rare earth metals lanthanum (La), cerium (Ce), and praseodymium (Pr) were used for transalkylation reaction. The modified beta zeolites were characterized by EDS, XRD, BET, FTIR, and TPD. The effect of various process parameters like metal loading (2–10 wt%), catalyst loading (1.44–8.63 w/w%), temperature (448–573 K),reactant ratio 1–15, and space time (3.2–9.29 kg h/kmol)on the conversion of reactant and selectivity of product was studied. Catalytic performance of praseodymium-modified beta zeolite shows highest cumene conversion (86.4 wt%)and cymene selectivity (65.7 wt%) compared to other zeolites. The maximum cumene conversion and cymene selectivity were obtained at 523 K, toluene-to-cumene ratio of 9:1, and a space time of 9.29 kg h/kmol. Kinetic modelling of the reaction was done to estimate the reaction kinetic constants and adsorption constants. The activation energy of the transalkylation was found to be 61.44 kJ/mol.
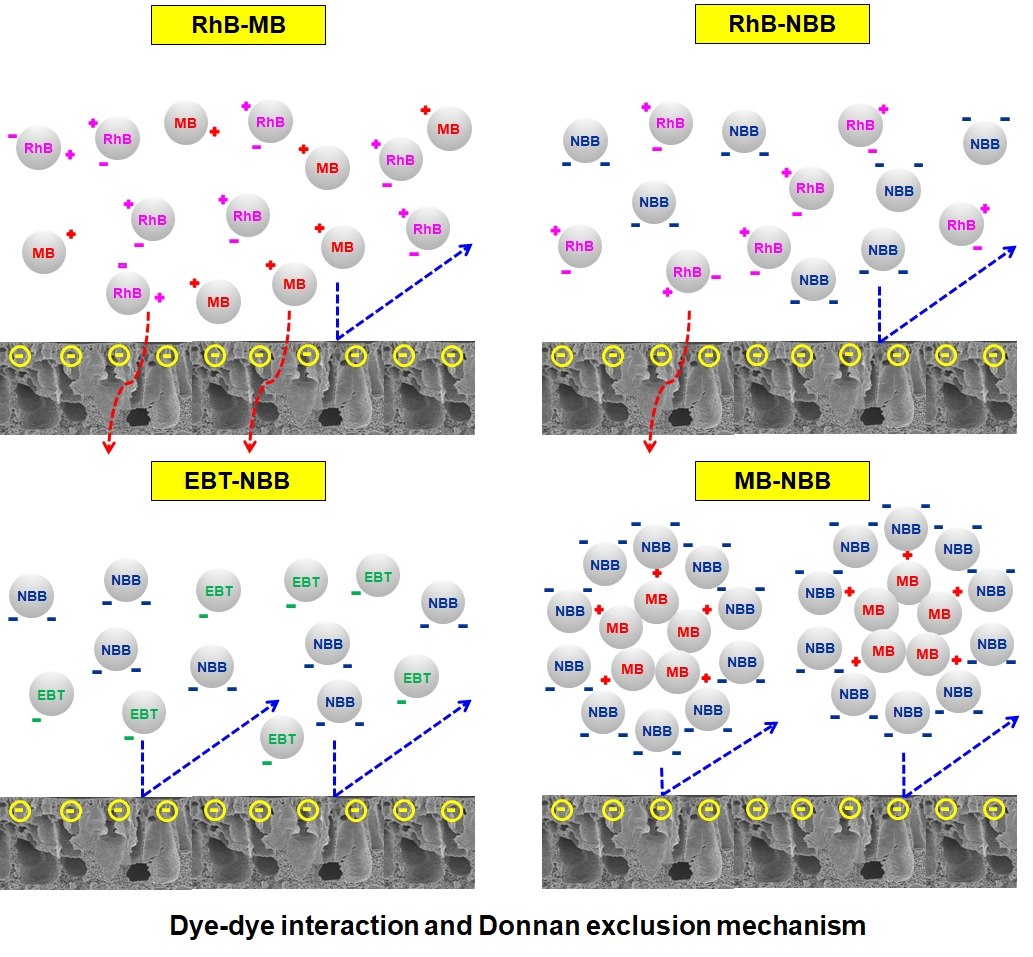
The objective of our research was to investigate the impact of dye-dye interaction on the separation performance of dual dye systems through an ultrafiltration (UF) membrane. Four representative charged dyes were chosen for single experiments, including rhodamine B (RhB) - a zwitterionic dye, methylene blue (MB) - a cationic dye, and eriochrome black T (EBT) and naphthol blue black (NBB) - anionic dyes. Four binary dye mixtures (RhB-MB, RhB-NBB, EBT-NBB and MB-NBB) were then studied in mixture experiments. A microporous poly(vinylidene fluoride) (PVDF) UF membrane was prepared via phase inversion method for the dye separation. The Donnan exclusion effect was proven as the predominant dye removal mechanism. The PVDF membrane surface was negatively charged in almost all experimental dye solutions (pH > 4.5). Therefore, zwitterionic RhB had nearly no rejection (1.1%), cationic MB exhibited low rejection (45.6%), whereas anionic EBT and NBB achieved high removal rates (83.5% and 89.1%, respectively). In dye mixtures, the mixed zwitterionic or same-charged dye solutions (RhB-MB, RhB-NBB and EBT-NBB) showed rejection efficiencies similar to their components' single dye solutions and filtrate permeance values in the range of the constituents' single dye solutions. On the contrary, in the case of the mixed opposite-charged dye solution (MB-NBB), intermolecular interaction induced the formation of micron-scale dye aggregates which provided stronger negative surface charge and were readily repulsed by the membrane. Hence, the MB-NBB mixture attained almost complete dye removal (98.0%) along with an enhanced permeation rate (111.1 L m-2 h-1 MPa-1). The excellent performance stability for all the dye feeds was demonstrated during three-cycle filtration tests. The separation mechanism proposed in this work can be applied to real dye wastewater systems of multi-components. The filtration performance may be predicted once the molecular interactions are known, and the surface zeta potentials of the solutes and membrane are identified.
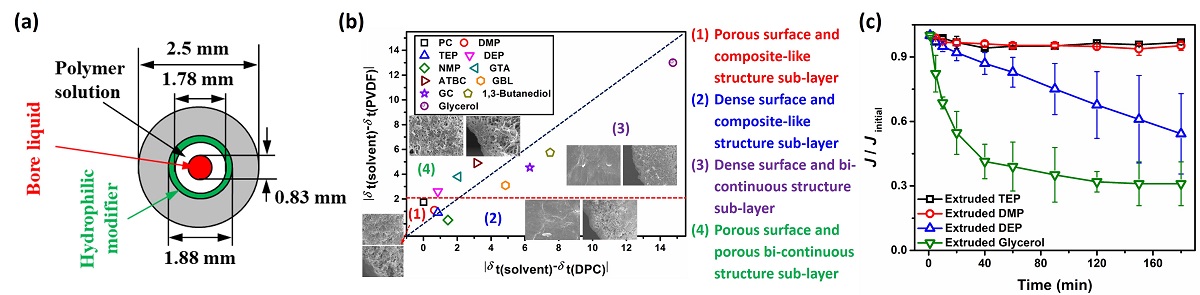
In this work, we demonstrated for the first time that by extruding solvents at the outermost layers of the extruded polymer solutions in the thermally induced liquid-liquid phase separation by using a triple orifice spinneret, both the surface and sub-layer structures of hollow fiber membranes were effectively tailored. Hence, four essential characteristics of the membranes, permeability, rejection, permeation stability and mechanical strength were balanced here simultaneously.
The ternary interactions among the extruded solvents, diluent and polymer played a substantial role in tailoring the surface pore size and permeation stability of TIPS-prepared membranes. At the interfaces between extruded solvents and polymer solutions, apart from the segregations of diluent or polymer that control the surface pores size, composite-like structure (spherules connected by the bicontinuous network) at the sub-layer substantially affected the membrane permeation stability. Regarding the importance of the ternary interaction in tailoring the surface pore size and the permeation stability of the prepared membrane in TIPS process, turn on a spotlight for selecting appropriate extruded solvents to obtain hollow fiber membranes with high water permeability, permeation stability, controlled pore size, and satisfying mechanical strength.
Jatiluhur dam is one of the biggest dams in Indonesia which serves water for various activities of millions of West Java population such as for fisheries, agricultures, recreations and also as a source of clean water and drinking water. The water comes from Citarum River which passing through different areas thus dissolves various impurities during their travel along the river body. One of the impurities that usually occur in surface water is natural organic matter (NOM) that extracted either from the soil or decaying of plants or animals contacted with the water body. NOM may harmful to human since during water treatment it will produce DBPs (Disinfection by Products) which are carcinogenic compounds.
This study is aimed to investigate the characteristics of NOM at Jatiluhur dam water and their removal efficiency using membrane. The water was obtained at 0.2 and 5 m depth and taken at two different seasons; rainy and dry season. Meanwhile, one commercial ultrafiltration membrane and two commercial nanofiltration membranes were investigated at various pressure and pH for NOM removal. The research found that the characteristics of NOM found on Jatiluhur dam were dominated by hydrophobic compounds either at rainy or dry season. The best permeability was obtained by using ultrafiltration membrane at pH 6 and 6 bar operated pressure. Whilst, higher rejection is obtained by nanofiltration membrane by around 73% of the NOM was rejected at pH 6 and 4 bar operated pressure.
Keywords: Membrane, NOM, Jatiluhur Dam, Drinking Water, Hydrophobicity
Energy conservation of distillation processes is still an important issue in process industries. Though the heat pump has been said to be a promising tool for energy saving, it has not been applied to many processes. One of the drawbacks that obstructs the installation is the high equipment cost of the custom-ordered compressor. In this paper, we propose to introduce a general-purpose heat pump system (G-HP) to the distillation processes. G-HP is superior to a custom-ordered heat pump in the cost and the maintainability, that is very important feature for the long term operation.
The G-HP proposed in this research uses water as the working fluid in the heat pump. The coefficient of performance (COP) decreases with the increase of the temperature difference between the heat source and the heat sink. Thus, it is preferable to select the heating and cooling stages that has small temperature difference. On the other hand, the efficiency of distillation is highest when the heat is supplied to the reboiler (highest temperature position) and removed from at the condenser (lowest temperature position). It means that the proper selection of the heating and cooling places is important.
In this study, by taking the simplicity of the process structure into account, the vapor to the condenser is selected as the heat source. The required compress ratio depends on the selection of the heat supply stage and affects COP. We executed the simulations of the cases having various VLE curves and product specifications, and derived the best location of the heating stage and the condition suitable for the introduction of G-HP. The effectiveness of the structures having double heat pumps that supplies heat to the middle of the column and the reboiler, and those having double feed streams is also discussed.
Since methanol is an attractive fuel and chemical for various applications, its demand will continue to increase in energy year. Most methanol is currently produced from fuel such as natural gas through steam reforming. A methanol–water mixture is produced from the carbon monoxide or carbon dioxide and hydrogen in the presence of a catalyst and distilled to acquire pure methanol.
Self-heat recuperation was recently developed as an energy-saving process design, in which overall-process internal energy caused by condition changes is recirculated within the process without the need for heat addition, leading to a reduction in the energy requirements of several chemical processes.
In this study, the feasibility of applying self-heat recuperation technology to the industrial methanol synthesis process which possesses both temperature and pressure changes, a reactor and separators was investigated from energy-saving and process design points of view, based on actual plant data. Based on simulation results, it was concluded that the use of self-heat recuperation technology is an attractive alternative for sustainable future development of the methanol synthesis process.
Mass transfer was both experimentally and theoretically investigated based on shell model for a packed distillation column equipped with wire-mesh structured packing. The experimental column consists of three packed beds stacked in series and total bed height is 5,320 mm. In order to observe local variation of HETP, thermocouples were embedded at an equal interval on the column centerline. Each thermocouple can be considered to measure the temperature of internal reflux liquid owing to its large heat capacity. Distillation test was conducted at normal pressure under total reflux condition with three kinds of binary systems. Local variation of HETP in each bed region was determined by comparing the observed temperatures with the calculated ones due to the process simulator package. After obtaining the corresponding overall HTU from the experimentally obtained HETP, the vapor-phase and liquid-phase film coefficients were calculated by solving a simultaneous equation deduced between two vertical locations. However since the stripping factor remains almost unchanged in the top and bottom regions, it was considerably difficult to obtain an appropriate simultaneous equation without approximation. On the other hand, it has been found that those film resistances can be easily determined by assuming that the tie-line and VLE equilibrium curve should have an orthogonal relation at an arbitrary theoretical stage.
As a result, this orthogonal model successfully gave comprehensive correlation of vapor-phase and liquid-phase HTUs with the corresponding F-factor and superficial liquid load.
Although this investigation is still in the process of analysis, the orthogonal model has a possibility to lead to further progress in distillation mass transfer from a view point of transport science.
The development of the industrial systems for the year 2050 has been well defined in the recent Research Agenda with many strategic sectors, such as water, energy, food, health, etc. The drive towards greater sustainability has prompted process industries to search for opportunities to decrease their production costs, energy consumption, equipment size, and environment impact as well as improve the raw material yields, remote control, and process flexibility. Distillation process as a dominant player among all separation technologies are typically energy and cost intensive. One of the major challenges in distillation process industry is thus to improve the energy efficiency of existing and/or new processes through economic and ecological strategies. Integration, intensification and hybrid approach of distillation have become the main trend to achieve green and sustainable chemical process. Process integration and intensification is defined as a set of innovative principles applied to the design of processes and equipment to satisfy those concerns about energy and ecology impact of the process. The energy efficiency of a distillation process could be maximum by optimizing the design and operational parameters. However, as distillation processes become more complex in structure and operation through integration, intensification and hybrid, to find their optimal design and operation conditions drawing out its full potential is also becoming more challenging. This presentation will review briefly applications and trend of integration, intensification, and hybrid of distillation processes with their optimization. The potential and reliability of these technologies are addressed briefly, which will enable distillation process to achieve higher efficiency and high capacity. The recent developments in current research are summarized to highlight the importance as well as the effects, challenges, and future prospects of distillation process integration, intensification, and hybridization.
Orthogonal model of interphase mass transfer in a packed column distillation process
Kunio Kataoka, Goro Nishimura, Hideo Noda and Hiroshi Yamaji
Kansai Chemical Engineering Co., Ltd
2-9-7 Minami-nanamatsu-cho, Amagasaki, Hyogo 660-0053., Japan
Mass transfer model was investigated for a distillation column which consisted of three packed beds of wire-mesh structured packing stacked in series. Thirteen thermocouples for local liquid temperature observation were embedded at an equal interval on the centerline of the packed beds. The distillation experiment was conducted with a binary solution of methanol and ethanol under total-reflux conditions. The F-factor was varied as the control parameter by changing the heat duty of the reboiler. The shell balance model was based on a cylindrical control volume having a local HETP as the shell height. The process simulation analysis was done by using a simulator package in the same condition as the experiment. Local variation of HETP, HTU, and volumetric overall mass transfer coefficient were analyzed by comparing the experimental data with the process simulation results,
In order to analyze interphase mass transfer based on the two-film theory, an orthogonal relation between the tie-line and the vapor-liquid equilibrium curve was assumed, so that the vapor and liquid compositions can be evaluated at the vapor-liquid contacting interface. This orthogonal assumption definitely determined vapor-phase and liquid-phase volumetric film coefficients of mass transfer. By means of dimensional analysis, the vapor-phase and liquid-phase mass transfer coefficients defined in the form of j-factor data were well correlated with the corresponding Reynolds number.
In the middle bed where the accuracy of local HETP observation is the best, a set of correlation functions were obtained by least square.
By virtue of the orthogonal assumption. the Reynolds number dependency of the mass transfer model in the form of j-factor was improved, especially in the liquid-phase j-factor correlation.
These days, many chemical engineers pay much attention to apply heat pump system for improving the energy efficiency of industrial separation processes. In distillation process, heat pump system can save energy significantly by upgrading the low-temperature waste heat to high temperature and utilizing it instead of steam for supplying heat to the reboiler. In this study, a blower based heat pump system was proposed to improve the energy efficiency of separation and purification processes in chemical industry. This paper showed the advantages and disadvantages of a blower based heat pump system and figured out when it can be applied. Blower based heat pump system is mainly used when there is only a small temperature difference between the hot and cold streams, where small pressure ratio and consequently smaller blower duty are needed. Several important industrial cases have been investigated to demonstrate the proposed configuration. By applying blower based heat pump systems, the latent heat can be circulated during the process, leading to a substantial improvement in energy efficiency. Notably, the operating costs can be reduced by up to 78%, 64% and 82% for the C3 splitter, C4 splitter and refrigerant separation processes, respectively.
Acknowledgement
This project is supported by the R&D Center for Reduction of Non-CO2 Greenhouse Gases (201700240008) funded by the Ministry of Environment as a ‘Global Top Environment R&D Program' and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1A2B6001566) and by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A6A1031189).
In order to improve the economics of the ETBE production, binary and ternary VLE data for ETBE(1)+Ethanol(2)+TBA(3) system are measured and correlated using NRTL-RK model. According to the obtained thermodynamic model, pressure dependence of the azeotropic point between ETBE(1)+Ethanol(2) system is evaluated using the Residue Curve Map. The separation feasible flow sheets are derived from the Residue curve maps and modeled using Aspen Plus. The process economics and performance are evaluated with those of the existing Uhde Process.
Since fossil fuel combustion systems will still be used to meet the energy demand of the future, capture of CO2 represents a global environmental challenge. Simultaneously, removal of hazardous chemicals is a critical issue from environmental standpoints. Therefore, environmental regulations for industries from acceptable levels of human exposure become continuously more stringent to cope with the emission of CO2 gas as well as to protect humans from toxic chemicals. For practical applications, the developed sorbents should be structured into pellets or beads, satisfying the stability, working capacity, shape, etc.
Herein, MgO was prepared by various synthesis methods of nano-sized mesoporous MgO to optimize the platform material for MgO composite for sulfur removal and CO2 sorption. To improve the removal and decomposition efficiency of sulfur compounds, various MgO composites were developed: MgO-SiO2, MgO-Fe3O4, carbon-coated MgO, etc. The developed MgO composite showed higher performance than commercial activated carbon in sulfur removal. Then we exploit new synthesis methods for the development of porous MgO composite beads. The as-prepared spherical beads featured diameters of hundreds of micrometres, hierarchical porosity, and high surface areas. With respect to pre-combustion CO2 capture, the sorption working capacity of the salt-promoted MgO composite beads is comparable to that of powder MgO, with remarkable cyclic stability. However, at more real regeneration condition using CO2 flow, stable operation was achieved after several cycles, where the working capacity was 1.3 and 1.6 times higher than that of the corresponding powder and pressed-pellet sorbents.
Finally, in the presentation, a platform simulation model of a dual fluidized-bed system for CO2 capture and sorption enhanced reaction, which consists of a fast fluidized-bed carbonator and a bubbling fluidized-bed regenerator, will be also introduced.
This simulation study utilizes zeolite 5A to separate oxygen from air in order to compare the performance of semicylindrical adsorber with that of traditional cylindrical adsorber for pressure swing adsorption (PSA). The semicylindrical adsorbers are designed to contact and exchange heat to each other by lateral surface area. Therefore, semicylindrical adsorber can have better heat compensation during adsorption and desorption, which increases the oxygen purity in Skarstrom cycle.
The air composition is simplified to 21% oxygen, 1% argon and 78% nitrogen. The extended Langmuir isotherm model is used to describe adsorption isotherms of gas components. Linear driving force model is used to describe the mass transfer resistance between gas and solid phase. Furthermore, the simulation program is verified with experimental data from literature. The results show the reliability of program and parameters. Then, we built a PSA process with semicylindrical adsorbers under appropriate operating conditions and compared it with a PSA process with traditional cylindrical adsorbers.
From the results of discussing the operating variables, heat compensation of semicylindrical adsorber does increase oxygen purity for oxygen separation from air, once the adsorber changed from cylindrical one into semicylindrical one. The benefit of heat compensation is more obvious for larger adsorber, higher operating and surrounding temperature, and higher feed pressure in the range of this study.
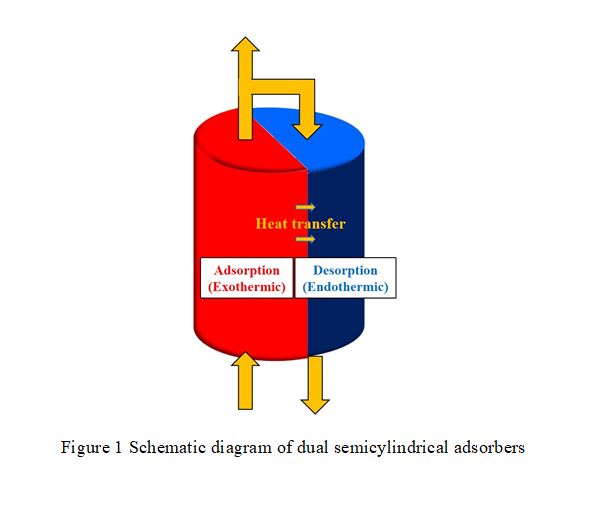
Low-grade fuel (e.g. coal and heavy oil) is one of the main sources of NOx and SOx at a time of combustion. The conventional processes for removing NOx and SOx, such as selective catalytic reduction and limestone-gypsum flue gas desulfurization, have a low NOx removal efficiency and require high cost. In our laboratory, we developed an absorption equipment having glass fiber filter, and found it attained good NOx removal performance. When the high contacting area and perfect mixing property of absorbent in this equipment are applied to simultaneous removal of NOx and SOx, there are a lot of merits, such as simplification of treatment process, reduction in operating cost, resource recycling for nitric acid and sulfuric acid, for gas treatment process. The objective of this thesis was to develop a new small model of absorption equipment for simultaneous removal of NOx and SOx, and evaluate its performance. In addition, the absorption kinetics of NOx and SOx in the equipment were analyzes. We developed new absorption equipment and indicated that NOx and SOx could be efficiently absorbed into water by using the equipment. Additionally, mixing characteristics of fluids or mass transfer characteristics in the equipment were suggested.
Up to now, many adsorbents were used for CO and CO2 separation through corporation Cu(I) to suppports. However, it is difficult to achieve high CO/CO2 separation factor, excellent CO uptake amount and large CO working capacity, simultaneously. In this work, the adsorbents were prepared by doping double metal ions consisting of divalent copper ion and various metal ion (K+, Al3+, Zn2+, Ni2+) onto MIL-100(Fe) support via impregnation method. Remarkably, the obtained adsorbent doped with copper (40 wt%) and zinc (5 wt%) showed higher CO/CO2 separation factor than the other adsorbents doped with the same concentration of copper and other metal ions (K+, Al3+ or Ni2+). The effect of zinc concentration was systematically investigated. These results proved that Cu-Zn@MIL-100(Fe) adsorbent promisses as a well adsorbent, and reveal a new route to prepare a adsorbent applied for CO/CO2 separation effectively.
Adipic acid is one of the basic materials to produce synthetic fibers like nylon-6/6. The effluent gas of adipic acid production mainly consists of nitrous oxide (N2O), oxygen, nitrogen, carbon dioxide, and water. A significant amount of N2O is emitted from the production process. Since N2O is one of the representative non-CO2 greenhouse gases, it should be recovered from the effluent gases for the mitigation of global climate change. Furthermore, the recovered N2O can be widely used in many industrial areas such as medical, automotive, and food and beverage. In particular, high purity N2O is a high value-added chemical used in chemical vapor deposition (CVD) process in semiconductor production.
Proper adsorbent selection is required for the design of adsorption separation process. In this study, to evaluate the performance of adsorbents in terms of equilibrium separation and kinetic separation, adsorption isotherms of N2O, O2 and N2 are measured on various carbon-based (activated carbon and carbon molecular sieve) and silica-based (zeolite and silica) adsorbents by a volumetric method. Adsorption amount, adsorption affinity and working capacity were compared among the adsorbents with respect to equilibrium separation. And adsorption kinetics for each adsorbent was also evaluated by applying either non-isothermal model or isothermal dual resistance model to experimental uptake curves. The physical properties and adsorption characteristics of each adsorbent-adsorbate system were used to each model. The validity of the equilibrium and kinetic results was confirmed by comparing the experimental breakthrough curves with the dynamic simulation results using the obtained values.
ACKNOWLEDGEMENT This research was supported by the Design Technology Development of Hydrogen Production Systems (100,300 Nm3/h) for Hydrogen Refueling Station (RD2017-0421) funded by KOGAS research institute.
Metal-organic frameworks (MOFs) offer many interesting opportunities in adsorption technology, with unprecedented capacities and chemical and structural tunabilities. A great deal of effort has been made to develop a method of synthesizing MOFs, including solvothermal, microwave-assisted, sonochemical, mechanochemical, and electrochemical techniques. Among the many MOFs, zeolitic imidazolate framework (ZIF) is undoubtedly the most extensively studied because of its facile synthesis coupled with its exceptional chemical and thermal stabilities. Controlling the crystal size and shape is crucial for regulating the structural flexibilities and mass transport properties.
Herein, we describe highly versatile and effective strategies to produce polycrystalline ZIF particles and membranes. The spray-drying method has been applied to fabricate the polycrystalline ZIF particles. The polycrystals with grain boundary structure enhanced both adsorption capacity and uptake rate. The support-surface activation has been applied to fabricate the polycrystalline ZIF membranes without the use of seed crystals. The flexibility tuning related with grain size could control the membrane separation performances.
The proposed synthesis strategies open new opportunities for creation and morphology-control of new MOFs.
Multi-functional sorbent derived from biodegradable lignocellulose aerogels have been successfully prepared from coir fibers with sulfur–free method and NaOH-urea system. Sulfur was avoided during pretreatment because of its environmentally harmful and disadvantageous for sorption applications. Interestingly, these pretreatments had strong effect on the physical properties of the aerogels produced. Excellent physical properties of lignocellulose aerogel can be obtained when the Kappa number, i.e., the lignin content, in the pulp is lower than 14.8. The aerogel has a macroporous structure, ultralight density, high porosity, good durability, and thermal stability. The lignocellulose aerogels were capable of absorbing approximately 22 times their dry weight of water and approximately 18 times their dry weight of oil. For dye adsorption, the cellulose aerogel could absorb methylene blue molecules up to 62 g/g aerogel, hundred times higher than those of synthesized from natural-origin adsorbents. Therefore, the prepared lignocellulose aerogels have great potential for various applications including liquid absorbents and dye adsorbents.
