Formic acid (FA) is considered as a promising liquid organic hydrogen carrier for the fuel cells. FA is well-known compound to transfer into H2/CO2. With its properties, we concerned FA as the source of high-pressure CO2/H2 without any compressing procedures. These high-pressure gases can utilize for not only the high-pressure H2 for FCVs, but also various chemicals as solvents and reagents. In our presentation, we show the development of the continuous production of high-pressure H2/CO2 over 150 MPa, and easy separation from the generated gases under high-pressure conditions.
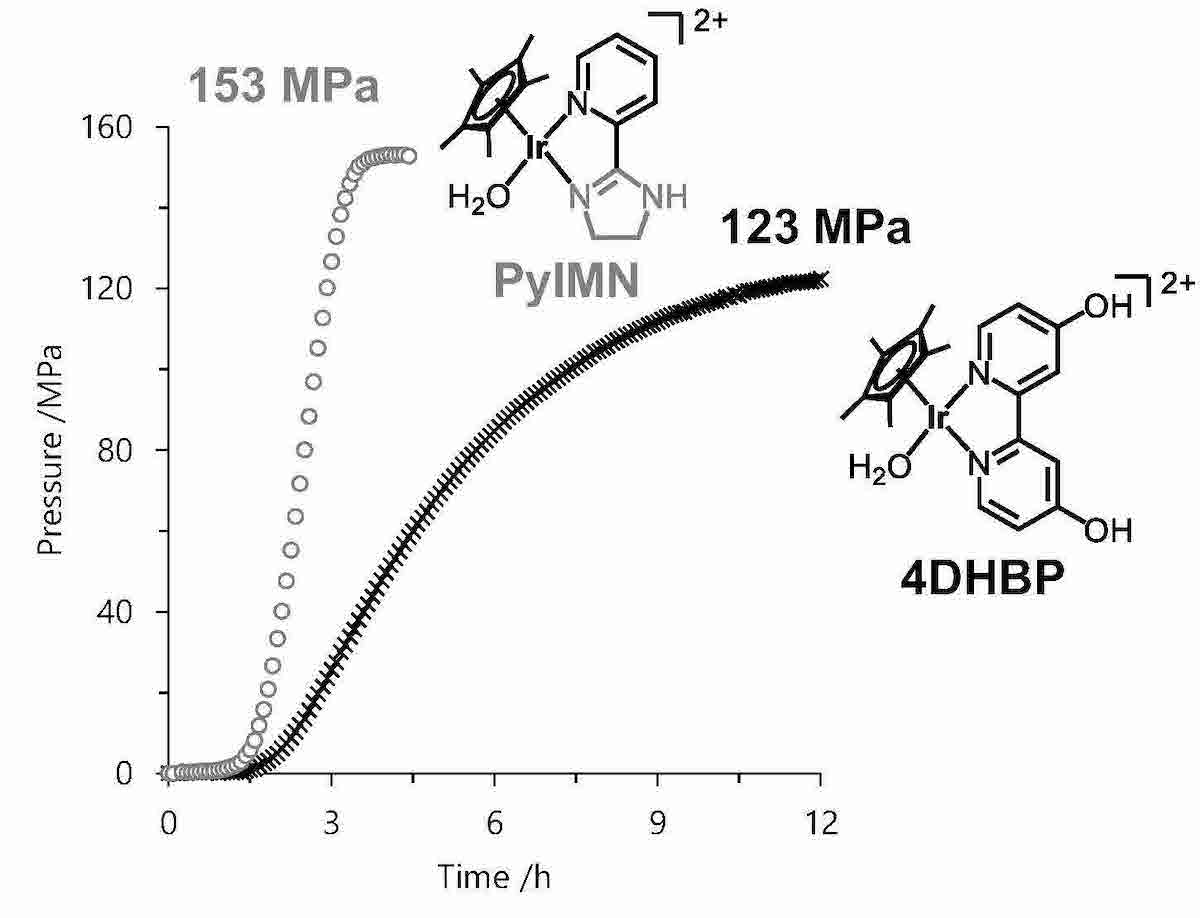
For the development of the high-pressure gas generation by the FA dehydrogenation, we used the Ir complex as a homogeneous catalyst. The catalyst can successfully produce the high-pressure gas with a high rate (TOF = 2500 h-1 at 30 MPa) at the temperature of 80oC. Whereas in the high-pressure conditions, the catalyst was deactivated gradually with decreasing the reaction rate. To overcome this, we developed higher durable catalyst by modification of the ligand, and the modified catalyst can generate high-pressure CO2/H2 for longer time. For the further development, we found that the iridium aqua complex coordinated with a pyridyl-imidazoline ligand showed the higher performances on the FA dehydrogenation at the pressure as high as 153 MPa.
We also demonstrated the high-pressure H2/CO2 gas separation by cooling the gas to change the supercritical to gas-liquid phase. Using our system, more than 99 mol% of H2 (96 mol% of purity) and 94 mol% of CO2 (99 mol% of purity) were separately obtained as a gas and liquid, respectively, under the high-pressure conditions.
Ours system has a potential to be used as a simple and easy to handle system for the generation of high-pressure CO2/H2 and these separated high-pressure gases can be used for the variety of application such as fuel cell vehicles.
Carotenoids exist in plants usually as the (all-E)-configuration which have higher crystallinity and lower solubility in organic solvents compared to the Z-isomers. When they are converted to Z-isomers (cis forms), bioavailability increases due to the lower crystallinity and higher solubility [1, 2]. We have studied Z-isomerization of carotenoids such as lycopene and beta-carotene and behavior of the isomers in extraction process and fine particle formation process. Since Z-isomers have higher solubility in supercritical CO2, extraction of carotenoids from plant materials is enhanced. Fine particle formation process is also influenced due to the difference in the crystallinity as well as the solubility.
Z-isomerization behavior of lycopene in the oleoresin was studied by adding various vegetable oils and the key component to enhance the isomerization was revealed. When the plant materials were pretreated to convert Z-isomers, the extraction rate of carotenoids by supercritical CO2 or subcritical dimethyl ether increased considerably [3].
For particle formation by SAS process, smaller particle was obtained by Z-isomerization pretreatment, whereas (all-E)-form of carotenoid produced larger crystal form of particles [4].
Reference
[1] K. Murakami, M. Honda, R. Takemura, T. Fukaya, M.Kubota, Wahyudiono, H. Kanda, M. Goto, Biochem. Biophys. Res. Commun., 491, 317, 2017
[2] M. Honda, Yo Watanabe, Kazuya Murakami, Nguyen Ngoc Hoang, Wahyudiono, H. Kanda, M. Goto, Eur. J. Lipid Sci. Technol., 2017, 1700293 (1-8), 2017
[3] K. Murakami, M. Honda, Wahyudiono, H. Kanda, M. Goto, Sep. Sci. Technol., 52, 2573-2582, 2017
[4] T. Kodama, M. Honda, R. Takemura, T. Fukaya, C. Uemori, Wahyudiono, H. Kanda, M. Goto, J. Supercritical Fluids, 133, 291-296, 2018
The history, principles and potential of Process Intensification (PI) will be introduced. A range of “tools” that are particularly suited for the development of PI-based processes will then be described, with particular reference to the re-engineering of pharmaceuticals to improve bioavailability, and to provide targeted response and controlled release modalities. Examples of PI success stories and “not-so-successful” case studies will be presented, as well as some lessons learned from the coal-face during my travels along the Journey to Technology Transfer & Commercialisation.
Curcuma xanthorrizha Roxb, known as Temulawak or Javanese ginger, is a plant species. Its rhizomes are used as a medicinal herb. It contains curcumin as an active compound and ethereal oils mainly consisted of sesquiterpenes. In this work, Curcuma xanthorrizha Roxb ethanolic extract was micronized with an addition of PVP using supercritical antisolvent (SAS) method. The ethanolic extract was obtained from dried Curcuma xanthorrizha Roxb using soxhletation. For the micronization, the extracted compound solvent was a mixture of acetone and ethanol (90:10 (v/v)), while the supercritical CO2 was used as an antisolvent. The SAS process was carried out at temperatures of 40, 60, and 80oC, pressures of 8, 10, and 12 MPa, and PVP concentrations of 0, 2, and 4%. The produced particles were analysed by SEM (Scanning Electron Microscopy), FTIR (Fourier Transform Infrared Spectroscopy) and HPLC (High Performance Liquid Chromatography). The effect of operating conditions on the particles size and morphology was evaluated. Based on the HPLC analysis, the extract mainly contained curcumin. By using this process, the extract microparticle had smaller size than standard curcumin (6.42 ± 3.45 μm) and PVP (33.25 ± 18.83 μm). Microparticle extract with size ranging from 0.6 – 5.95 μm were successfully formed. The particle size decreased as the temperature increased, whereas pressure did not significantly affect the particle size or morphology. The best result was predicted at a pressure of 12 MPa, temperature of 40oC, and 2% PVP concentration in solution. Extract microparticles have amorphous form and it does not form crystal. From the experimental result, it can be concluded that SAS method with an addition of PVP is very effective to reduce the size of particles and increase the bioavailability of extract.
Keywords: Micronization; Curcuma xanthorrizha Roxb; Supercritical antisolvent; PVP; Curcumin;
A mixture of CO2 and a co-solvent is widely employed as a solvent or mobile phase for supercritical fluid extraction, chemical reaction, supercritical fluid chromatography etc. Kinematic viscosity is one of important transport properties, and is required for designing chemical process as well as estimating dimensionless numbers such as Reynolds number, Schmidt number, Prandtl number [1]. A more accurate estimation method for kinematic viscosity is increasingly demanded. The authors have proposed the estimation method using modified Eyring and activity coefficient equations [2-5].
This paper deals with the estimation of kinematic viscosities for CO2 + alkane (decane, tetradecane, hexadecane, octadecane) systems at high pressures using the modified Eyring and Wilson-VISCO model by the following equations [6].
where
The correlated results are compared with those of McAllister model [5] based on Eyring model..
where
The kinematic viscosities for ternary systems (CO2 + alkane + alkane) were then predicted using binary parameters.
