
One of the essential thermal heat storage is phase change material which is methyl palmitate in this work because of high latent heat and suitable transition temperature range. However, the major problem is supercooling an undesired property in terms of crystallization during cooling. Moreover, microencapsulation preventing liquid form to leak makes phase change material suffer more. Microencapsulated methyl palmitate was synthesized by sol-gel method using tetraethyl orthosilicate as shell initiator, and spray drying process developed it into powder form. Palmitic acid was used as a nucleating agent. Differential scanning calorimetry (DSC) determined what supercooling degree of microcapsules was, and morphology structure and particle size distribution were characterized using scanning electron microscopy (SEM) and laser particle size distribution analyzer (PSD), respectively. The present study aimed to determine if factors of each production process are associated with microcapsules' supercooling degree. They delved deeply in my research consisted of HCl quantity, sol-gel reaction time, types of emulsifiers, homogenizer speed and duration, nucleating agent quantity, and spray dryer's inlet air temperature. The results showed that increasing HCl quantity didn't significantly affect to the supercooling degree, but made latent heat decrease. As for sol-gel reaction time, the more reaction time spent, the less degree of supercooling happened; on the other hand, the more latent heat had. The positive charge of emulsifiers was better microcapsules properties than negative. Supercooling degree at homogenizer speed of 8000 rpm was lesser than of 6000 and 10000 rpm; nonetheless, latent heat was the lowest of them, and three minutes of homogenization is the best. Furthermore, the supercooling degree was decreased by palmitic acid as a nucleating agent, and at an inlet air temperature of 160 °C, it was appropriate for applying to the spray drying process.
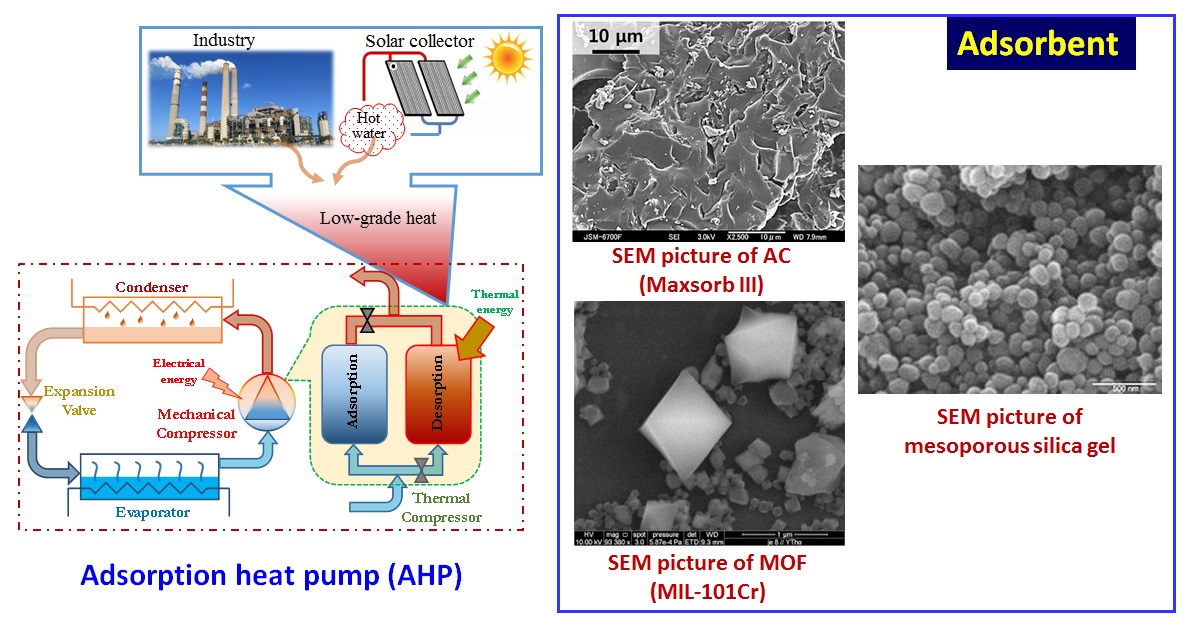
The research on adsorption cooling cycles got momentum after observing the worldwide energy crisis along with the obligation of international protocols which limits the production and utilization of CFCs and HCFCs as refrigerants. This paper presents the synthesis procedure of high grade activated carbons (ACs) derived from biomass precursors, namely waste palm trunk (WPT), and mangrove (M) which are abundantly available in nature. The thermophysical properties and adsorption characteristics of WPT and M activated carbons along with silica gel and metal organic frameworks (MOFs) are presented from the perspective of performance in cooling applications.
Various thermal energy storage (TES) systems have been studied in an effort to increase energy efficiency. Among these are phase change materials (PCMs) which have the advantage of high energy density from their latent heats. Research developments have progressed so far as to identify fatty acids and their mixtures to be materials that have great potential for this technology – given their chemical stability, non-toxicity, reproducible freezing and melting characteristics, low corrosion activity, and the renewable raw materials from which they are derived. The Capric-Lauric eutectic mixture is a PCM that has been thoroughly studied, and has theoretically been proven to have great potential for TES. The study investigates the thermal energy storage performance of the binary eutectic mixture of fatty acids embedded in gypsum wallboards, and experimentally verifies its viability for actual space cooling application. The small-scale test space unit is found to prolong the maintenance of cool internal temperatures (18 °C - 26 °C) for periods two to three times as long with the incorporation of C-L acid in the wallboards of the unit.
In order to utilize the renewable energy such as waste heat emitted from plant and solar heat more efficiently, compact and high performance heat storage system is one of the bottleneck technologies. Heat storage tank filled with ceramic sensible heat storage body have been used as effective utilization for high temperature waste heat > 500 °C from an industrial furnace such as regenerative burner system. However, the development and design of the heat storage body optimized for the temperature range and the heat storage and release rate is not performed yet. In prevalent ceramic heat storage body such as Al2O3 sphere, there were problems that the inside of Al2O3 sphere was not effectively utilized for heat storage. To solve these problems, we proposed newly non-spherical shell structure made of fine ceramics as effective heat storage body. In this research, one-step fabrication of the tetrapod shell structure ceramics body by slip-casting method was introduced. And the heat storage property was evaluated experimentally using the heat storage tank filled with tetrapod shell structure ceramic body.

Energy and water access for off-grid islands has been actively studied due to the difficulty of supplying these resources from the main land. In line with Sustainable Development Goals 6 and 7, the feasibility of supplying an off-grid island with renewable energy and water is often studied. Freshwater can be supplied to off-gird islands by purifying surrounding saltwater via desalination. However, recent studies have shown that there are tradeoffs between the production energy and water. In this work, the feasibility of implementing mechanical vapor compression (MVC), multi-effect distillation (MED), multi-stage flash (MSF), electrodialysis (ED), and reverse osmosis (RO) desalination technologies was investigated on an off-grid island with solar PV, diesel, and lithium ion battery storage. The water-energy system was simulated using the Island System LCOEmin Algorithm (ISLA) software, which has been validated with HOMER Pro. Principles in chemical engineering was used by the software to determine the energy consumed by the desalination technologies per unit of water produced. The distribution of energy between generation, storage, and desalination units was determined via energy balance. The optimum configuration was determined by iteratively using different component sizes. The optimum component sizes, levelized cost of electricity (LCOE), levelized cost of water (LCOW), and power flows were analyzed and policy recommendations on desalination implementation were made. PV+Diesel systems produced the lowest LCOE and LCOW, which is ideal because many off-grid islands are transitioning to this setup. Reverse osmosis plants produced the lowest LCOW.
A novel rotating coil-shaped spiral gas-solid contacting device was proposed. The authors propose to make this spiral tube of 180 degree elbows which are commercially produced according industrial standard as piping parts. This device is considered to be suitable for biomass gasification because this enables good contact between volatile matter and solids so that tar can be decomposed over solids. Also external heating through the wall can be carried out with less thermal expansion stress. The solids in the spiral can be mechanically transported with rotation of the spiral, thus sticky material can be handled. Cold models of spiral structure were made by combining 180 degree elbows of half octagonal shape using ID 24 mm transparent plastic tube. A 1.5-cycle model which was driven by hand and a 5-cycle model which was continuously driven by a motor were used. As solid materials, three types of spherical glass beads and two types of spherical silica-gel particles were employed. In addition, one kind of non-spherical plastic pellet was used. The solids transportation rate by volume per rotation was determined to be about 30 cm3 for spherical particles irrespective of particle density, but the rate for non-spherical particles was lower. Solid back-mixing was evaluated by adding a batch of tracer particles with different color at the inlet of the 5-cycle model. The number of tracer particles was counted after taking the samples at the exit of rotating spiral reactor for each rotation. As an index to describe the back-mixing, the probability (p) of particles moving from one unit to the next with one rotation was proposed. The probability was higher than 0.9 for all particles. Average residence time and residence time distribution were derived from the probability.