The separation of protein, which influences on the purity and the production cost of downstream processing, plays a critical role in the protein production process. The affinity separation method using a ligand is often used for separation of protein. In this study, a functionalized membrane was prepared by immobilizing cibacron blue (CB), a dye with an affinity for specific proteins, on a polyvinyl alcohol (PVA) nanofiber nonwoven membrane produced by an electrospinning method. To evaluate its separation performance, the adsorption test of bovine serum albumin (BSA), a kind of proteins, was conducted using it. The CB molecules were immobilized by covalent bonding of hydroxyl group of PVA and chlorinated triazine ring of CB under alkaline condition. The adsorption test of CB-enhanced affinity PVA nanofiber membrane was conducted by soaking it into the BSA solution as an adsorbate. After immersing the PVA nanofiber membrane in 15 ml of the BSA solution and shaking for 4 hours, the adsorption amount of BSA was obtained from the reduction in the concentration. Furthermore, the dependence of pH was also examined by testing the BSA adsorption amount under various pH conditions. As a result, after enhancing affinity by CB, the BSA adsorption amount of the PVA nanofiber membrane indicates 2.28~3.35 times increase due to functionalization. The adsorption amount of BSA became a maximum at pH 5.0, which is the isoelectric point of BSA. In conclusion, the BSA adsorption performance of the PVA nanofiber membrane could be greatly improved by the modification with CB. Moreover, the adsorption characteristics were heavily dependent on the solution environment.
The main aim of this study is to investigate the removal of phenolic compounds as pollutants in palm oil mill effluent (POME), the wastewater from palm oil industry, by palm kernel shell activated carbon (PKSAC). In the first, palm kernel shell (PKS), one of the wastes from the industry, obtained from Thailand was characterized by proximate and ultimate analyses, and FTIR one. Secondly, PKS was thermally treated to produce PKSAC by chemical activation with orthophosphoric acid. The yield of PKSAC ranged from 0.36 up to 0.72 and decreased with increasing temperature or time in the thermal treatment. The specific surface area of PKSAC (SPKSAC) was 400×103-800×103 m2 kg-PKSAC–1 and the highest at 673 K of thermal treatment temperature. The longer treatment time reduced SPKSAC. The types of functional groups on PKSAC surface were less than those on PKS surface and new functional groups of phosphorus species formed. Then, batch equilibrium adsorption of the model POME was conducted to remove phenolic compounds in POME using the PKSAC. The PKSAC could adsorbed phenol and m-cresol in the model POME and remove them from the solutions successfully. The adsorption isotherms of both phenolic compounds followed the Langmuir model. The saturated adsorbed amount of m-cresol was higher than that of phenol. This may result from higher hydrophobicity, i.e., lower solubility in water of m-cresol than phenol. The saturated adsorbed amounts of phenolic compounds increased with SPKSAC. The effect of the functional group types on PKSAC surface on the phenolic compound adsorption was unclear. Finally, the treatment process of POME with the PKSAC was discussed with the above results based on simple relationship of material balance and adsorption equilibrium. The treatment process would be expected as a promising treatment method since the PKSAC gave high saturated adsorbed amount with high yield production.
Biosorption is an eco-friendly, low-cost method and suitable for heavy metal adsorption among conventional methods of metal ion removal. Au(III) and Cu(II) adsorption from aqueous solution were carried out using low cost, biosorbent materials of sheep wool and chemically treated sheep wool. Sheep wool is a keratin-based natural fibrous material and contains many active functional groups. NaOH, Na2S, NaHSO3, and NaBH4 were used as chemical reagents for the treatment of the wool. The morphological and chemical characterization of the chemically treated wool and wool samples were performed by the Scanning Electron Microscopy and Fourier Transform Infrared Spectroscopy. The fibrous wool was transformed into a film like structures after the chemical treatment of Na2S. The functional group of amine was determined as being the main active site for the adsorption of Au(III) by FTIR analysis. The sheep wool and the chemically treated sheep wool samples adsorbed Au(III) from Au-Cu binary aqueous solution and the presence of copper ion had no effect on the Au(III) adsorption. Au(III) adsorbed substantially at low pH range. The experimental data fitted well with the model of pseudo-second-order kinetic and the adsorption amount of Au(III) adsorption increased with time and reached a plateau after 12 h. The rate determining step of Au adsorption would be defined as chemisorption due to the formation of a monolayer on the wool surface. Sheep wool is a low cost, eco-friendly material, and has a high capacity of adsorption, and could be used as a biosorbent for precious and heavy metals by modification of appropriate chemical treatment.
This paper investigates the separation characteristics of membrane + distillation hybrid processes for azeotropic ternary mixtures using simulation programs developed by the authors. When azeotropic mixtures are separated with azeotropic or extractive distillation, a solvent which is not in a feed mixture must be used. This sometimes results in contamination of the products.
The hybrid processes consisting of membrane and distillation have been studied as an alternative energy saving processes. However, they also have an advantage in that it is possible to separate the azeotropic mixture without a solvent. The separation performance of the hybrid process is maximized when the membrane module and the distillation column perform and cooperate properly.
In this paper, we conduct numerical simulations of the distillation process showed in Fig.1 with a VP or PV module using the numerical examples of ternary separations which include binary azeotropic mixtures. The results are summarized and the conditions for obtaining better performance in the hybrid processes are explained from the following viewpoints.
- Effect of feed compositions: the composition profiles of the distillation column will be affected.
- Effect of operating temperature: the compositions of the permeate and the retentate will be affected.
Hydrate-based gas separation is often investigated with using batch or semi-batch operations. In order to increase the throughput of gas mixture without increasing the apparatus volume, it is preferable to perform a continuous operation of hydrate-based gas separation. Therefore, we proposed a flow-type apparatus for performing continuous formation with passing gaseous mixture and subsequently decomposition with passing gas hydrate particles. Characteristics of multiple fluid and heat and mass transfer of hydrate slurry are essential for efficient operation of the apparatus. In this study, we focused on heat transfer characteristic in presence of bubbles in water and surfactant solution. First an apparent overall heat transfer coefficient under pressure during steady operation of the apparatus was calculated on a simple assumption. Next, to control hydrate amount and occurring position of hydrate-decomposition and hydrate-formation in apparatus, we focused on temperature profile of the inside fluid. A heat transfer model with using heat balance of defining heat of hydrate-formation and heat transfer of agitation of fluid was created for hydrate-based gas separation apparatus. To evaluate validity of the heat transfer model, a calculation value is compared with an experimental value. We are going to report in detail on the results of this study.
Hydrated-based gas separation is a method capable of selectively separating and recovering greenhouse gases. Although a conventional hydrate-based gas separation apparatus is a batch or a semi-batch system, continuous operation is preferable in order to increase the throughput of gas without changing the apparatus volume. In previous studies, we have considered a gas- liquid-hydrate slurry three-phase as a gas-hydrate slurry pseudo two-phase gas absorption apparatus, and investigated about effects of fluidity and solubility on mass transfer characteristics of hydrate-based gas absorber with using a column type apparatus. Recently, we proposed a flow type apparatus to allow continuous operation of hydrate formation (absorption) and subsequent decomposition (desorption). The aim of this study is to investigate mass transfer characteristics of the continuous apparatus with using HFC134a-N2 mixed gas system. The volumetric mass transfer coefficient was calculated especially during steady state of gas absorption. In addition, we investigated effects of hydrate particles and flow behavior on the volumetric mass transfer coefficient, and compared mass transfer performance between the hydrate-based gas absorber and a conventional bubble column. Sodium dodecyl sulfate was used as a hydrate dispersant. In the flow type apparatus, the gas-liquid contact was good and the hydrate slurry state was observed during hydrate formation. The experimental volumetric mass transfer coefficient was almost constant independent of HFC134a mole fraction in feed gas. In surfactant solution, bubble-hydrate slurry pseudo two-phase was formed easily because bubble size was decreased and hydrate formed on bubble surface was released by surfactant. As a result, the volumetric mass transfer coefficient increased in comparison with that in water. The volumetric mass transfer coefficient with hydrate was about 10 to 100 times higher than that of the bubble column. These results suggest that hydrate formation improve the gas absorption performance.
Refrigerant reclamation is the act of processing used refrigerant gas, which has previously been used in some types of cooling loop, such that it meets stringent purity levels before re-entering the marketplace. This process contribute to prevent the ozone depletion in the upper atmosphere. In this study, an existing batch distillation was used for reclaiming dichlorodifluoromethane CCl2F2 (R-12 or Freon-12), which is the most common representative of chlorofluorocarbons (CFCs), from waste refrigerant mixture. Since this is the existing batch distillation column, many designs variables are needed designing and many constraints, such as operating temperature and pressure of existing batch distillation or operating temperature of existing cooling and heating medium, are needed checking during design and optimization. Thus, a new systematic methodology was suggested to design and optimization the separation using an existing batch distillation. The details of a systematic methodology are described through the results from an industrial case study of R-12 recovery from a waste refrigerant mixture. Based on the comparison Txy diagram with experiment data, REFPROP can be selected as the thermodynamic models for the VLE behaviors. After design and optimization process using shortcut and rigorous methodologies, a real operation using industrial-scale batch distillation unit was carried out and compared with simulation results. Simulation results using Aspen Batch Modeler software are in good agreement with experiments carried out in a commercial batch distillation column at 8 bar.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and was funded by the Ministry of Education (2018R1A2B6001566), Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A6A1031189), and the R&D Center for Reduction of Non-CO2 Greenhouse Gases (201700240008) funded by the Ministry of Environment as a ‘Global Top Environment R&D Program'.
In this study, a continuous distillation column was proposed for reclaiming refrigerant. One of the challenges is that the distillation column has to be able to handle many different feed mixtures instead of building up many distillation columns. The experimental data of VLE behaviors of several mixtures were compared with simulation result to find the suitable thermodynamic models. Aspen Hysys V.10 was used to carry out all simulations in this study. Based on the comparison Txy diagram with experiment data, REFPROP can be selected as the thermodynamic models for the VLE behaviors. Three popular mixtures including R-22 and R-410a, R-32 and R-125, and R-12 and R-134a were used to design and optimize the distillation process via shortcut and rigorous methodologies. Then, the hydraulic performance of the proposed column was tested to verify whether the column can be used for each specific mixture or not.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and was funded by the Ministry of Education (2018R1A2B6001566), Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A6A1031189), and the R&D Center for Reduction of Non-CO2 Greenhouse Gases (201700240008) funded by the Ministry of Environment as a ‘Global Top Environment R&D Program'.
The main cause of the global warming is considered to be the emission of carbon dioxide. The utilization of carbon dioxide for agriculture achieves an increment of crop yield and an enhancement of quality of agricultural products. Exhausted gas contains 10-20 volume percentage of carbon dioxide and also contains toxic nitrogen oxides. Carbon dioxide in exhausted gas cannot directly utilize for agriculture. Therefore, expensive and purified carbon dioxide has been utilizing for agriculture. In our previous work, nitrogen oxides in exhausted gas decreased in 10ppm by flat glass-fiber filter NOx absorption equipment and treated carbon dioxide in exhausted gas from Kikugawa biogas power plant utilized in tomato cultivation factory (Bell Farm). For utilization of carbon dioxide in exhausted gas, storage and transportation of carbon dioxide became a problem. This was because the agricultural utilization was half day in spite of full day carbon dioxide production and the carbon dioxide production plant was far from carbon dioxide consumption place. To overcome this problem, we propose a completely exchange of potassium hydroxide for potassium carbonate with flat glass-fiber filter (GF) equipment. The merit of this method is that NOx removal and storage of carbon dioxide simultaneously achieve. The absorbent, saturated aqueous solution of potassium carbonate is transported to the farm using a tank lorry and carbon dioxide is desorbed with nitric acid produced by NOx absorption.
GF equipment had the characteristics of long residence time, near perfect mixing flow and large surface area. The gas-liquid contact area was 1.14×102 m2·m–3. From the estimation of mass transfer and chemical reaction, the averaged diameter of gas bubbles was about 1.35 mm in the filter. GF equipment achieved completely exchange of potassium hydroxide for potassium carbonate.
Distillation is a widely used in the chemical process industries. It was consumed a large amount of energy. Therefore, Energy saving of distillation technology is important. Several studies on energy saving distillation technologies have been reported such as VRCs and HIDiCs. Recently, the distillation-membrane hybrid separation technologies have been proposed. This technology has a high energy saving performance. However, this process have not been evaluated economic performances. In this study, we evaluate the economics of distillation process, membrane separation process, VRC, membrane-distillation hybrid process via model-based approach.
Extractive distillation technology have been attention to apply to the ethanol dehydration due to its nature of lower energy consumption compare to the traditional azeotropic distillation technology. However due to the increase of design parameters such as the solvent feed (S/F) ratio, solvent / feed location in the column, the optimization of the design of extractive distillation was quite challenging. In this work, we investigate a) the required solvent characteristics, b) the numerical analysis of the extractive distillation behavior in various S/F ratio and the reflux ratio, and c) numerical design method of the extractive distillation column. Based on the novel numerical design method, the example of the the extractive distillation coulmn for ethanol dehydration using ethylene-glycol will be presented.
Reactive distillation has advantages such as energy and capital saving, compact device, high reactant conversion since reaction and separation can be simultaneously performed in one column. However, the design and operation procedure of reactive distillation is more complicated than that of a conventional process due to the interaction between chemical reaction and distillation separation. As a result, multiple steady states occur. In a multiple steady state conditions, reaction conversion at the upper branch was the highest. High reaction conversion lead to not only a large amount of product, but also small amounts of unreacted components. If the decrease in the energy consumption of the recovery distillation column is larger than the increase in energy consumption of the reactive distillation column, the energy consumption of the reactive distillation process becomes energy-saving. This study evaluates the energy consumption of reactive distillation process for tert-amyl methyl ether (TAME) synthesis through multiple steady state conditions using steady-state simulation. Bifurcation analysis revealed that the multiple steady states did not exist under a reflux ratio 1, but existed under reflux ratios 2 and 3. Although the reboiler duty of reactive distillation column with high reflux ratio became large, the reaction conversion became also high. Due to decrease unreacted component, the reboiler duties of the recovery distillation columns at the multiple steady state conditions were lower than that at the reflux ratio 1. Compared to the energy input per mole of TAME product at reflux ratio 1, the energy inputs per at reflux ratio 2 and 3 were reduced by 17 and 12%, respectively.
An enzymatic membrane bioreactor system using the non-purified culture supernatant of white-rot fungus Trametes versicolor was developed to perform the decolorization of dye wastewater. The culture supernatant was periodically obtained from fungal cultures cultivated in cost-effective natural medium, which uses blackstrap molasses typically present in food waste, by gravitational sedimentation. The model dye wastewater containing an anthraquinone type dye and the supernatant exhibiting dye decolorization activity were mixed in a continuously stirred tank membrane reactor with an ultrafiltration membrane that permitted enzyme recycling in the reactor. The results obtained from the enzymatic membrane bioreactor showed that approximately 80% of decolorization and approximately constant permeation resistance were maintained for a few days of continuous treatment. The decolorization behaviors were well-described using a combined equation based on mass balance for continuous stirred tank membrane reactor and kinetics of enzymatic decolorization of the dye. In addition, dehydration of excess fungus that grows in the process of the decolorization treatment was carried out by mechanical expression. It should be noted that the moisture content in the compressed cake of fungus was finally reduced to 20 wt% under pressure of 7 MPa. The kinetics of expression such as the time variation of the moisture content in the compressed cake were accurately described on the basis of the multi-stage creep model. Furthermore, we confirmed that the squeezed liquid from the fungus body contains physiologically active substances such as superoxide dismutase-like materials.
Flocculation is a widely used process for particle removal in water/wastewater treatment. Recently, cationic cross linked polymer flocculant has been proposed. In this study, fundamental aspects of flocculation process using the cationic cross linked polymer flocculant are investigated. Suspension of kaolin (multidisperse) and PMMA particle (monodisperse) are used for flocculation tests under various conditions. When a little excess of cross linked polymer flocculant was added, a massive floc was formed. Cross linked polymer flocculant molecules cause the microflocs to form a massive floc. Interestingly, under conditions of low ionic strength, a massive floc was not formed. Extended cross linked polymer flocculant molecules are effective for junction of microflocs.
In response to the request of advanced industrial use of fine particles, a technique for uniformizing particle size efficiently has become increasingly important. One of the promising classification techniques is a microfiltration (MF) method using a sieving function of the membrane. However, a rapid decrease in the filtration rate caused by the membrane fouling during MF process makes efficient classification difficult. In this study, we focused on the ultrasonic irradiation and verified its effect to solve this problem of MF method. Colloidal silica was used as sample fine particle in the MF experiments. This particle had a mass-based size distribution of 0.1 to 1.0 μm and a surface mean diameter of 0.37 μm. The MF experiments were carried out under the condition of constant pressure using a membrane with a pore size of 0.45 μm. The ultrasonic irradiation with a frequency of 28 kHz was applied continuously to the filter during the MF experiment. The filtration rate and the particle concentration in the filtrate were measured through the MF experiment. The effect of the ultrasonic irradiation was confirmed by comparing with the experimental data obtained under the non-irradiation condition. As a result, when ultrasonic irradiation was performed, the filtration rate increased about twice, and the particle concentration in the filtrate also increased to a concentration at which the particle size distribution could be measured. Also, considering that only particles with less than 0.45 μm were present in the filtrate, effective particle classification was achieved by the ultrasonic irradiation. In conclusion, the ultrasonic irradiation can contribute to the suppression of the membrane fouling and the improvement of the filtration rate. Moreover, it enables effective classification of the fine particles through a MF membrane.
Excess sludge production by wastewater treatment plants represents a serious environmental problem that necessitates the development of effective sludge treatment/disposal methods. Excess sludge contains valuable materials such as extracellular polymer substances (EPSs) accounting for 10–40% of the dry weight of excess sludge, and their recovery is of great practical importance. In previous study (Cao et al., Chem. Eng. J., 2018, 354: 866–874.), we demonstrated that the EPSs recovered from excess sludge can be used as adsorbents for the removal of diverse heavy metal ions from wastewater, exhibiting performances that are on par with those of commercial adsorbents. In order to achieve the separation of EPSs with the adsorbed heavy metal ions from wastewater, combining EPSs recovery and heavy metal ions adsorption into a dead-end ultrafiltration, the ultrafiltration of EPSs solution was carried out to obtain the cake on the membrane and once this process was completed, heavy metal-containing wastewater was filtered through the cake. The removal rate could achieve more than 90% for typical heavy metal ions by EPSs cake when the certain concentration rates of EPSs and heavy metal ions were used. Calcium ion and filtration-aid (diatomite) could reduce the filtration resistance and had no effect on the removal of heavy metal ions.
Dynamic filtration plays an important role in the food production process and waste water treatment since the stable filtration operation can be maintained by removing the filter cake hydrodynamically. In this study, we developed a dynamic filtration method that jets the feed slurry tangentially toward the rotating drum filter medium. The tangential flow mode is more effective than the vertical one because of higher shear stress to the filter medium and more efficient cake removal. In order to evaluate the filtration performance of this dynamic filter, the constant rate filtration experiments were conducted using a corn starch suspension and the pressure variations in the filter were measured over time. The effects of the operational parameters such as volumetric flow rate Q of the feed slurry, rotational speed ω of the drum filter medium, and opening width w of the supply slit were investigated to find the optimum conditions for stable filtration operation. As a result, the stable filtration process with low pressure loss was attained under the conditions of ω = 1 rpm, Q = 50 L/min, and w = 2.85 mm. The experimental data also demonstrated that the filtration performance was much more efficient when the linear feed velocity obtained by dividing the volumetric flow rate by the slit area was about 1.7 m/s under low rotational speed. In conclusion, the dynamic filter with the tangential jet feed toward the rotating filter medium developed in this study is very useful as one of efficient and stable dynamic filtration systems.
In this study, the gold ore sand was sorted by the hydrocyclone, and the gold ore sand would be sorted into overflow and underflow. Both overflow and underflow then treat by chemical solvent to strip the gold from gold ore sand to get the result. The research is to figure out which hydrocyclone have the best separation effect to gold ore sand, that is, what kind of hydrocyclone can sort the gold ore sand with the lowest gold content concentration at the overflow and the highest gold content concentration at the underflow. The gold ore sand at the overflow would be removed, then we only need to strip the gold from the underflow to get the most portion of the gold.
In series A and series B of hydrocyclone experiments were carried out under a fixed 5% gold ore sand solution. The experimental results show that as the cone length of the hydrocyclone increases, the concentration and solid content of the overflow decrease. This is due to fact that when the cone length increase, gold ore sand can be separated more effectively in the hydrocyclone, the gold ore sand with large density and large particle size is discharged from the underflow with the main spiral vortex, and decrease the portion of large particles that carried gold from taken away by overflow spiral vortex.
It has been reported that when a diluted slurry is filtered at a constant rate by a precoat layer prepared by water containing fine bubbles (FBs), the filtration capacity of the layer becomes more than twice larger than using a precoat layer prepared by water without FBs. This study aims to clarify the reason why the addition of FBs to the precoat layer increases the filtration capacity. Precoat layer of kieselguhr was prepared by tap water containing FBs or tap water without FBs, followed by tap water permeation through the layer. In case of “with FBs,” FBs caught in the precoat layer grow during the water permeation period, and a part of them leave from the layer, resulting in a change of the surface of the layer from smooth to rough. The figure shows the effect of water permeation time on filtration capacity which is defined as the amount of captured particles per unit area of a filter medium until filtration pressure reaches a predetermined value. Filtration capacity increases with water permeation time until the capacity reaches a maximum value at 10 min. Up to the time 10 min, FBs grow, and a part of them leave from the precoat layer, increasing porosity and roughness of the layer. The increase of filtration capacity seems to be in accord with the increase of the roughness of the layer. When the permeation time is longer than 10 min, the structure of the layer is collapsed by over-grown bubbles leaving from the layer. It seems that several filtration mechanisms proceed successively or simultaneously. The degree of improvement by addition of FB water in preparation of the precoat layer can be evaluated in terms of an increase of effective filter area.
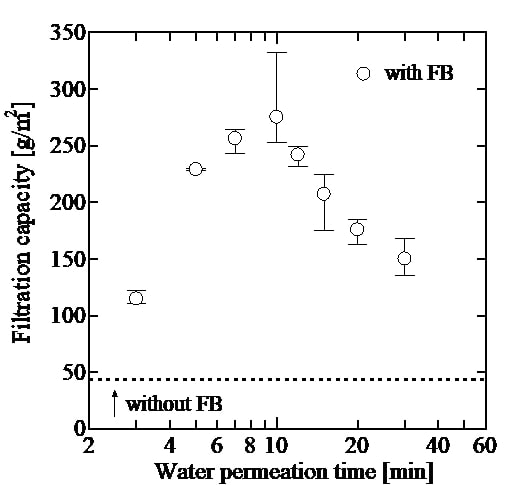
Poly(L-lactic acid) (PLLA) is one of the major biodegradable biomass plastics to reduce the use of fossil resources and the plastic wastes in the natural environment. We have been developing some microfiltration membranes of PLLA to reduce the wastes of filter media in filtration process in industries because the polymer is degradable under the hot and humid conditions in compost machines after use [1-5]. In this paper we developed microfiltration membranes of PLLA by using γ-butyrolactone (GBL) and surfactants via phase separation methods. PLLA was dissolved at 120 oC in GBL containing surfactants (none, Tween 20, Tween 40, Tween 80, polyoxyethylene (20) oleyl ether, and Span 80). The solutions were cast on glass plates with a 0.5 mm-thick frame of polytetrafluoroethylene at 70 oC and immersed in a 25 oC purified water to induce phase separation. Filtration characteristics were examined by dead-end filtration with purified water and 0.3 μm polystyrene latex beads. Asymmetric filtration membranes were formed when Tween 20, Tween 40 and Tween 80 were used as surfactants. The retention of the latex beads was more than 97% when the concentrations of PLLA and Tween 80 in GBL were 10-15% and 5-10%, respectively. The membrane resistance prepared from 10% PLLA solution in GBL containing 10% Tween 80 was about 1/20 of that prepared from 10% PLLA solution in 1,4-dioxane containing 15% Tween 80. The membrane will be one of the candidates of the compostable filtration membranes.
References
1) T. Tanaka, D.R. Lloyd, J. Membr. Sci., 238, 65–73 (2004).
2) T. Tanaka et al., J. Chem. Eng. Jpn., 39, 144–153 (2006).
3) T. Tanaka et al., J. Chem. Eng. Jpn., 44, 467–475 (2011).
4) T. Tanaka et al., J. Membr. Sci., 396, 101–109 (2012).
5) H. Minbu et al., J. Membr. Sci., 479, 85–94 (2015).
When a non-uniform electric field is applied to the particle dispersion, the particles move by receiving a force along the electric field gradient due to the magnitude of the polarization of the dispersion medium and the particles. This phenomenon is called dielectrophoresis, which can be useful from particle recovery from dispersion. When dielectrophoresis occur, particle aggregation simultaneously occurs. This phenomenon also can be useful for particle separation because it can enhance sedimentation of fine particles in liquid.
The objectives of the present work is to investigate the conditions in the application of the abovementioned aggregation phenomenon to the removal of silica fine particles from water. For this purposes, we tried to design the electrode structures that can give appropriate electric field profiles for high removal efficiency. Using the newly designed electrodes, the effects of the applied voltage, its frequency, and the voltage application time on the separation efficiency were observed.
In the experiment, water dispersed with silica particles (diameter = 20 nanometers) at 0.04 wt% was used as target dispersion. DC and AC voltage of 100 V with 0.1 ~ 1000 Hz frequency was applied to the dispersion, and the light absorbance of the dispersion were measured before and after the voltage application to evaluate the progress of particle aggregation and the removal. Figure1 shows the change in absorbance with respect to the change in frequency of the applied voltage. Note that the dispersion of nanoparticles is nearly transparent, the absorbance increases when the particles were aggregated. Such phenomenon may occur when AC voltage was applied, but the aggregation degree may not be high enough to enhance sedimentation. In contrast, the absorbance decreased when DC voltage was applied, suggesting that the DC voltage is effective to enhance the precipitation of silica particles than the AC voltage.
Post-combustion carbon capture from flue gas after the fossil fuel combustion is important to mitigate the global warming. CO2 capture by regenerable sorbents is a promising technology for post-combustion technologies because of its potential of low energy requirement and low operating temperature [1].