References:
1) B. E. Poling, J. M. Prausnitz, J. P. O'Connell: The Properties of GASES AND LIQUIDS, fifth edition, McGraw Hill (2001)
2) K. Tochigi, K. Yoshino, V. K. Rattan: Int. J. Thermophys., 26, 413 (2005)
3) K. Tochigi, T. Okamura, V. K. Rattan: Fluid Phase Equilib. 257, 228 (2007)
4) H. Matsuda, K. Kurihara, K. Tochigi, T. Funazukuri, V. K. Rattan: ibid., 470, 188-192 (2018)
5) H. Matsuda, K. Tochigi, K. Kurihara, T. Funazukuri, V.K. Rattan: ibid., 492, 137-144 (2019)
6) K. Tochigi et al.: AIChE 2018 annual meeting, Oct.28 – Nov. 2, Pittsburgh (2018)
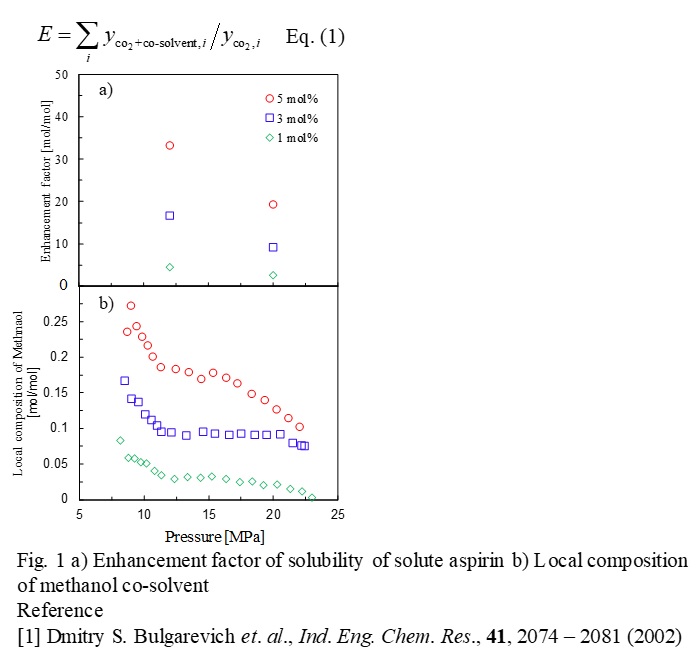
Supercritical carbon dioxide (scCO2) extractions and fractionations with co-solvents can provide many advantages for pharmaceutical and natural foods, which are no solvent residual, mild critical conditions and capability of tuning solvent property by varying pressure and temperature. Solubility data of pharmaceutical compounds are important information to design scCO2 extraction and fractionation processes. The objective of this work is to acquire the solubility data of pharmaceutical compounds in scCO2 with co-solvent and to analyze their behavior of co-solvent adding effect in term of enhancement factors (E: Eq(1)) based on local compositions. Solubility of aspirin derivatives in scCO2 with a methanol were measured at 333 K and 12.0-20.0 MPa by changing the methanol contents. The enhancement factor (E) were calculated from the measured data and correlated with thermodynamic models. Local compositions of MeOH co-solvent (xLMeOH ) were evaluated by considering the similarity of the local composition to the cybotactic region in spectroscopic measurements around an indicator, in co-solvent and CO2 system. The UV-Vis spectral data of the co-solvent with CO2 were obtained from literatures [1]. Figures 1a and 1b show the E and xLMeOH values as a function of pressure. At lower pressures (P < 10 MP), interactions of aspirin with co-solvent methanol were dominant due to low polarity of CO2. The contribution of local composition (xLMeOH ) was significant (Fig.1b). With increasing pressure, solubility of aspirin in CO2 generally increased, however, the E values (Fig. 1a) tended to decrease. The reasons for this may be attributable to a decrease in xLMeOH values (Fig. 1b). Polarity of CO2 becomes stronger at higher pressures and consequently strengthen the interact with the solute, leading to a reduction in xLMeOH values (Fig. 1b). Thus, analysis of solubility based on local composition provides better understanding in molecular interactions.
Activated carbon has been widely used to prevent emissions of volatile organic compounds (VOCs) in industrial processes because of its high adsorption ability. Regeneration of used activated carbon is required for its reuse in the industrial processes with the aim of SDGs. Supercritical carbon dioxide (scCO2) is a promising solvent for the regeneration of activated carbon because of its high diffusivity into the microstructure and ability to operate at a moderate temperature (critical point : 304 K) resulting in less damage to the microstructure of the adsorbent.
We have studied the scCO2 regeneration of the activated carbon used in exhaust facilities of semiconductor production factories, suggesting long used activated carbon could not be regenerated because of high boiling point adsorbates [1]. The experimental results show these high boiling point adsorbates were not removed from activated carbon by the scCO2 regeneration, and these adsorbates were caused by propylene glycol monomethyl ether (PGME) and propylene glycol monomethyl ether acetate (PGMEA) as main VOC components at practical semiconductor manufacturing processes [1]. The activated carbon with PGME and PGMEA was influenced by heat treatments (200-500 °C) at the exhaust facility, and high boiling point adsorbates were observed in the case of heating temperature of higher than 250 °C, which suggested that heating temperature of 200 °C prevented high boiling point adsorbates.
In this study, practical applications of the scCO2 regeneration process of activated carbon used at the exhaust facility of a semiconductor production factory were studied while considering the performance of the exhaust processor with the scCO2 regenerated activated carbon and reduction effects of industrial wastes and CO2 emissions.
Reference
[1] Ito, Y., I. Ushiki, Y. Sato and H. Inomata, Kagaku Kogaku Ronbunshu 45, 29-34(2019)
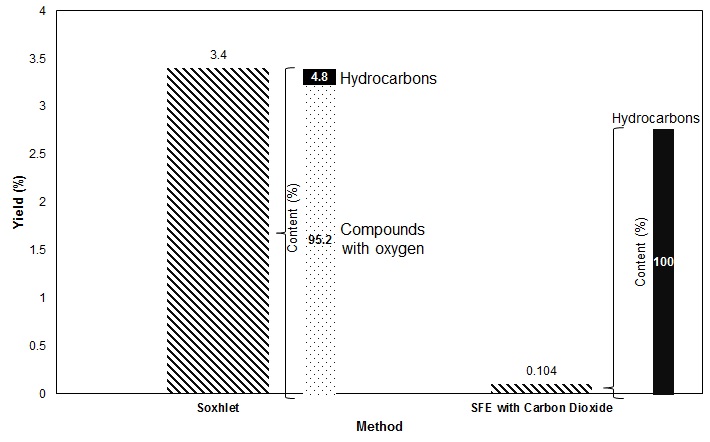
High Density Polyethylene (HDPE) is a type of polyethylene used in a lot of products, from containers for high purity chemicals, package for food to bottles for water. It is impact resistant, long lasting, and easily molded. This type of polyethylene is manufactured at low temperatures and pressures, using Ziegler-Natta and metallocene catalysts or activated chromium oxide (known as a Philip catalyst). In this process, impurities like oligomers, additives, and products of side reactions are generated. These impurities increase the toxicity of the polyethylene, and may migrate to the packed product if found in high concentrations. For the purpose of extracting them, many extraction methods like soxhlet, accelerated solvent extraction, and microwave extraction have been known in the literature. However, these methods have many disadvantages like long extraction times, use a lot of solvent, and require further clean-up of the solvent. To overcome these problems, supercritical fluids have become an alternative because of their tunable solvating properties by changing pressure and temperature. In this research, we use supercritical carbon dioxide because it does not require further clean up, has an easy achievable critical point and functions as a good swelling agent for polymers, which facilitates extraction. We succeeded on extracting hydrocarbons from C16 to C22 at 22 MPa and 60 °C using 1 L/min flow rate and studied the effect of time on the extracted yield. These results were also compared to soxhlet which selectivity for hydrocarbons was found to be less than the shown by supercritical carbon dioxide extraction (see Fig. 1)
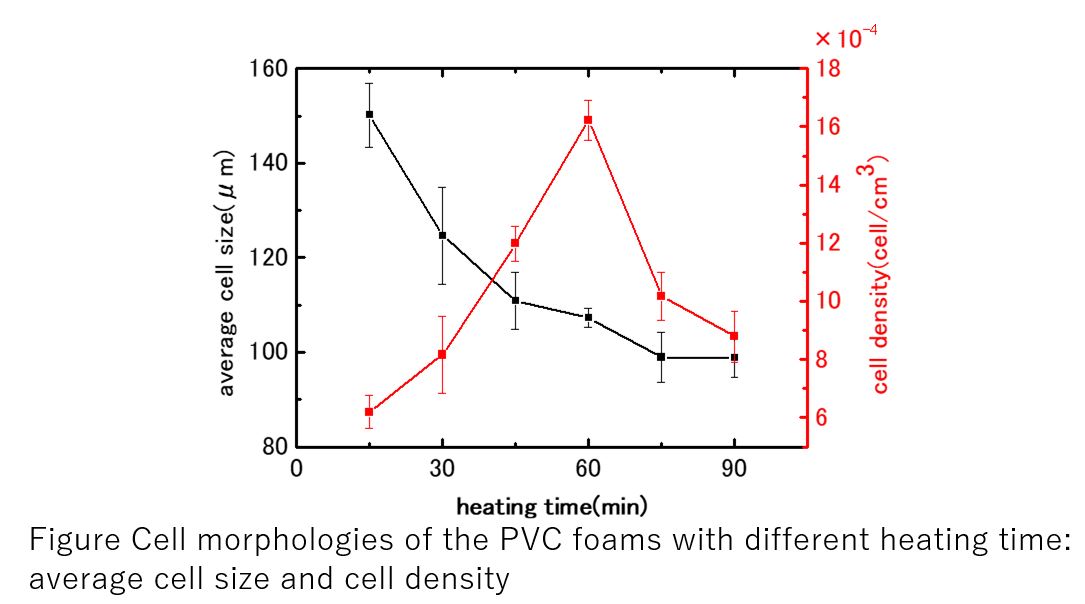
Polyvinyl chloride (PVC) consumes less petroleum, which is an exhaustible resource, and is cheaper than other plastics. Foaming of the PVC is also a subject of interests in engineering because the foam is superior in thermal insulation, cushioning and light weight property. In the forming, use of carbon dioxide (CO2) as plasticizer/blowing reagent has been attracting attentions due to its characteristics which form microcellular plastics. Unfortunately, in the case of the PVC, the PVC does not dissolve CO2, and use of CO2 as plasticizer/blowing reagent is impossible.
In the PVC molding process, the mixture of PVC, organic plasticizer, and stabilizer (antioxidant reagent) was heated up because PVC is a thermosetting plastic. In this study, we are focused on the plasticizer instead of PVC as a CO2 reservoir for the foaming of the PVC during the heating stage. In theory, solubility of the CO2 into the plasticizer is high at low temperature. The CO2 would separate from the PVC mixture during the heating stage which result in the formation of bubbles in the PVC mixture matrix. As the separation of the CO2 and the thermal curing take place at the same time, the control of the foaming process would be difficult.