Figure: Photograph of coil-shaped spiral reactor conveying blue silica-gel particles

Electrocatalytic hydrogen evolution reaction (HER) is used as a hydrogen production technology from electricity. Although platinum cathode is known to exhibit the highest HER activity at acidic condition, development of alternative cathode materials is expected from the viewpoint of resource constraints. Recently, metallic MoS2 nanosheets have attracted attention as a candidate of the cathode material. However, synthesis of metallic MoS2 is difficult due to its metastable phase and hence only a few of the bottom-up synthesis method has been reported. In this study, we fabricated metallic MoS2 thin film on glassy carbon (GC) substrate for HER cathode by hydrothermal electrodeoisition and conducted the detailed characterization.
To deposit metallic MoS2 thin film on GC, a mixed aqueous solution of (NH4)2MoS4 and KCl as the electrolyte was injected into the specially designed reactor with two electrodes system (cathode GC and anode gold foil). The reaction condition was set to 200 °C and 10 MPa, and a voltage was applied at 1.5 V for 1 minute.
The SEM observation showed an edge-exposed MoS2 thin film. XPS analysis revealed the formation of metallic MoS2 because the binding energy of Mo3d was shifted to the low energy side by about 1 eV. Although a hydrothermal synthesis of metallic MoS2 in a previous report required 12 hours of the reaction time, our synthesis requires only 1 minute-electrolysis. This can be attributed to the electrochemical reaction promotes the reaction to generate MoS2 from MoS3. In order to ascertain HER activity, we carried out linear sweep voltammetry with the three-electrode method (working: fabricated MoS2, counter: Pt and reference: reversible hydrogen electrode). As a result, we obtained high HER activity corresponding to those of the previous report. Particularly, the value of Tafel slope was much lower than that of stable MoS2 semiconductor, also suggesting that metallic MoS2 was synthesized.
Scheelite-monoclinic BiVO4 serving as a photoanode for photoelectrochemical water oxidation is known for its fast electron-hole recombination and slow kinetics, thus limiting its efficiency. In this study, a highly active oxygen evolution reaction catalyst, FeOx, is successfully deposited on BiVO4 by a simple and low energy needed photochemical metal organic deposition (PMOD) process under ambient environment. During the PMOD process, metal organic precursor goes through ligand-to-metal charge transfer, triggered by UV irradiation, and then react with oxygen to form a thin amorphous FeOx catalyst layer. This FeOx coated BiVO4 photoanode boosts the hole injection efficiency to ~100% which is almost triple comparing to the bare BiVO4. Furthermore, with FeOx layer, the onset potential shifts cathodically for about 0.4 V and the photocurrent density at 1.23 V vs. reversible hydrogen electrode (RHE) reaches 1.1 mA/cm2 which is 2.5 times of a bare BiVO4 photoanode. The stability of the photoanode is also improved. The FeOx coated BiVO4 demonstrates a substantial enhancement of the incident photon to current efficiency (IPCE) and the absorbed photon to current efficiency (APCE) due to its ability in increasing the hole injection efficiency and oxygen evolution reaction (OER) kinetics.
At present, thermal power still accounts for more than half of the power generation. Therefore, the shortage of fossil fuel caused by coal combustion and the air pollution caused by carbon dioxide released after combustion are still issues that we should consider. At the same time, study on the use of renewable energy and the hydrogen supply chain had begun to attract international attention. But in China today, there are still relatively few studies on hydrogen power generation, and the efficiency of the hydrogen energy supply chain is still under research and development.In this research, we investigated an energy supply system in China based on compressed hydrogen derived from renewable energy. Here, we will use the existed natural gas pipelines to transport compressed hydrogen. And we will build an energy and excergy flow chart for the hydrogen energy supply chain to calculate the energy efficiency of the energy system.By analyzing the energy and excergy loss of every device in system, the energy efficiency based on the calorific value was 34.5% when the output of renewable energy was set to 100%, the total electrical power efficiency was about 18% and the efficiency of the heat supply was 16.5%.
Hydrogen-bonding solid crystals of methane hydrate have recently gained a great deal of attention as a globally recognized new energy resource for the next generation. Despite its popularity, the phase change processes of methane hydrate are not well understood.
During the decomposition of methane hydrate, long-term stable phenomena occur under temperature and pressure conditions (around 240K) where the material is expected to be inherently unstable. Although a full degradation mechanism has not yet been elucidated, it is hypothesized that super-cooled water suppresses the decomposition reaction [1].
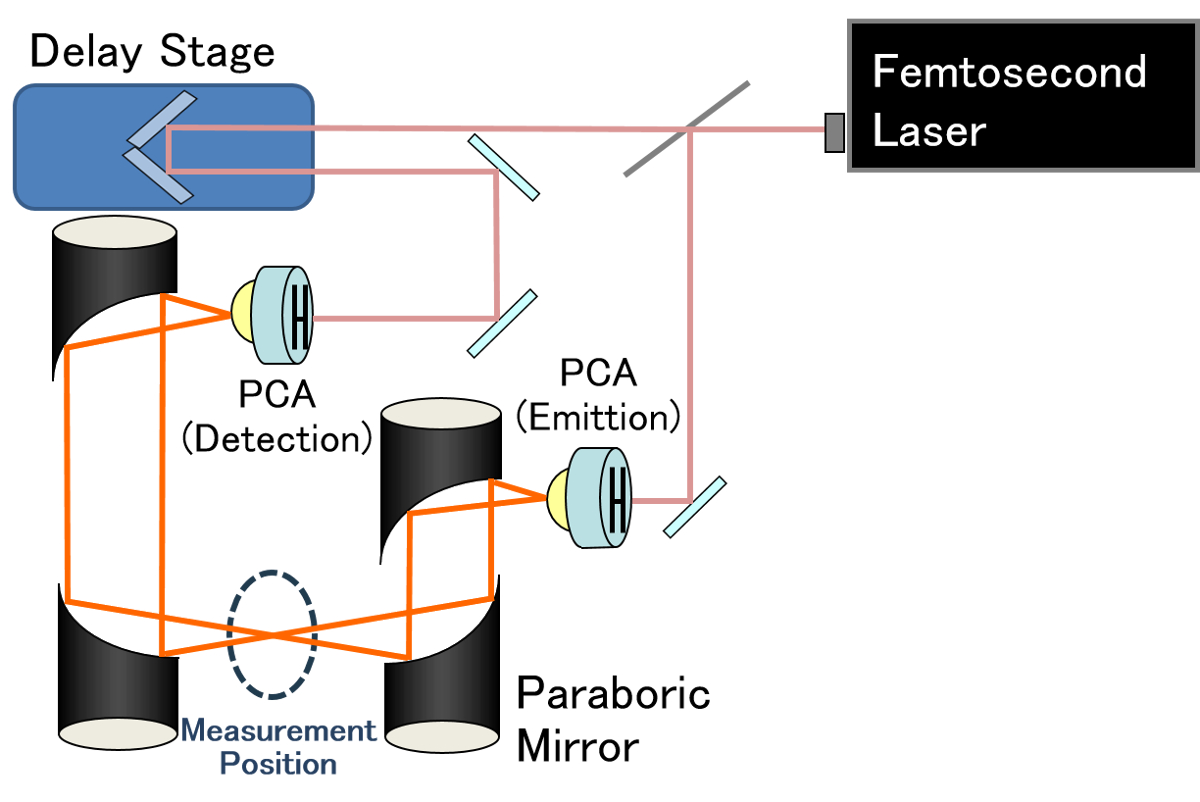
In this study, we observed phase changes in methane hydrate using terahertz wave spectroscopy. Terahertz radiation boasts the material permeability of radio waves and the ease of handling of light. In addition, terahertz waves are strongly absorbed by water, making them especially well-suited for detecting liquid water.
The terahertz time-domain spectroscopic (THz-TDS) system shown in Figure was used to observe phase changes in methane hydrate. This system can simultaneously observe absorption due to the presence of water and phase changes in the methane hydrate material. Experiments were conducted at 210K to 260K while maintaining approximately 3 hours at each temperature.
An increase in absorption and a leftward shift in the time waveform were observed at temperatures greater than 240K, indicating distinct changes in the sample at this temperature. In addition, this temperature coincides with the temperature at which super-cooled water exists, strongly implying an active role of super-cooled water in the phase change activity of methane hydrate.