In this study, the post-combustion CO2 capture process in a dual fluidized-bed system has been investigated by developing a rigorous dynamic model for the system with K2CO3-based sorbent. The dual fluidized-bed CO2 capture process consists of a fast fluidized-bed carbonator and a bubbling fluidized-bed regenerator. As a case study, the K2CO3-based sorbent was used to sorb CO2 selectively from the flue gas by carbonation reaction. A ternary gas (CO2:H2O:N2=11:12:77 mol %) as an effluent gas from the fossil fuel-fired power plant and a binary gas (CO2:N2=95:5 mol %) as a regeneration gas were applied to the dual fluidized-bed process. The sorption and regeneration kinetic models were developed and applied to the carbonator and regenerator model. The developed model including the rigorous kinetic models was validated by comparing the experimental data [2] with the simulation results at various operating conditions. By using the developed model, the dynamic behaviors inside the carbonator and regenerator were investigated. The CO2 removal performance and the energy consumption for the regeneration of the sorbent were also evaluated at various conditions.
References
[1] Jayakumar A, Gomez A, Mahinpey N. Post-combustion CO2 capture using solid K2CO3: Discovering the carbonation reaction mechanism. Applied energy. 2016;179:531-543.
[2] Yi C-K, Jo S-H, Seo Y, Lee J-B, Ryu C-K. Continuous operation of the potassium-based dry sorbent CO2 capture process with two fluidized-bed reactors. International Journal of Greenhouse Gas Control. 2007;1:31-36
In this study, the experimental research work was conducted for the purpose to develop a higher performance removal agent for ethylene fabricated from the waste plum seeds of pickled Ume.
Plum seeds with residual pulp (O.ume) and pre-treated plum seeds (D.ume), which were removed pulp, washed and dried, were used as the raw materials for activated carbon. Using O.ume and D.ume, the activated carbons which were called O.AC-ume and D.AC-ume were prepared by a one-step-preparation method by using superheated steam, respectively. Predetermined amounts of Pd were loaded on the D.AC-ume to prepare activated carbons supporting Pd.
As results of the ethylene removal experiments using the various prepared ethylene removal agents, ethylene concentration in gas phase decreased with time by all the removal agents (Fig.1). There was no significant difference in the removal ability of ethylene between O.AC-ume and D.AC-ume. The removal ability would be markedly improved by loading Pd on D.AC-ume. In addition, as the loading amount of Pd increased, the ethylene concentration in gas phase decreased in a shorter time. Time course of change in the ethylene concentration in gas phase by O.AC-ume, D. AC-ume, and AC-Pd could be correlated by the pseudo-first-order kinetic equation, respectively.
SEM images of D.AC-ume and AC-Pd were taken by a scanning electron microscopy. As results of the quantitative elemental analysis and element mapping by EDS, it would be cleared Pd was almost uniformly supported on the surface of each AC-Pd.
Recognition of lectin by microfibers with sialic acids
Takumi Inoue and Kazuya Uezu
Influenza viruses are significant human respiratory pathogens that cause both seasonal, endemic infections and periodic, unpredictable pandemics. The virus recognizes specifically sialic acids on the surface of human throat cells with the lectin, hemagglutinin, on the virus surface. Lectins are "proteins showing binding activity to sugar chains". To prepare the functional materials capturing the influenza viruses, we have been immobilizing sialic acids to the polymer brush on the surface of microfiber nonwoven fabric to mimick the interaction between sugar and lectin. An epoxy-group-containing monomer, glycidyl methacrylate (GMA) was grafted onto a nonwoven fabric made of polypropylene, and N-acetylneuraminic acid (NANA) was immobilized by the reaction after introducing the coupled monomer of iminodiacetic acid (IDA) and 1-ethyl-3-carbodiimide hydrochloride (EDC) on GMA polymer brush. We used a wheat germ-derived lectin (WGA) with specificity for NANA. In this study, the lectin adsorption experiment was conducted in gas phase. The lectin solution was aerosolized to be droplets. While WGA did not adsorb on GMA polymer brush, it adsorbed on IDA-EDC and NANA immobilized nonwoven fabric. The adsorption amount of WGA on NANA was more than twice compared to on IDA-EDC. These observations indicate that the microfiber nonwoven fabric having sialic acids recognized sialic acid-specific lectins in aerosol.
Chromium (Cr) and its compounds are widely used in elecroplating and leather industries, which tends to be a main source of Cr(VI) pollution in water. The expose of Cr(VI) is causes of health problems like liver damage, lung cancer and so on. Therefore, removal of Cr(VI) from water environment has received more and more attention. Adsorption is one of the preferable methods for the removal of Cr(VI) in the water environment. In addition, reduction of Cr(VI) to Cr(III) is effective for detoxication, since the toxicity of Cr(III) is much lower than that of Cr(VI). In present work, reductive adsorption of Cr(VI) has been investigated by using activated carbon (AC).
Coal-based activated carbon, M010 (Mitsubishi Chemical Calgon), was used as an adsorbent. Adsorption was carried out by shaking mixture of M010 and aqueous feed solution. After filtration, concentration of Cr species in aqueous solution was determined by ICP-AES and colorimetry.
Figure 1 shows the effect of pH on the adsorption of Cr(VI) on AC. The adsorption of Cr(VI) was highly influenced by pH of aqueous solution, and high adsorption amount was obtained pH range of 4 - 6. This is because the species distribution is affected by pH. In addition, the reduction of Cr(VI) to Cr(III) occurred during the adsorption processing, and thus Cr(III) appears in the aqueous solution after adsorption, especially at acidic pH region. Reductive adsorption of Cr(VI) is therefore achieved with the AC.
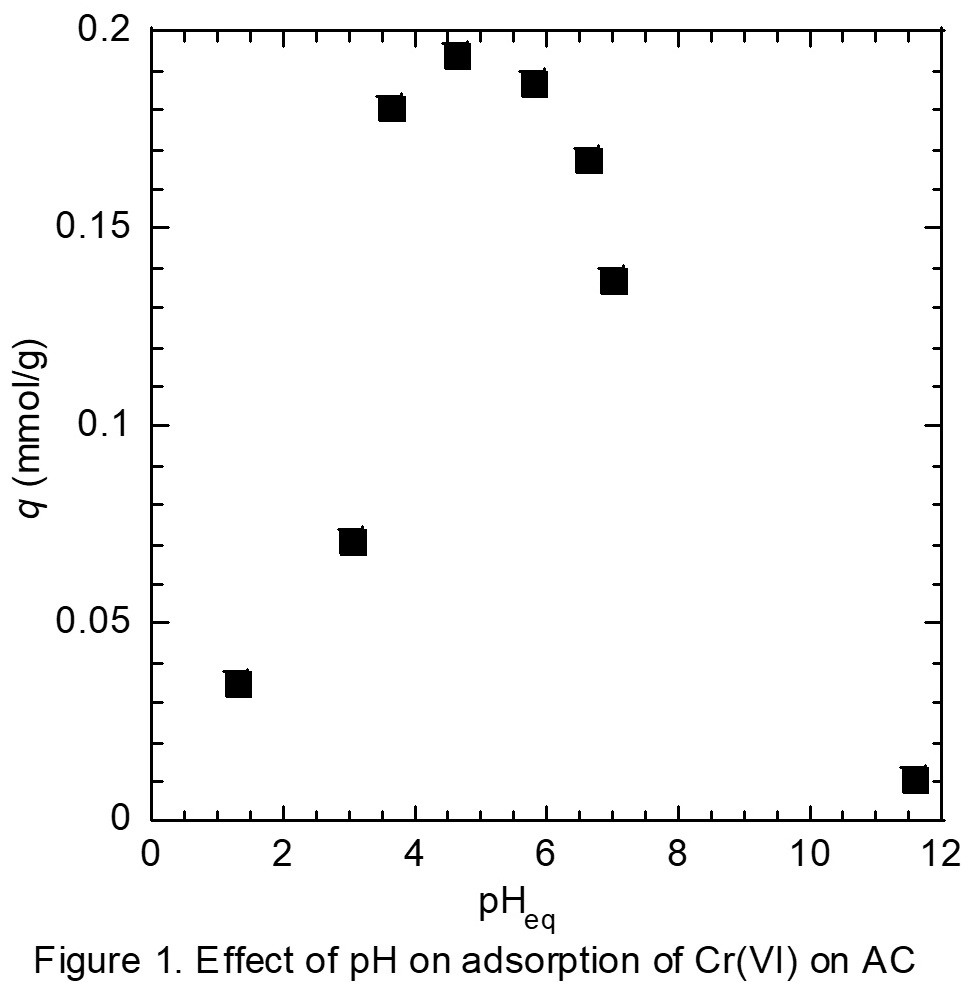
Plating waste sludge contains a large amount of valuable metals and need to develop an industrially sound technology for their treatments. In this study ion exchange/chelate fibers were used to develop the separation and recovery of Ni and Sn from the sludge. Adsorption equilibrium and separation characteristics were examined to understand the optimum conditions. The solid sludge as well as standard Ni and Sn ions were dissolved in 3M HCl solution. “Weak base type chelate fiber” containing a polyamine groups (called KC-31) as well as three other fibers were used in this study. The equilibrium data were in good agreement with the Langmuir isotherm for single-component system when using standard Sn individually of mixture with Ni, the equilibrium data were found to be in good agreement with the Langmuir isotherm. Fig.1 shows the results of the separation of Ni and Sn ions from the sludge solution. As shown, Ni and Sn ions can be completely separated from each other. The calculated maximum adsorption capacities for Sn from the sludge was 1.03 mol/kg when KC-31 fiber was used, while the adsorption capacity for Ni at the same conditions was zero. The water and or HCl 0.1~1M solutions were found as effective eluent to desorb the adsorbed Sn on the fiber. The adsorption breakthrough curve and desorption curve were scaled up from batch to a fixed bed reactor. When the flow rate was 1 mL/min, the adsorption breakthrough time and desorption completion times were 9 and 20 min, respectively. Obviously, it was revealed that the breakthrough time and the desorption time become longer when the flow rate reduced. As a main conclusion, it was found that Sn and Ni can be entirely and successfully separated and recovered from waste plating sludge by adsorption and consequently desorption using the fiber KC-31.
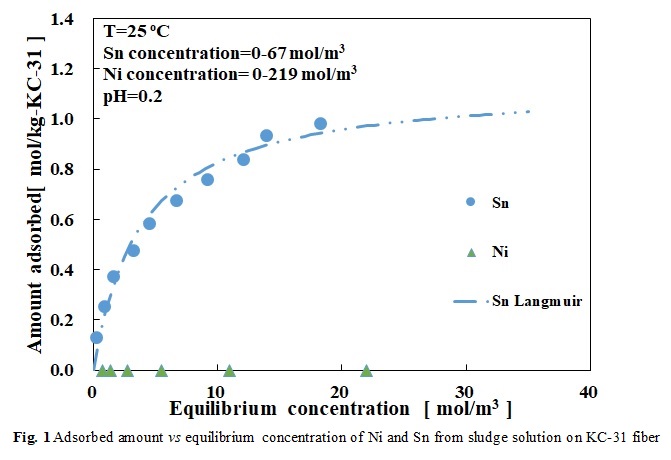
Separation of propane and propylene has a significant value in the petrochemical industry because propylene is an important feedstock for polypropylene, acrylonitrile, acrylic acid, and other many poly-gas chemicals [1]. Cryogenic distillation method is a general method to separate them, but it is one of the energy-intensive processes in petrochemical industries [2]. Therefore, the adsorptive separation process may be an alternative in economic and energy perspective. One of the main problems in developing the process was the difficulty of regeneration for adsorption bed because of the high affinity of propylene on adsorbents.
In this study, desorbent swing adsorption(DSA) separation of propane and propylene in zeolite 13X has been carried out. As a desorbent, carbon dioxide and helium were chosen. And adsorptive behaviour, depending on desorbent, temperature and pressure, was investigated by breakthrough experiments. To estimate the separation performance, the breakthrough experiments, composed of adsorption, rinse, desorption, and re-adsorption were conducted to design a 4-step DSA process.
The dynamic simulator was developed to predict the breakthrough results at various conditions. The required data for the simulation were used from the previous study [3] and the validation of the model was confirmed through the comparison between the model and experimental breakthrough results. The simulated separation performance of the DSA process will also be presented.
[1] M.C. Campo, A.M. Ribeiro, A. Ferreira, J.C. Santos, C. Lutz, J.M. Loureiro, A.E. Rodrigues, Separation and purification technology, 103 (2013) 60-70.
[2] C.A. Grande, A.E. Rodrigues, Propane/propylene separation by pressure swing adsorption using zeolite 4A, Industrial & Engineering Chemistry Research, 44 (2005) 8815-8829.
[3] J.J. Kim, S.J. Lim, C.H. Lee, Microporous and Mesoporous Materials, 274 (2019) 286-298.
Several methods of CO2 capture and storage have been investigated, and adsorption separation is known to be one of the key technologies. Zeolites are often used as adsorbents for CO2 separation due to its high CO2 adsorption capacity. However, zeolites adsorb H2O selectively rather than CO2 under humid conditions. Since flue gas from industries generally contains H2O, dehumidification of flue gas is necessary for present CO2 adsorbents, but it consumes extremely large amount of energy.
In this study, core-shell structured chabazite consisting of a chabazite aluminosilicate core coated with thin layer of hydrophobic pure silica chabazite was synthesized, and its CO2 adsorption property under humid conditions was evaluated.
A chabazite aluminosilicate core was synthesized by hydrothermal synthesis. Then, a pure silica chabazite coating was performed by a secondary growth of a core chabazite in fluoride solution under hydrothermal condition. CO2 adsorption property of the products under humid conditions were assessed by means of breakthrough measurements.
Crystal size was slightly increased after core-shell structuring, and the Si/Al ratio values of aluminosilicate core and core-shell structured sample were 5 and 8, respectively. This indicates the thin layer of hydrophobic pure silica chabazite was successfully grown on a chabazite aluminosilicate core. CO2 adsorption amount under the humid condition was assessed by adsorption-desorption cycles of CO2 with H2O vapor at 313 K. Figure shows the relative change of CO2 adsorption amount during adsorption-desorption cycles without heat treatment. CO2 adsorption amount on the chabazite aluminosilicate core was gradually decreased with cycles due to co-existing H2O vapor. In contrast, the negative effect of co-existing H2O vapor was suppressed on the core-shell structured chabazite. It was experimentally demonstrated that core-shell structuring on chabazite zeolites was effective for CO2 adsorption under humid conditions.
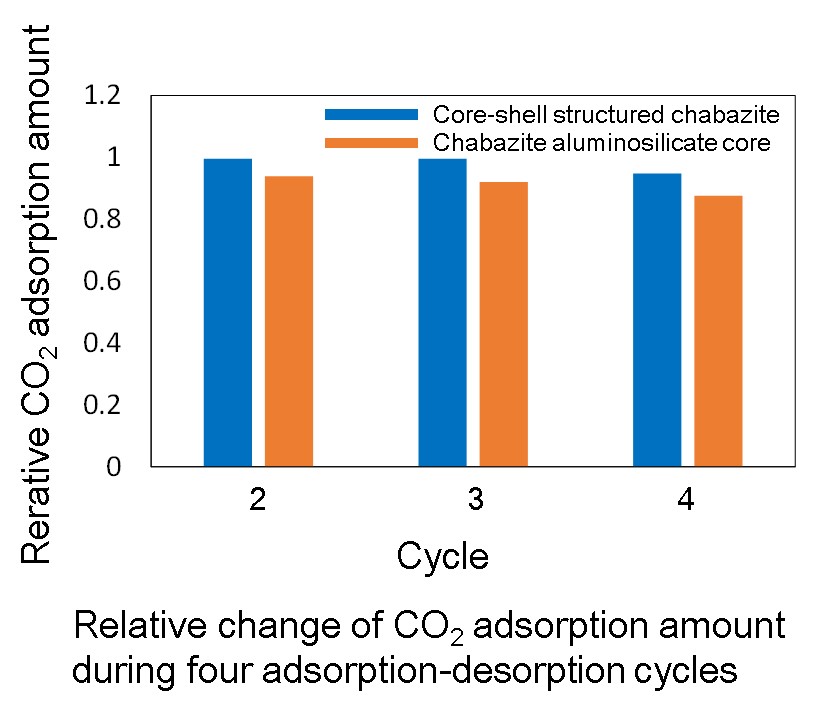
In this study, we developed the simulated moving bed (SMB) process that was customized for the continuous-mode separation of fucose and 2,3-butanediol (BD) with high purities. As a first step for this task, an effective adsorbent for the targeted separation was selected through a series of pulse tests for several potential resins that are commercially available. On the basis of selected adsorbent, the intrinsic parameters of fucose and BD were estimated through a multiple frontal analysis method. The estimated parameters were then used in the optimization of the fucose-BD separation SMB process. To experimentally verify the separation performance of the optimized SMB process, the relevant SMB experiment was carried out. Finally, we devised two potential strategies to make a further improvement in product concentrations and/or desorbent usage while keeping the purities and yields of fucose and BD almost unchanged. The results of this study will be helpful in making a substantial improvement in the economical efficiency of the microalgae-based fucose production process that utilizes the Klebsiella oxytoca fermentation as a final step for fucose purification.
In recent years, arrangements for reducing greenhouse gases have been reviewed with the rise in global average temperature. In fact, the Paris Agreement, new agreement about climate change suppression agreed in 2015, aim for holding the increase in the global average temperature to below 2 oC above pre-industrial levels, so greenhouse gases capture methods are much in demand. Hence, we focus on CO2 one of greenhouse gases and have been developing CO2 capture using amine-containing hydrogel film. This material is able to maintain the performance of CO2 capacity even under high humidity environment (R.H. > 95%) compared to other CO2 capture materials such as activated carbon, zeolite and so on. However, CO2 absorption kinetic of this material is not enough to use CO2 capture because ionic diffusion rate of HCO3- dissolved hydrogel film is too slow. In contrast, HCO3- diffusion in liquid phase is 10,000 times slower than CO2 diffusion in gas phase. In this study, we designed new type of hydrogel film with micro channels for gas diffusion, namely aeroporous hydrogel, for fast CO2 capture (Figure 1). We could prepare this material by mixing amine-containing hydrogel particles (GPs) and hydrophobic fillers like carbon black via dry process, molding film by uniaxial press, and humidifying under R.H. > 95%. After mixing these materials, SEM images showed GPs coated with hydrophobic filler. Then, we observed some void that can be gas diffusion phase in aeroporous hydrogel film by TEM images. CO2 absorption kinetics of this film are several times larger than that of conventional hydrogel film. Additionally, we discovered that CO2 absorption kinetics differed according to filler characters such as hydrophobicity and particle size because these affected formation of micro channels. Therefore, we expect that more improvement of CO2 capture efficiency can be achieved by using this material.
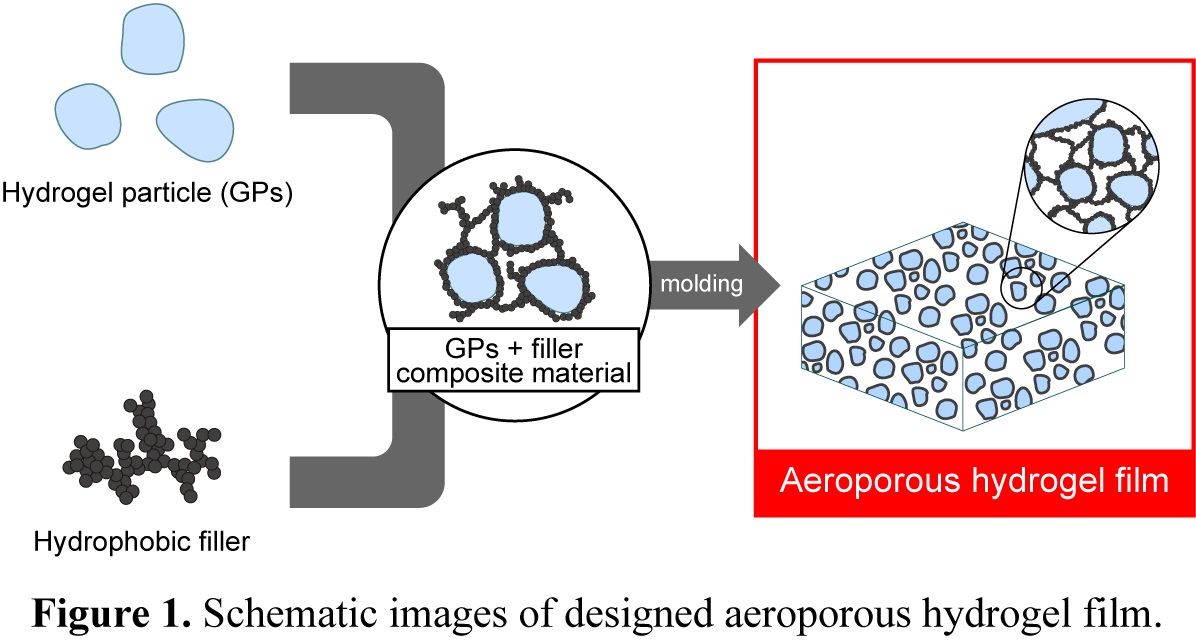
Nitrogen monoxide and dioxide are referred to together as oxides of nitrogen (NOx). Since NOx is toxic for human beings and may cause acid rain, both amount and concentration of NOx in exhaust gas are restricted to low levels by domestic or international legal control. In our laboratory, removal of NOx by adsorption against a zeolite and NOx recycling process for a production of nitric acid have been studied. Low concentration of NOx in exhaust gas can be concentrated by adsorption and thermal desorption by using zeolite. When concentrated NOx is absorbed to water, unable saturated nitric acid can be produced. In this study, we analyzed adsorption-desorption and oxidation mechanism on NOx concentration process for nitric acid production from NOx in exhaust gas. It was found that NOx (NO occupying the majority) is adsorbed on the zeolite and desorbed from zeolite as NO2 by thermal desorption. 100% of NO can be oxidized in NO2 by zeolite. NO2 is easily absorbed to water. This is very useful for NOx recycling process for a production of nitric acid.
Drying is in widespread use in wide a variety of industries, including the pharmaceutical, food, and organic chemical sectors. Vacuum foam drying has advantages, in that the drying process is rapid and heating can be eliminated. The process is now used in producing certain types of viruses and amorphous solid dispersion preparations. While the foaming of a solution is difficult to control under conditions of low vacuum, the application of a high vacuum above a certain level causes instantaneous foaming without any time lag. In our studies, we found that, when the volume of a solution is reduced to a certain extent and is then punctured with a steel needle (needle-stimulation), drying restarts under conditions of decompression (~1000 Pa) and forming is typically induced with a significantly high probability. Hence, the feasibility of vacuum foam drying under conditions of a low vacuum with the aid of the needle-stimulation-based induction of foaming was investigated. The sample solution, comprised of a sugar or a polymer as a solute and alcohol as the solvent, was vacuum dried in the two steps (initial and secondary drying), between which needle-stimulation was conducted. The probability of inducing solution foaming was estimated as a function of the initial drying period. The relationships between the initial drying period and the probability of inducing foaming were compared among different solute/solvent combinations and different needle-stimulation conditions. The changes in the solution viscosity during the initial drying period were also measured as a factor determining the probability of inducing foaming. Based on the obtained findings, the mechanism for how needle-stimulation induced the foaming was investigated.
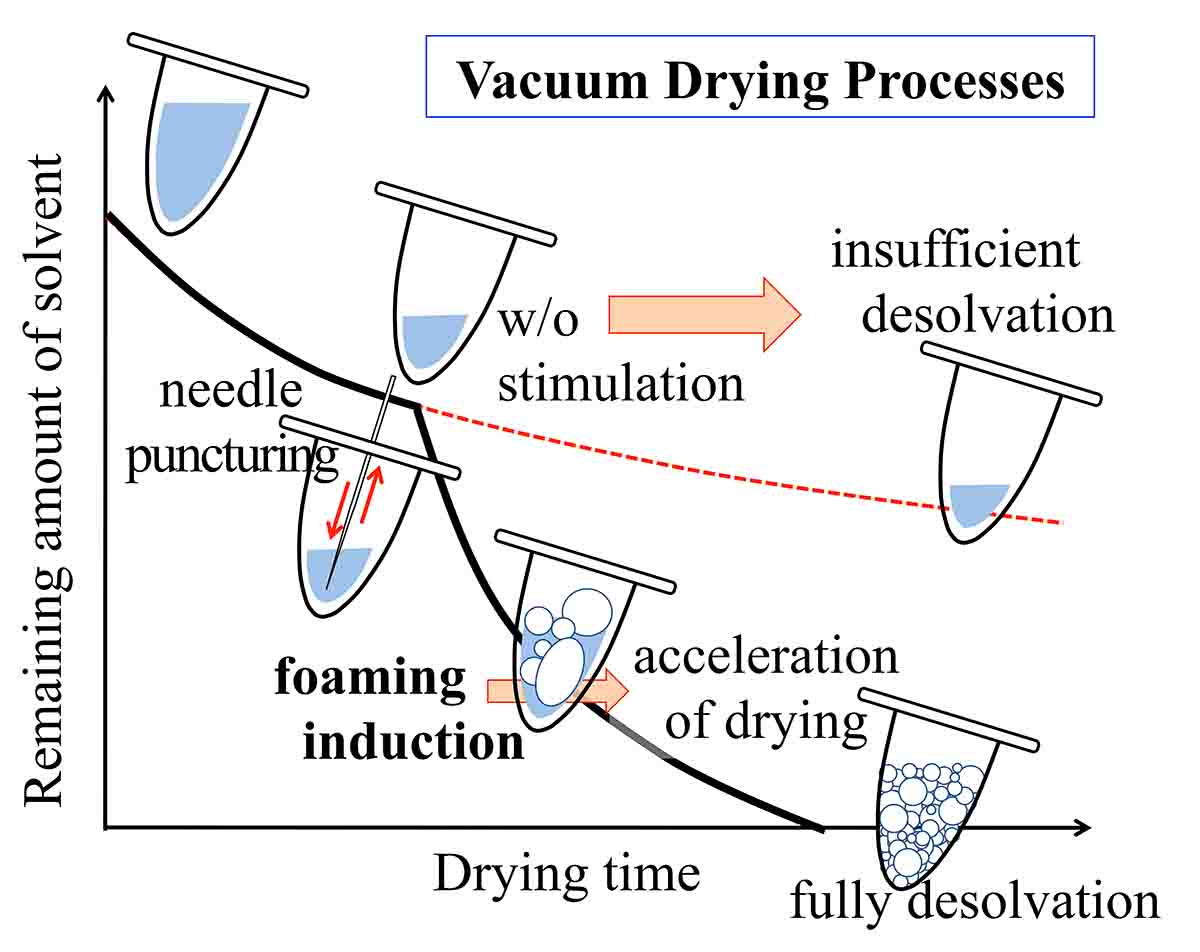
Chitosan is a superior metal adsorbent and is expected to be used in the removal of metallic ions in wastewater. Its adsorption capacity and selectivity can be enhanced by chemical modification. However, chitosan is easily soluble in dilute acidic solutions because of the protonation of the amino group. In most cases, crosslinking among amino groups of chitosan is carried out for insolubilization. The metal adsorption capacity generally decreases with an increase in the extent of crosslinking because amino groups on chitosan bind the metal ions. Recently, the preparation of chitosan-cellulose hydrogel beads enhanced the mechanical and chemical strength. The mechanically prepared gel beads were also soluble in the acidic solution and crosslinking of the gels was needed as an adsorbent. Recently, because ionic liquid can dissolve such polysaccharides as cellulose and chitosan, chitosan-cellulose beads by regeneration from ionic liquid 1-butyl-3-methyl imidazolium chloride were prepared.