In this study, we examined the preparation of PVC foam using carbon dioxide as a blowing agent, Diisononyl phthalate (DINP) as plasticizer, and LOX-13 as a stabilizer by a batch process. As described above, foaming and thermal curing occurs at the same time, the structure of the PVC foam strongly depends on the heating time and temperature. So, the relationships between foam characteristics and the heating time and temperature were examined.
Fast flow liquid-liquid extraction process using CO2 microemulsion has been developed to extract and separate hydrophobic compounds from liquid. The process can be adapted to aqueous ethanol solution of a biaryl compound, which is a model of solution obtained after Suzuki-Miyaura cross coupling reaction. Ethanol aqueous solution of the biaryl with salts and CO2 are mixed in a micromixer at 40 °C and 20 MPa, which forms microemulsion of CO2-rich phase/water-rich phase. Because of large interface area of phases fast extraction can be achieved. After extraction, CO2-rich phase and water-rich phase are separated in a separating cell. The level of water-rich phase is maintained by controlling the aperture of a control valve in the bottom outlet line to keep the differential pressure between lines from bottom and top of the cell at constant. Pressure is controlled by a backpressure regulator equipped in the top outlet line. The above process can extract more than 80% biaryl continuously to CO2-rich phase.
This study investigated effects of water/ethanol ratio of the model liquid and extraction pressure on biaryl extraction ratio at 40 °C. Biaryl extraction ratio increased with increasing water/ethanol ratio at 20 MPa. It is probably because the solubility of biaryl in liquid decreased with increasing water/ethanol ratio since the biaryl is a hydrophobic substance. Biaryl extraction ratio increased with increasing extraction pressure. Carbon dioxide density increases with increasing pressure under a constant temperature, so the solubility of the biaryl should increase at higher pressure conditions. Thus, it is considered that the higher solubility in CO2-rich phase resulted in higher biaryl extraction ratio under higher pressure conditions. In the presentation, relationship between biaryl solubilities and water/ethanol ratio or extraction pressure will be discussed based on the biaryl solubility data.
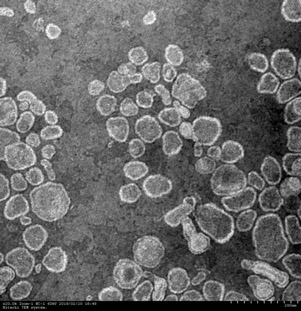
Liposome is widely recognized as an ideal drug carrier for its unique characteristics such as possession of internal space, bio-compatibility and sub-microstructure. In the conventional production methods, multi-step processing and batch production restrict production efficiency. To overcome this problem, flow production method using supercritical carbon dioxide and microfluidic system was proposed in this study.
Supercritical carbon dioxide was used as a main solvent for lipids because of its high bio-compatibility, tunable properties by temperature and pressure and low critical temperature, which is beneficial for the production of thermo-sensitive products. Moreover, supercritical carbon dioxide can be thoroughly eliminated after the process simply by depressurization and therefore, solvent separation step can be omitted from the process. Liposome was produced in the micro-channel, where slug flow of supercritical carbon dioxide phase and liquid phase is formed. Each liquid slug in the flow behaves as a tiny container separated by supercritical slugs and steadily flows along micro-channel with a constant space time. This regularity of the slug flow guarantees that the product properties have no deviation. After the production of liposome, supercritical phase and liquid phase were separated by gravitational force and continuously flows out through metering valves.
Obtained liposome was observed by transmission electron microscope (see figure). Nearly spherical structure less than 1 μm can be recognized. The size of this structure was measured by dynamic light scattering method and confirmed to be controllable in the range of 100 – 300 nm by changing flow rate of raw materials. Drug encapsulation, which defined by the ratio of total drug used in the process and entrapped drug in the structure, was measured by high performance liquid chromatograph. Furthermore, surface functionalization of liposome by chitosan is also achieved using the same method. The external surface of liposome was confirmed to be positively-charged after functionalization by dynamic light scattering method.
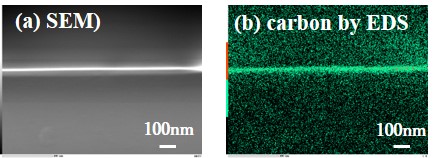
Organic photovoltaics (OPV) gains attentions because of various advantages including light-weight, flexibility, and low-cost. However, OPV suffers from low power conversion efficiency (PCE). To improve PCE, ordered bulk heterojunction (OBHJ) has been proposed as an ideal structure, in which p/n-types organic semiconductors are deeply interdigitated in nano-scale. Nevertheless, conventional technologies can fill only shallow features with the aspect ratio below 3. Meanwhile, we have developed temperature-driven supercritical fluid deposition (TSFD), which successfully filled micrometer-scale trenches (aspect ratio of 10) with anthracene. Accordingly, TSFD will be the only solution if it can fill the nano-scale high-aspect-ratio features with organic semiconductors. In TSFD, organic semiconductor is dissolved in hot supercritical CO2 and transferred to a cooled substrate under the constant pressure. The temperature drop causes supersaturation, resulting in precipitation and crystallization of the solute on the substrate. By carefully controlling the temperature drop, deposition can proceed in quasi-equilibrium, ending up with excellent crystallinity. High concentration supply of the solute leads to relatively high growth rate. As the process should start with ultra-thin and continuous film formation, followed by filling the remaining features, initial growth behavior during TSFD of tetracene was examined in this work. Dependences of supersaturation, supply concentration, and substrate temperature were studied. Small grains were found on the substrate besides the film, but completely eliminated by suppressing the flow rate fluctuation and adjusting the substrate temperature. Ultra-thin tetracene film was therefore formed with no grains. Under ultraviolet light irradiation, green emission from tetracene film was observed on the entire substrate surface. The thickness was 30 nm as shown in the figure. X-ray diffraction revealed the strong (001) orientation. The lateral size of the film was several orders of magnitude larger than those fabricated by the conventional methods, which is preferable for high PCE. Filling nano-scale high-aspect-ratio features will be realized.
High-temperature and high-pressure water has been recognized as a green chemical medium for monosaccharide reactions because dehydration and/or retro-aldol condensation can proceed without the use of a catalyst. There are some reports on the monosaccharide containing the hydroxyl groups (-OH) such as glucose and fructose. We previously reported the reaction of N-acetyl glucosamine, which contains an acetoamide group (-NHCOCH3). In this work, we investigated the reaction of sialic acid and fucose, which are monosaccharides; however, there is no report on these two monosaccharides previously. Sialic acid contains both an acetoamide group (-NHCOCH3) and a carboxyl group (-COOH). Fucose contains a methyl group (-CH3). The dehydrations of sialic acid and fucose were proceeded in high-temperature and high-pressure water. In addition, the chemical structures of the dehydration products were affected by the functional group contained in sialic acid and fucose. From the comparison of the sialic acid, fucose, and other monosaccharide, we will discuss the effect of functional groups on monosaccharide reactions in high-temperature and high-pressure water.
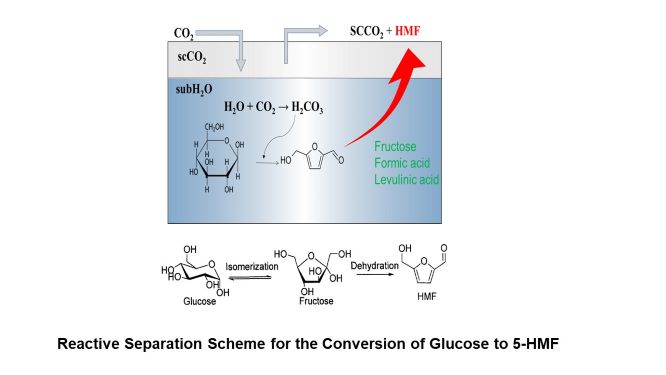
The declining amount of petroleum resources has prompted researchers worldwide to explore various alternatives including biomass as feedstocks. In this context, 5-hydroxymethylfurfural (5-HMF) has attracted growing interest because it can be produced from biomass-derived sugars, and has the potential to be further converted into fuels and fine chemicals. Acid-catalyzed conversion of fructose to 5-HMF has been widely investigated. However, glucose is more cost-effective and readily available. This research focuses on the conversion of glucose into 5-HMF employing the synergy of subcritical H2O and supercritical CO2. Carbonic acid resulting from the synergy of supercritical CO2 and subcritical H2O could acted as an acid catalyst. The operating conditions were optimized, and addition of alcohol such as isopropanol and low transition temperature mixtures was also investigated. In addition, combined reactive separation technique was also employed to further increase the yield. In this approach, since 5-HMF has low polarity, scCO2 dissolve 5-HMF. Thus, continuous CO2 flow enables simultaneous reaction and separation, and could also suppress the formation of by-products.
The highest yield (30.4%) and selectivity (62.8%) was obtained under the condition of 2 wt%, 200 o C, 20 MPa, 1.5 h. This was likely due to the higher solubility of 5-HMF in scCO2 at higher pressure. Addition of isopropanol also had positive effect on the yield of 5-HMF. These results suggest the applicability of adding isopropanol and the use of reactive separation approach employing the synergy of subcritical H2O and supercritical CO2 to the conversion of glucose to 5-HMF.
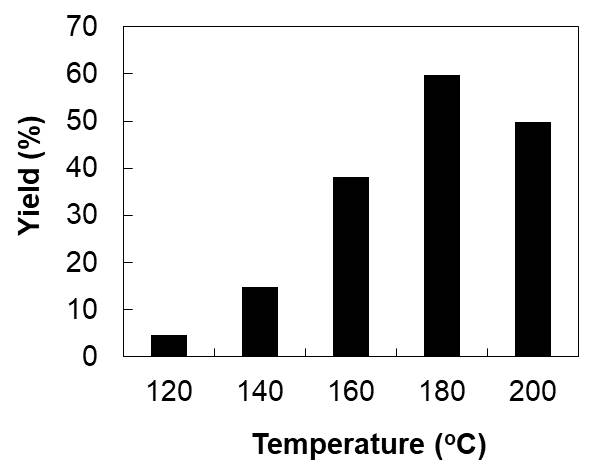
Rutin (quercetin-3-O-rutinoside) is a flavonoid glycoside which is widely distributed in many plants such as buckwheat and onion. It has several functional properties such as antioxidant, anti-inflammatory, anticarcinogenic and other effects in human body. However, it has low absorption efficiency in the body and lower antioxidant activity and bioavailability compare with its aglycon, quercetin. Quercetin can be obtained via hydrolysis of the glycosidic bond between quercetin regidue and rutinose one. Conventionally, a liquid acid catalyst such as hydrochloric acid is used for the hydrolysis. Although the high yield of quercetin can be obtained by using the conventional method, there are some problems such as high impact to the environment and high cost due to considerable after treatments such as neutralization. Therefore, in this study, solid acid catalysts like graphene oxides (GOs) were employed as alternative catalysts and microwave (MW) irradiation was used as a tool of rapid heating of the starting material. The objectives of this study are to explore suitable operating conditions for high efficient hydrolysis of rutin and to develop a new isolation process of quercetin after the hydrolysis of rutin under hydrothermal conditions. As a result, we found that relatively high yield of quercetin was achieved at hydrothermal conditions (160-180oC) for 15-30 min in the presence of graphene oxide with microwave irradiation as shown in Fig. 1. In the conference, a suitable reaction and separation conditions for higher yield of quercetin by using this technique.