References
[1] K. Takeya, K. Nango, T. Sugahara, K. Ohgaki, A. Tani J. Phys. Chem. B 109 (2005) 21086
Recently, the replacement of natural gas hydrates using CO2 injection has been considered as a promising natural gas production and CO2 sequestration method. This method differs from thermal stimulation and depressurization technologies in that it is non-destructive and environmentally friendly because natural gas hydrates do not need to be dissociated for production and CO2 is safely stored in the form of gas hydrates. In this study, sI and sH hydrates which were formed with methane and methane + methylcyclopentane (MCP), respectively, were used for replacement using pure CO2. To examine the effect of CO2 injecting pressure on the replacement behavior, the experiment was conducted within pressure conditions where pure CO2 hydrate is thermodynamically stable. To identify replacement efficiency, the compositions of the hydrate phase were measured by gas chromatography (GC) after dissociating the replaced hydrates. In addition, 13C NMR, powder X-ray diffraction (PXRD), and Raman spectroscopy were used to examine the structural information of gas hydrates and the changes in cage occupancy of guest molecules before and after the replacement. For sI hydrate, the replacement efficiency was found to be about 70 %, which was independent of CO2 injecting pressures. It was found from PXRD patterns that cage occupancy of guest molecules after replacement in sI hydrate were almost the same at lower and higher CO2 injecting pressures. However, for sH hydrate, the replacement efficiency was increased up to 80% with increasing the CO2 injecting pressure, which was attributed to a larger portion of a structural transition to sI hydrate at higher CO2 injecting pressure. In addition, time-dependent Raman spectra demonstrated that the structural transition of sH to sI hydrate was more significant at higher CO2 injecting pressure.
CO2 activation is an attractive pathway to utilize CO2 obtained either from large industrial point sources such as heavy industry and power plants, or captured directly from air. CO2 can be activated by reacting it with methane to obtain CO or syngas as a product. An often suggested process to do so is the dry reforming process.
CH4 + CO2 → 2 CO + 2 H2
However, the dry reforming process produces a mixture of CO and H2 and can thus not be operated flexibly to adjust the H2/CO ratio. Furthermore, only one mole of CO2 can be activated per mole of CH4 used. These issues can be addressed by aiming at the production of only CO and water from the same reactants instead.
CH4 + 3 CO2 → 4 CO+2 H2O
A promising implementation of this net reaction is to combine methane cracking and metal oxide redox reactions in a two-reactor moving bed process. In the ideal embodiment of this process, methane is first cracked to solid carbon and hydrogen on an iron substrate in the first reactor. The produced hydrogen is used to reduce an iron oxide material to the aforementioned iron substrate, producing H2O. The iron and the carbon deposited on its surface are then exposed in a second reactor to CO2, yielding a CO/CO2 product gas mixture and regenerating the iron oxide. Here, we report on the gas production and operating parameters of this process in a one-reactor laboratory-scale moving bed, in which this two-reactor concept is emulated.
In this study, we efforted to CCU that converse from CO2 to CO using the reverse cycle chemical looping process. Reaction rate by TGA was calculated, and product gas was analyzed by gas chromatography in FBFR.In addition, oxygen carrier before and after use were analyzed by XRD and SEM, and changes in composition and surface observed. At a reaction temperature of 1123 K or more, it was observed by XRD that the calcium ferrite structure was changed.Forthermore, even if redox reaction were performed multiple cycles, there was no difference in reaction rate in any cycles. At reaction temperature of 1023 K or less, calcium ferrite changes mixure of CaCO3 and Fe3O4. At any tempture, used calcium ferrite surface has been changed to be rough when observed by SEM, so that, surface area is expected to increase.
Two major problems that arise along with economic and population growth in the World are the increase of energy consumption and amount of plastic waste. Recently, crude oil is used as one of energy sources. However, it is not renewable energy. Therefore, alternative renewable energy sources such as plastic waste are needed. Plastic waste is composed of long-chain hydrocarbon compound, so it could to be converted into liquid fuels via catalytic pyrolysis method. Catalytic pyrolysis is the process decomposition of organic materials using catalyst in the absence of oxygen. The plastic waste used in this research is Polypropylene (PP). Fuel generated from PP plastic pyrolysis process, is expected to increase energy supply and reduce the amount of plastic waste in the World, especially in Indonesia. The purpose of this research is to study the catalytic pyrolysis of PP plastic and to refine products of catalytic pyrolysis of PP plastic with distillation methode. And also to study the effect of temperature on the liquid fuels, the effect of adding the catalyst material zeolite on the products of PP plastics pyrolysis and determine physical and chemical properties liquid fuels of pyrolysis products before and after distillation. Pyrolysis is operated at a temperature of 350OC and 450OC during 60 minutes and using natural zeolite catalysts 60 and 100 mesh.
The GC-MS analysis showed that the pyrolysis liquid oils from all samples mainly consisted of hydrocarbon compounds such as C5H12 and C8H16. The produced liquid oils have density, viscosity and high heating values (HHV) of 0.78 - 0.79 g/ml, 2.15 – 2.52 and 10540 – 10650 cal/g respectively, which are similar to conventional gasoline. The liquid oil has potential to be used as an alternative source of energy or fuel production
It is well known that ammonia is produced through a catalytic reaction at high temperature and pressure from pure nitrogen and hydrogen. This catalytic chemical process is a massive and high energy-consuming process, but a very important one for nitrogen fixation.
Here, we show a non-catalyzed one-step synthesis of ammonia from atmospheric air (nitrogen source) and water (hydrogen source), based on an interfacial reaction between the air plasma gas phase and the water phase, at 25 °C and atmospheric pressure. In the plasma/liquid interfacial reaction (P/L reaction), atomic nitrogen, which is produced through electro-discharge, abstracts hydrogen from the H2O molecules on water phase surface at the P/L interface, and then NH is produced without any catalyst. Formed NH is reduced further in the water, affording NH3, which then dissolves in the water phase.
In the presentation, the mechanism of this reaction was considered from the relationship between the quantitative result of atomic nitrogen and the amount of ammonia production by the P/L reaction. In addition, we have obtained results suggesting that the yield of P/L reaction is greatly improved by devising the discharger to produce excited nitrogen more efficiently.
The PL reaction doesn't require hydrogen gas, and the only sources are nitrogen and water. It is a reaction system at normal temperature and normal pressure, and all that is required is electricity. Therefore, it is considered to be suitable for small-scale ammonia water production regardless of the production site.
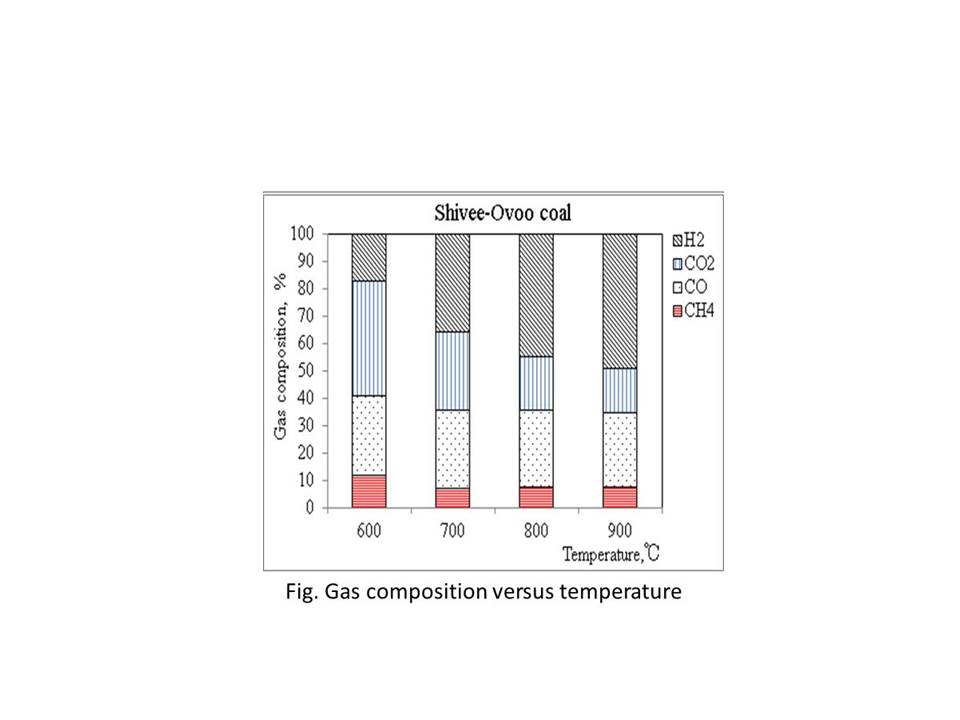
In this study, pyrolysis of Mongolian three different brown coals was investigated in the fixed bed reactor and fluidized bed reactor. In the fluidized bed reactor, the porous alumina and quartz sand were used as bed material for studying the influence of bed material. Size range of bed materials (quartz sand and alumina) was -250μm +212μm and the gasifier temperature between 600 and 900oC. The main components of the obtained syngas were CO, H2, CH4 and CO2, which were measured by Gas chromatograph. The gas and tar yields were reported in more detail at temperature from 600oC to 900oC. Also, the composition of char was determined at given temperatures.