In this study, chitosan-cellulose gel beads were regenerated from ionic liquid and their application to metal adsorbent was examined. The chitosan-cellulose gel beads, which were prepared from 1-ethyl-3-methyl imidazolium acetate, were more stable in the acidic and basic aqueous solutions than cellulose or chitosan. The adsorption of copper on chitosan-cellulose gel beads was examined and the adsorption amount of copper ion increased with pH, suggesting that amino groups on chitosan were related to adsorption. The adsorption isotherm of copper, zinc and nickel ions was well described by the Langmuir model and the adsorption ability of these metal ions obeyed the Irving-Williams series. Fe(III), Mn(II), and Co(II) from a buffer solution and Pt(IV), Au(III), and Pd(II) from a hydrochloric acid solution were not adsorbed on the chitosan-cellulose gel beads.
Industrial wastewater often contains heavy metals such as lead, copper and iron, which are used for various purposes. It is essential to utilize rare metals, especially precious metals, and the shortage of scarce metal resources is a problem and the collection of precious metals from the wastes is also important. Recovery of the metals in wastewater has been carried out mainly by precipitation method and adsorption method. The precipitation method is suitable for treating a large amount of wastewater of high concentration, however, a neutralization treatment is required and a large amount of high concentration alkaline waste liquid is generated. However, heavy metals are likely to be accumulated in the body and health damage often occurs since metabolic pathways are not established in the human body. New metal ion recovery method with hydrogels as a reaction and separation media has been investigated. Hydrogels based on N-3-(Dimethylamino)propyl acrylamide (DMAPAA) or (3-Acrylamidopropyl)trimethylammonium chloride (DMAPAA-Q) were prepared. The DMAPAA hydrogel exhibited high pH value inside when they swelled in water because the tertiary amino group was protonated by water and produced hydroxide ion retained in the hydrogel. When the hydrogel was immersed in the metal ion aqueous solution, the metal ion diffused into the hydrogels to produce metal hydroxide in the hydrogel. On the other hand, DMAPAA-Q hydrogel modified by various anions such as chloride, sulfide, and carbonate can produce metal salt inside the hydrogel, too. The metal salt in those hydrogels were easily separated from the solution. The selective recovery of a certain metal ion from the mixed metal ion solution was attained by using hydrogels with variety of anions according with metal ions. The results obtained here suggest the possibilities of the novel selective metal ion recovery method replacing the coagulation and/or adsorption methods.
Japan is one of the resource-rich countries in the world from the standpoint of "urban mine", and the establishment of simple, low-cost and environment-friendly technology for noble metal recovery is the urgent issue to be solved. In this study, the fabric membrane which can adsorb gold ions selectively was developed using electrospun nanofiber with extremely high specific surface area as an adsorbent. Prior to the development of nanofiber membranes, the nanofiber material useful for gold ion adsorption was explored, and as a result, it was found out that nylon 6 has a great affinity for gold ions. The objective of this study is to investigate the gold ion adsorption ability and selectivity of the nylon 6 nanofiber membrane. The nanofiber membrane was prepared by depositing nylon 6 nanofibers produced by electrospinning method. The mean diameters of nanofibers produced in this study were 143 and 206 nm at the nylon 6 concentrations of 10 and 20 wt%, respectively. As a result of the gold ion adsorption tests in a highly acidic environment of 2 M hydrochloric acid, the adsorption behaviors could be expressed by the Langmuir's adsorption isotherm, and the saturated adsorption amounts were estimated at 909 mg/g for 10 wt%-fabric membrane and 769 mg/g for 20 wt%-fabric membrane. The fabric membrane with thinner fibers has higher adsorption capacity due to larger specific surface area. The adsorption test of a mixture of Au3+, Cu2+, Al3+ and Fe3+ was also performed. As a result, only gold ions could be selectively adsorbed to the nylon 6 nanofiber membrane. In addition, the amount of gold ion adsorption tended to increase with an increase in hydrochloric acid concentration. Moreover, it was also observed that gold ions could be desorbed by immersing the gold ion-adsorbed fabric membrane in a neutral solution.
Selenium in aqueous solution exits predominantly as two chemical species: selenite (SeO32-, Se(IV)) and selenate (SeO42- , Se(VI)). Both oxyanions are known to bioaccumulate and could result in health problems. The value of a drinking water standard of selenium, 10 μg/dm3 was set by the World Health Organization to minimize the health risks. In this study, a series of iron-yttrium composite oxides were prepared by coprecipitation. The iron-yttrium composite oxides prepared were evaluated by XRD, SEM, BET surface area measurement and zeta potential analyser. The iron-yttrium composite oxides were formed via aggregation from primary nanoparticles. The specific surface area and the point of zero charge (PZC) increased with increasing yttrium content in the iron-yttrium composite oxides. The adsorption of Se(IV) and Se(VI) by the iron-yttrium composite oxides were investigated, and the adsorption mechanisms of Se(IV) and Se(VI) were discussed. The adsorption of Se(IV) and Se(VI) depended on pH and decreased with increasing pH. The adsorption isotherms of Se(IV) and Se(VI) at neutral pH fit well to Langmuir adsorption model. The maximum adsorption capacities of Se(IV) and Se(VI) were 2.04 and 0.418 mmol/g, respectively, which were higher than those of adsorbents based on on binary oxide previously reported. The adsorption of Se(IV) and Se(VI) was affected by ionic strength and decreased with increase in ionic strength. After adsorption of Se(IV) and Se(VI), the surface charges of the iron-yttrium composite oxides slightly decreased at lower pH than PZC, but the PZC did not shift. It was suggested that Se(IV) and Se(VI) were adsorbed on the iron-yttrium composite oxides by the formation of outer-sphere complexes. The results indicate that the iron-yttrium composite oxides could be attractive adsorbents for Se(IV) and Se(VI).
Mesoporous metal oxide particles have been widely used as adsorbents for VOCs adsorption, as they have high surface area, high thermal and chemical stability. In this study, mesoporous alumina, silica, and titania particles were synthesized by spray pyrolysis combined with sol-gel process. The spray pyrolysis method has advantages of synthesizing spherical micron-sized particles in one step and controlling the size and morphology of the product particles. Also, mass production is possible with this method as it is a continuous reaction process. In order to control specific surface area and pore structures, the particles were prepared by adding CTAB as a template and to improve abilities of VOCs adsorption, some transition metals were added. The product particles were analyzed by BET, XRD and FE-SEM.
In this work, MIL-100(Fe) framework was successfully synthesized by using a continuous tubular reactor under microwave irradiation for a short reaction time (20 - 50 min) and low temperature (80 - 110 °C). The effects of experimental conditions such as temperature and reaction time were systematically investigated. Compared to other approaches for preparation of MIL-100(Fe), this method can produce large quantity of MIL-100(Fe) for a shorter time and lower temperature. The obtained MIL-100(Fe) at optimum conditions were used for CO and CO2 adsorption after loading with various transition metals. The results showed that Cu(I)-containing MIL-100(Fe) was a potential adsorbent for CO separation due to its high CO adsorption capacity and high CO/CO2 selectivity, superior to some other adsorbents such as CuCo/13X, Cu(I)/Y, Cu(I)/AC, Cu(I)@SNW, etc.
By optimizing the raw material ratio of Ethylenediaminetetraacetic acid-type chitosan (EDTA-type chitosan), the extraction rate of rare earth elements were improved. The synthesis with a lower concentration of chitosan-acetic acid than in the previously reported preparation method, it was confirmed by IR measurement that no peaks attributable to the carboxyl group of EDTA were observed and the amide bond reaction between chitosan and EDTA was more progressed. By the optimizing preparation conditions, the extraction ratio of Nd, Dy, Sm, Tb was improved about twice. In the preparation method of this work, the amide bond reaction could be advanced over the competitive chitosan acetylation reaction. In addition, it was shown that the separation factor of rare earths did not change, and that there was nothing other than adsorption by EDTA-type chitosan. In the presentation, the results of mutual separation of rare earths by column method are also reported.
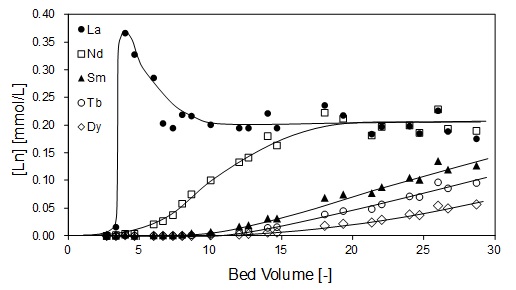
The inverted-capacitive deionization (i-CDI) technique usually employ carbon materials modified with permanently negative and positive charges for anode and cathode, respectively, where ion adsorption results from cell discharging and vice versa. Similar to the typical CDI systems, the permanently charged surface in the i-CDI cell repels ions of the same charge when the applied electric field is absent, which reduces the ion removal efficiency. In this work, MnO2 and polypyrrole-coated activated carbon (PPy@AC) with pseudocapacitive characteristics are employed as “memorized” anode and cathode in the i-CDI cell. The salt adsorption capacity (SAC) of MnO2//PPy@AC highly depends on the discharging time and the highest SAC of 27.55 mg g-1 is obtained at the 60-min discharging time. The SAC retention of this unique cell is 100% after 10 charge/discharge cycles at 1.2/0 V. The memoried desalination effect of MnO2//PPy@AC is confirmed by the unchanged conductivity of the cell when the applied cell voltage is interrupted, which has been demonstrated for water deionization and salt concentration applications.
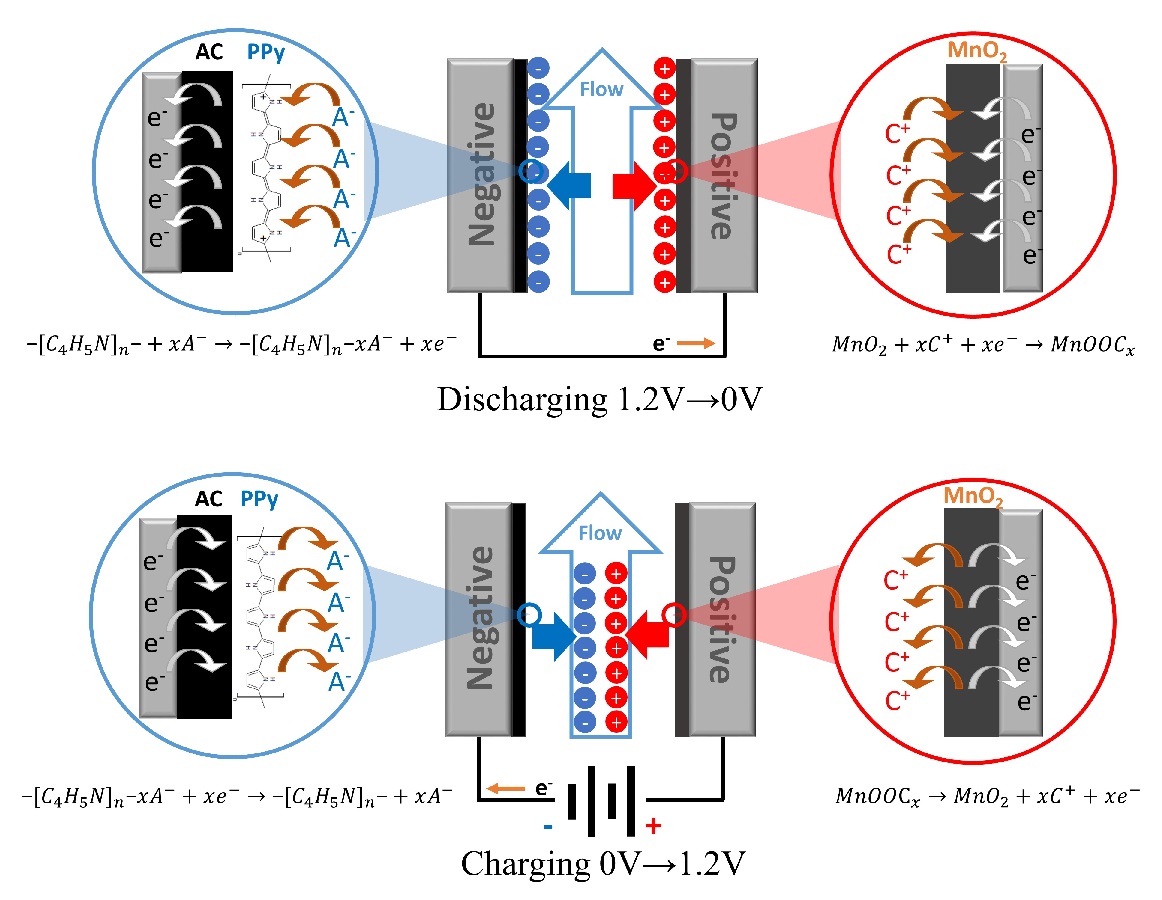
Propylene glycol is used in various foods such as coffee-based drinks, carbonated beverages and ice cream and also used as a solvent in flavoring substance. A simple method was developed for the analysis of propylene glycol (PG) in foods using gas chromatography with flame ionization detector (GC-FID). PG in foods were extracted with water-acetone (30:70) mixture solution, followed by centrifugation. The resulting solution was filtered using 0.45 μm membrane syringe filter and injected into GC. The chromatographic separation of PG was performed on DB-WAX UI column (30 mm x 0.25 mm, 0.25 mm). The calibration curves of PG were linear in the range of 0.5 to 100 μg ml-1, with good correlation coefficients (r2 > 0.999). Recoveries ranged from 83% to 119% for spiking levels of 10, 50, and 100 mg/kg PG in five samples (coffee beverage, dumpling, candy, chocolate, and snack), with relative standard deviation values below 9%. The limit of detections (LOD) and limit of quantifications (LOQ) were 0.16 mg/kg and 0.49 mg/kg, respectively. The proposed method was successfully applied to the determination of PG in foods. The detection level ranged from ‘not detected (ND)' to 2,389.6 mg kg-1. This method is suitable for routine monitoring and exposure assessment of PG.
Adiabatic gas adsorption rises the temperature of adsorption column because of the heat of adsorption, which results in the suppression of adsorption capacity and gas separation performance. We have thus focused on mixing capsules with phase change material (PCM) as a latent heat storage medium into adsorbent materials. To effectively utilize the PCM, it is essential to determine the amount of PCM. Therefore, we developed a detailed column model based on a set of partial differential equations along the column axis: each equation is based on the mass balance, heat balance, and adsorption rate equation for each component, and the adsorption amount, temperature, mole fraction of the fluid eluted from the adsorption column were obtained as a function of time.
In this study, we assumed butane adsorption on activated carbon, and the obtained temperature at the end of the adsorption column and the mole fraction of the butane eluted from the adsorption column as a function of time are shown in Figures 1(a) and 1(b). We assumed that an inert gas is used as a carrier gas and the mole fraction of butane was set to 0.0625. The adsorption column without PCM shows a sharp temperature rise due to the heat of adsorption and gradual temperature decrease due to the heat exchange with the gas flowing inside the column (Figure 1(a)), which results in an unusual breakthrough curve of butane (Figure 1(b)). On the other hand, when the capsules with PCM are uniformly dispersed in the adsorption column by the volume fraction of 0.5, the temperature becomes almost constant and the breakthrough curve of butane shows a sharp step, which suggests that the net heat of adsorption was successfully suppressed by PCM and the breakthrough behavior can be controlled by dispersing the capsules with PCM.
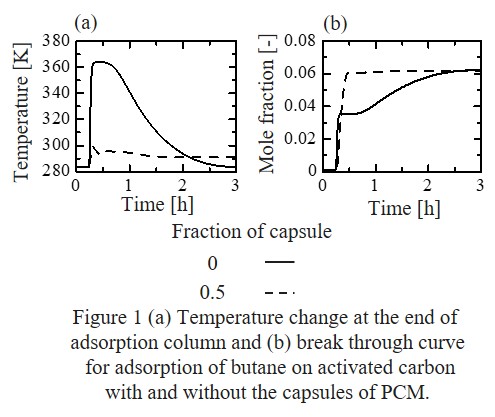
1. Introduction
The reverse osmosis (RO) membranes have been developed as polymeric membranes. Here, we have paid attention to inorganic materials such as zeolites to improve chemical resistance. Zeolite is a porous aluminosilicate with ion exchange properties. The pore sizes of zeolite can be controlled by the ion exchange. In this study, Faujasite (FAU) zeolite membranes had prepared for RO permeation. The membrane properties were controlled by the ion exchange procedures of LiCl.
2. Experiments
FAU membranes were prepared by using a secondary growth method on α- alumina substrates. Seed crystals were coated on the α-alumina substrates using a dip method. The molar ratio of the parent gel was SiO2:Al2O3:Na2O:H2O= 10.7:1:18.7:850. Hydrothermal synthesis was carried out at 90°C for 20h. Ion exchange procedures were performed in a 0.1M LiCl or NaCl solution for 16 h at room temperature. Water permeation test were performed in a 500ppm LiCl or NaCl, PEG6000 solution.
3. Results and discussions
Figure shows the time course of the permeation results of NaCl, LiCl and PEG6000 by changing the cations in the FAU zeolite membrane. The rejection through the as-made membrane (Na type) was 58% with the flux of 1.1 kg m-2h-1. While the rejection through the Li type membrane was only 3% with the flux of 6 kg m-2h-1. The membrane was not broken because the rejection increased at 45% after the ion exchange for Na type. The molecule size of PEG6000 is much larger than that FAU pores showing that the PEG6000 was permeate through the grain boundary of the FAU membrane. The size of the grain boundary must increase by the ion exchange procedure of LiCl to permeate the PEG6000. The possibility to control the permeation properties by the ion exchange procedures was shown by this experimental.
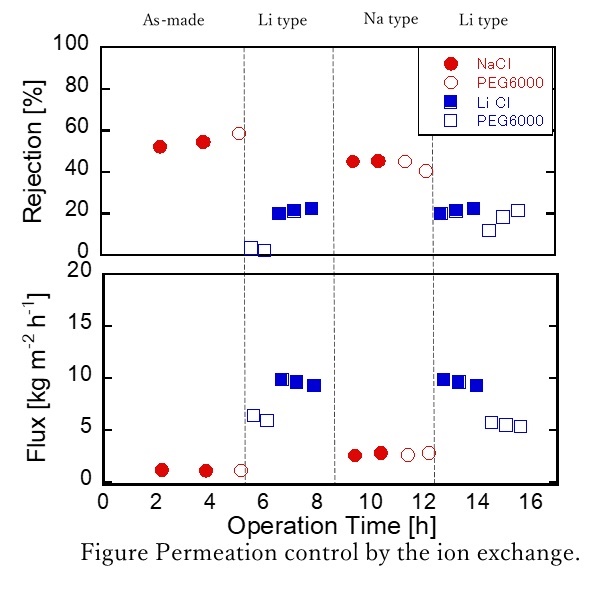
Bis(triethoxysilyl)propane (BTESP)-derived organosilica membranes have attracted a lot of attention in the high performances for hydrogen over large molecules such as C3H6 and C3H8. In the present study, the effect of acid molar ratio (AR) in sol and calcination temperature on network pore size and gas permeation property was evaluated. BTESP-derived sols were prepared with AR=10-2, 10-1, 100 and 10, and calcination temperature was controlled at 350-800oC. The effect of AR and calcination temperature on BTESP structure was evaluated by FT-IR, NMR, and N2 adsorption measurement.
Single gas permeation experiment of BTESP membranes showed high H2 permeances and high H2/SF6 selectivities. The pore size became smaller as AR decreased because condensation of much number of Si-OH groups in sol during calcination process densified the network pores. On the other hand, the pore size became larger as the calcination temperature increased because of a change in carbon structure of linking unit. Characterizations of the carbon structure by 13C-NMR measurement showed that the propylene groups were pyrolyzed and the proportion of methyl groups increased at high calcination temperature above 600oC.
In conclusions, pore size of BTESP-derived membranes can be controlled by acid molar ratio in sol and calcination temperature; dense BTESP network for separation of H2 over N2 was designed when BTESP membrane was calcined at low temperature or utilizing sol with low AR.
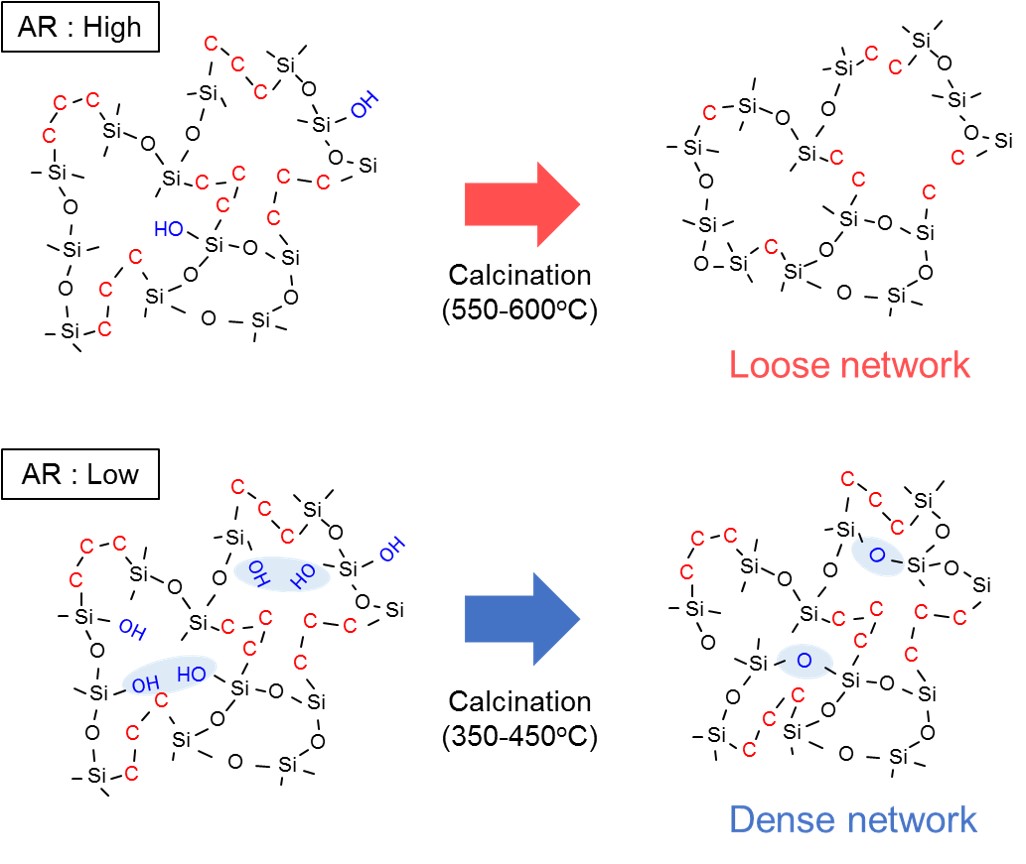
Platinum group metals (PGMs) are used not only for jewelries but also in various industrial scenes such as a catalyst, electronic components and so on. Among them, platinum, palladium and rhodium are used as an automotive exhaust catalyst for the purification of exhaust gases owing to their excellent catalytic properties. However, these resources are unevenly distributed (mostly in South Africa) and their reserves are limited. It is important to develop the recovery technique for selective separation of these metals from a spent automotive catalyst. The conventional recovery method for these metals is solvent extraction. The advantages of this method are high metal selectivity and easy back-extraction. In contrast, the use of huge amount of organic solvent is a critical environmental issue. To solve this problem, we focused on the membrane separation method. A polymer inclusion membrane (PIM) is a promising alternative of solvent extraction, which spends less organic solvent and extractant.
In our previous study, it was found that trioctyl (dodecyl) phosphonium chloride (P88812Cl) has high selectivity for platinum, palladium and rhodium as an extractant in the solvent extraction method. In this study, we prepared a PIM containing P88812Cl as an effective PGM carrier. The PIM was fabricated with the composition of 50% PVDF-HFP as a base polymer, 30% P88812Cl as a carrier and 20% 2NPOE as a plasticizer to obtain a self-standing transparent membrane. Batch adsorption and desorption experiments for Rh(III) were carried out to examine the optimum operational conditions for the membrane transport. In the membrane transport trial, the selective transport of Rh(III) over Fe(III) was conducted by using an ammonium chloride solution as the receiving phase. As a result, 60% of Rh(III) was selectively transported to the receiving phase and Fe(III) was hardly transported.
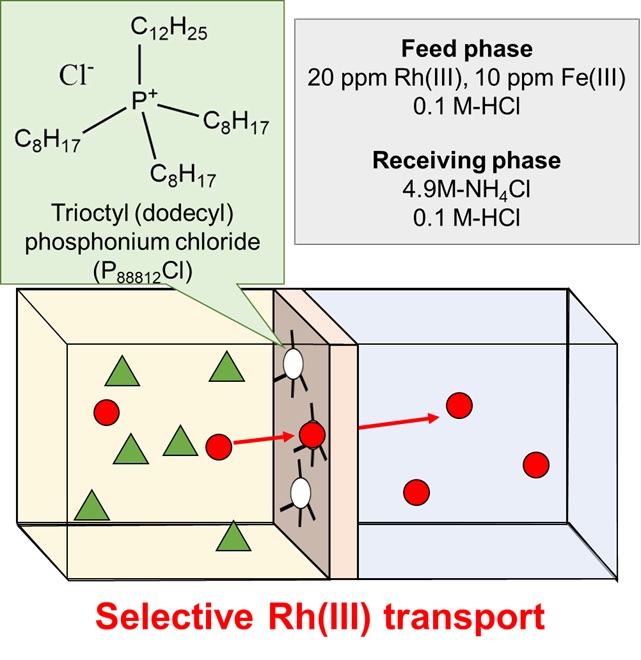
1.Introduction
Activated carbon is usually used as an adsorbent for general organic molecules in the water purifiers.However, the detachment properties of the activated carbon should be improved especially for the high temperature conditions.In this research, detachment properties of the zeolites were investigated. In order to suppress the detachment amounts, surface of the MFI zeolite particles was coated.