Development of drug delivery systems (DDS) and regenerative medicines (RM) are important in improving the quality of life (QOL) of the patients in medical care. Hyalurinic acid (HA) is a biocompatible polymer with high molecular weight (~600 KDa) alternating co-polymer consisted of two saccharide repeating units. HA have properties such as strong water retaining ability, high viscosity, gelation, biocompatibility and biodegradability which makes it a promising material for drug delivery systems and regenerative medicines. It is known that desirable forms and functions can be designed by controlling the molecular weight of the HA. Current methods for degrading the HA are by enzymes or acid or base degradation which require multiple steps, toxic chemicals and long reaction times (hours). In this work, we studied the decomposition of HA in high temperature water without any catalysts using batch type and flow type reactors. Results revealed that the hydrothermal treatment of HA with long reaction times lead to low molecular weight HA with different chemical structure from the initial HA and undesirable decomposion products. On the other hand, the hydrothermal treatment of HA conducted at short reaction times (below 10 s) using the flow reactor lead to low molecular weight HA having the initial chemical structure and no decomposition products. The estimation of molecular weight of the hydrothermal treated HA was possible by a kinetic model assuming random depolymerization. Hydrothermal treatment using a flow reactor system is an efficient method to obtain low molecular weight HA without chemical changes in the product HA and undesirable degradation products.
The catalytic activities of metal/metal oxide depend strongly on their exposed crystal facet. However, the active facet is thermodynamically unstable and the catalyst deteriorates with morphological change during the usage. Therefore, the regeneration process of facet-controlled catalyst has significant importance in the chemical engineering.
In previous study, it is reported that supercritical hydrothermal treatment with carboxylic acid enables the morphological control of AlOOH during the dissolution-recrystallization process [T. Fujii et al. Crystal Growth and Design, 16, 1996–2001 (2016)]. In this study, to develop the method for reactivation of degenerated catalyst, the change of exposed facet for ceria nanoparticles under supercritical hydrothermal condition with carboxylic acid is investigated.
In the experiment, spherical ceria nanoparticles was treated under supercritical hydrothermal condition at 400 °C with decanoic acid in batch-type reactor. As the modifier concentration increased, the morphology of nanoparticles changed from spherical to cubic shape exposing (100) facet. Moreover, the size distribution became narrower and the nanoparticles below 4 nm disappeared after supercritical hydrothermal treatment at higher modifier concentration. This narrowing of the size distribution accompanying with the morphological change from spherical to cubic implies that the morphological change was induced by the dissolution-recrystallization process affected by the organic modifier. Our previous study revealed that the in-situ surface modification by organic molecules during nanoparticles formation in supercritical hydrothermal condition enables the formation of ceria nanocubes exposing (100) facet, having high catalytic activity [J. Zhang et al. Advanced Materials, 19, 203–206 (2007).]. These results indicate that morphology and exposed facet can be controlled not only by in-situ surface modification, but by the post-treatment with organic modifier.
In APPChe 2019, we will discuss on the detailed mechanism of morphological change during the supercritical hydrothermal treatment and the feasibility of this method for the reactivation of facet-controlled catalysts.
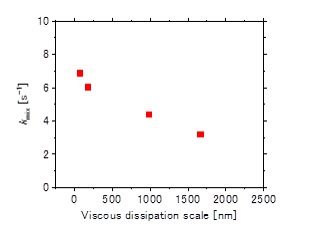
Reaction kinetics of hydrothermal synthesis was evaluated through the formation of nickel oxide nanoparticle as a model in the wide range of high-temperature compressed water. Mixing state in supercritical hydrothermal synthesis was considered to find reaction control conditions. Rapid mixing can be achieved by low kinetic viscosity with small eddy size. Figure 1 shows the relationship between the mixing rate and Kolmogorov scale at mixing point; rapid mixing achieved with short mixing length in supercritical water. Based on the mixing study, the reaction rate of nickel nitrate was conducted changing pressure and temperature. Acceleration of reaction rate in the supercritical region was observed and investigated in terms of the solvent effects with the changes in dielectric constant using Kirkwood model. The role of water in terms of reactant was also investigated, and it was found that the reaction is retarded under dilute conditions where the water dissociation suppressed such as high temperature or low pressure. The fundamental kinetics study would pave the way for the practical use of supercritical water as a medium of nanomaterials synthesis.
With the advent of electric cars that do not consume any petroleum fuel and the commercialisation of shale oil, the petrochemical industry is facing a big turning point. There is a need for an economically feasible process for petroleum fuels, which is less burdensome to the environment, and at the same time, makes it possible for a direct chemical conversion to the olefins. Upgrading bitumen through subcritical hydrothermal processing is a promising process. By using physicochemical properties of water at near critical conditions, it is possible to crack the asphaltene to light oils. Besides, since the operating temperature is lower than other processes at a high temperature, there is an advantage of suppression for the coking formation. However, since the operating temperature is relatively low, it is necessary to advance the reaction sufficiently. Therefore, a highly active catalyst capable of activating the reaction at a relatively low temperature is required.
In this study, highly concentrated Cr-substituted CeO2 nanoparticles were synthesised by the nonequilibrium reaction using a flow-type reactor under sub/supercritical hydrothermal condition. The oxygen storage capacity (OSC) of Cr-substituted CeO2 nanoparticles was compared according to the loading amount of Cr and the presence of the surface modifier. In a batch reaction, Cr substitution hardly proceeded, however in the flow-type reaction, more than 30 mol% of Cr was substituted. Moreover, the activity of Cr substituted CeO2 nanoparticles was evaluated using the bitumen upgrading under subcritical water conditions. The weight fraction of asphaltene decreased, and the gases and light oils increased with increasing the concentration of substituted Cr. Finally, by analysing the correlation between the OSC and the product of the nanocatalyst, the possibility of upgrading the low-temperature bitumen is suggested.
In this work, cerium-zirconium oxides particles were synthesized by solvothermal and hydrothermal method at low temperature in order to be utilized as catalyst in the delignification of wood biomass. The experiments were conducted at temperatures of 150oC – 250oC and pressures of 2 MPa – 5 MPa for 2 h in an autoclave made of SUS 316 with internal volume of 100 mL. Water, mixed of water and ethanol (70/30 vol/vol), and mixed of water and ethylene glycol (70/30 vol/vol) were used as solvent, while Ce(NO3)3 and ZrO(NO3)2 with 0.06 M concentration were used as precursor. The synthesized products were dried at 60 oC for 6 and then calcined at 500 oC for 6 h. The particle products were characterized using SEM and XRD. Furthermore, the particle products were used for hydrothermally delignification process of wood biomass. The addition of cerium-zirconium particles dramatically increased % lignin removal of rapeseed wood up to 97.6%. Based on the result, cerium-zirconium oxides particles are effective for pre-treatment of wood biomass in bio-refinery application. Moreover, cerium-zirconium oxides may reduce the use of chemicals in the delignification process.
Plant derived biomass has been focused as chemical feedstocks as well as an alternative fuel. Cellulose can be converted to glucose and cellooligosaccharides, which are ingredients for chemicals, pharmaceutical, dietary foods etc. However, due to its high crystallinity caused by inter- and intra-molecular hydrogen bonding it is not easy to convert to monosaccharide and oligosaccharides efficiently. Thus, a new efficient process to convert cellulose to monosaccharide and oligosaccharides is demanded. Hydrothermal conversion is one of the promising methods. In this study, filter paper as a cellulosic sample was hydrolyzed using a batch reactor under hydrothermal conditions. The effects of temperature, alkali species and the concentration on product distribution, reaction rates and the degree of polymerization (DP), crystallinity index (CrI) of hydrolysis residue were investigated.
In a batch reactor (volume 3.5 mL) ca. 0.05 g- filter paper and ca. 2.5 g- alkali solution were loaded at room temperature. Alkalis used were methylamine, dimethylamine, trimethylamine, ethylamine, diethylamine, triethylamine, propylamine, n-butylamine, s-butylamine, t-butylamine, pyridine, sodium hydroxide, aniline, benzylamine and ammonia. The hydrolysis experiments were carried out from 513 to 553 K. The product yields were measured by high performance anion exchange chromatography. The major products were glucose, cellobiose, fructose, levoglucosan and 5-hydroxymethylfurfural. Total organic carbon in the solution was also measured by a TC analyzer. The hydrolysis residues were analyzed by X-ray diffraction and CrI. The CrI values were calculated from the equation of segal1).
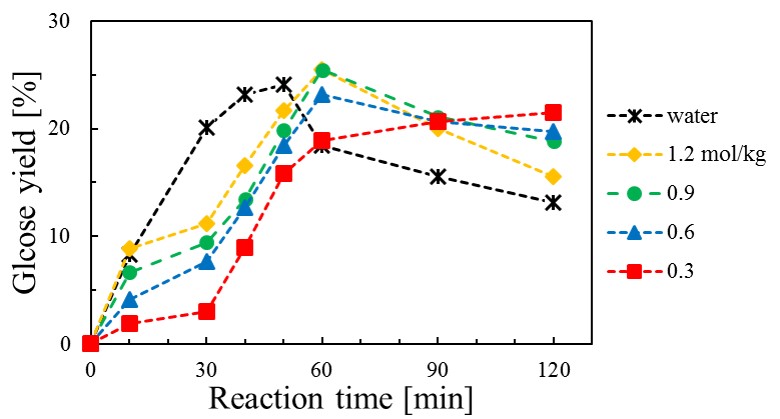
Fig.1 shows the effect of pyridine concentration on the glucose yield at 533 K. Glucose yield decreased after 60 min at pyridine concentrations above 0.6 mol/kg. We conclude that further decomposition products such as levoglucosan and 5-hydroxymethylfurfural were produced after 60 min.