The coal and gas fired power generation are defined as significant power sources, however it is necessary to reduce CO2 emissions from thermal power generation. In this session, I will introduce the development trends and prospects of next-generation thermal power generation technology in Japan.
Spontaneous ignition of coal stockpile is a serious economic and safety problem.
Theorefore, coal oxidation has been received great attention and many studies has been carried out.
Although coal oxidation at actual stock yard proceeds under the condition of low temperature and humid atmosuphere, almost all of previous study has been carried out under the condition of high temperature (>100 °C) or dry atomosphere.
In this study, coal oxidation under the condition of low temperature and humid atmosphere was investigated.
CO and CO2 were the major products and the formation rate of CO2 was larger than that of CO.
The heat generation from coal was also observed and that was followed by the formation of carbon oxides.
Therefore, it was considered that the main factor of heat generation was oxygen absorption which is initial step of coal oxidation.
Heat generation rate under dry atmosphere was almost the same as that under humid atmosphere.
So water would have little influence on heat generation at this condition.
CO formation rate under the dry atmosphere also almost the same as that under humid atmosphere.
On the other hand, CO2 formation rate under the dry atmosphere is much smaller than that under the humid atmosphere.
As a result, water has pronounced effect on CO2 formation reaction.
Coke serves as fuel, reductant and support material to allow continuous flows of gas and liquid in blast furnace of ironmaking industries. Over the last few decades, since the scale and production ability of blast furnace kept increasing, mechanical strength of coke became more and more important. However, when raw coking coal is exposed to air, it would be weathered, which leads the coke made from it can not obtain enough strength to meet the requirement of ironmaking industries. It is necessary to investigate the influence of weathering on the coke strength quantitatively.
Oxidation at relatively low temperature is believed to be one of the main reasons for weathering. In this study the influences of the oxidation level of raw coal at low temperature on the softening and melting ability of raw coal and the mechanical strength of coke were investigated from the viewpoint of oxidation reaction rate.
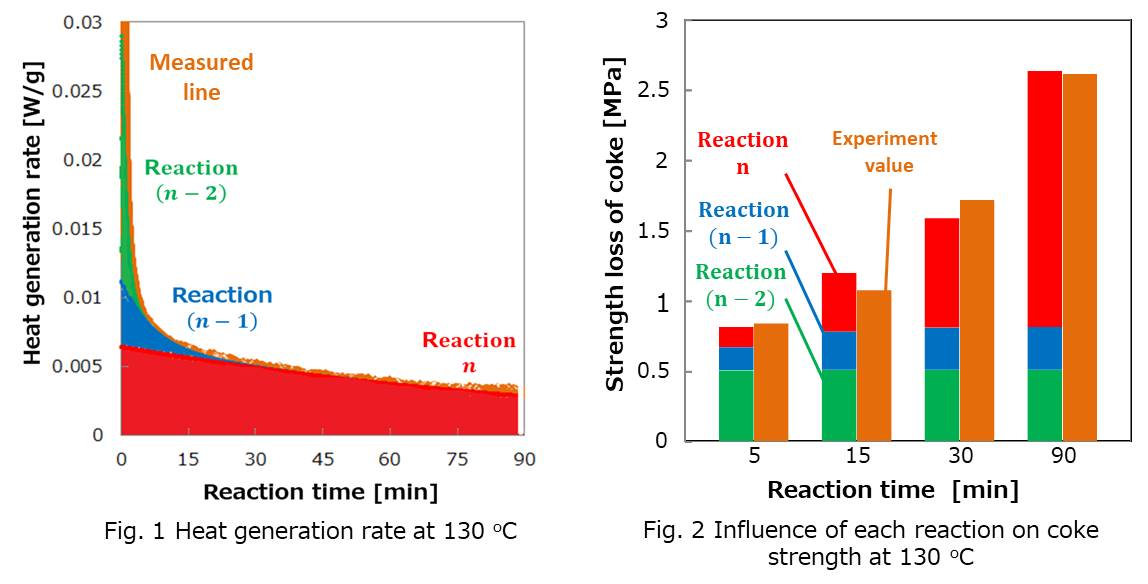
Since the weathering takes several months, simulated weathered coal was prepared by heating coking coal in air at 120 oC to 150 oC, where oxidation reactions were accelerated, for 5 to 90 minutes. Then coke was prepared from the simulated weathered coal. The coke obtained had less mechanical strength as the treatment temperature and time increased. The heat generation rate in the oxidation reaction at each temperature was measured by differential scanning calorimetry. Reaction rate analysis showed that the oxidation reaction of raw coal could be divided into three parallel first-order reactions (Fig.1). It was found that in the long run, the reaction which has the lowest reaction rate constant had the most significant influence on coke strength (Fig.2).
Biomass waste is a renewable source for utilizing to generate energy products, particularly in Thailand, large amounts of biomass wastes are generated every day. Moreover, the torrefied product from biomass wastes is regarded as candidates instead of coal. However, the energy yields of the torrefied product lost so much during torrefaction. Therefore, it is essential to develop a method to increase the energy yield of the torrefied product during the torrefaction of biomass wastes. In this study, Leucaena, which is woody biomass, was pressurised at around 70 MPa at room temperature, called cold press in this work, to prepare biomass pellet. Leucaena was also pre-treated under the mechanical pressure of around 70 MPa at the temperature range of 25oC to around 250oC, called hot press in this work. Then, both cold press and hot press pellet were subjected to torrefaction process at 260, 280 and 300oC. It was found that the char yield of cold press pellet was 20.7 wt%, while the char yield of powder leucaena was only 19.2 wt%. On the other hand, the char yield of the hot press was surprisingly increased to 28.1 wt%. The energy yield of the hot press pellet torrefied at 300 oC was as high as 96.7%, while the energy yield of the cold press and powder leucaena were 89.1% and 89.5%, respectively. From the detail analyses of gas formation during the pyrolysis in TGA, it was found that the dehydration reaction was accelerated by hot press carbonization and the mechanical pressure suppresses the evolution of tar components. Therefore, the mechanical pressure is judged to be effective to increase the energy yield through the torrefaction of biomass.
Biomass waste is a renewable sources and its effective utilization is indispensable, particularly in Thailand, where massive amounts of biomass wastes are generated. Moreover, charcoal from biomass wastes is regarded as candidates for low priced raw materials for activated carbons. However, the yield of charcoal from biomass wastes is low in general. So, it is essential to develop a method to increase the yield of charcoal. In this study, Leucaena, which is a woody biomass, was pressurized at around 500 MPa at room temperature, called cold press in this work, to prepare biomass pellet. Leucaena was torrefied under the mechanical pressure of around 10 MPa at the temperature range of 25 °C to around 250 °C, called hot press in this work. Moreover, Leucaena was also torrfied under gas pressure at 250 °C in a tube-bomb reactor, and was called gas pressure sample in this study. Then, the cold press pellet, hot press pellet, and gas pressure sample were subjected to carbonization at around 900 °C by using a TGA. It was found that the char at 900 °C yield of the cold press pellet was 20.7 wt%, while the char yield of powder leucaena was only 19.2 wt%. On the other hand, the char yield of the hot press pellet was surprisingly increased to 28.1 wt%. The char yield at 900 °C of the gas pressure sample was increased to 23.1 wt%. From the detail analyses of gas formation during the carbonization, it was found that the dehydration reaction was accelerated by torrefaction under mechanical pressure and the mechanical pressure suppresses the evolution of tar components. Therefore, the mechanical pressure is judged to be effective to increase the char yield through the carbonization of biomass.