2. Experimental
The MFI precursor was crystallized from the parent gel of TPAOH: NaOH: TEOS: H2O: EtOH = 4.4: 0.1: 25: 756: 100 at 100 °C for 72 h.
The parent gel for MFI seed was prepared at a molar ratio of SiO2 : TPABr : NaOH : H2O : Al = 1.0: 0.1: 0.15: 50: 0.0 to 0.005. Colloidal silica was used as a silica source.After stirring at room temperature for 3 h with 1.0 g of the MFI precursor, hydro-thermal synthesis was carried out at 180 °C for 20 h in a 100 cc autoclave. The composition of the coating gel solution was a molar ratio of TPAOH: TEOS: Al: H2O =0.2:1:0.04:2501. The detachment properties were evaluated by a Thermal-weight analyzer.
3. Results and discussions
The effect of the coating on the added amount of MFI was examined. When the amount of MFI added to the coating solution was increased, the adsorption amount tended to increase. When the amount of MFI to be added was small, it was suggested that MFI crystals were dissolved during processing of the coating solution due to the effect of aluminum sulfate used as Al source.
4. Conclusion
MFI zeolite was coated by the MFI layer with low detachment properties. The amounts of seed crystals in the coating gel was important.
1.Introduction
Membrane separation is an important technique for saving energy. Heat and chemical resistance for liquid separation polymeric membranes should be improved to apply new application. Silica hybrid membrane have been developed by using a counter diffusion chemical vapor deposition (CVD) method. The pore sizes have been controlled by changing structure of silica precursor. The deposited membrane prepared by using Diphenyldimethoxysilane (DPhDMOS) as a silica precursor showed the H2SO4 rejection of 92%. In this study, the new silica precursor (3,3,3-Trifluoropropyltrimethoxysilane:TFPrTMOS) for the CVD was employed. The ion separation performances were evaluated for the silica membranes.
2.Experimental
A porous γ-alumina tube substrate was placed in a reactor. Deposition of TFPrTMOS and O3 was carried out for 60 min at 100~250 °C. O2 supplied at the inside of the substrate at 200 mL min-1. TFPrTMOS was supplied at the outside of the substrate by N2 bubbling at 200 mL min-1. Membrane performance after the deposition measured by liquid permeation test by using 100ppm of NaCl, Na2SO4, MgCl2, MgSO4 solution.
3.Results and Discussion
Fig.1 shows the relationship between rejection and diameter of the permeation molecules. Rejections of glucose were 60% and 33% through the TFPrTMOS and PrTMOS membranes, respectively. The neutral molecules such as glucose are separated by the molecular sieving mechanism. The difference of pore size can be explained by the higher reactivity of TFPrTMOS.
Rejections of ions were higher than those of the neutral molecules. The rejections of the divalent anions were higher than those of monovalent anions. This permeation mechanism should be based on the negatively charged for both PrTMOS and TFPrTMOS membranes. The negative charge for the TFPrTMOS membrane was lower than that for the PrTMOS membrane.The TFPrTMOS membrane showed higher glucose rejection of 60% with lower ion rejections.
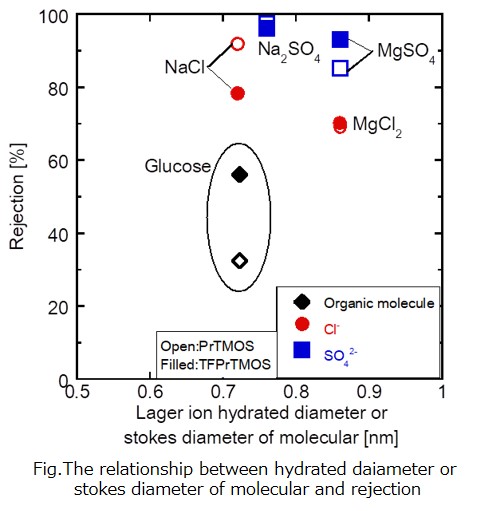
Metal-organic frameworks (MOFs) have been attracting attentions as materials for gas separation membranes. Mixed matrix membrane (MMM) is one of the methods to prepare composite membrane composed with polymer matrix and MOF particles as filler. In the case of stacking two-dimensional shape fillers horizontally in the plane of MMM, high gas selectivity can be obtained by reducing the permeation of non-permeable component. Zeolitic Imidazolate Frameworks-L (ZIF-L) is one of the two-dimensional MOFs formed by coordination bonding with zinc ion and 2-methylimidazole. In previous studies, the preparation of ZIF-L based MMMs and the increase of H2/CO2 selectivity were reported. The size of ZIF-L particles is expected to have influence on the gas permeation properties of MMMs. However, there have been no reports on the preparation and the gas permeation properties of MMMs using ZIF-L particles with different sizes.
In this study, the effect of the ZIF-L particle sizes on structures and gas permeation properties of MMM was investigated. First, ZIF-L particles with different sizes were prepared by changing the reaction time. Then, MMMs were prepared by suction-filtration of ZIF-L suspension with porous membrane support followed by complexation with polymer solution. Structural analyses of ZIF-L particles were performed using powder X-ray diffraction measurement and scanning electron microscope. Structure of the fabricated MMMs were analyzed using scanning electron microscope, and gas permeation properties are evaluated by single component gas permeation measurements using hydrogen, carbon dioxide, oxygen, nitrogen, and methane. The details will be reported in the presentation.
Silica membranes shows hydrogen permselectivity with thermal resistance and acid stability. Counter diffusion chemical vapor deposition (CVD) method is one of the method of preparing silica membranes where silica precursors and oxidants were supplied to the opposite side of the porous substrate. Membrane pore size can be controlled by changing structure of the precursor. We have been developing hydrocarbon selective or liquid separation membrane by using this technique. However, membrane deposition mechanism is not clear.
In this study, in situ gas analysis was conducted during CVD. Mass spectrometer was connected to the outlet of module of oxidant flow side. Membrane formation was evaluated by bubbling gas diffusion condition during CVD of each silica precursor.
A γ-alumina tubular support (φ:10 mm, L: 95 mm) was used as substrate. Deposition was conducted using silica precursor supplied by N2 bubbling to the outer side of substrate. Simultaneously, oxidant was supplied to the inner side of it and flow outlet was connected to the mass spectroscopy. Diffusion condition of bubbling gas was analyzed. Alkoxysilane tetramethoxysilane, tetraethoxysilane or alkyl alkoxysilane ethyltrimethoxysilane, butyltrimethoxysilane, hexyltrimethoxysilane were used as silica precursors. Deposition temperature was 270-600 °C, deposition period was 3-180 min.
The intensity of the molecular weight of 28 in mass spectrometer analysis should be N2 concentration. Diffusion amounts of N2 decreased by increasing the deposition periods. By changing silica precursor vapor concentration, membrane formation time was changed. The time was proportional to silica precursor concentration. Apparent activation energies were calculated from the decreasing rate of N2 diffusion. Activation energies of tetramethoxysilane and tetraethoxysilane were 40.9 kJ mol-1 and 74.1 kJ mol-1, respectively. The activation energies of alkylalkoxysilanes such as ethyltrimethoxysilane, butyltrimethoxysilane, hexyltrimethoxysilane were observed between 43-46 kJ mol-1 indicating the similar reaction mechanism for the tetramethoxysilane. Deposition reaction must be affected by the alkoxy group of silica precursor.
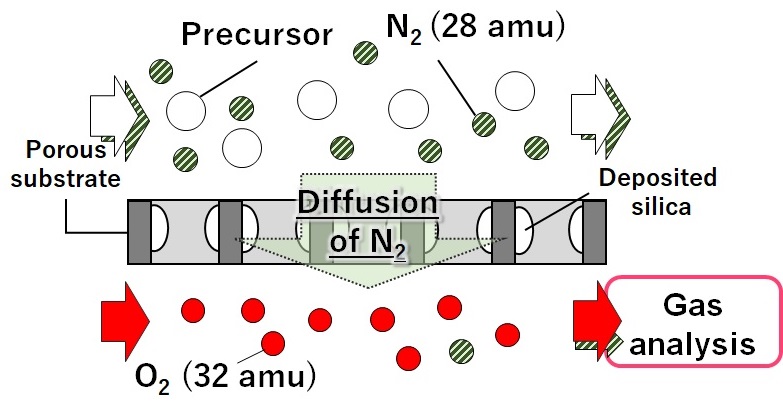
The sol-gel method was applied for the fabrication of amorphous silica membranes, which showed high H2 permselectivity. However, hydrothermal stability is not so high due to the condensation of high Si-OH density in amorphous structure. The incorporation of Si-F bounds in amorphous silica structure is an effective method to eliminate the Si-OH groups. In the present study, a molecular sieving membrane for H2 separation was fabricated using triethoxyfluorosilane (TEFS), which contains Si–F bonds and is categorized as a pendant-type alkoxysilane. The thermal and hydrothermal stability for TEFS membrane was evaluated. Tetraethoxysilane (TEOS) was used as a Si precursor to discuss the effect of Si precursor on hydrothermal stability and network pore size of silica membrane. TEFS membranes showed high H2 permeance (>1.0 × 10-6 mol m-2 s-1 Pa-1) and approximately the same pore size distribution, irrespective of calcination temperature (350, 550 and 750oC) due to the low Si-OH density in the amorphous structure, which could prevent the densification of network caused by the condensation of Si-OH groups during calcination process. TEOS membrane showed drastic decrease in H2 permeance of 80% and did not reach a steady-state even under mild steam condition (300oC, partial pressure of steam : 30 kPa). On the other hand TEFS membrane showed a decrease in H2 permeance less than 20% and reached a steady-state. In addition to this, TEFS membrane showed high H2 permeance (>1.0 × 10-6 mol m-2 s-1 Pa-1) and stable membrane performance even under severe steam condition (500oC, partial pressure of steam : 90 kPa). In conclusions, the hydrothermal stability of fluorine-SiO2 membrane was dramatically enhanced due to smaller number of Si-OH groups in the amorphous structure.
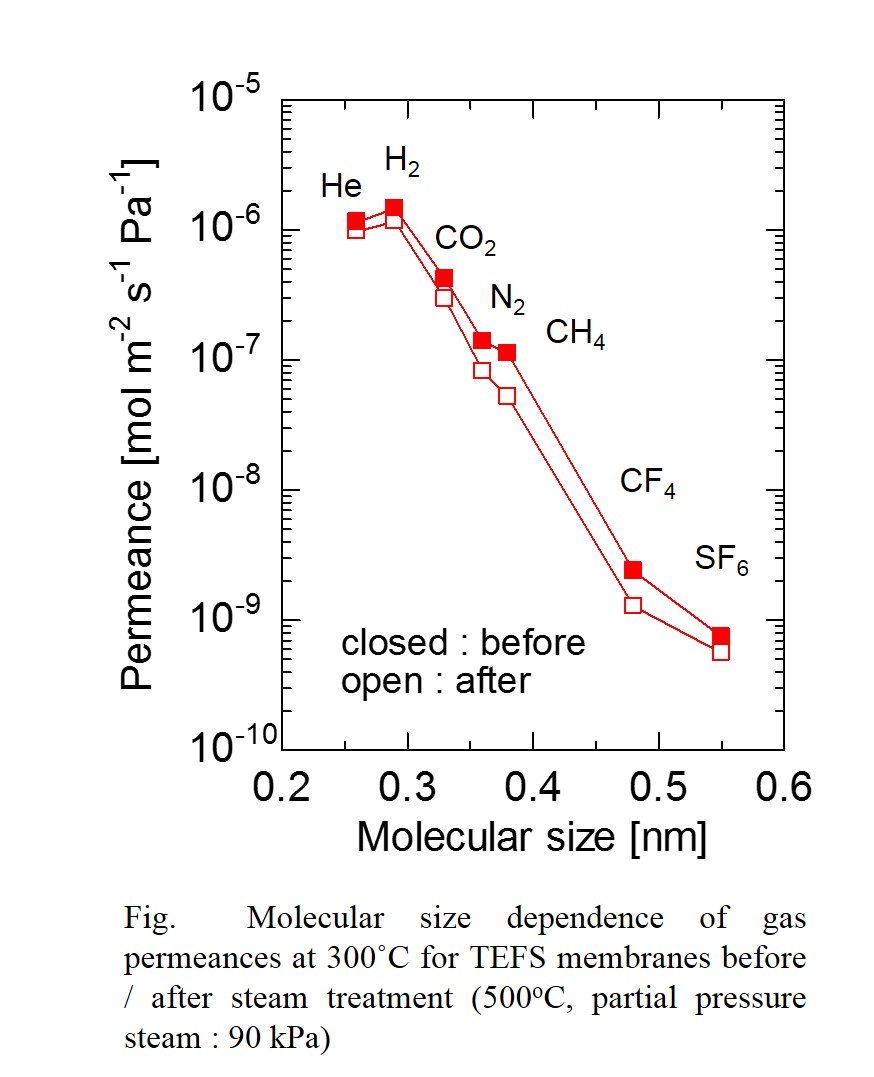
This study introduced a new synthetic route for fabrication of organosilica membranes on porous substrates by in-situ polymerization of liquid precursors initiated by atmospheric-pressure plasma jet. In this work, porous substrates were impregnated with 1,2-bis(triethoxysilyl)ethane (BTESE) as a monomer of organosilica, and then irradiated by the atmospheric-pressure plasma jet from upper surface of the substrates to polymerize the liquid monomers. Since reactive species generated in the plasma are supplied from the gas phase, the polymerization of liquid monomer can occur only at the gas-liquid interface. Therefore, it is expected that a very thin polymerized-layer can be obtained on substrates. Furthermore, in this process, not only volatile precursors but also non-volatile precursors can be used, which enables to control structure using various precursors. The chemical structure and the morphology of the resultant organosilica thin layer were evaluated by Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM), respectively. The gas permeation property was investigated using pure gases of different molecular sizes. After plasma irradiation, the polymerized layer with thickness of 200 nm was obtained on the outermost surface of the substrates, and all gas permeance decreased and permeance ratio increased. This result indicates that the polymerized layer is effective for gas separation. The permeance ratios for He/N2 and He/SF6 were found to be 25 and 1500, respectively.
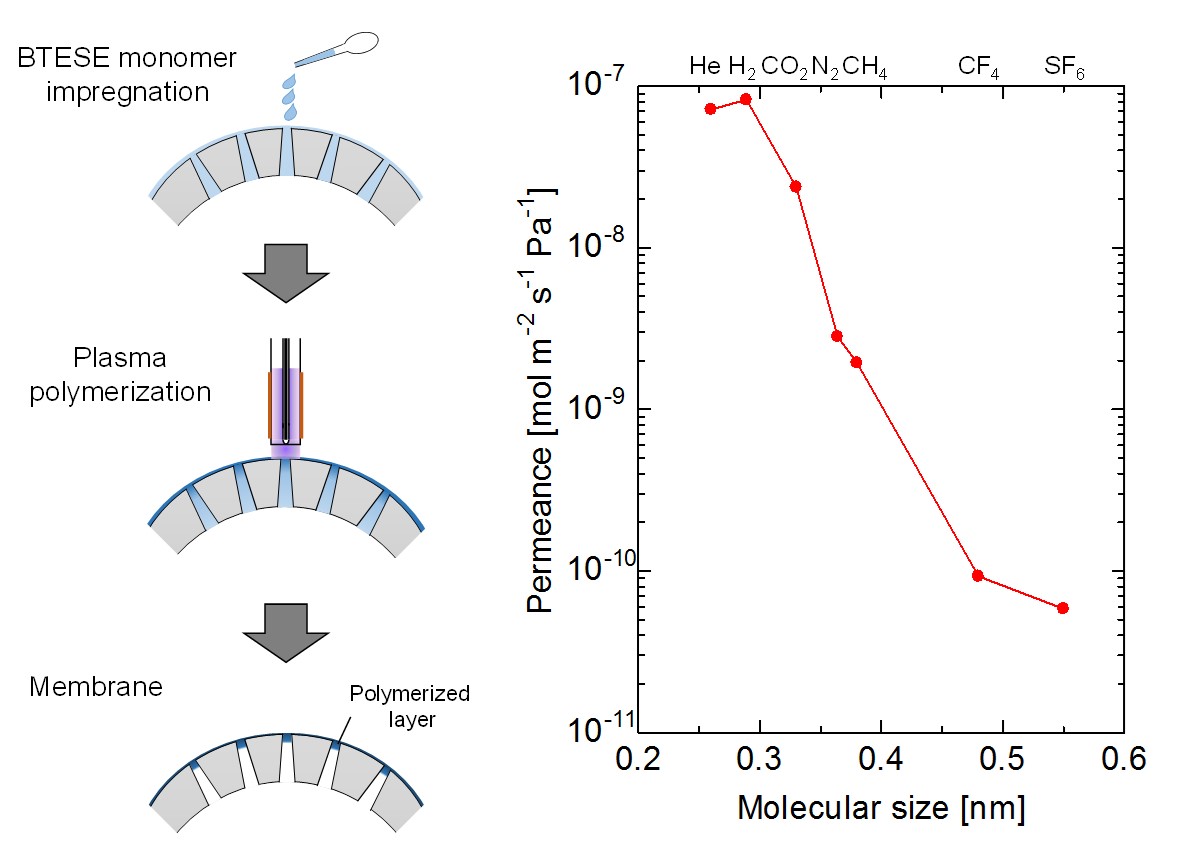
The Normalized Knudsen-based Permeance (NKP) method was useful for evaluation of sub-nano scale pore size in a range of 0.3-0.6 nm on microporous ceramic membranes for gas separation.1). However, the calculated pore size by the NKP method is affected by a small amount of larger pores or pinholes because this method can evaluate only average pore size. In this study, we evaluated relatively larger micropores around 1 nm based on the micropore filling phase permeation.2) Gas permeation properties thorough a micropore at temperatures below critical temperature, Tc were examined. Even if the bulk pressure is lower than a saturation pressure of the permeation gas, a potential field in a micropore would effectively work to compress the permeant and a liquid-like high density phase would be formed in pores. This phenomenon is ‘micropore filling phase permeation'. The formation of such a micropore filling phase leads to decrease the permeance. According to the Polanyi's adsorption potential theorem, the relation between the filling pressure pf and the potential energy EP is given by Eq. 1 (Fig. 1a). We calculated pore size dependence of EP and evaluated pore size distribution from pressure dependence of condensable gas permeance based on the Polanyi's adsorption potential theorem3). Several types of condensable gas species were employed as permeating and filling gases. Fig. 1b shows an example of pressure dependence of SF6 permeance. The pore size distribution was calculated from the slope of permeance as a function of pressure based on micropore filling model. These results suggested that large pores of 1.0-1.2 nm could be well evaluated by this method.
References
[1] T. Yoshioka, et al., AIChE J., 59, 2179 (2013), [2] T. Yoshioka, et al., J. Chem. Eng. Jpn., 46, 659 (2013), [3] T. Yoshioka, et al., Trans. Mater. Res. Soc. Jpn., 29, 3247 (2004).
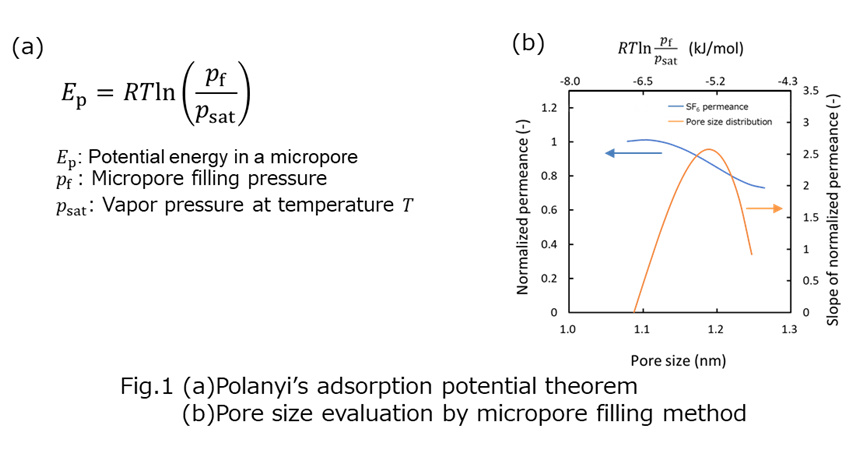
Currently, heavy metal contamination has become a severe environmental issue, because of the exponential increase in the use of heavy metal compounds in various industrial processes. Removal of toxic heavy metal ions has become a top priority in wastewater treatment. Various membrane-based technologies have been applied for this purpose and forward osmosis (FO) is the latest technology. The FO is more promising than others due to several advantages. In the present study, FO technology has been applied for removing chromium (Cr) from the water to elucidate the comparing the removal efficiency.
FO was conducted with under various Cr concentration in the feed solution for each experiment. NaCl solution (0.5 M – 2 M) was used as draw solution of FO. After the system was stabilized (~ 30 min), the samples were collected in 60 min intervals for 5 h from a 0.5 L reservoir of the feed and draw solution to measure the total Cr concentration.
The effect of pH on the removal efficiency of Cr(III) and Cr(VI), and water flux (Jw) was shown in Figure 1. Due to the change of the ionic forms, the water flux for Cr(VI) was slightly bell shape against pH.. The water flux for Cr(VI) was higher than that for Cr(III). Rejection of Cr(VI) was increased with increase in pH, because of electrostatic repulsion and large hydrated radii (0.461 nm) of Cr(VI). In the case of Cr(III), much higher rejection ca be obtained, because its hydrated radii is larger than that of Cr(VI).
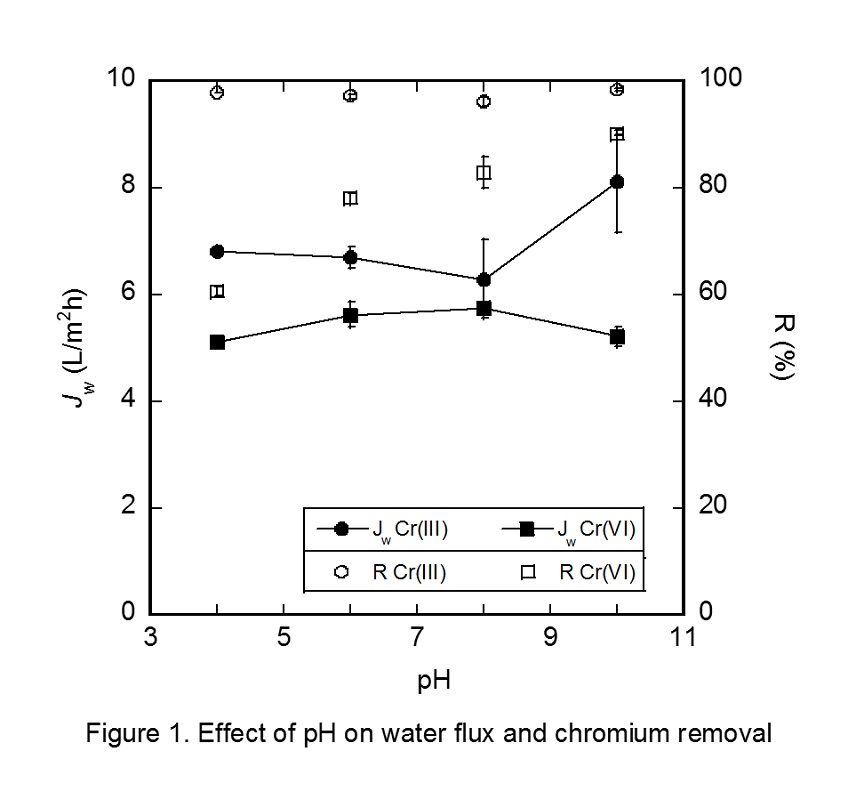
A new type of indium recovery method from acid leach solution of waste liquid crystal by using bipolar membrane electrodialysis (BPED) was developed. A chelating agent such as EDTA is added to an acid leach solution containing indium, tin, and aluminum ions, under which indium ions selectively form a chelate complex. The solution is then introduced to BPED system; only indium-EDTA complexes, negatively charged, are transported towards the anode side, while other metal cations are transported towards the cathode side. We examined the effects of operation conditions of BPED on the indium separation performances from a model acid leach solution, to clarify optimum operation conditions.
A laboratory-scale BPED setup used is a stack of five three-cell units composed of two bipolar membranes, an anion exchange membrane, and a cation exchange membrane. A model acid leach solution containing In(NO3)3 (0.02 M) and Al(NO3)3 (0.02 M) with EDTA was supplied to the feed cell and circulated. HNO3 (0.01 M) was circulated through the recovery cells of anions and cations, respectively. The effects initial pH, initial [EDTA]0/[In]0 ratio of the feed solution, and the voltage on the indium recovery performances, recovery ratio and selectivity, were examined.
For the constant [EDTA]0/[In]0 = 1 and the voltage at 20 V, the highest selectivity and the highest recovery ratio were obtained when the initial pH was about 2.5. Under the constant voltage at 20 V and the initial pH at 2.5, the recovery ratio increased with increasing the [EDTA]0/[In]0 ratio up to 2, but the selectivity was highest for [EDTA]0/[In]0 = 1. For the constant [EDTA]0/[In]0 = 1 and the initial pH at 2.5, the higher voltage resulted in the higher selectivity and recovery ratio up to 30 V. However, the specific power consumption for indium recovery was lowest for the voltage at 5 V.
Two-dimensional (2D) stacked nanosheet membranes have been expected as next generation membrane because of the ultrathin thickness and unique nanochannel. These membranes are formed by assembling single molecular sheets on support membrane by vacuum filtration etc. . Graphene oxide (GO) is one of the 2D material with specific property including good mechanical strength and surface charge. Therefore, GO is mostly used as nanosheet membranes and the fabricated GO membranes show high rejection for anionic molecules. However the instability of GO membrane in water is serious problem for their application. Recently, we have developed stable stacked nanosheet membrane using niobium oxide (NbO) nanosheets. The stacked NbO membranes had a dense structure formed by the chemical cross-linking between nanosheets and showed high stability during water filtration test. In this study, the composite membranes including two different layered structure utilizing NbO and GO nanosheets were fabricated to improve the performance for nanofiltration membrane. The weight ratio of NbO to GO (NbO / GO) was 55/45. The membrane having GO layer on NbO layer (Layered type) showed over 90% rejection of Na2SO4. That value was about 2 times higher rejection compared with that for NbO membranes with similar water permeability (Fig.1). This composite membrane also showed higher rejection of Na2SO4 than GO membranes with cross-linking agent. In additions, this membrane also showed higher rejection of NaCl than NbO and GO membranes. The differences of surface charge and structure of nanochannel between these membranes is discussed.