1) L. Calvo et al., Ind. Eng. Chem. Res., 41, 6503 (2002).
Keratin is a protein contained in human hair. The keratin contains 17% of the sulfur-containing amino acid cysteine, which could form disulfide bridges. Recently, low-molecular-weight keratin peptides are receiving a lot of attention in cosmetics and hair repair agents because of their high affinity to the skin and hair. At present, the low-molecular-weight keratin peptides are produced by the enzymatic hydrolysis of the keratin raw materials such as feathers. However, the enzymatic hydrolysis of the human hair keratin is difficult because the cuticle keratin protects the surface of the human hair.
In this work, we conducted the hydrothermal treatment of the human hair to hydrolyze keratin proteins. Since the hydrothermal treatment uses only water, the separation of the enzyme and the peptide is not needed and the peptide is safe as a hair repair material. The influence of reaction conditions (temperature and treatment time) on the molecular weight was studied.
0.1g of human hair and 1 mL of water was loaded into a stainless 316 tube reactor (inner volume 6 cm3). The rector was immersed into a sand bath at a temperature of 150, 180, and 250 °C. After 5~30 min, the reactor was removed from the sand bath and rapidly quenched in a water bath to room temperature. After solid and liquid products were separated by a filtration, the molecular weight of the products in the liquid phase was measured by the size-exclusion chromatography (SEC).
The low-molecular-weight keratin peptides could be obtained from human hair by the hydrothermal treatment. The low-molecular-weight keratin peptide with a peak at around 3.0 kDa was obtained at 150 and 180 °C. At 250 °C, the peptide with a peak at around 1.0 kDa was produced. The effect of the molecular weight of the keratin peptides on the repair capacity is also studied.
Chitin is the second most abundance resources after cellulose and mainly contained in the exoskeletons of crustaceans such as crabs and squid pens. Chitin nanofiber (NF) has attracted research interest in medical materials and healthy foods in recent years because chitin NF has large specific surface area and high biocompatibility. Furthermore, there are two types of chitin depending on crystalline structure. α-Chitin has antiparallel molecular chains with hydrogen bonds between molecular chains. β-Chitin has parallel molecular chains without hydrogen bonds between intermolecular sheets. Therefore, nanofibrillation of β-chitin is easier than that of α-chitin because of the absence of hydrogen bonds. In our previous report, we have prepared β-chitin NF dispersions with high viscosity and high transmittance from β-chitin powder; however, α-chitin could not be disintegrated into such NF dispersion due to many hydrogen bonds. In addition, we have obtained self-sustaining hydrogels from β-chitin NF dispersions by hydrothermal treatment; however, it is difficult in the case of α-chitin. If the gelation of α-chitin NF can be achieved, mass production for commercialization will be realized because α-chitin is more abundant in nature than β-chitin. Thus, it is important to establish the gelation method of α-chitin NF by hydrothermal treatment.
In this work, we obtained the α-chitin NF dispersions with high viscosity and high transmittance by reducing degree of N-acetylation and adding acids. The disintegration of α-chitin powder into NF was conducted with a water jet machine (Star Burst System, Sugino Machine Co, Ltd, Japan). The prepared α-chitin NF dispersions showed high viscosity and high transmittance. In addition, the hydrothermal treatment of the prepared α-chitin NF dispersions was conducted at 160 °C for 60 min by using a sealed reactor. After the hydrothermal treatment,α-chitin hydrogels with high mechanical strength could be obtained.
Supercritical carbon dioxide (scCO2) dyeing for textile and polymer processing is considered as a green and energy-saving process for view-points of environmental friendly and sustainable development. We developed a compact apparatus dyeing for plastic buttons of acrylic, polyester, nylon, and casein in SC-CO2 and carried out dyeing plastic buttons with C. I. Disperse Red 22. We could successfully dye the plastic buttons without cracks, bubbles, and black spots over the temperature ranges of 313.15 to 328.15 K and pressures of 8 to 14 MPa for acryl, the temperatures of 383.15 to 398.15 K and pressures 10 to 16 MPa for polyester, and 353.15 to 383.15 K and pressures of 8 to 14 MPa for casein with dyeing treatment time of 30 to 60 min, and the temperatures of 383.15 to 403.15 K and pressures of 21 to 29 MPa for nylon with dyeing treatment time of 60 to 100 min. The dyeing color depth of dye sorption in the buttons were measured by a spectral color difference meter. Also we measured the solubility of C. I. Disperse Red 22 over the temperature and pressure ranges of 353.15 to 398.15 K and 15 to 25 MPa. The K/S values for plastic buttons were correlated by a regression model, which was formulated by combining Fick diffusion equation with Henry-Langmuir type of surface adsorption of buttons in the present work. Using the model, we examined to represent the experimental K/S in terms of temperature, pressure, density, solubility of dye, and dyeing time. Finally, we compared K/S value by the regression model with those obtained by a multiple regression model expressed in terms of temperature, pressure, density, solubility of dye, and dyeing time and the range analysis of experimental design method previously reported.
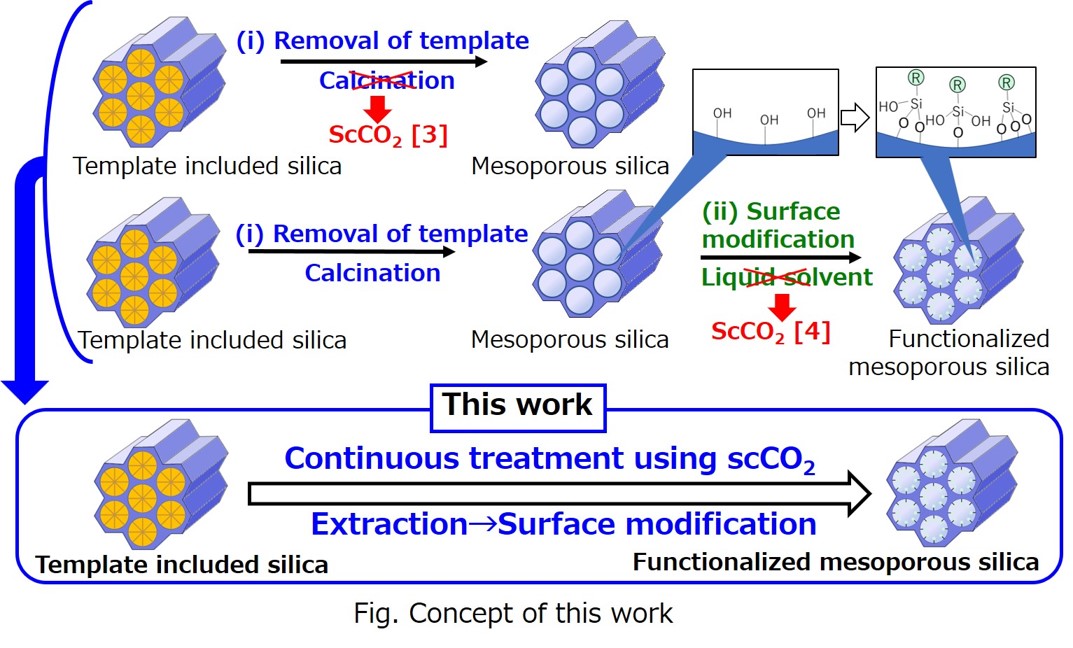
Mesoporous silica materials such as MCM-41 [1] and SBA-15 [2] have been widely used in various applications because of its uniform pore structure, large pore volume, and large specific surface area. Mesoporous silica materials are generally prepared by removing template agents by calcination processes from the template included silica (step (i)). Then, functionalized mesoporous silica can be obtained by the surface modification of silanol groups on the surface of the material using silane coupling reagents (step (ii)) with liquid solvents. However, these conventional methods have some drawbacks such as the possible shrinking of pore structures, disappearance of silanol groups, and low penetrability of solvents into the mesopores.
To overcome these drawbacks, in the present study, the novel preparation method of functionalized MCM-41 mesoporous silica was developed using supercritical carbon dioxide based on our previous works [3, 4], which can achieve continuous treatment of extraction of the template (step (i)) and surface modification of mesoporous silica (step (ii)). The functionalized MCM-41 prepared by the continuous treatment method demonstrated more uniform pore size distribution and higher amount of modification by silane coupling reagents in comparison with the conventional methods.
References
[1] C.T. Kresge et al., Nature, 359 (1992) 710-712.
[2] D. Zhao et al., Science, 279 (1998) 548-552.
[3] I. Ushiki et al., Kagaku Kogaku Ronbunshu, 37 (2011) 512-517.
[4] I. Ushiki et al., Kagaku Kogaku Ronbunshu, 38 (2012) 391-396.
Spent coffee ground (SCG) is regarded as a food waste. However, SCG contains various kinds of high-value phytochemicals such as diterpenes, polyphenols and lipids. Diterpenes present beneficial effects towards human health. Diterpenes can protect skin from the harm of UV, and they are anticarcinogenic, anti-inflammatory and antioxidant. These features of diterpenes make them valuable in both cosmetics and pharmaceutical industries. In this research, we extracted cafestol and kahweol from SCG using supercritical CO2 with ethanol. To maintain stability of the extracts and also to increase their solubility and dissolution rate in water, we studied the micronization of extracts containing cafestol and kahweol with PVP using supercritical CO2. As cafestol and kahweol are sparingly soluble in water, combining it with PVP (water-soluble polymer) and transforming it into small particles can improve the solubilities in water to enhance their absorption in human body. The main objectives of this work are to extract cafestol and kahweol from SCG and to micronize it with PVP using supercritical CO2. The response surface methodology (RSM) with Box-Behnken design was employed to study the effects of extraction parameters on the extracts. The ranges of three parameters are temperatures (40-60 °C), pressures (10-30 MPa), and ethanol flow rates (0-0.5 ml/min). The predicted optimum total diterpenes amount is 65.81 mg/g-extract at temperature of 80 °C, under operating pressure of 30 MPa, and with the ethanol flow rate at 0.3 ml/min. The micronization process was carried out by supercritical anti-solvent process under 40 °C, 25 MPa, CO2 flow rate (15 ml/min) and solution flow rate (0.25 ml/min). Cafestol and kahweol were successfully encapsulated into PVP particles with diameters ranging from 0.25 - 0.05 μm.