I. Introduction
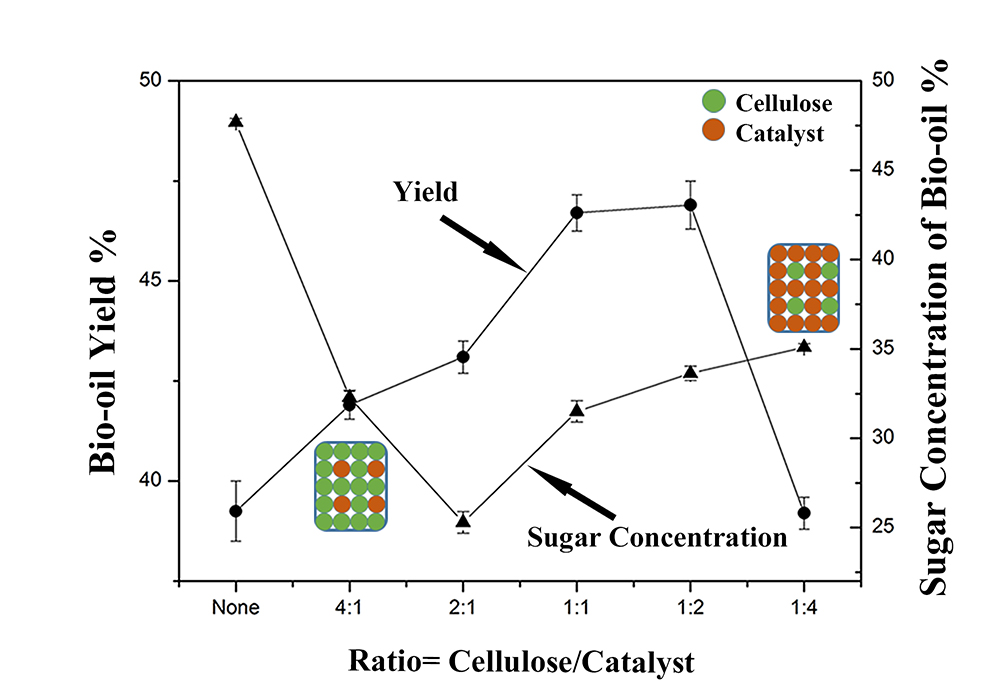
Carbon neutral bio-oil is needed to replace liquid fossil fuels in order to reduce CO2 emissions and global warming. However, bio-oil produced by pyrolysis of biomass has a high oxygen content (e.g.sugars), low energy density, and is also expensive to produce. Based on previous research results it was shown that inexpensive Ni, Fe catalyst clusters can improve the quantity or quality of bio-oil produced from the biomass, but to our knowledge, very few studies have actually investigated the mixing cellulose (biomass model compound) and catalyst directly together followed by pyrolysis to produce bio-oil. In this study, Ni2Fe3 catalyst was mixed with cellulose in five different ratios in a pyrolysis batch reactor to investigate its impact on the bio-oil yield and sugar content.
Experiment
The catalyst was prepared by sol-gel method and heat-treated in flowing H2/N2 gas. Pyrolysis experiments were conducted using a fixed bed reactor, flowing 100ml/min N2 gas, at 450 °C with five different catalyst/cellulose ratios, where cellulose mass was fixed at 4g, with the addition of catalyst of 1g, 2g, 4g, 8g, and 16g.
II. Results & Discussion
In Figure 1, bio-oil yield and sugar concentration measured by GC-MC is plotted versus cellulose/catalyst ratios, the yield increase as the catalyst is added initially and then decrease. The sugar content decreased initially as the catalyst is added and then increased slightly. The reason is that when the cellulose ratio is low, the metal catalyst can help transfer heat uniformly and provide sufficient reactive surface (figure inset). However, when the catalyst ratio is high, the catalyst has little physical contact with the cellulose, which auto-aggregates at high temperature. A high catalyst ratio not only decreases the yield of bio-oil but influences the overall catalyst acitivity. Research work is continuing on evaluating the catalyst reusability.
Recently, many biomass gasification plants are operated as local power plants. However, the management relies on FIT because the energy is the cheapest product. To establish the economically reasonable technology for biomass conversion, it is required to develop a new scheme for producing valuable chemicals selectively with simple operation. To realize it, we propose a multi-stage pyrolysis with instant selective separation scheme. Utilizing the difference in the decomposition temperatures of main biomass three components, hemi-cellulose, cellulose, and lignin, biomass was pyrolyzed sequentially at three step optimal temperatures for recovering volatiles selectively in vapour phase. Thus produced volatiles, which contained saccharides derivatives, were further converted into valuable chemicals by installed second higher-temperature zone in each stage pyrolyzer. In this study, the effective pyrolysis conditions for the selective valuable chemicals production were examined using pulverised cypress to clarify the validity of proposed method.
As the first step, pyrolysis was conducted at 300 °C for the selective decomposition of hemicellulose into saccharides derivative volatile. Thus produced volatile was immediately heated up to 650 °C, and furan was selectively obtained at the yield of 0.623 g/g-(hemicellulose in biomass). As the second step, the obtained char was subsequently pyrolyzed at 370 °C. Through the second-step pyrolysis, cellulose was selectively decomposed and the anhydrosugar of levoglucosan was obtained as the product. The second-step pyrolysis avoided lignin decomposition, which resulted in avoiding undesirable heavy tar production. The remaining solid product, char, in the second step pyrolysis can be used high calorific carbon materials for power plant. Thus, the validity of the proposed scheme for selective co-production of valuable chemicals and energy was clarified.
<Gasification is known as one of the most promising processes for utilization of coal and biomass, e.g. for production of fuels and chemicals. During the gasification, besides gases, a number of volatile hydrocarbons are discharged together with tar. Tar is a complex mixture of components, typically poly-aromatic. How to deal with the viscous tar is one of the major issues in the biomass/coal gasification process since it can cause quite a few problems in the different applications.
A large number of catalysts have been researched to eliminate tar in gas products. The two most catalysts are Ni-based catalysts and dolomites. When Ni-based catalysts are used, tar concentration in the product gas can be reduced significantly by means of reforming but quite rapid catalyst deactivation (both by coke fouling on Ni catalyst surface and by sintering). Dolomite, a kind of inexpensive and abundant natural mineral, is very attractive as a tar cracking catalyst and it was found to be effective on coking resistance during tar steam reforming.
In this study, tar was treated in a two-step reactor, where catalyst sample was filled in the secondary step. Tar gasification using calcined dolomite was discussed at different temperatures and various introduction gases. The combined use of dolomite and nickel-based catalyst was also compared. Gas yield and carbon deposit formations were determined. Additionally, changes of the catalyst pore feature, surface area were analyzed.>
Microalgal biofuel was produced by a continuous hydrothermal liquefaction (HTL) of native microalgae using a bench-scale tubular reactor. Native microalgae cultivated and harvested with high biomass productivity (> 29 g/m2/day) in a cool temperate zone (Minamisoma, Fukushima, Japan) were used. The lipid content of harvested native microalgae was 5.0 – 16.9 wt%. The usage of native microalgae as a biomass offers benefits in terms of stable culture and productivity with easy maintenance. The slurry of native algae was continuously injected to a bench-scale tubular reactor by high-pressure pumps, and then gradually heated up to the reaction temperature through preheating tube, resulting in the production of biocrude. Produced biocrude was collected, and then the bio-oil was extracted from the biocrude using organic solvents. Both biocrude and bio-oil products as microalgal biofuel were characterized by a thermogravimetric analysis. Among the examined conditions, HTL processing at algal biomass concentration of 19.4 wt% at 350 °C showed reasonable biocrude yield and highest bio-oil yield (8.6 % by hexane, 24.9 % by chloroform-methanol mixture). Bio-oil products obtained at this condition were composed more of light oils substances compared to heavy oils, useful regarding biofuel production. Thermogravimetric analysis showed that some of the heavy oil components in microalgal biomass was converted into the light oil substances. Thus, native microalgae could be successfully converted to biofuel by HTL processing using a continuous bench-scale tubular reactor.
Supercritical water gasification is gasification of biomass in hot compressed water. It is suitable for wet biomass treatment because it uses water as reaction medium and does not require costly and energy consuming drying pretreatment. Because of the high temperature, water is very reactive, and almost complete gasification is possible. However, it cannot be free from tarry material production. Detail study on tarry material production showed that tarry material can be produced by both ionic and radical reactions. To suppress ion tar, rapid heating up of feedstock was found to be effective. To suppress radical tar, radical scavenger addition was found to be effective. In this study, food processing waste was processed in continuous supercritical water gasification experimentally. The result of the operation is presented. This study was supported by NEDO.
The Philippines being an agricultural country generates bio-based residues that can be utilized as low-cost fuel for biomass plants to generate electricity. The aim of this study is to assess the amount of bio-based residues together with their theoretical bio-energy potential (BEP) on each of the regions of the Philippines. The residues considered include agricultural and agro-industrial residues, animal by-products, woody biomass and marine biomass residues. Considering the limitations in the recovery these residues and the technological limitations on existing direct combustion power generation facilities, their available and recoverable bio-energy potentials are also estimated, respectively. According to the estimate, biomass residues have a theoretical bio-energy potential ~660 PJ/yr with majority contributed by agricultural and agro-industrial residues (~66%). While approximately half of this potential is available for recovery, only ~74 PJ/yr (3,300 MW) can be possibly harnessed as electrical power considering the efficiencies of co-generation plants. This potential, of which 6% is already harnessed, could possibly contribute to 15% of the current national power generation capacity and can potentially displace 15 million tons (Mt) of coal annually. Western and Central Visayas have the highest recoverable BEP among 17 regions with 14.2 and 11.3 PJ/yr, respectively.