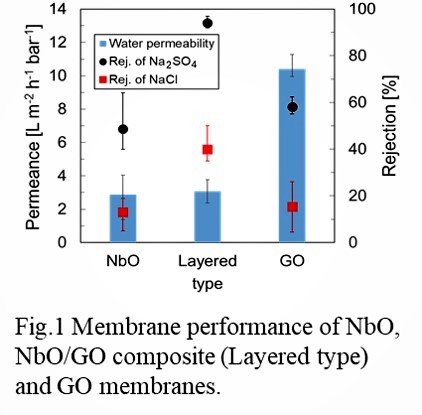
Organic solvent nanofiltration (OSN) is one of the emerging separation technologies in extensive processes for the purification or separation of organic solvents. However, OSN membranes are rarely applied in many chemical processes, because OSN membranes have poor stability and permeability in a variety of strong organic solvents. In order to overcome these drawbacks, OSN membranes were fabricated by thermally induced phase separation (TIPS) method using polyamide 6 (PA 6). OSN membranes are expected to be used in many organic solvents. Therefore, the relation between solvent property and filtration performance was investigated using various solvents. The result showed correlation between permeability and solvent property such as viscosity, absorbed amount in PA 6 (Fig.1). In conclusion, the permeability performance in PA 6 membrane could be estimated before filtration.
Metal organic frameworks (MOFs) have attracted attention as novel materials for catalysis, adsorption and membrane separation. Zr-based UiO-66 families are known as MOFs with remarkably high thermal and chemical stability. UiO-66-(COOH)2 consisting of pyromellitic acid (BTeC) and Zr cluster was predicted to be a suitable material for CO2 separation. However, compared to UiO-66 using telephthalic acid as organic linker, the extra two carboxyl groups of BTeC also form coordination bonds, inhibiting to form framework properly. This would make successful synthesis of UiO-66-(COOH)2 with high crystallinity difficult. Thus, no publication has reported successful synthesis of UiO-66-(COOH)2 membrane so far. Here, we investigated a preparation method of UiO-66-(COOH)2 membrane and evaluated its gas permeation property.
UiO-66-(COOH)2 membrane was prepared on a porous α-alumina support by secondary growth method. The support was immersed in a Zr source solution, and then UiO-66-(COOH)2 seed crystals were prepared on the surface of the support by solvothermal synthesis. The seed crystals were secondarily grown to a membrane under solvothermal condition. The gas permeation property of the membrane was evaluated by single-gas-permeation test.
Monocarboxylic acids such as acetic acid (AcOH) can compete with organic linkers and produce crystal growth in a preferred direction, because they have only one coordination site. Thus, the effect of AcOH as a solvent on the membrane preparation was investigated, and as a result, a continuous UiO-66-(COOH)2 membrane could be successfully synthesized as shown in Figure. From the single-gas-permeation test at 293 K, ideal separation factors of H2/N2 and CO2/N2 were found to be 4.41 and 0.93, respectively. The higher ideal separation factor of H2/N2 than the theoretical value for Knudsen diffusion control implies the membrane had few defects in the separation function layer. On the other hand, the ideal separation factor of CO2/N2 indicates this membrane was slightly CO2 selective.
Silicon carbide (SiC)-based membranes were by coating allylhydoridopolycarbosilane (AHPCS) as a precursor of the separation layer, followed by sintering at 300-800 °C. SiC-based membranes showed molecular sieving performances for gas separation. A membrane pyrolyzed at 700 °C showed high permeance and permeance ratio, as H2 permeance of 3.3 × 10-6 mol / (m2 s Pa) and H2/SF6 permeance ratio of >10000. On the other hand, SiC-membranes pyrolyzed at 800 °C showed lower permenace and permeance ratio, due to densification of SiC porous networks. In addition, we found that SiC-based membranes pyrolyzed at high temperature showed high thermal stability and oxidation resistance. This is probably because by pyrolysis at high temperature, hydrosilyl group (Si-H) formed silicon-carbon group (Si-C) and Si-Si bonds which have thermal stability and oxidation resistance.
In recent years, forward osmosis (FO) process is attracting research attention to overcome a global water shortage. FO is a remarkable membrane separation technology utilizing an osmotic pressure gradient between solutions across a semipermeable membrane. FO processes have great advantages of lower energy consumption and less membrane fouling compared with pressure-driven membrane processes such as reverse osmosis (RO). In FO processes, it is important to develop a superior draw solution with high osmotic pressure, easy regeneration and low solute leakage. Our research group has been searching for better materials as a draw solute to put FO process into practical use in a few years. In this study, block co-oligomers with different numbers of branches were developed as a novel thermoresponsive draw solute for FO (Figure).
A series of the block co-oligomers were systematically designed and synthesized. The average degrees of polymerization of ethylene oxide and butylene oxide units of the block co-oligomers were varied to control the hydrophilic/hydrophobic balance of the molecules. The numbers of branch of the block co-oligomer was also controlled by changing the center molecule. Most of them showed lower critical solution temperature type phase separation at temperatures below 60 °C.
Aqueous solutions of the block co-oligomers were evaluated in terms of their phase diagrams, osmotic pressures and viscosities. The osmotic pressure of the dense phase the block co-oligomers were high enough to draw water from 0.6 M NaCl (model seawater) with high flux. Moreover, the leakage of branched block co-oligomers were much lower than that of conventional draw solutes due to the steric molecular structure. Meanwhile, the osmotic pressure of the dilute phase of the block co-oligomers solution were very low and reduced energy consumption during post-processing by low-pressure RO to collect pure water.
Organic chemical hydrides such as cyclohexane, methylcyclohexane, and decaline are promising hydrogen storage transport and production materials. Their dehydrogenation reactions have been extensively investigated to produce highly pure hydrogen supplied for polymer electrolyte fuel cells. Highly efficient hydrogen purification systems are thus required, and membrane separation technology is expected as an energy conservation technology in hydrogen purification from such hydrides. Carbon membranes are one of the strong candidates applied for these separations due to the good gas separation performance and the high chemical resistance. We have investigated carbon hollow fiber membranes based on poly(phenylene oxide) (PPO) and its derivatives as a novel polymeric precursors. Recently, the development of flexible carbon hollow fiber membranes was successfully achieved using sulfonated PPO (SPPO) as a precursor polymer.
In this study, the separation of hydrogen and toluene converted from methylcyclohexane as the organic chemical hydride was investigated using SPPO carbon hollow fiber membranes. Highly hydrogen selective carbon membranes were prepared by using one-step chemical vapor deposition (CVD) method. In addition, energy requirement for ultrapure hydrogen purification process by means of carbon membranes was evaluated and compared with that of pressure swing adsorption (PSA) method.
Removal of heavy metal ions in environmental water is an important issue because of its high bioaccumulation potential and high toxicity. The insolubilized alginate membrane was obtained by drying the soluble alginate solution, followed by crosslinking with calcium ion. The alginate membrane had smooth surface and applicable mechanical strength for practical use. Isothermal ion-exchange adsorption of Cu2+ in batch process was conducted on alginate membranes obtained. The result was consistent with the Langmuir type isotherm, and the Cu2+ ion-exchange capacities were estimated by fitting of the experimental data. It was found that the ion-exchange capacity of the alginate membrane was equivalent to alginate gel beads. For an improvement of water permeability, polyethylene glycol was added in the casting solution before drying process, and then, was removed from the prepared membrane. The permeation flux of the membrane was determined from the amount of pure water permeated under pressure and its value increased with the amount of polyethylene glycol added in the casting solution. An in-line removal experiment using the prepared membranes was also attempted and succeeded in removing Cu2+ ion under flowing condition. In addition, novel calcium alginate membrane embedded with zeolite as an ion-exchange material was successfully prepared for heavy metal ions removal from aqueous phase. The zeolite-embedded membranes were also subjected to ion-exchange tests in a batch process, because of its applicable mechanical strength. As a result, ion-exchange capacities of membrane were increased compared to the alginate membranes mentioned above, though ion-exchange of zeolite contained in the membrane was slightly inhibited by inclusion in the membrane. The biocompatibility of alginate is widely confirmed in vitro as well as in vitro. Therefore, the membrane is expected to behave well in various water purification processes, particularly for water resources and drinking products.
Ceramic membranes have the advantage of high mechanical strength, chemical resistance and thermal resistance. Development and many research works of silica (SiO2) membranes, titania (TiO2) membranes and zirconia (ZrO2) membranes have been conducted [1,2]. Amorphous SiO2 membranes can have suitably controlled pore size for gas separation. However, it has been reported that SiO2 was dissolved and densified by being exposed to water, resulting in degradation of membrane performance. On the other hand, ceramic materials such as TiO2 and ZrO2 have excellent resistance in water , and have been put to practical use for various waste liquid treatments as ultrafiltraton (UF) membranes and nanofiltration (NF) membranes. However, since these are crystalline materials, it is difficult to control fine pore size for gas separation.
In this study, in order to control pore size, we focused on Gallate-based organic chelate ligands having ester groups of various lengths in the side chain of the benzene ring in the TiO2-ZrO2 material. In particular, we aimed at more precise pore size control of TiO2-ZrO2 composite membrane by using Methyl Gallate (MG) with the shortest side chain among them. TiO2-ZrO2 colloidal sols whose colloid size was well controlled to be nano-scale were prepared. The sol was coated on α-alumina support and fired to obtain TiO2-ZrO2 ceramic membranes. TiO2-ZrO2-MG composite membranes were prepared, and the permeation characteristics of various gases were evaluated using a pressurized gas permeation apparatus. As the results, end of the ester group of MG was to be decomposed with increasing temperature. Differences in gas permeance of TiO2-ZrO2-MG membranes with varying porous structure were observed.(Fig.)
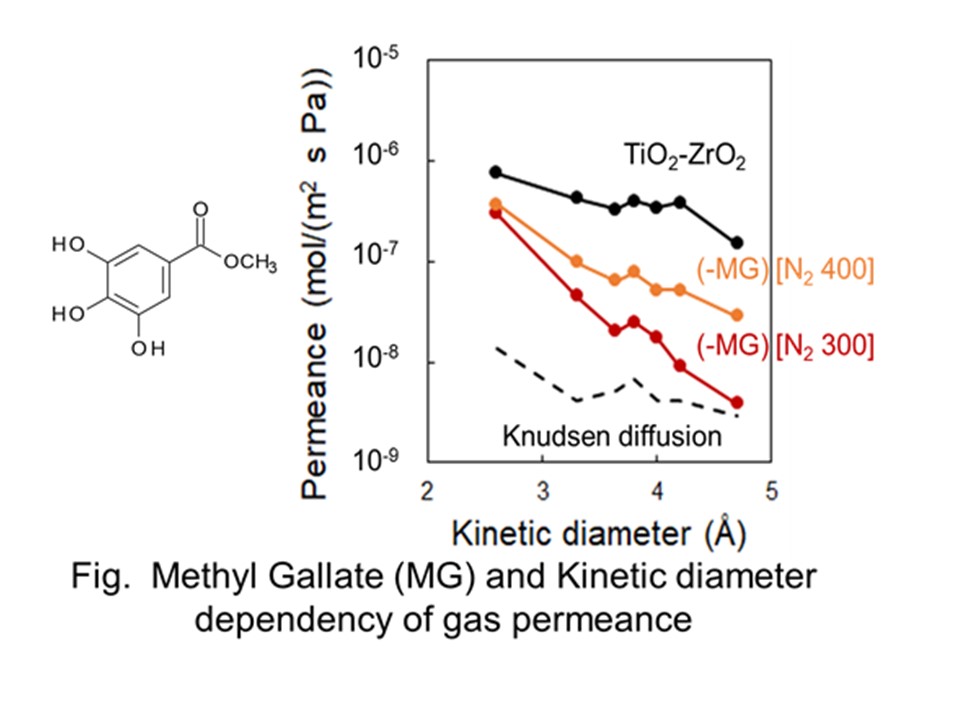
High silica Y-type zeolite (USY) are industrially used as catalysts for fluid catalytic cracking (FCC) of heavy gas oil. Products are generally gasoline fractions through a series of reactions such as cracking and isomerization, and recently the severity of decomposition has often been increased to increase the propylene yield. In comparison with conventional catalytic reactions, the contact time can be shortened by using catalyst membrane where reaction occurs in the course of the permeation of feed through a membrane. Thus, one can anticipate to improve selectivities to intermediate products during catalytic cracking with catalytic membrane reactors. In this study, we investigated synthesis of high silica Y-type zeolite membrane and its catalytic performance for n-hexadecane cracking.
Y-type zeolite membrane was synthesized on a tubular porous α-alumina support by a secondary growth method using seed crystals. Thereafter, the resulting Y-type membrane was subjected to be dealuminated by combining steaming and EDTA treatment. Dealuminated treatment was performed by steaming at 300°C for 30 min and successive EDTA treatment in an ice bath for 30 min. This treatment was repeated multiple times. Pt was loaded on Y-type membrane by an ion exchange method to obtain Pt-Y membrane. Cracking was carried out at 573 K through Pt-Y membrane for a mixture of H2 and n-hexadecane (9:1).The dealuminated treatment increased Si/Al ratio of Y-type membrane from 1.86 to 2.24. Table 1 shows the catalytic test result using n-hexadecane through Pt-Y membrane. Interestingly, C6 spices were only obtained with a high selectivity above 99% in the liquid component at 48.9% of the level of conversion of n-hexadecane. A trace amount of C4 spaces were observed in the gas product.
It was suggested that excess decomposition was suppressed when the contact time was shortened by using catalytic membrane.
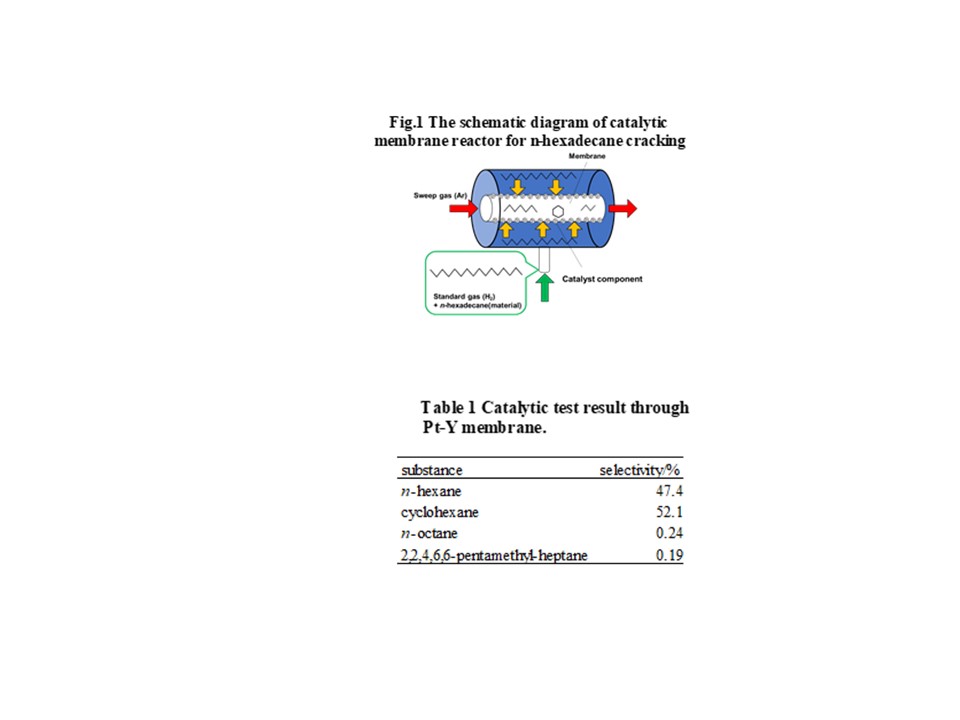
Global demand of platinum group metals (PGMs) has been continuously increased year by year, however, the resources are strictly limited. To address this issue, recycling of end-of-life products was often carried out in industrial processes, but the separation among PGMs is one of the difficult challenges. Metal recycling requires a separation process to ensure high recovery and purity. Membrane transport is one of the most effective methods for metal separation. This technique may provide a cheaper and more environmentally benign process compare to the conventional hydrometallurgy process i.e. solvent extraction. In this work, we show a successful separation of palladium(II) and rhodium(III) in a chloride solution using a polymer inclusion membrane (PIM) transport system. The PIM consists of three components, which are poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-co-HFP) as a polymer base, 2-Nitrophenyloctyl ether (2NPOE) as a plasticizer and an ionic liquid namely trioctyl(dodecyl) phosphonium chloride (P88812Cl) as a carrier, where P88812Cl was recently developed as an extraction solvent for PGMs. The performance of separation and recovery of the metals was examined with a transport apparatus. The effects of PIM composition and experimental conditions on the separation efficiency were systematically investigated. By properly adjusting the HCl concentration in the feed solution and the concentration of thiourea used for Pd(II) stripping in the receiving solution, separation of Pd(II) from Rh(III) with 98% recovery and 99% purity was achieved. Membrane stability is essential to the success of membrane separation. We demonstrate the high stability of the PIM by 7-cycles of membrane transport experiments.
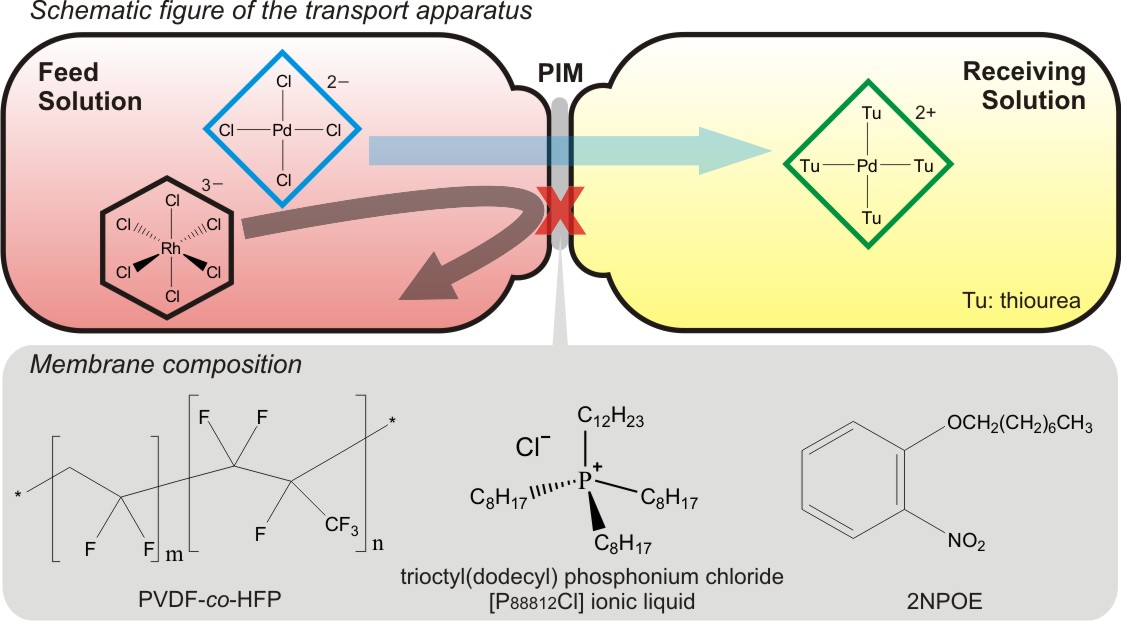
In recent years, the use of bio-alcohol, which is one of the renewable energy sources, has been recommended as an approach to depletion of fossil resources and global warming. However, the concentration of bio-alcohol produced by fermentation is too low, and concentration and dehydration are required to use it for industrial application. Moreover, since the separation process by distillation currently used requires a large amount of heat energy, energy saving is needed.
The membrane separation technique has attractive attention as an energy-saving separation technology to replace distillation. Membranes can selectively permeate the target molecular, and the separation properties are controlled by preparation material. Recently, zeolite has attracted attention as a membrane material because of the characteristic properties; uniform pores at the molecular level and adsorption property controlled by the composition of the framework component (Si/Al ratio). Therefore by using a hydrophobic zeolite composed of pure silica (Si/Al=∞) as the membrane material, it is possible to develop a separation membrane that selectively recovers only hydrophobic molecules such as bio-alcohol.
However, the permeability of existing hydrophobic zeolite membranes, MFI-type zeolite membranes, is generally low. Therefore, many membranes need to be used in order to gain throughput, which leads to high separation costs. Since the zeolite membrane separates through the pores of zeolite, its permeability is strongly dependent on the pore size. So, we focused attention on *BEA-type zeolite with larger pore size than MFI-type zeolite and the influence of synthesis conditions was investigated in detail. As the result, a pure silica *BEA-type zeolite membrane were successfully prepared, and it exhibits PV performance with a separation factor of 229 and flux of 0.62 kg m-2 h-1 for 1.0 wt.% n-butanol/water mixtures at 318 K, and is higher than that using pure silica MFI-type zeolite membrane.
Blend membranes of poly (vinyl chloride) (PVC) and poly (vinyl alcohol) (PVA) were prepared, and applied to the separation of liquid mixtures of ethyl acetate and toluene. A solution of PVC and PVA in N, N-dimethylacetamide was casted onto a glass plate, and dried under vacuum to obtain a dry membrane (thickness ~ 100 μm). The weight ratio of the polymers in the casting solution was fixed at 8 wt%, and the ratio of PVC to PVA was changed. The membranes were applied to the pervaporation (PV) experiments; the feed solution was an equal-weight (50/50) mixture of ethyl acetate and toluene, which are major volatile organic compounds (VOCs) used in printing industries. The PV was conducted at room temperature, and the downstream side was kept vacuum (~ 5 mbar). Almost no flux was obtained for the pure PVA membrane, while the pure PVC membrane was dissolved in the feed solution. With increasing the PVC ratio in the PVC/PVA blend membrane, the flux increased as shown in Fig. 1. The PVC/PVA blend membranes were ethyl acetate selective, and the weight fraction of ethyl acetate in the permeate increased with increasing PVC ratio. However, the PVC/PVA blend membranes with the PVC ratio higher than 40% were dissolved in the feed solution. The flux and the weight fraction of ethyl acetate in the permeate for the PVC/PVA blend membrane (PVC/PVA = 40/60) were, 142.5 g h-1 m-2, and 70%. According to the Hansen's solubility parameters, the distance between PVC and toluene (7.4) is larger than that between PVC and ethyl acetate (4.9). The distance between PVA and toluene is also larger (18.2) than that of PVA and ethyl acetate (12.0). This is consistent with PVC/PVA blend membranes being ethyl acetate selective, and the increase in the flux with increasing the PVC ratio in the PVC/PVA blend membranes.
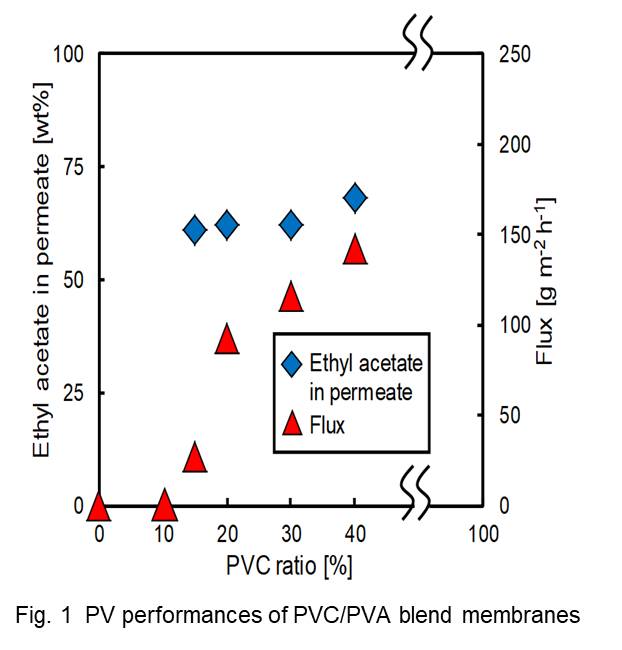
Membrane separation of organic solvents is expected as a low energy cost separation. Currently, polymer membranes are widely used for water treatment, and have the advantages of being inexpensive and easy to fabricate. However, polymer membranes have the possibility of dissolution and swelling in organic solvent conditions, so it is necessary to study the improvement of organic solvent resistance. We reported polyamide (PA) thin film composite (TFC) membrane using polyketone (PK) support membrane with organic solvent resistance. However, this support membrane is applied to the forward osmosis (FO) membrane method, and it is also necessary to improve the pressure resistance in order to use it in the reverse osmosis (RO) membrane method. The pressure resistance improvement was achieved by controlling the preparation conditions of the PK support membrane and combining the non-woven fabric. Further, the PA-TFC membrane was fabricated by forming a PA active layer by an interfacial polymerization on this non-woven composite PK support membrane. The membrane performance of water permeability and NaCl rejection was evaluated by RO test. This membrane had commercial RO membrane level performance and pressure resistance up to 30 bar. In addition, the permeability of each of the following pure solvents was evaluated by RO test (Fig. 1) : water, methanol (MeOH) , ethanol (EtOH) , 1-propanol, 2-propanol, N-Methyl-2-pyrrolidone (NMP) , and dimethyl sulfoxide (DMSO) . 1-propanol and 2-propanol did not permeate through the PA-TFC membrane. Also, the permeability was not correlated with the reciprocal of the viscosity (1/η) .