Bio-oil is often extracted from biomass by liquid solvent or supercritical carbon dioxide. However these extraction methods have several disadvantages. Liquid solvent extraction using hexane can be operated at an atmospheric pressure, but the extracted oil includes the impurities such as phospholipid and wax owing to the strong solvent power of hexane. On the other hand, supercritical carbon dioxide can extract bio-oil without impurities, but it requires high pressure condition. In this study, the expanded hexane with carbon dioxide is examined as a new extraction solvent. This solvent is diluted liquid hexane with carbon dioxide and it has less solvent power and higher selectivity of neutral lipid than normal liquid hexane does. Therefore the expanded hexane is expected to dissolve nonpolar oil and not to dissolve polar oil such as phospholipids.
The objective of this research is the development of new extraction technique of bio-oil from biomass using expanded hexane with carbon dioxide, where rice bran is used as a target biomass. The extraction was carried out at 25 oC, 4-5 MPa and 0.8-0.95 of molar fraction of carbon dioxide in expanded hexane with carbon dioxide. At 25 oC, 5 MPa, 0.86 of molar fraction of carbon dioxide, the oil yield was 1.2 times greater than that with normal liquid hexane because of the low viscosity and the high permeability of expanded hexane. Furthermore the phosphorus concentration in extracted oil was 6 ppm. It was very low level, comparing with that in rice bran, approximately 230ppm. This was because the solvent power of expanded hexane to neutral lipid decreased a little and that to polar components decreased much. As the results, expanded hexane with carbon dioxide could extract the phosphorus-free bio-oil with high yield under mild conditions.
Strawberry is one of the most popular fruits in Japan and its production is about 160 thousand tons per year. The leaf of strawberry is dumped after harvest. However, the leaf has high antioxidant property. Therefore, the extraction of antioxidants from the leaf of strawberry is expected as a production of new healthy food and effective utilization of biomass. In this study, the leaf of strawberry was extracted with a mixture of supercritical carbon dioxide and water that are non-toxic and harmless solvent.
The extraction experiments were conducted with semi-batch extraction system. At first, the leaf was introduced in the extractor, and then carbon dioxide and entrainer was supplied to the extractor. The extraction pressure was controlled with a back pressure regulator. The extract recovered was dried and weighted. The antioxidant capacity was evaluated by DPPH (2,2-diphenyl-1-picrylhydrazyl) radical-scavenging method on the basis of the amount of 2,6-di-tert-butyl-p-cresol (BHT), and the total phenolic content was evaluated with Folin-Ciocalteu method on the basis of the amount of gallic acid (GAE). The effect of entrainer was evaluated at 308 K and 20 MPa. The existence of water as an entrainer gave higher antioxidant capacity and the amount of total phenolic content of the extract than those in the absence of entrainer. At 20 MPa, antioxidant capacity and amount of total phenolic content tended to increase with increasing temperature. The extraction of antioxidants enhanced in high temperature region. At 313 K, the antioxidant capacity and the amount of total phenolic content tended to decrease with increasing pressure. The optimal condition for antioxidant capacity and the amount of total phenolic content was at 313 K and 20 MPa up to 333 K and 30 MPa.
Recently, attention is now focused on effective utilization of biomass, which is abundant in resources as a substitute resource for fossil resources. It is desirable to use woody biomass which is non-food biomass since the population is increasing, the use of food-based biomass may have new food problems. However, it is difficult to convert woody biomass to useful substances, and because high temperature processes are frequently used, a problem as high energy cost occurs. In recent years, therefore, attention is focused on chemical conversion of woody biomass using supercritical fluid technology. Based on the above background, attention was paid to tea fruit residue which is waste that don't cause food competition and capable of extracting useful components such as lipids or fatty acids in the previous research. The purpose of this work was to search conditions for high yield of lipids by extracting useful components from tea fruit residue on supercritical carbon dioxide. In supercritical carbon dioxide extraction, the effects of pressure, temperature and flow rate of carbon dioxide on the extraction yield of oil and lipid class were investigated.
Fluorochemicals have various unique characteristics [1] such as heat, chemical and non-adhesiveness/water repellency. Thus, fluorochemicals are used for various products to apply these characteristics. However, most of fluorochemical wastes are disposed by landfill and incineration above 1000 °C because of C-F bonds having high bond energy and electrical stability [2]. Prior to the disposal fluorochemical wastes should be made because toxic compounds could possibly be emitted in the course of their disposal. Namely, the incineration emits toxic compounds produced such as gaseous hydrogen fluoride, and the landfill elutes fluorine in environment [3]. Thus, a new effective defluorination process has been demanded. Many efforts have been made to remove fluorine from fluorochemical materials: by defluorination under hydrothermal conditions [3], electrochemical [4], and photocatalytic methods [3]. Our previous research found that the addition of ammonia and ammonia or sodium hydroxide were effective for hydrothermal dechlorination of PVC [5]. The objective of this study was to demonstrate the effectiveness of using aqueous basic solution in the hydrothermal defluorination of 4-fluoronitrobenzene. Certain amount of 4-fluoronitrobenzene and a basic aqueous solution were loaded in a small bomb-type bath reactor, and the reactor was heated in a molten salt or oil bath. Various basic species were tested, and sodium hydroxide was one of the most effective among them. The conversion rapidly increased in the early stage, and thereafter did slowly. The reaction rate will be discussed in the presentation.
[1] Maruzen Publishing Co., Ltd., Handbook of Chemistry: Applied Chemistry, 6th ed., 757 (2003)
[2] Moriwaki H. et al., Env. Sci. Tec., 39, 3388 (2005).
[3] M. Nozoe, et al., Env. Eng. Res., 43, 105 (2006)
[4] C. Schaefer, et al., Env. Sci. Technol., 52, 10689 (2018)
[5] K. Hashimoto et al., J. Mat. Sci. 43, 2457 (2008).
This study reports the separation of raw petroleum pitch materials by applying the supercritical fluid technology. The aim is to obtain the mesophase pitch, which is an important intermediate compound for various applications. Those applications include the battery manufacturing and artificial carbon materials. It is required to remove the low molecular weight and complex polycyclic aromatic hydrocarbons (PAHs) compounds from the raw pitch before the production of mesophase pitch. The purposes of this research are: (1) to develop a cleaner separation method for PAHs by using the supercritical fluid technology, and (2) to develop a relatively simple analysis scheme on determining the molecular weight and polydispersity by using the gel-permeation chromatography (GPC) with dual RI and UV detectors.
The separation process included two parts. The first part was the supercritical carbon dioxide extraction of the raw pitch sample. A stage-wise supercritical extraction process was investigated. Three extraction stages were operated at different temperatures. The second separation part used the residual pitch after the above extraction as the input materials. The subcritical anti-solvent (SAS) treatment was employed. In this SAS process, the relatively lower molecular weight part of the residual pitch was further removed. The final carbon dioxide insoluble pitch was obtained by using modified SAS equipment and novel experimental procedures. This final product was examined for its molecular weight and polydispersity. This final residual solid was ready for the next thermal condensation polymerization process. The most appropriate SAS operation condition was reported in this presentation.
A simple and effective analysis approach was reported in this study. The GPC with dual RI and UV detectors were investigated. A satisfactory calibration curve was obtained to determine the molecular weight after each separation stage in this study. The consistency between this analysis result with that from the complex NMR data was demonstrated.
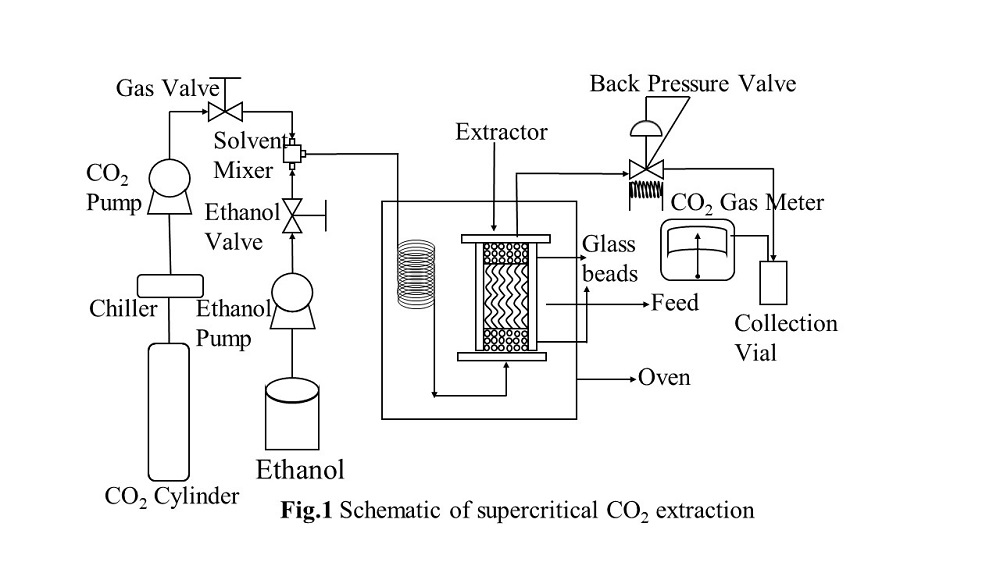
Utilization of microfluidic systems for supercritical CO2 (ScCO2) extraction from the aqueous solution has gotten much attention to be applied for wide industrial fields. This is because the extractions in the microfluidic systems can achieve the high heat and mass transfer rates between ScCO2 and water phases, consequently improving the extraction efficiency. In the microfluidic system, understanding of the fluid flow patterns can be an important approach to clearly explain the extraction behaviour and to design the ScCO2 microfluidic system.
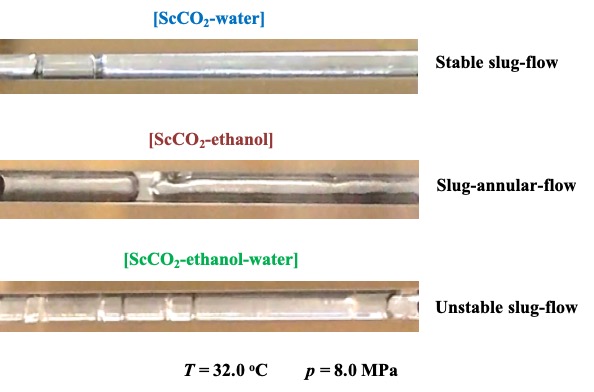
In this study, flow patterns of ScCO2-water and ScCO2-ethanol binary systems, and ScCO2-ethanol-water ternary system, are experimentally visualized in 1 mm of hydraulic diameter of window-square-channel. The flow patterns of the fluids after a micro-swirl-mixing technique are captured under different temperatures (32.0 to 60.0) °C and pressures (8.0 to 20.0) MPa. At near critical condition of ScCO2 (32.0 °C and 8.0 MPa), the mixture of ScCO2 and water formed a stable slug-flow. The flow pattern for ScCO2-ethanol system forms a slug-annular flow. The ternary system of ScCO2, ethanol and water resulted in the unstable slug-flow near the critical point (See Figure). In addition, at all other studied conditions, stable slug-flow with water slug formation are observed under ScCO2-water binary system. However, by changing the temperatures and pressures, different flow patterns are generated under ScCO2-ethanol binary and ScCO2-ethanol-water ternary systems. Wavy-flow, unstable slug-flow and single-phase flow are observed under ScCO2-ethanol binary system. While, ScCO2-ethanol-water ternary system forms the slug-annular-flow, wedging-slug-flow, unstable slug-flow and stable slug-flow at various temperatures and pressures. The formation of these flow patterns can be explained with respect to the phase equilibrium diagrams of ScCO2-ethanol binary and ScCO2-ethanol-water ternary systems.