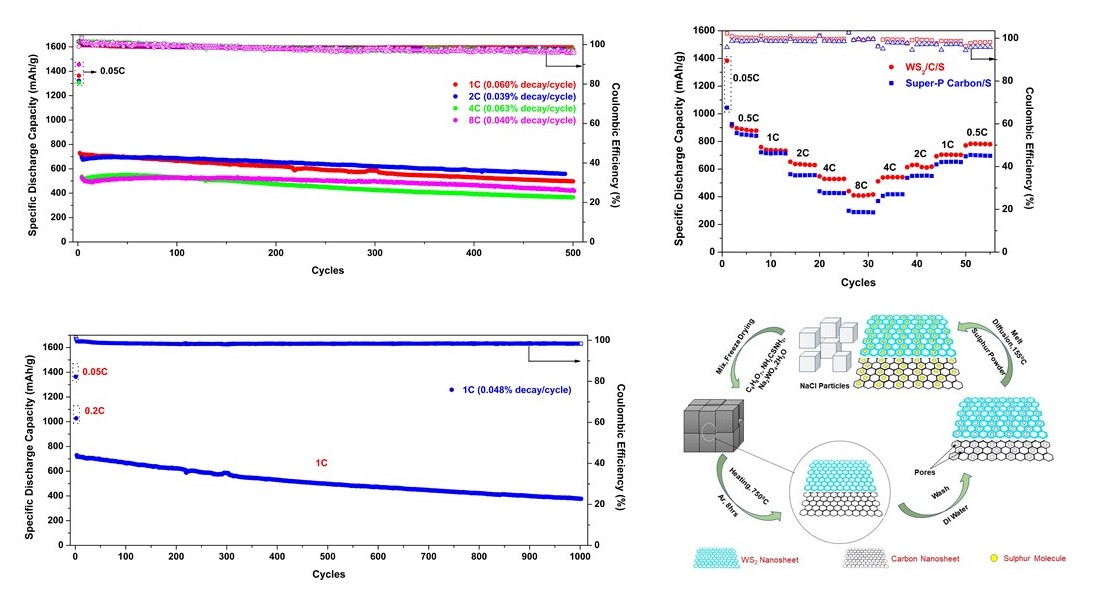
Mesoporous sulfur host consisting of tungsten disulfide (WS2) and carbon nanosheets has been synthesized in situ by using sodium chloride (NaCl) particles as the template. Two-dimensional architecture of WS2/C composite provided large specific surface area (643 m2g-1) and facilitated the rapid diffusion of lithium-ions during cycling of lithium sulfur batteries. The WS2/C porous nanosheets served dual function by providing physical barrier and strong chemical affinity to restrict the shuttling of intermediate lithium polysulfides. The sulfur rich cathode showed an excellent cycling performance by maintaining low capacity decay rate of 0.048% per cycle for 1000 cycles at 1C rate (1C=1675 mAh g-1). Moreover, it revealed an impressive rate performance by delivering high specific discharge capacity of 419 mAh g-1 at 8C after 500 cycles (80% capacity retention). At high areal loading of sulfur, 4.7 mg cm-2, were the battery delivered a high areal capacity of 3.4 mAh cm-2 after 100 cycles at 0.5C rate. Self-discharge analysis was also carried out to investigate the effect of polysulfide shuttling while the batteries were rested. It greatly suppressed self-discharge capacity loss by maintaining 94.5% of its initial capacity after 10 days of resting. The electrochemical impedance spectroscopy (EIS) analysis illustrated that even after 400 cycles, the interfacial and charge transfer resistance only increased by 1.2 and 1.7 Ω, respectively, explaining faster electrochemical kinetics and inhibiting the formation of insulating layer of lithium sulfide (Li2S) on the surface of the electrodes. The facile synthesis procedure of porous WS2/C nanosheets and its superior electrochemical performance as a host provide valuable options to engineer practical sulfur cathode for high performance lithium sulfur batteries.
Acknowledgement: Financial support provided by Hong Kong Research Grant Council, National Natural Science Foundation, China (NSFC, N_PolyU601/16) and the Hong Kong Polytechnic University (1-ZE30).
Oxygen reduction reaction (ORR) is the most demanding process in the commercialization of fuel cell. To replace expensive rare earth metals in electrocatalyst, carbonized metal-organic frameworks became an emerging candidate. Zeolitic imidazolate frameworks (ZIFs), a sub-class of MOFs, is a hybrid crystalline material consisting of metal nodes and organic linkers. It has a big advantage on easy production and high specific surface area. If the ZIFs are synthesized in hollow structure, they have notable advantage to reduce the mass transfer resistance. However, the conventional synthesis method to make hollow structure requires multi-steps in small-scale. In this work, Co-N-C hollow-spheres were prepared from CoZn-ZIFs hollow-sphere in a facile method. Co/ZnO was prepared in a spherical form by ultrasonic spray pyrolysis, which has a big advantage in mass production. Then, it was converted into CoZn-ZIFs in ligand solution with simple stirring, followed by the carbonization to Co-NC hollow-sphere. The ORR activity for the final product was tested and it showed outstanding performance comparing to the materials in the literatures.
Annual production of crude steel reached 1.8 billion tons in 2018, and is projected to grow further. A major portion of iron is currently produced from iron ore in blast furnace. Because it uses coal as fuel and reductant, significant amount of CO2 is produced along with the steel. Therefore, iron and steel industry is considered to be in a category of "difficult to eliminate emissions". The blast furnace of Japan, the second biggest steel-making country, is the world-leading clean process. Nevertheless, CO2 emission from iron and steel industry accounts for nearly 15% of the total domestic CO2 emission. In view of global concern on the earth's warming, alternative green approaches must be introduced for the iron-making. To address this issue, herein a new concept for iron-making process is proposed. The iron ore reduction process is consisted of three steps: dissolution of iron ore using organic acids, photochemical reaction of the dissolved iron complex for the reduction and recovery as solid, and then pyrolytic recovery of iron under reductive atmosphere. The reactions in each step occur at temperatures much lower than that of conventional process. Although CO2 is formed at the reduction process, if it is recovered, converted to organic acids, and recycled to the process, the stoichiometry of overall process can be written without carbon. In other words, the process has a potential to be net-zero emission. The present study explains details of chemistry involved in the process, and discuss current status of technologies for key reactions with information from past studies to find technical barriers and challenges. Moreover, the iron-making process (iron ore reduction part) is experimentally demonstrated in a diluted reaction system.
Evolution profiles of Hg during sintering was investigated with flow-type fixed bed reactor at 10 °C/min up to 950 °C in air. Five types of iron ores were used in this study, and Hg contents ranged from 60 to 180 ppbw. Hg content depended on the iron ore types, and there was no correlation between Hg content and composition of iron ores. Hg released from iron ores as a Hgo and Hg2+ during the sintering, and mass balance ranged within 100±10%. Proportion of total gaseous-Hg and remained-Hg in solid after sintering were 20-70% and 30-70%, respectively. Hg evolution started beyond 100 °C and continued until 950 °C. Main and shoulder peaks of Hg evolution profiles were observed at 150-250, 300-400, 450-550, 500-700 and 700-900 °C, and the evolution profiles depended on the iron ore types. The temperature-programmed-desorption (TPD) experiments of physical mixtures of Hg compounds (HgCl, HgCl2, HgO, HgS and HgSO4) and Fe2O3 were carried out to investigate Hg form in iron ore under above-mentioned conditions. When pure Hg compounds were heated in air, the main peaks of Hg evolution profile were observed at 140-715 °C. However, Fe2O3 and HgCl2, HgCl, HgS, HgO or HgSO4 mixture showed the Hg evolution profile having the main peaks at 150, 165, 390, 510 or 565 °C, respectively. With the mixture samples, all peaks shifted to the low temperature compared with those of pure Hg compounds, and new peaks appeared at 347 or 434 °C for the mixture of Fe2O3 and HgCl or HgCl2. This result shows the occurrence of interactions between Hg compounds and Fe2O3 during sintering of the mixture. Therefore, Hg evolution below 600 °C may occur from HgCl2, HgS, HgO, and HgSO4 in iron ores.
Coal pulverization is essential for coal combustion systems due to leading to enhance the efficiency of coal combustion. Coal consists of components as macerals and minerals, so that coal particles generated by pulverization have different grindability. The difference of grindability between the coal particles generated results in the difficulty of predicting the performance of coal pulverizer. Generally, the grindability of coal is evaluated by a Hardgrove grindability index referred to as HGI. The problem of HGI is to assume a uniform particle and evaluate an initial stage in a grinding process. Therefore, a semi-continuous test by an HGI test equipment were conducted. Based on the semi-continuous test, grinding rate constant distribution was derived from a multicomponent consecutive grinding model. The grinding rate constant distributions of high ash coal and low ash coal having approximately same HGI showed to be different. The grinding rate constant distribution of coal provided grinding information in detail.
In this study, pyrolysis characteristics of Mongolian brown coals were investigated in a fluidized bed reactor under a nitrogen atmosphere at temperature up to 1173 K. 1) The porous alumina and quartz sand were used as bed material for investigating the influence of bed material on the products yield. 2) The effect of the temperature on the properties of pyrolysis products (gas and char) was characterized.