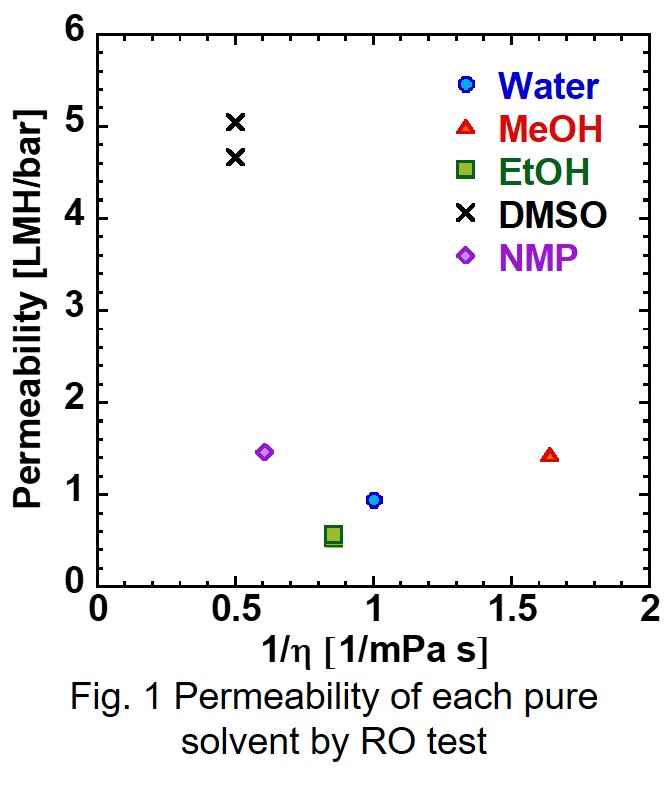
Recently we developed novel facilitated transport membranes (FTMs) with metal-containing ionic liquids (MCILs) as an O2 carrier. The FTM had higher O2 permeability than conventional facilitated O2 transport membranes. However, the O2/N2 selectivity of the MCILs-based FTMs were still low. In this research, we tried to propose a guideline to improve the O2/N2 selectivity of the FTMs. We synthesized several MCILs and prepared the supported ionic liquid membranes (SILMs). The O2 and N2 permeabilities of the SILMs were measured and compared with those estimated from the gas absorption amounts and the diffusion coefficients of the gases and O2 complexes in the MCILs (Figure). The diffusion coefficients were determined by Wilke-Chang equation, which is useful to estimate diffusion coefficient of solutes in many kinds of solvents. As shown in Figure, the experimentally determined permeabilities of O2 and N2 were much higher than the estimated ones. These results suggested that the diffusion mechanism of a solute in the MCILs were different from that in a conventional solvent. To clarify the diffusion mechanism of solutes in the MCILs, we analyzed the diffusion coefficients according to free volume theory, which is useful to describe the diffusion phenomenon of a solute in polymer materials. As the result, based on the free volume theory, it was found that the diffusion coefficients of the gas molecules and the O2 complexes were depended on the free volume of the MCILs. This means, even though the MCILs were liquid, they acted as a solid because of their high viscosity (20000 – 90000 cp). To improve the O2/N2 selectivity, switching the diffusion mechanism of gases and the O2 complex from that in a solid-like MCIL to that in a liquid MCIL, which could be achieved by decreasing the viscosity of the MCILs, could be effective.
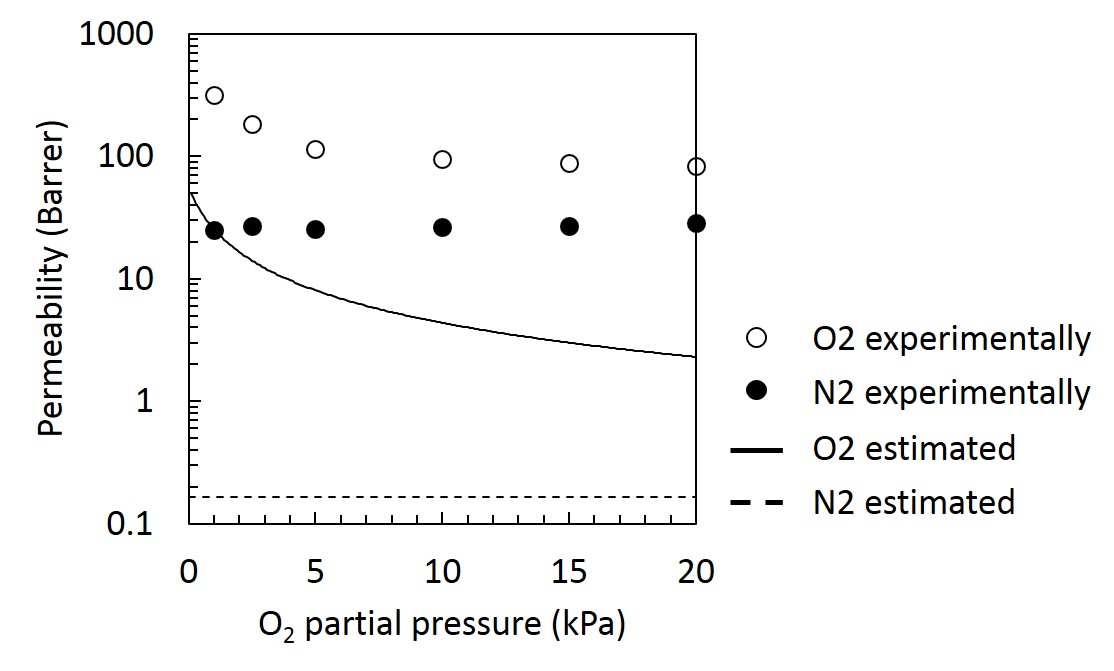
Membrane separation of organic solvents is attracting attention as an energy-saving process. Therefore a high-permeability nanofiltration membrane for demonstration is required1). However, the permeation phenomena of liquids in nanopores cannot be correctly predicted by the Hagen-Poeisuille law, and the permeability decreases as decreasing pore size2). This is thought to be due to the strong influence of pore wall potential and intermolecular interactions on the permeating molecules in the nanospace 3,4). In this work, the influence of molecular properties such as shape, size and interaction with pore walls on diffusion and permeation phenomena was analyzed using molecular dynamics (MD) method.
The interaction between the membrane material and solvent molecules was studied by utilizing a commercial software (BIOVIA Materials Studio 2018). Pores were formed in the crystalline structure of TiO2, and the pore surface was modified with -OH groups. The solvent molecules were filled in the pores, and the diffusivity was calculated. (Fig. 1). It was shown that methanol interacts with the pore wall more strongly than hexane, resulting in limited diffusivity of methanol. We have also adopted the Lennard-Jones potential and the Coulombic interaction, for modeling solvent molecules in nanopores. The interaction parameters were decided to express actual physical properties reported in literature. Non-equilibrium MD simulations of liquid permeation through nanopores on the above-mentioned TiO2 membrane structure were conducted as shown in Fig. 2. The transport behaviors of liquid-stated organic solvents were studied by the fluctuating wall MD technique2).
1) R. P. Lively et al., Nature Materials., 16, 276-279 (2017).
2) H. Takaba et al., J. Chem. Phys., 127, 054703 (2007).
3) J. A. Thomas et al., Inter. J. Therm. Sci., 49, 281-289 (2012).
4) T. Yoshioka et al., Sep. Purif. Technol., 220, 259-267 (2019).
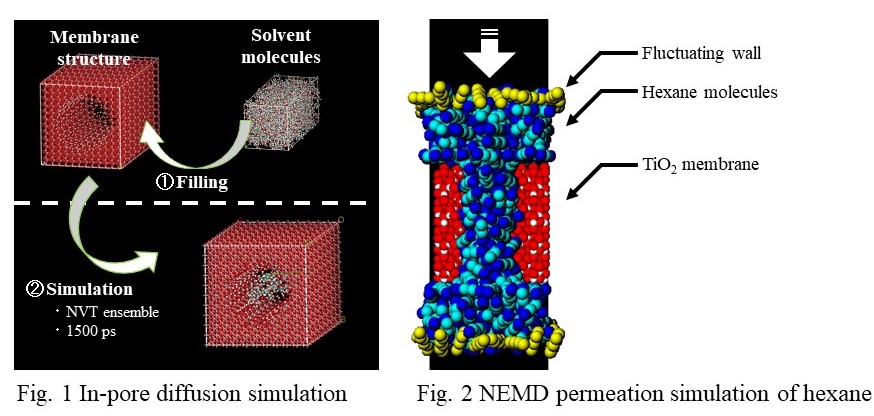
Silicalite-1 zeolite membranes have attracted great interest owing to their hydrophobicity, uniform pore structure, and high thermal and chemical stability, and thus have significant potential for use in the separation organic molecules from organic/water mixtures. Silicalite-1 membranes are generally obtained by hydrothermal synthesis (HT), the synthetic gel is prepared and immersing the seeded support in this gel to synthesize the membrane. Thus, although HT is an established method for the synthesis of zeolite membranes, it involves the use and subsequent treatment of large amounts of the synthetic gel, which is an alkali-mixed solution containing an expensive SDA.
Here, we report the preparation of silicalite-1 membranes on a silica-particle-coated tubular silica support using a gel-free steam-assisted conversion (SAC) method. The effects of the silica-particle layer formed on the top surface of the silica support and the physical properties of the silica particles themselves on the membrane-formation process were investigated. The silica particles coated served as the additional silica source for growing the silicalite-1 seed crystal layer into the silicalite-1 membrane. As a result, it was possible to form a dense and continuous membrane even under gel-free conditions. Furthermore, it was found that the properties of the silica particles, such as their primary particle diameter, had a determining effect on their solubility during the steam treatment, that is, on the supply rate of the silica source. The silicalite-1 membrane obtained using the spherical-silica-particle-coated support had an approximately 9-μm-thick separation layer and showed very high pervaporation performance, exhibiting a separation factor of 105 and flux of 3.72 kg m–2 h–1 for a 10 wt% ethanol/water mixture at 323 K. Thus, the gel-free SAC method can be used with a silica support coated with silica particles to readily prepare high-performance membranes without producing any chemical waste.
A molecularly imprinted polymer (MIP) is a polymer that has been processed using the molecular imprinting technique which leaves cavities in the polymer matrix with an affinity for a chosen "template" molecule. Permeability of a MIP film is sensitive to the specific binding with the template. This phenomenon called “gate effect” is applicable for biosensors. However, the mechanism of morphological change of MIP due to the specific binding is still unknown. A molecularly imprinted self-supporting membrane (MISSM) was developed for analysis the change in the internal structure and the permeability by the specific interaction with the template. The MISSM was prepared by copolymerization of mixed crosslinkers of triethyleneglycol dimethacrylate (TEDMA) and polyethyleneglycol diacrylate (PEDA) and mixed functional monomers of 2-vinylpridine (2-VPy) and methacrylic acid (MAA) in the presence of D-phenylalanine as a template with thickness 50 μm. (The membrane was defined as D-Phe imprinted membrane (DIM)).
DIM (TEDMA: PEDA=215:10 in molar ratio) was impregnated with fluorescein-labeled polyethyleneglycol (MW: 2,000), and the fluorescence intensity distribution in the depth direction of DIM (TEDMA: PEDA=215:10) was observed by confocal laser microscopy. The fluorescent intensity at the dense domain in the bulk of the membrane was increased to 1.5 times by the addition of the template. Those results indicate that the specific interaction with the template increases the porosity of the dense domain of the membrane.
The methanol aqueous solution was filtrated across the membrane in order to evaluate the convectional permeability. The filtration rate of DIM (TEDMA: PEDA=215:10) was increased to 3.4 times by the addition of the template, but rather was decreased by addition of the enantiomer.
As a conclusion, the blending ratio of the two crosslinkers contributes to the gate effect where the internal porosity of the dense domain and diffusive and convectional permeability of the molecularly imprinted membrane.
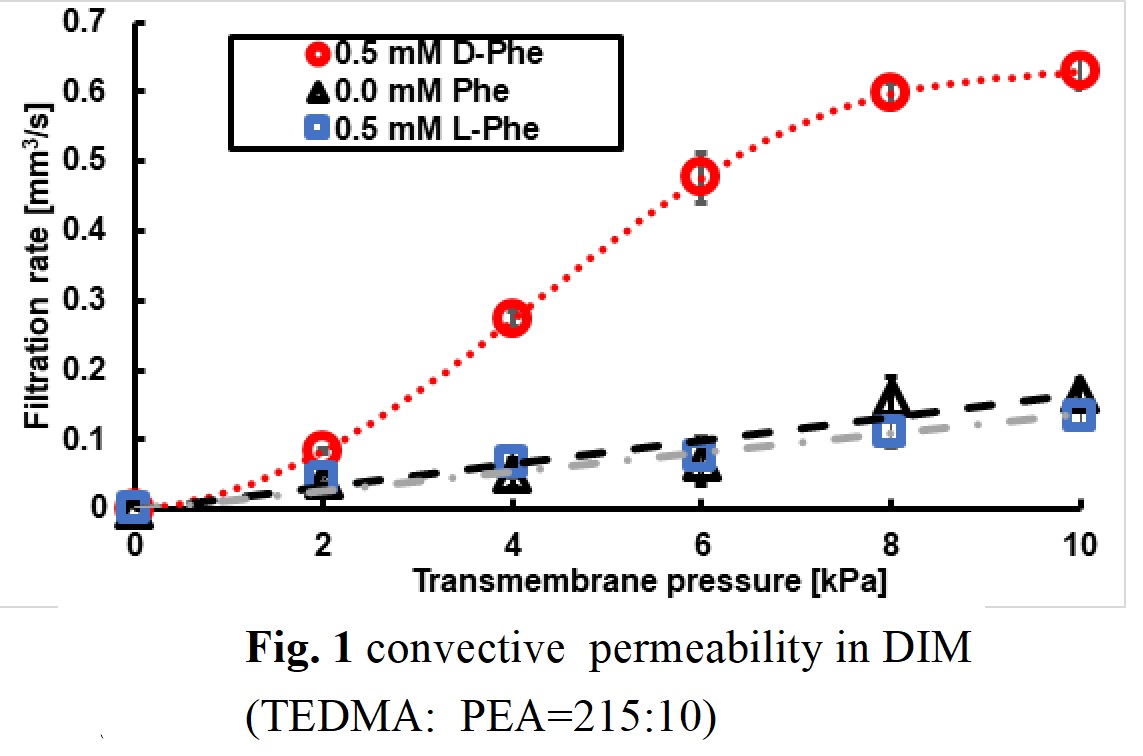
Forward osmosis (FO) process has received much attention because of their versatile potential application in water treatment. The development of FO membranes with both high pressure resistance as well as high water flux has been required because FO membranes can also be utilized under hydraulic pressurized conditions such as pressure-retarded osmosis (PRO). Recently, we have prepared aliphatic polyketone (PK) based polyamide thin-film composite (TFC) membranes and the potential of the PK based membranes as FO membrane has been demonstrated [1]. However, the highly porous structure of the PK support results in low mechanical strength of the support membrane. In addition, it was reported that the high pressure resistance and water flux are trade-off in FO membrane [1].That is, improvement in pressure resistance is required while keeping water flux. In this study, PK support membranes were treated with m-phenylenediamine (MPD) to control the membrane structure in order to enhance the mechanical strength. After the thermal treatment of PK support with MPD, the bottom surface structure became denser with the increase of treatment temperature (Fig 1a). As a result, it was found that the pressure resistance of PK based TFC membrane was improved over 20 bar under the condition of 110 °C (Fig 1b). The fabricated TFC membrane is expected to be applied for PRO process.
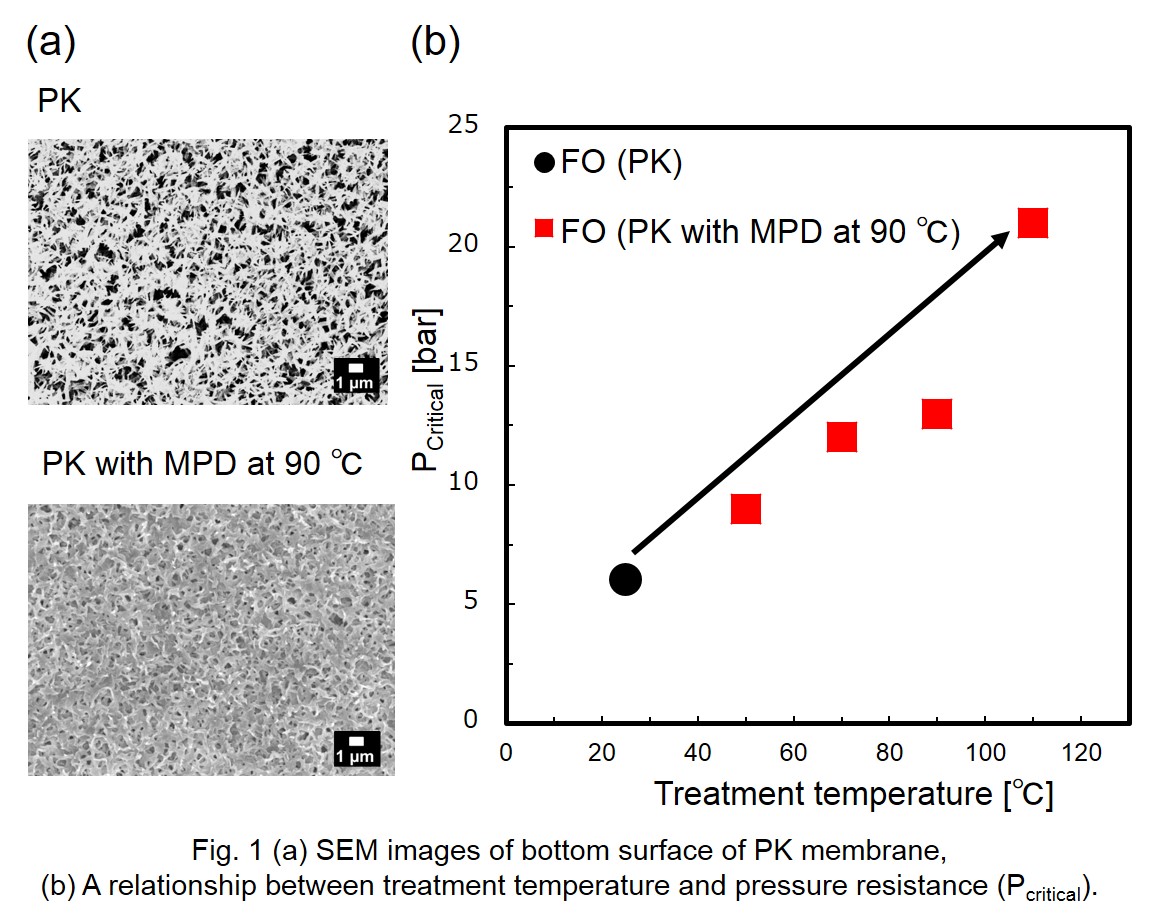
Graphene oxide (GO) nanosheets membranes have been extensively studied because their unique molecular sieving properties with fast water permeation, simple fabrication method as well as surface hydrophilic properties.. Vacuum assisted method is widely used for fabricating GO nanosheet membrane, but low regularity of laminated structure of nanosheets is a problem. In this study, GO nanosheet membranes were fabricated by pressure assisted method which enables to fabricate more stable laminated structure. The influence of the surface treatment on their layered structure and performance of GO membrane were examined.
In this study, GO nanosheets were fabricated by Improve Hummers method. GO membranes were fabricated by laminating GO nanosheets on porous α-alumina support under 1 bar (GO membrane). The heat treatment at 200 °C to reduce the oxygen functional group was performed (rGO membrane). Permeability was calculated from the weight of permeated water by dead-end system. Rejection was calculated from the absorbance of the permeate by using two dyes, Evans blue (EB, Mw:960.81) and Acid red (AR, Mw:635.59). Fig.1 shows the membrane performances of the GO and rGO membrane. As shown in Fig.1 (a), the permeability of GO membrane is around 10 times higher than that of rGO membrane. Fig.1 (b) shows the dye rejection of GO and rGO membrane. The rejection of GO membrane was about 90 % for EB and about 30 % for AR whereas the rejection of rGO membrane showed about 70 % for EB and about 10 % in AR. The reduction of the GO surface and structural changes of the layered structure would affect the membrane performances.
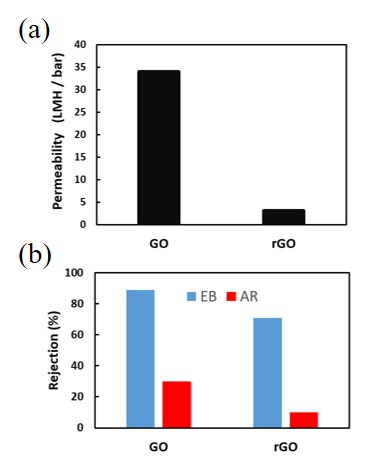
Conventional wastewater treatment process cannot completely remove trace pharmaceutical and personal care products (Cao et al., Chem. Eng. J., 2019, 370: 684–694.), and an advanced wastewater treatment process is expected. Forward osmosis (FO) relies on the osmotic pressure difference on both sides of the membrane as the driving force to realize the spontaneous diffusion of water molecules. Because of low energy consumption and low membrane fouling, FO has become a novel membrane separation technology in water treatment field. This study investigated the interaction between typical pharmaceuticals (ciprofloxacin, moxifloxacin and acetaminophen) in FO. The results showed that the factors affecting pharmaceuticals rejection rate included: 1) number of pharmaceutical molecules determining the collision probability between molecules; 2) pH influencing the charge on membrane surface and the patterns of pharmaceutical molecules; 3) size ratio of membrane pore and pharmaceutical molecule determining membrane pore blockage; 4) charge neutralization or electrostatic repulsion existing between pharmaceutical molecules.
In recent years, a facilitated transport membrane1) in which a polymer gel membrane is impregnated with an ionic liquid, are attracting attention as a core technology for the next generation carbon dioxide separation. By applying this to conventional carbon dioxide emission sources such as steel plants and thermal power plants, it is possible to reduce carbon dioxide emissions. However, ionic liquids have the disadvantage of low diffusivity because they have very high viscosity, and it is essential to improve the diffusivity of ionic liquids for practical use.
In this study, the diffusivity and liquid structures of several types of ionic liquid molecules with a primary or secondary amine group ([P4444][Ala], [aP4443][Pro]) were analyzed by molecular dynamics (MD) simulation. These ionic liquids have already been known to exhibit high carbon dioxide absorption. It was confirmed that the diffusion coefficient of [Ala-CO2] complex was higher than [Pro-CO2] (Fig. 1). We also evaluated the interaction and clarified the relationship between the interaction and the molecular structure. [Pro-CO2] was highly structured with other [Pro] molecules, while [Ala-CO2] showed no structured peaks in the radial distribution function (RDF) (Fig. 2). The diffusivity was very low in an ionic liquid having a structure that easily caused interaction with other molecules. It was suggested that ionic liquid molecules that formed intramolecular hydrogen bonds exhibited high diffusivity by reducing the interaction with other molecules. By designing a new ionic liquid based on the obtained knowledge, it is expected to develop a carbon dioxide separation process suitable for practical use.
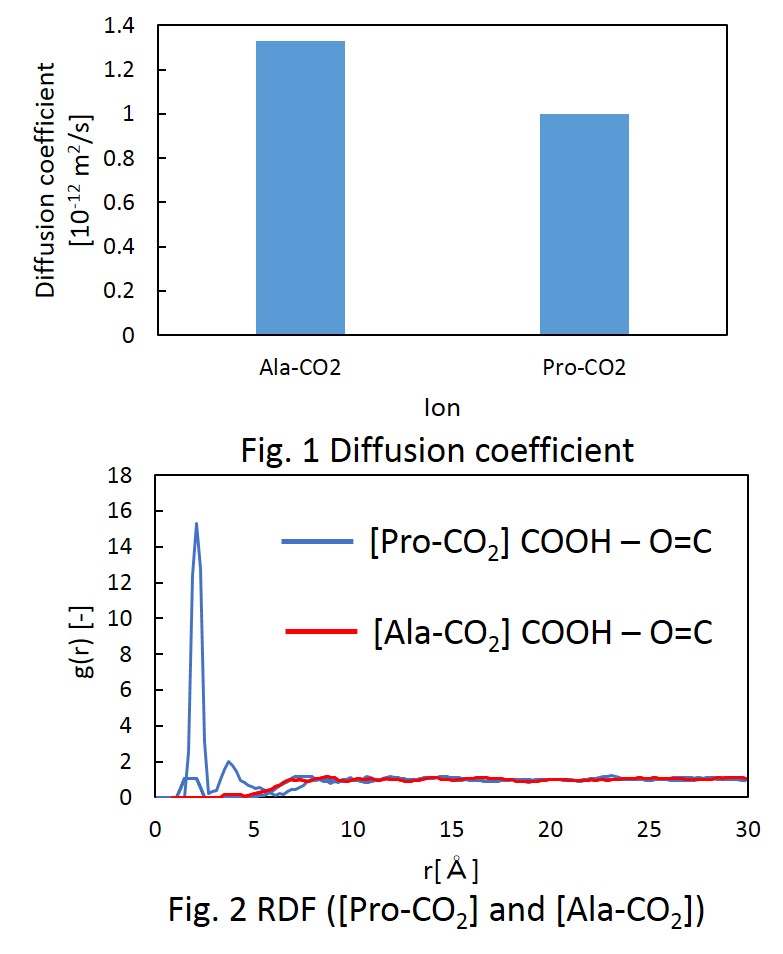
Volatile organic compounds (VOCs) are widely used in industries such as painting and printing as solvents or adhesives. A large portion of used VOCs used in the industries is discharged into the atmosphere to cause air pollutions. Membrane separation process can be applied to recover and separate VOCs from waste gas or liquid streams. In this study, polydimethylsiloxane (PDMS) membranes were examined for the pervaporation separation of mixtures of VOCs including toluene/isopropyl alcohol (IPA), which are major VOCs discharged from printing and painting industries.
Commercially available PDMS membranes with different hardness (20, 50, 70) were used as base membranes. The base PDMS membranes were chemically modified with tetraethyl orthosilicate (TEOS) and other silane coupling agents to control the affinity to the target VOCs. The membranes were applied to the pervaporation experiments under vacuum at the permeate side.
The base PDMS membranes were toluene selective against ethyl acetate for all the hardness, but the PDMS membranes with the hardness at 50 and 70 showed higher toluene selectivity (~80% in permeate) and lower flux (~400 g/m2/h) than the PDMS membrane with the hardness at 20 (~54 % and 2280 g/m2/h). The toluene selectivity was greatly improved by the chemical modification with TEOS as shown in Figure. The toluene fraction in permeate was ~77%, and the total flux was 567 g/m2/h for the treatment for 18 h without hydrochloric acid. The treatment conditions such as the treatment time and the addition of hydrochloric acid as an acid catalyst has slight effect on the pervaporation performances. Compared with the base PDMS membranes with the hardness at 50 or 70, the toluene selectivity of the treated PDMS membrane with the hardness at 20 was almost equivalent, and the permeation flux was 20% higher.