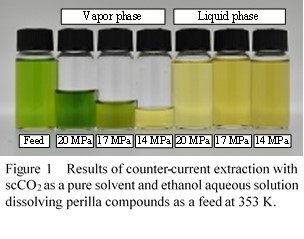
Supercritical CO2 (scCO2) extraction is suitable for the separation/fraction of functional compounds in natural products because it has various advantages of low temperature and non-toxic solvent residue. A co-solvent can be added to increase polarity and density of CO2 solvent, leading to an increase in extraction yield. Extraction using a co-solvent has been mostly operated via a semi-batch type apparatus. Recently, studies on a continuous extraction have attracted much attention from the viewpoint of throughput and operability by contacting scCO2 with natural compound solution. However, there have been reported a few studies related on the continuous extraction for multi-components systems.
In this work, ethanol and water were used as co-solvents for extraction/separation of perilla components via continuous counter-current scCO2 extraction. Among functional compounds contained in perilla, we focused on perillaldehyde, caryophyllene, squalene and rosmarinic acid, and target compounds in separation of perilla-extract are set to chlorophyll and rosmarinic acid. The former is undesirable compound in final products for its unique color, the latter is valuable compound with anti-dementia effect[1]. Figure 1 shows the results of counter-current extraction at 353 K and 14.0 - 20.0 MPa. The results showed that chlorophyll was more extracted in vapor phase as pressure increased, but rosmarinic acid was not found in vapor phase in this pressure range. Thus, chlorophyll could be separated from rosmarinic acid at high pressure condition.
[1] K. Ono et al., J. Biol. Chem., 287, 14631 (2012)
Wastewater including organic solvent is discharged from the cleaning and drying process in semiconductor manufacturing. It is generally treated by the activated sludge method. However, this method has problems such as a long treatment time, the necessity of pretreatment and aftertreatment, the generation of excess sludge. Therefore, the wastewater treatment technique with a short time and less environmental load is expected. In this study, we focused on catalytic oxidation in superheated steam.
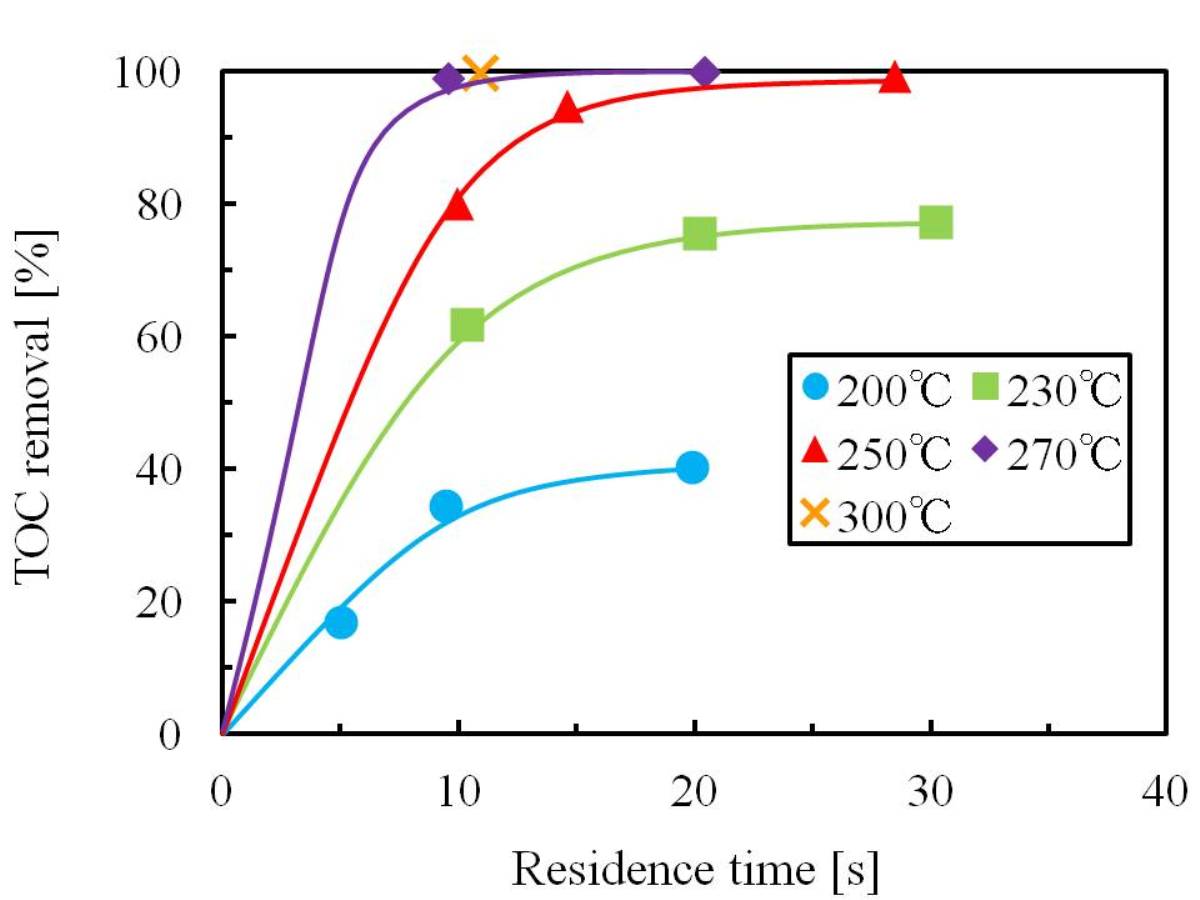
The purpose of this research is the development of wastewater treatment technique by catalytic oxidation in superheated steam. The target substance was isopropyl alcohol (IPA). The experiment was carried out using a plug-flow reactor filled with MnO2 catalyst. The optimum decomposition condition was determined and the kinetics of catalytic oxidation reaction was analyzed. The experimental results were expressed as total organic carbon (TOC) removal.
The dependences of TOC removal on the reaction temperature and residence time were investigated. TOC removal increased with the reaction temperature and residence time. It achieved 99.8 % at 300 °C, 0.9MPa, 11 s and 1.2 of oxidant ratio. On the other hand, TOC removal was constant with long residence time at the low reaction temperatures of 200 °C and 230 °C. It was because acetone and acetic acid were produced by oxidation of IPA, and they were detected as TOC.
The reaction kinetics was examined using the pseudo-first order reaction model, and TOC removal correlated well using this model. Based on the results of this kinetics, the activation energy (Ea) and the frequency factor (A) for TOC decomposition were determined from the Arrhenius equation. As a result, Ea was 56.1 kJ/mol and A was 6.17×104 s-1.
From there results, the catalytic oxidation in superheated steam is useful for wastewater treatment.
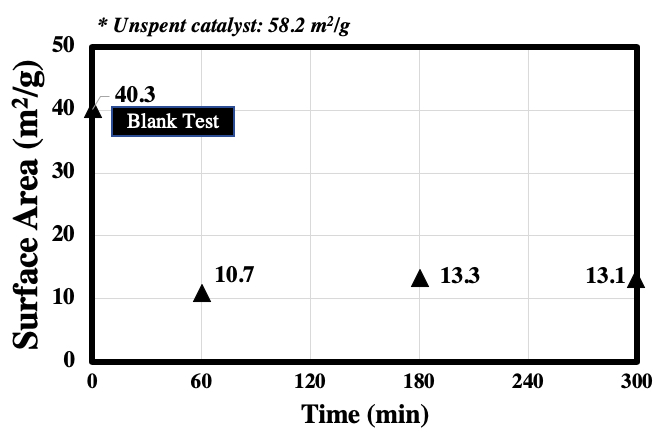
Supercritical water gasification (SCWG) process transforms biomass with high water content into H2-rich gaseous products by utilizing the unique properties of supercritical water at T ≥ 374 °C and P ≥ 22.1 MPa. From the viewpoint of food sustainability, utilizing non-food biomass such as waste cooking oil (WCO) is desirable. So far, its known treatment method is conversion to biodiesel through transesterification and hydrodeoxygenation. However, these processes suffer from low product conversion due to WCO's high water and free fatty acid content. Therefore, SCWG is an attractive option to achieve WCO treatment and bioenergy conversion at the same time. Due to SCWG's high operating requirement, a suitable hydrothermal catalyst is often required. However, the efficiency of this process is currently limited by catalyst deactivation. This study aimed to determine the mechanism of catalyst deactivation in SCWG. Vegetable oil was used as a representative compound for WCO. Experiments were conducted by gasifying vegetable oil using nickel catalysts in lab-scale flow type reactor made of stainless steel tubing. Experimental conditions employed were fixed at 400 °C and 25 MPa at variable feed concentration (2 – 5 wt%) and reaction time (60 – 300 min). The performance of the process was evaluated according to gas yields and efficiencies. Catalyst characterization was carried out before and after the reaction using N2 gas sorption analysis. To probe the catalyst surface, XRD and SEM-EDX analysis were conducted. The results of the study showed that catalyst performance can be observed with the system's ability to convert the biomass into desired gaseous products. Carbon deposition and loss of active catalytic surface area (Figure 1) due to particle sintering were considered as the main causes of nickel catalyst deactivation in the SCWG of vegetable oil.
Food allergy is caused by a specific amino acid sequence of allergen protein. In order to reduce allergenicity of allergen protein, we must destroy specific amino acid sequence by hydrolysis or thermal treatment. Therefore, there are many researches that focus on the amino acid sequence of the protein. On the other hand, it is reported that the molecular weight of the protein determines allergenicity. This is based on a fact that most allergen proteins have a certain range of molecular weight, but there is no scientific basis.