The results have shown that the pyrolysis temperature affects significantly the product yield and distribution. With the increase of the pyrolysis temperature, the char yield decreases and the gas yield increases. Elemental composition of formed chars is similar with high rank coals at higher temperatures. Molar ratios of oxygen and hydrogen to carbon were analyzed with a coalification diagram.
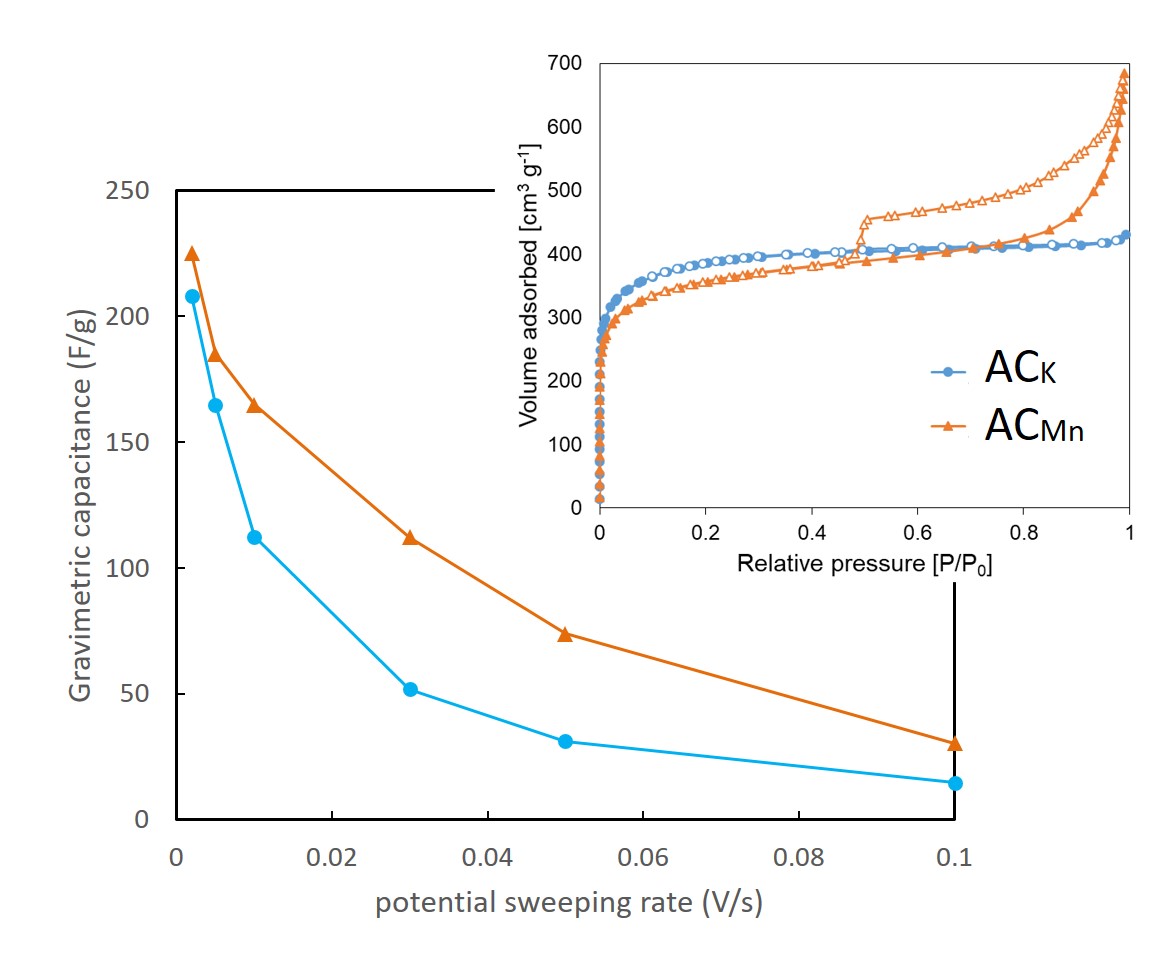
In this work, activated carbons (ACK and ACMn) were developed from bamboo powder by pyrolysis carbonization and KOH or KMnO4 activation process using one-step strategy. N2 adsorption-desorption, X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to investigate the characteristics of the produced activated carbons. The electrochemical performance of the activated carbons as electrode materials for supercapacitors were studied by electrochemical measurements. The as-prepared ACK and ACMn samples using KOH and KMnO4 respectively possessed a porous structure with a large specific surface area of 930 and 915 m2/g and abundant micropores. However, the ACMn sample has more mesopores and macropores than the ACK has. Further electrochemical measurements revealed that the ACMn electrode exhibited a specific capacitance of 75.0 F/g at a potential sweeping rate of 0.05 V/s, which was higher than that of the ACK electrode (35.0 F/g). Moreover, it was observed that when the potential sweeping rate was increased, the capacitance of both ACK and ACMn electrode tends to decrease, but the decreasing rate by ACK was more severe than ACMn. This is considered that the KMnO4 activation process of the bamboo powder resulted in the enhancement of the meso-macro structure, and improved the storage capacity and transport behavior. This one-step strategy using bamboo powder and KMnO4 activation process can be highly expected to achieve low cost, green and industrial-grade production of renewable biomass-derived carbon materials for advanced energy storage applications in the future.
Torrefaction is a thermal pretreatment method at relative low temperature range of 200 – 300 °C in an inert atmosphere, which aims to increase the energy density and to decrease the grinding energy of biomass. In this study, the torrefaction of two types of wood pellets, which are rubber wood pellet and leucaena pellet, was carried out in the temperature range of 260 – 300 °C for 30 min residence time. The products yield, its compositions and fuel properties at various torrefaction temperatures were also determined. The increase in temperature from 260 to 300 °C reduced the torrefied pellet yield from 87.4 % to 63.5 % for rubber wood pellet. The heating value (21.4 MJ/kg) and the volumetric energy density (17.5 GJ/m3) of torrefied pellets at 300 °C were comparable to that of coal. The increase in torrefaction temperature also improved the hydrophobicity (resistance to water uptake). Moreover, the combustion behavior of torrefied pellets show some improvement compare to raw pellets. These results showed that the torrefaction of wood pellets (TAP process) is a potential pathway to produce energy-dense pellets as a tradable solid biofuel to replace coal.
There are energy issues such as carbon dioxide emissions and depletion of fossil fuel, energy supply not depending on fossil fuel would be required. So increasing of biomass and waste biomass using for energy would be able to solve in these problems. There is carbonization of sewage sludge as one of methods of waste biomass using. And in the case of carbonizing sludge with mixing coal, high quality fuel better than sludge could be manufactured. Char manufactured from both materials is called co-char in this paper. In this study, gasification rate of co-char was measured, and effect of co-carbonization of both materials on co-char reactivity was evaluated by comparing reactivity between co-char and single char made from a material. In the experiment, char was heated up in reactive gas agent flow, weight loss by reacting char and gas was measured by thermo-balance. As a result, weight loss rate in gasification of char was low speed with increasing coal ratio in co-char. In arrhenius plot calculated from gasification model, activation energy of coal char (coal ratio in co-char is 100 %) was largest, and activation energy of sludge char (coal ratio in co-char is 0 %) was lowest. And activation energy of co-char was in ranging between coal char's value and sludge char's value. On the other hand, graphite structure of co-char was developed with increasing coal ratio from measurement of raman spectrum. So, carbon structure in co-char was different in every coal ratio, it was considered changing of carbon structure affected reactivity of co-char in gasification.
Biomass have been attracting much attention as a renewable and sustainable energy resource to substitute fossil fuels. Thermochemical treatments of biomass such as pyrolysis and gasification are general technologies to utilize biomass energy. However, these technologies have the common problem of the high temperature required for the reaction, which results in a low energy efficiency. Therefore, a method for reducing the activation energy of the biomass pyrolysis reaction and promoting the reaction at a low temperature is needed. One effective method is the use of catalyst. Although a lot of researches have been dedicated to develop efficient catalysts, this study focuses on various kinds of non-precious metal oxide catalysts including semiconductor and non-semiconductor materials. Then, the purpose of this study is to clarify the influence of such metal oxide catalysts on pyrolysis behavior of woody biomass.