In recent years, researches on stacked nanosheet membranes fabricated using two-dimensional (2D) nanosheets such as graphene oxide (GO) membranes have attracted much attention because of the ultrathin thickness, easy fabrication methods, and their potential of high selectivity in water treatment and gas separation. However, the molecules permeate through the 2D interlayer channels formed between nanosheets for the stacked nanosheet membranes, which results in a long permeation pathway. On the other hand, nanosheets of MOF with a porous structure and functional surface groups are considered as excellent candidates for ultrapermeable and highly selective 2D membranes due to their nanoscale thickness, extreme low mass-transport resistance and 2D morphology. Gascon et al [1] have fabricated CuBDC/polyimide nanocomposite membranes, which showed a remarkable improvement in gas separation. In this study, the stacked CuBDC MOF nanosheets membranes were fabricated by vacuum filtration method. The addition effects of cross-linker into the stacked nanosheet membranes on their stability in the solvents were investigated.In the results of XRD pattern, two peaks at 2 theta = 17.09 and 34.18 °corresponding to the (201) and (402) crystallographic planes of the CuBDC structure were observed, which imply the formation of pore openings nanosheets (Fig.1). The structural stability was evaluated by immersing the stacked nanosheets membranes into water and organic solvents. It was found that the crystal structure of CuBDC was relatively stable in DMF and methanol as compared with water. For the permeation test using a cross-flow membrane filtration system, it was found that the addition of cross linkers such as triethanolamine stabilized the stacked nanosheet membranes. The effects of the cross linkers on their solvent permeation and separation performances will be compared.
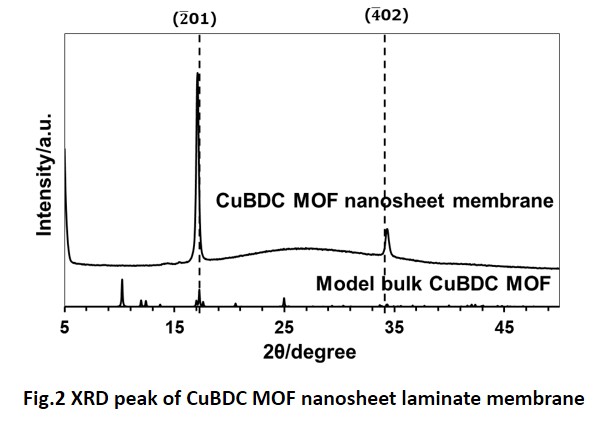
Methanol is an important material for chemical products. Conventionally, methanol is synthesized from synthesis gas. On the other hand, direct partial oxidation method from methane has been widely studied, as one good option to replace the conventional method, but the yield is still low. To solve this problem, we propose to use a membrane reactor. The membrane reactor is a physical device that combines a chemical conversion process with a membrane separation process to remove products of the reaction. Produced methanol is separated from the reactor by the membrane, leading to prevent further reactions of methanol to other chemicals including CO2. However, it is difficult to separate methanol and methane through molecular sieve membranes, because of their similar kinetic diameter. Recently, we have developed a new concept of chemically stabilized ionic liquid (IL) membranes, which are organosilica membranes prepared from silylated ILs.
The figure below shows the effect of temperature on permeation and separation performance in methanol/methane binary mixture system. The prepared membrane shows high selectivity toward methanol against methane. Separation factor was increased as high as 219 at 100 °C. Apparent activation energies (Ep) for permeation of methanol and methane were calculated to be 2.6 kJ/mol for methanol and 31 kJ/mol for methane using the Arrhenius equation. The obtained Ep of methanol was much smaller than that of methane, regardless of their similar molecular size. The obtained results indicated that the ILs group strongly affected the methanol permeation even at high temperature up to 200 °C. The silylated IL-derived organosilica membrane showed the great potential for separation of methanol from methane at high temperature.
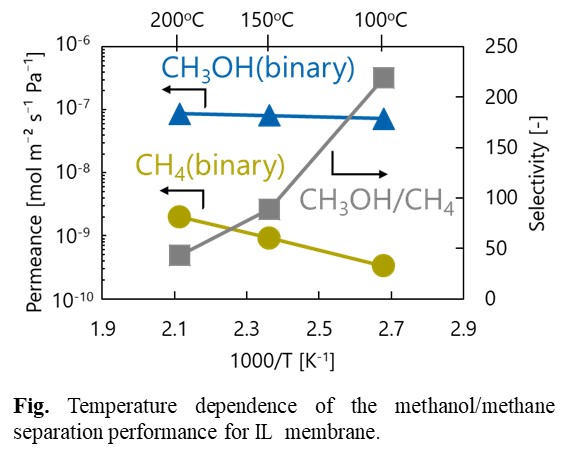
Zirconium based UiO-66, consisting of zirconium oxocluster (metal nodes) and terephthalic acid (organic linker), is known as the most chemically and thermally stable material among the metal organic frameworks. Moreover, it has been reported that UiO-66 membrane has good separation performance for CO2/N2 separation, which is necessary to reduce greenhouse effect. However, synthesizing continuous and defect-free UiO-66 membrane is a challenging work, because UiO-66 has no interaction with the supports. In this work, UiO-66 membranes were synthesized on the gamma alumina coated alpha alumina support by in situ growth method. Gamma alumina layer was prepared from the sol-gel coating of boehmite sol to increase the interaction between UiO-66 and support. Synthesized membrane was analyzed by XRD, SEM, FT-IR and CO2/N2 gas separation test. Gas separation result showed that UiO-66 membrane is a promising candidate for CO2/N2 separation.
Separation of propylene/propane mixtures is an important and challenging task in petrochemical industry, which is currently performed by energy-intensive cryogenic distillation. If effective membranes are developed, membrane technology may provide substantial energy and capital cost savings. In this study, the continuous and compact ZIF-67 membranes were synthesized on alumina supports by a counter diffusion-based in situ methods for propylene/propane separation. X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), and energy dispersive X-ray (EDX) mapping were used for the characterizations. After three synthesis cycles, the defects of the ZIF-67 membranes were completely healed, showing high quality of ZIF-67 membranes. The effects of the solvothermal synthesis cycles and time were investigated. Gas permeation tests revealed that the membrane prepared with three synthesis cycles at 120oC for 4 h had well-intergrown and compact ZIF-67 structure, showing propylene/propane separation factor of 73.82 with a C3H8 permeance of 1.04x10-10 mol m-2 s-1 Pa-1.
Poly[2-(dimethylamino)ethyl methacrylate] (PDMAEMA) has been paid great attention as CO2-switchable polymer, because various applications are recently expected, such as extractants of organic solvent and draw solutes of forward osmosis desalination. PDMAEMA can react with CO2 in water, which can increase drastically its lower critical solution temperature (LCST), characterizing the coil-to-globule transition from a soluble to an insoluble state. The transition certainly results from the protonation–deprotonation of the tertiary amine group in PDMAEMA side chains. However, it is still challenging to investigate the conformational transition of PDMAEMA in water at the atomistic levels. To provide detailed insights into the origin of the transition, it is essential to evaluate the microscopic interactions between the PDMAEMA side chains and water molecules. In this study, the coil-to-globule transition of DMAEMA oligomers is investigated by using molecular dynamics simulations. We calculate the radial distribution functions (RDFs) between the DMAEMA moieties and the surrounding water molecules to investigate the influence of the hydration structures in the vicinity of DMAEMA side chains on the CO2-switchable property. We also calculate the time evolution of the radius of gyration (Rg) to evaluate the temperature dependence of the coil-to-globule transition. The Rg is calculated by averaging the mass-weighted distance of each constituent atom of the oligomer from the center-of-mass position of the constituent atoms. Consequently, the values of Rg distinctly depend on the protonated/deprotonated states and the temperatures, indicating that the DMAEMA oligomer shows the conformational transition at the atomistic levels. We also confirm that the predicted LCST values from our simulations are correlated with the previously reported experimental LCST values. In our presentation, further details are shown, including the RDFs and the values of Rg at varied simulation conditions.
Protein chromatography is an important technique to separate and purify the protein of interest based on protein's size, hydrophobicity, and total charge. For purifying the protein with required level, loss of the purified protein is associated due to the multiple chromatography steps involved. Also, in some cases, high concentration of salt used for protein adsorption/desorption causes protein denaturation.
Recently, an ion recognition gating membrane grafting N-n-propylacrylamide (NPAM) and Benzo[18]crown-6-acrylamide (BCAm) is developed which can control its pore opening with response to low concentration of K+ ion at ambient temperature [1]. The NPAM moiety of the membrane can change its hydrophilic/hydrophobic property at certain temperature, and this membrane further controls the charge state by capturing K+ ion to BCAm moiety. Hence this grafted membrane has the potential to be applied for protein chromatography that can overcome its present problems. Herein, we evaluate the protein adsorption/desorption properties of this grafted membrane with response to specific ion.
The NPAM and BCAm monomers were grafted to the pores of polyethylene membranes by plasma-graft polymerization and the grafting is confirmed by FT-IR. A feed solution containing negatively charged γ-globulin and K+ ion was continuously supplied to the membrane followed by buffer solution, and concentration of γ-globulin in permeated solution was evaluated by Bradford assay in each steps. During γ-globulin solution supply, γ-globulins were gradually adsorbed on the grafted polymer capturing K+ ion. After the buffer supply, the adsorbed γ-globulins were desorbed from the membrane due to the K+ ion release from the grafted polymer (Fig. 1). Although protein adsorption/desorption was successfully controlled with response to K+ ion, the amount of desorbed γ-globulin is low due to the nonspecific adsorption of the γ-globulins to the membrane. We are currently considering monomer component of grafted polymer to suppress this nonspecific adsorption of the protein.
[1] Y. Asano, et al., SCEJ 49th autumn meeting, PA242, 2017
The use of plastic packaging for foods and beverages caused a serious environmental problem since most plastic waste is not recycled. One of the most common plastic materials for packaging is polyethylene terephthalate (PET). Million tons of PET bottles are produced every year, and discarded by consumers after use, resulting in a huge amount of plastic waste. The recycle of used PET bottles is a big challenge in the environmental conservation. In this study, used PET bottles were utilized as a raw material to prepare ultrafiltration membranes with the aim to obtain membranes which can be used for water treatment. Used PET bottles were cut into pieces and dissolved in various solvents. Polyethylene glycol was added as an additive to the PET solution. The polymer solution was then casted onto a glass plate using a casting knife and then immersed in a coagulation bath containing non-solvent. The effect of the type of solvent and non-solvent, as well as the concentration of the additive, on the membrane morphology was studied using Scanning Electron Microscopy. The ultrafiltration experiment was carried out using pure water and aqueous solution containing bovine serum albumin at a pressure of 1 bar to investigate the permeate flux and the rejection of the membranes. It was observed that the permeate flux increased when various hydroalcoholic solutions was used as non-solvent with the sequence water-ethanol, water-propanol, and water-butanol solution. The addition of more polyethylene glycol as the additive resulted in an increase in the permeate flux. The permeate fluxes ranged between 1.72 kg/m2h and 41.67 kg/m2h, depending on the preparation condition of the membrane. The membrane showed a rejection of bovine serum albumin of 90.6%. This result showed that used PET bottles can be used as a raw material to fabricate ultrafiltration membranes for water treatment.
MeOH synthesis from syngas is one of the most important reaction in industry. Because the conversion level of MeOH synthesis at equilibrium is thermodynamically limited, membrane reactor is expected to be effective for shifting the conversion level beyond equilibrium. MeOH synthesis is currently performed at 473-573 K and 5-10 MPa, and thereby steam partial pressure in the reactor reaches up to 0.6 MPa. Na-ZSM-5 membrane is a candidate to use in the membrane reactor for MeOH synthesis1). It is known that steam causes deterioration of zeolite structure at higher temperature. In this study, we focused on the stability of Na-ZSM-5 membrane against steam at high temperature.
Na-ZSM-5 membrane was synthesized on a tubular porous α-alumina support by a secondary growth method. The membrane was exposed to steam under conditions of 573 K and 0.6 MPa of steam. After the steam exposure, the separation performance for MeOH/CO2 and MeOH/H2 mixtures were evaluated.
Fig. 1 shows the permeation test results at 473 K. In the MeOH/CO2 permeation test, CO2 permeance increased and the separation factor decreased from 127 to 101 by steam exposure in the early stage up to 50 h. However, no significant change in the permeance and separation factor was observed up to 300 h. The MeOH/H2 permeation test gave similar results. According to XRD results, decrease in the crystallinity of ZSM-5 structure was hardly observed even after steam exposure for 300 h.
As a result, we concluded that Na-ZSM-5 membrane is very stable at 573 K and 0.6 MPa steam.
Acknowledgment
This work was financially supported by the Artificial Photosynthesis Project, NEDO.
Reference
1) K. Sawamura et al., Chem. Asian J., 4(7) (2009) 1070-1077.
Rhodium (Rh) is the most expensive of the platinum group metals (PGMs) and is primarily used in automobile catalysts, along with platinum (Pt) and palladium (Pd). To date, no commercial solvent extraction reagent exists for Rh. In this study, new monoamide-containing quaternary phosphonium-based ionic liquid with chloride anion was developed for recovery of Rh. New ionic liquid [2-(di(2-ethylhexyl)amino)-2-oxoethyl]tributylphosphonium chloride (Fig. 1) was synthesized by simple two step reactions in our laboratory. Extraction of Rh(III) using novel ionic liquid was conducted by the batchwise method. An aqueous feed solution was prepared by diluting the standard solution of Rh(III) in 1.0 mol dm–3 HCl. An extracting organic phase was prepared by dissolving novel ionic liquid in toluene. Equal volumes (3 cm3) of the aqueous and organic solutions were mixed in a centrifuge tube and then shaken gently for 24 hours to attain extraction equilibrium. All extraction experiments were performed at 25 °C. After centrifugation, the initial and equilibrium concentrations of Rh(III) in the aqueous phases were measured by ICP-AES, to determine the degree of extraction. The effect of concentration of ionic liquid on the degree of extraction of Rh(III) was investigated. As expected, the degree of extraction increased with the concentration of ionic liquid and reached 70% using 200 mmol dm–3 ionic liquid.
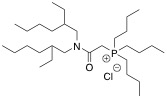
In the near future, it is expected that a large amount of wastes of used solar panels containing In(III), Ga(III) and Zn(II) is discharged. These metal electronic wastes are significant metal resources for Japan with a few metal resources.
Solvent extraction involves the distribution of a solute between two immiscible liquid phases in contact with each other. We are concerned with the extraction equilibrium and dynamic of the distribution and its use scientifically and industrially for separation of solute mixtures. Here, we examined the extraction selectivity for In(III), Ga(III) and Zn(II) with N-oleoylsarcosine in dodecane from 1 M (=mol/dm3) aqueous ammonium chloride contain- ing these metals, respectively. For comparison, commercially available, carboxylic acid (Versatic 10, VA10) also was used to extraction selectivity for In(III), Ga(III) and Zn(II).
In the case of VA10, the extraction order was Ga>In>>Zn in the pH region. This is due to electrostatic force between carboxylic acid and metal ions. In the case of N-oleoylsarcosine, the extraction order was Ga(III)>In(III)>>Zn(II) in the pH region. The extraction tendency of these results a similar to that of VA10, although the extraction pH region with VA10 is higher than that with N-oleoylsarcosine. This is due to the difference of acid dissociation constants both extractants. In addition, carboxylic acids with a long alkyl chain were chemically modified, the hydroxamic acid was synthesized for comparison. The extraction selectivity for these metals between carboxylic acids and alkylhydroxamic acid was discussed.
One method for removing radioactive cesium in an aqueous solution is the solvent extraction in which cesium salts are formed using extractant. Generally, benzene, cyclohexane or the like are used as a solvent, but they are flammable liquid having a high boiling point. In addition, cesium aqueous solution is generated again after stripping with acid for solvent recovery and recycling. Therefore, in order to simultaneously achieve the separation and the volume reduction of radioactive cesium in the aqueous solution, we proposed the solvent extraction using a fluorinated solvent which is nonflammable liquid having a low boiling point and easy to distill regeneration. The aim of this study is to determine the optimum conditions for single extraction, to perform multiple extractions aiming to improve the removal rate, and to create a flow type device. The extraction experiment was carried out by changing the pH of 5ppm non-radioactive cesium aqueous solution using bis(2-ethylhexyl)phosphoric acid (D2EHPA) as an extractant. The higher the pH of the aqueous phase and the D2EHPA concentration, the better the removal rate. The highest removal rate was about 60% under the pH = 13. From the experimental results, the optimum extraction condition was determined as pH = 13 and [D2EHPA] = 0.15 mol/L. Next multiple extraction test was carried out under this condition, 90% cesium removal could be achieved in 3 times extraction. Finally, the flow type device using a static mixer for mixing and extracting parts was proposed and removal test was performed. The cesium removal performance of this device was equivalent to the batch system.
In 1955, “Itai-itai disease” was caused by water pollution of zindu-river with Cd(II) in Japan, The WHO has warmed that Cd(II) causes cancer in humans. However, Cd(II) is contained in zinc ore and is useful as raw materials in the high technology industries such as Ni/Cd battery. But separation of Cd(II) from zinc mine is difficult, because Cd(II) is similar to Zn(II) on their chemical properties. The objective of this study is to develop highly selective extractant for Cd(II) over Zn(II).
1,4-Di[[di(2-ethylhexyl)carbamoyl]methyl]piperazine (DDCMP) has been newly synthesized to develop a selective extractant for Cd(II). DDCMP has been designed so as to exhibit high selectivity for Cd(II) through electrostatic force and coordination ability of the tertiary amine of piperazine. As we expected, DDCMP in toluene exhibited a high selectivity to Cd(II) over Zn(II) from hydrochloric acid. This is attributed to high coordination ability and electrostatic force of piperazine for Cd(II). The extraction of Cd(II) was significantly dependent on concentration of hydrochloric acid. Here, we have quantitatively discussed the extraction equilibrium of Cd(II) with DDCMP in toluene from hydrochloric acid. It was found that Cd(II) was extracted as a 1:2 complex formed through ion pair formation and coordination bonding. The high selectivity for Cd(II) over zinc(II) with DDCMP was explained by determining these extracted species.
Moreover the cellulose triacetate membrane (CTA) containing 2-nitrophenyl octyl ether (NPOE) as a plasticizer and DDCMP as a carrier was prepared, characterized and applied to the active transport of Cd(II) and Zn(II) mixture. The polymer inclusion membrane (PIMs) used in this study was very effective and highly selective to transport of Cd(II) from the Cd(II)-Zn(II) mixture feed solution to receiving solution.
Nitrogen oxides (NOx) is toxic for human beings and causes photochemical smog, acid rain and so on. The NOx removal in exhaust gas is quite difficult. This is because NOx has low water solubility and low reactivity. Therefore, common gas absorber such as packed or bubble tower cannot eliminate NOx efficiently. Gas absorber equipped with glass fiber filter as a packed material can remove NOx with high efficiency. The high wetting area or high coefficient of mass transfer in glass fiber filter would enhance NOx absorption into water. The objective of this work is to clarify mechanism of NOx absorption into water in gas absorber having glass fiber filter. As a first step, the contributions to NOx absorption of mass transfer coefficient KG (KL) and gas-liquid wetting area a were evaluated by the simulation. Reaction and equilibrium of NOx in both gas and water phase was very complicated. Since N2O3 and N2O4 were unstable and existed only a very small amount, we made an assumption of rapid equilibrium in gas phase and penetration theory was adopted in water phase. In addition to the simulation, we measured KG and a, experimentally. As a result, useful information for equipment design or optimization of operating conditions was obtained. It was suggested that rate-limiting step in the absorption of NOx is the association reaction of NOx in the gas phase. It was also found that main dissolved spices of NOx depend on the NOx concentration of the inlet gas.
In previous study, the interfacial tension during microwave irradiation was measured in the presence of the non-ionic surfactant, Triton X-100. If the surfactant is short, quick increase during the pulse irradiation was observed due to desorption caused by the quick thermal response of microwave. Although operation of microwave irradiation is useful for demulsification, combinations of two liquids and surfactant are various. Accordingly, optimization of microwave local heating is required for industrial application of this technique. In this study, desorption of longer surfactant, Triton X-405 as a result of microwave irradiation was investigated at different power, and dimensionless number for the local heating around the interface was proposed to estimate surfactant behaviour for the desorption and disorder quantitatively.
2-Ethylhexylthioglycolate(EHTG) and 1-Dodecanthiol(DDT) as extractants for selective separation of indium(III) and gallium(III) over Zn(II) were used in the extraction tests. To evaluate the extraction abilities of EHTG and DDT in toluene, liquid-liquid extraction was carried out using 1 M aqueous ammonium nitrate solution containing 1 mM metal ions. The extraction equilibria for metal ions were performed by a batchwise method at 303 K for 24 h. The initial and equilibrium concentrations of metal ions in the aqueous solution were determined using a flame atomic absorption spectrophotometer model Z-2310 (AAS, Hitachi Co., Tokyo, Japan). The metal concentration in the organic phase was calculated from the mass balance between the aqueous and organic phases. The extraction order for the metal ions with EHTG was In(III) > Ga(III) > Zn(II), while DDT hardly extract In(III) and Ga(III), and only Zn(II) was extracted in the region of pH = 6 around. These differences are due to the effect of oxygen atoms(=O). In the other, these results suggested that EHTG is possible to selectively separate In(III) from an aqueous solution containing In(III), Ga(III) and Zn(II). Therefore, membrane transport experiments were carried out using EHTG as the carrier of the polymer inclusion membranes(PIMs). As a result, it was possible to selectively transport only In(III) from the metal mixture solution. The PIMs containing EHTG was expected as a selective extractant of In(III) over Ga(III) and Zn(II) from such as wastes of solar panels.
As gold is used in electronic devices such as mobile phones, PCs, and laptops, recovery of gold from waste electrical and electronic equipment (WEEE) is important. Solvent extraction is an efficient separation technique for the recovery of precious metals leached using acidic chloride solution. Oxygen-containing compounds such as dibutyl carbitol (DBC) and methyl isobutyl ketone (MIBK) have been used as the extractants for Au(III). In the present study, extraction behavior for Au(III) using various aromatic diethers was compared to study the structural factors for the extraction.
The following aromatic diethers were synthesized: 1-ethoxy-2-methoxybenzene (o-EMB), 1-methoxy-2-propoxybenzene (o-MPB), 1-butoxy-2-methoxybenzene (o-BMB), and 1-methoxy-2-octoxybenzene (o-MOB). DBC and 1,2-dimethoxybenzene (o-DMB) were purchased and also used for the extraction test. Extraction test was carried out by contacting an aqueous solution containing 0.1 mM Au(III) in 0.1-8.0 M hydrochloric acid (5.0 mL) and the extractant (1.0 mL) without dilution. After shaking the mixture at 30 °C for 24 h, the phases were separated and the concentration of Au(III) in the aqueous phase was determined by FAAS to determine the percentage extraction.
Figure shows extraction profiles of Au(III) using the diethers and DBC as a function of hydrochloric acid concentration. The extraction using o-DMB is comparable to that using the commercial extractant DBC. The extraction percentage decreases as the increase of carbon numbers at side chain: o-DMB > o-EMB > o-MPB > o-BMB > o-MOB. The result suggests that relatively less hydrophobic diether compound is more favorable for the extraction of Au(III).
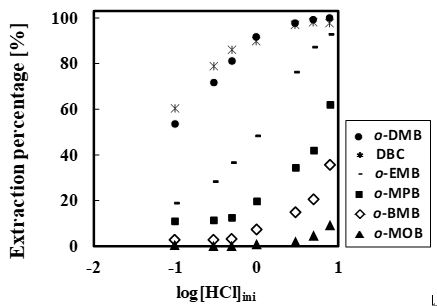
Due to the rapid expansion of worldwide lithium battery market, the issue for the recovery of lithium from waste battery or extraction of it from seawater and other aqueous solution has attracted great attention. Especially, there is virtually inexhaustible lithium resource in seawater, totalling approximately 250 billion tons. However, the concentration in is only about 0.17 mg L-1 so that it is difficult to extract Li+ ions from seawater. Thus, it is highly desirable to develop an efficient way to separate such a low concentration of lithium ion. In this study, two kinds of electroactive hybrid films were fabricated on the electrode by using a unipolar pulse electrodeposition technique. When the electrode was applied for the selective electrochemical extraction of low concentration of Li+ ions from aqueous solution via the electrochemically switched ion exchange (ESIX) process, both the Li+ ion adsorption capacities were over 35.2 mg g-1. The excellent ion separation performance of these hybrid film should be attributed to its low ion transfer resistance due to its porous structure and the high electric driving force during the ESIX process. It is expected that they can be applied as the promising electroactive material for effective separation of Li+ ions from seawater. In addition, a novel electric-field-accelerated ion-sieve membrane (EISM) system with potential-oscillation was designed for the continuous and selective separation of Li+ ions. (Acknowledgment: This work is supported by the JSPS KAKENHI Grant-in-Aid for Scientific Research C(Kiban C, 19K12395)
Macrocyclic compounds calixarenes have been used as platforms for the development of powerful extractants in solvent extraction system. However, most of calixarene derivatives is not soluble in typical organic solvents and toxic chlorinated hydrocarbons are used to dissolve calixarenes. In the present study, aromatic ethereal compounds have been synthesized as new solvents for calixarene derivatives.
Typically, 1-octoxy benzene (OB) was prepared by mixing 1-bromooctane and phenol in DMF under basic condition. Ten millimolar of a p-tert-octylcalix[6]arene hexacarboxylic acid derivative (tOct[6]CH2COOH) was soluble in OB. As the solubility of OB in water was less than 3.0×10-3 g/dm3, leak of OB into aqueous phase in solvent extraction is very small.
Extraction of L-tryptophan methyl ether (Trp-OMe), L-tryptophan ethyl ether (Trp-OEt), and L-tryptophan benzyl ether (Trp-OBz) was examined by contacting an aqueous solution containing Trp-OMe Trp-OEt, or Trp-OBz (0.2×10-3 mol/dm3, 10 cm3) and OB solution containing tOct[6]CH2COOH (1.0×10-2 mol/dm3, 2.0 cm3) at 30 °C for 24 h. Concentration of the amino acid esters in aqueous phase before and after extraction was determined using a UV-VIS spectrophotometer to calculate the extraction percentage. As the extraction is based on proton-exchange reaction, extraction of the amino acid esters increased with increasing pH. The order of the extraction depended on the hydrophobicity of the amino acid esters; Trp-OBz > Trp-OEt > Trp-OMe. The extraction behavior in OB agrees with that in chloroform, which was investigated in previous study.