In this study, we focused on the molecular weight of the allergen protein. If the molecular weight of the protein determines the allergenicity, the evaluation of the allergenicity for various proteins become possible because it does not depend on the amino acid sequence of protein.
In Japan, 70% of food allergy are caused by chicken eggs, milk and wheat. Therefore, we selected albumin (chicken egg), casein (milk) and gluten (wheat) as model of allergen proteins. The high-temperature and high-pressure water treatment was applied for the hydrolysis of these protein.
We used a batch reactor for high-temperature and high-pressure water treatment (150 ~ 225 °C and 15 ~ 180 min). The molecular weight was analyzed by size-exclusion chromatography (SEC). The allergenicity was evaluated by Enzyme-Linked ImmunoSorbent Assay (ELISA).
We investigated the relationship between the molecular weight of the hydrolyzed allergen protein and allergenicity. For all the proteins, the reduction of the allergenicity was confirmed with decreasing the molecular weight by high-temperature and high-pressure water treatment. The molecular weight of the hydrolysis product from casein is higher than that from gluten; however, the allergenicity of casein is lower than that of gluten. This is probably due to the difference in the amino acids sequence of the protein.
Extraction of value-added products from biomass such as agricultural residues and food wastes has attracted the attention of persons and organizations interested in establishing a sustainable society. In this study, rice bran and soybean were used for the production of value-added products via supercritical carbon dioxide (scCO2) extraction. The scCO2 extraction was performed at 20 MPa and 40 °C. In the case of rice bran, 15 weight % of dry rice bran was extracted via the scCO2 extraction; 30 weight % of dry rice bran was extracted via the extraction using organic solvent (mixture of toluene and ethanol).
Carbon nanodots (CDs) which are photoluminescent carbon-based nanomaterials doped with nitrogen have attracted much attention to replace inorganic quantum dots because of their excellent properties, such as low cost and low toxicity. For further applications as photoluminescent materials, full color tuning of CDs is required. In this work, CDs were synthesized under various solvothermal conditions with a batch reactor, and relevant parameters for determining the emission wavelength of CDs are discussed.
Phenylenediamines (o-,m- and p-PDAs) was used as the reactant. PDA was dissolved in ethanol at a concentration of 7.3×10-2 M, and sealed in a stainless-steel tube (~10 mL). The high-pressure vessel was placed in an electric furnace, and heated at 150–180 °C for 1–5 h. The crude solution of the product (oCDs, mCDs and pCDs) was diluted by 100, and the emission and excitation spectra and the absorption spectra were measured by a fluorescence spectrophotometer (F-2700, Hitachi). The particle size of CDs was determined from the TEM images. The FT-IR spectra of the CDs were obtained by a spectrometer (FT/IR-4200, Jasco).
The °CDs show green emission at ~540 nm for 350–500 nm excitation, and the pCDs show orange emission at ~580 nm for 370–550 nm excitation, irrespective of the reaction conditions. The mCDs show blue emission at ~500 nm and green emission at ~540 nm depending on the reaction degree. Whereas the particle diameter of these CDs was constant at 2.0–2.5 nm, it was suggested that the chemical structures affect the emission color; suppression of oxidation is related to emission at longer wavelength. The relationship between the optical properties and chemical structure of CDs is discussed in detail at the presentation.
A water-in-supercritical CO2 microemulsion, that is a thermodynamically-stable system of reverse micelles encapsulating aqueous nano-droplets in supercritical CO2, can be prepared by CO2-philic surfactants (e.g. fluorinated surfactants). Due to the solvent properties of water and supercritical CO2, it can be an environmentally friendly and universal solvent able to solubilize both nonpolar and polar substances. However, there are some issues caused by surfactant headgroups in applications of W/C MEs, for example, difficulty to remove surfactants from products, extracts and washings due to a strong surfactant headgroup interactions to those surfaces and compounds.
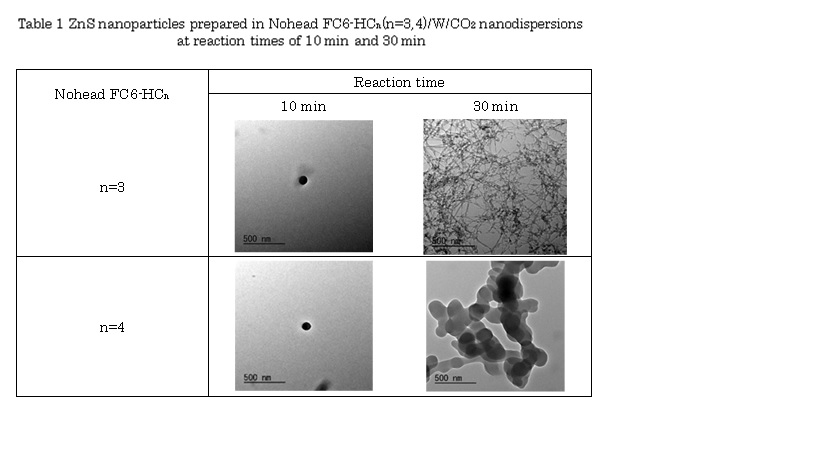
Recently, water-in-supercritical CO2 nanodispersions were successfully prepared by the headgroup-free fluorocarbon-hydrocarbon amphiphile Nohead FC6-HC4 (C6F13-Ph-COC5H11, Ph = phenylene group). The nanostructures of the dispersions were characterized by the high-pressure small-angle neutron scattering (SANS) technique. As the water clusters were able to dissolve water-soluble substances like an ionic dye and salts, it will be a universal green solvent without the issues caused by headgroups. Aiming to develop an effective, efficient and green nanoparticle production technology, this study tested synthesis of inorganic nanoparticles synthesis in water/CO2 nanodispersions with Nohead FC6-HCn (C6F13-Ph-COCH2-CnH2n+1, where n = 3 or 4). Table 1 shows are nanoparticles synthesized in the nanodispersions with 50 mM Nohead FC6-HCn at 200 bar and 45 °C. For ZnS nanoparticles synthesis, the nanoparticles were obtained with reaction times longer than 10 min. Those were spherical with diameters of ~ 100 nm at reaction time of 10 min for both amphiphiles. Interestingly, it became ZnS nanofibers with diameters of ~10 nm for Nohead FC6-HC3 at the reaction time of 30 min, and generated a network structure. Then water/CO2 nanodispersions were found to play a role of nanoreactor.
Nowadays, newly discovered drug candidates which are composed of high-molecular weight compounds have very low solubility in water, resulting in poor or erratic bioavailability. The bioavailability can be improved by reducing the particle sizes of drugs. Supercritical antisolvent recrystallization (SAS) using CO2 has been paid much attention as an effective and environmentally friendly particle design method. SAS process produces particles from a liquid solution by the addition of supercritical CO2 that is partially or totally miscible with the liquid solution and that is a non- or anti-solvent for the solutes. However, conventional SAS processes have problems such as a difficulty of production of small and uniform-sized particles because supersaturation heterogeneity of the solutes in a large volume crystallizer leads to a heterogeneity of nucleation rates and crystal growth rates, resulting in the production of large and ununiform-sized particles. Therefore, we proposed a new SAS using a micro device as a crystallizer (SAS-MD) to solve the problems.
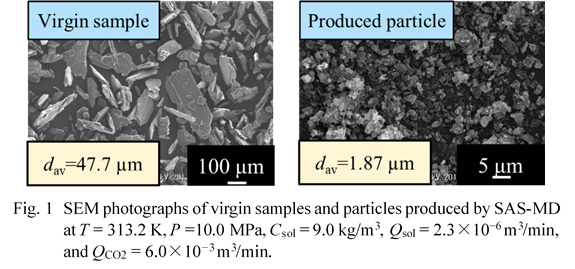
In this work, we aim to elucidate the effects of operation parameters including crystallizer temperatures (313.2–323.2 K), crystallizer pressures (10–19 MPa), liquid solution concentrations (4.0–14.3 kg/m3), liquid solution flow rates (2.3×10–6–2×10–5 m3/min) and CO2 flow rates (3.0×10–3–6.0×10–3 m3/min) on the production of microparticles of sulfathiazole by SAS-MD using a Y-type micro device (internal volume: 1.75×10–10 m3). Scanning Electron Microscope (SEM) images of virgin samples and those produced by SAS-MD are shown in Fig. 1. The particles produced were plate-like or spherical particles with mean sizes of around 2 μm which were nearly 1/22 of a mean size of the unprocessed plate-like samples. SAS-MD caused a polymorph transition from a mixture of form II and form IV to form I. Figure 1 shows the mean sizes of particles by SAS-MD were not mostly dependent on crystallizer temperatures.
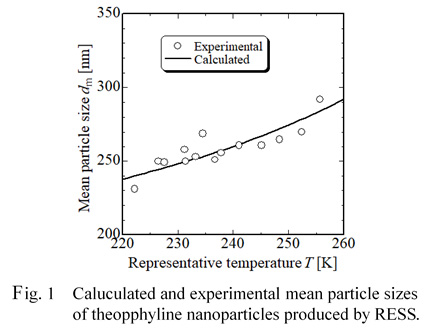
In the pharmaceutical industry, there are great interests in preparation of nanoparticles because particle sizes of drugs are of importance for an enhancement of bioavailability and the application to drug delivery systems (DDS). New process using supercritical fluids have been expected as an effective and environmentally friendly nanoparticle design process. The most well-known micronization technique based on supercritical techniques is the rapid expansion of supercritical solutions (RESS). In the RESS technique, a solute is dissolved in a supercritical fluid, and the solution is then expanded rapidly through an expansion nozzle. Because of the large rapid drop in both pressure and temperature, the solute dissolved becomes insoluble in a low-pressure gas and thus a large degree of supersaturation is created in the spray jet. Rapid nucleation and growth of crystalline particles occur, followed by production of micro- or nano-sized particles due to a very high supersaturation and a very small growth time. We have successfully produced nanoparticles with mean sizes ranging 200 to 700 nm of several drugs including ibuprofen, theophylline, caffeine and phenacetin, and have elucidated effects of several operation parameters in the RESS on the production of the nanoparticles. For practical application of this technique, it is necessary to establish particle design technique.
In this work, we report on a new and simple model to predict mean particle sizes of nanoparticles of several drugs produced by RESS. This model is based on both the mass balance in the RESS process and the classical nucleation theory and has two parameters including supersaturation and a representative temperature which is an average temperature between the temperature at inlet and that at outlet of the crystallization chamber. Figure 1 shows experimental and calculated results of mean particle sizes of theophylline nanoparticles produced by RESS and shows the model give good predicted results.