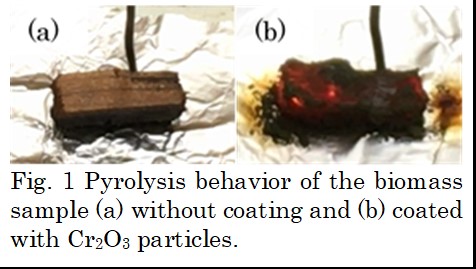
The pyrolysis behavior of a rectangular piece of cypress coated with different kinds of metal oxide particles was investigated on a hot plate in a room air. Al2O3, Cr2O3, Fe2O3 and TiO2 were tested. A sample without coating of any metal oxide was also used. As shown in Fig. 1, the sample pieces coated with Al2O3 and without catalyst coating were slowly carbonized with increasing the hot plate temperature, and finally charcoal remained at 500 °C. On the other hand, the sample pieces coated with Cr2O3, Fe2O3 and TiO2 began to glow red from the edge when the plate temperature exceeded around 300 °C, and then the glowing spread throughout the sample and the decomposition was rapidly completed. Only ash and the metal oxide particles remained at the end. The results suggest that semiconductor metal oxides have an effect of promoting biomass pyrolysis in the oxidizing atmosphere.
Although biomass has been attracting much attention as a sustainable energy resource, the biomass has the problems such as high moisture content and high transportation cost due to its low density. Then, we focused on carbonization as a pretreatment to solve these problems. Carbonization of biomass, however, reduces the yield and density by losing much mass and energy as tar. To overcome these drawbacks, this study proposes to increase the yield by tar recovery with the produced char during carbonization, and to densify the tar-containing char by compression molding.
A cylindrical piece of Japanese cypress was placed in the quartz glass tube installed in two tubular furnaces aligned vertically. The char particles, which were produced from the same biomass piece under the same condition in advance, were packed to form a layer for tar recovery around the boundary between the two furnaces above the biomass sample. The biomass sample was heated at 300-400 °C in the nitrogen flow and the generated tar was recovered by the packed char particles. After heating for 30 mins, the carbonization and tar recovery yields were calculated from the mass changes of the biomass sample and the packed char particles, respectively. The carbon contents and calorific values of the biomass sample, the produced char and the tar-containing char were measured to estimate the carbon and energy yields. Also, the tar-containing char was compressed in a mold to densify it.
The tar-containing char obtained at 350 °C carbonization had a high calorific value of 27.0 MJ/kg and a carbon content of 68.5 %. Both the energy and carbon yields exceeded 70%. The char particles were pelletized with the adsorbed tar as a binder by the compression molding. The density was 1 g/cm3 or more, which is about three times that of the raw biomass.
Background
Polyurethane (PU) was originally synthetized by Otto Bayer in 1937 in Germany. Usages of them cause significantly environmental impacts. This material is very stable on a lot of different applications, such as, transportation, packaging, footwear, paint, and insulation component for refrigerators and buildings. In 2016, approximately 18 million tons of PU will be produced and 3/4 of them were in foam format. Asian countries used most of them.
Methods
A new study on biomass-polyurethane co-gasification for syngas production was performed. The feed stock blending ratio, equivalent ratio (ER), steam to biomass ratio (SB), catalyst, and CO2 blending ratio were the key experimental design parameters and the experimental design was performe by the Taguchi L18 orthogonal array. The gasification temperature was set to be 800 °C of all experiments. The reaction was performed at a continuous feed fluidized bed gasifier. The conversion of carbon in the feed, yield of various products, and the low heating values of the effluent gas was analyzed.
Results
With the presence of the polyurethane and CO2 blending in the feed stream, the yield of the syn gas and the heating value of the effluent gas were increased. The carbon conversion of the feed was increased presence of the catalyst blended in the feed. This study suggested potential route for the energy production by blending the agriculture and municipal waste in the future.
Acknowledgement
Financial support: Ministry of Science and Technology in Taiwan, ROC (MOST 107-2218-E-035-017).
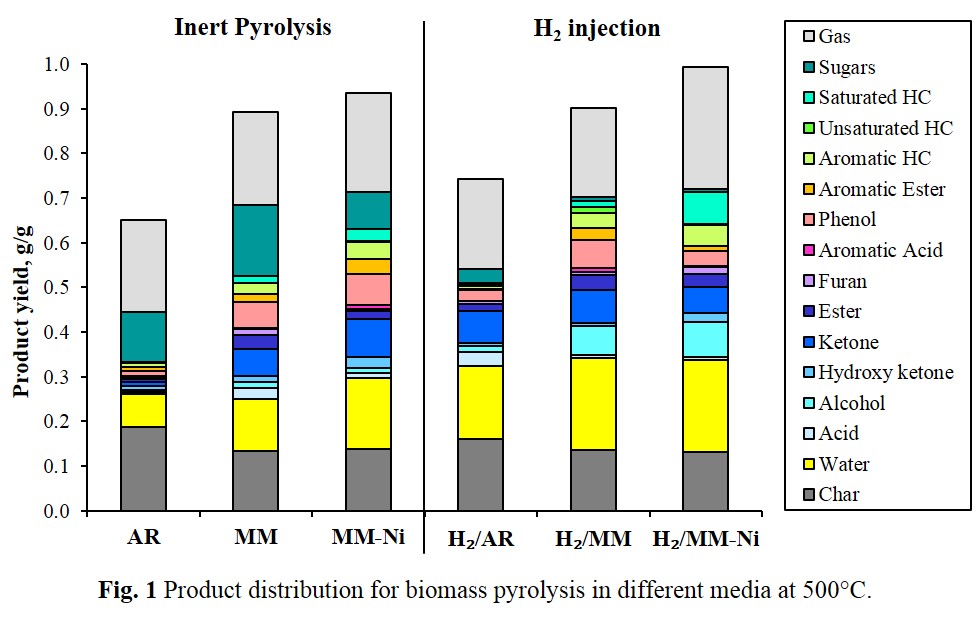
Rapid heat transfer in molten metal could potentially enhance biomass decomposition, and combining this with Ni-catalyst could also promote hydrogenation reactions. We have already indicated the effectiveness of Sn-Bi molten metal and its combination with Ni/Al2O3 for the gasification of biomass [1]. The aim of this work was to investigate the effects of these media with respect to bio-oil production. Aspen wood pyrolysis was performed at 500 °C at high heating rate in the molten metal medium (MM) and molten metal with catalyst medium (MM-Ni). Pyrolysis in the inert argon atmosphere (AR) was conducted as the control case. In addition, hydrogen injection was studied for hydro-deoxygenation of pyrolysis vapors. The results shown in Fig. 1 demonstrate that the biomass pyrolysis in the proposed media increased the yield of liquid products and reduced the char formation, thus improving carbon conversion efficiency. Fast heating rate in the media promoted the cracking of large molecules, which resulted in the production of the bio-oil having lower average molecular weight. Ni-catalyst aided dehydration, decarboxylation, and hydrogenation reactions, thereby increasing the hydrocarbon yields. Hydrogen injection further upgraded the bio-oil by successful oxygen removal and hydrogenation of unsaturated bonds. The proposed media were thus effective for the production of a higher grade bio-oil from lignocellulosic biomass.
[1] A. Arenova et al. J. Anal. Appl. Pyrolysis., 137, 61-69 (2019).
This study investigated the optimal reaction conditions for biodiesel production from soursop (Annona muricata) seeds. A high oil yield of 29.6% (w/w) could be obtained from soursop seeds. Oil extracted from soursop seeds was then converted into biodiesel through two-step transesterification process. A highest biodiesel yield of 97.02% was achieved under optimal acid-catalyzed esterification conditions (temperature: 65 degree C, 1% H2SO4, reaction time: 90 min, and a methanol:oil molar ratio: 10:1) and optimal alkali-catalyzed transesterification conditions (temperature: 65 degree C, reaction time: 30 min, 0.6% NaOH, and a methanol:oil molar ratio: 8:1). The properties of soursop biodiesel were determined and most were found to meet the European standard EN 14214 and American Society for Testing and Materials standard D6751. This study suggests that soursop seed oil is a promising biodiesel feedstock and that soursop biodiesel is a viable alternative to petrodiesel.
Saturated monoglyceride (SMG) is a main cause of precipitate formed above cloud point of biodiesel (B100), which leads to filter plugging in diesel engine. In this work, we studied the effect of SMG content (0.1–0.7 wt%) of palm biodiesel (PO-B100) on the cloud point of diesel fuels blended with PO-B100 at different concentrations (B0, B7, B10 and B20). Euro 4 and Euro 5 diesels with high and low aromatic content, respectively, were used. In case of B7, B10 and B20, the results show that the SMG content in the range of 0.015–0.020 wt% increased the cloud point for 10 oC, 5 oC and 4 oC, respectively. An adsorptive removal of SMG in PO-B100 was investigated by using natural zeolite as much cheaper adsorbents than commercial magnesium silicate (MS) and silica gel. The crystalline structure of natural zeolite and MS was studied by X-ray diffraction. Both MS and silica gel exhibited higher performance than natural zeolite in the SMG removal at 45 oC. To improve the SMG adsorption capacity, natural zeolite was treated with 1 M nitric acid solution at 60 oC. The resulting modified zeolite had an increased SiO2 content, as measured by X-ray fluorescence spectroscopy, due to dealumination effect. Moreover, it showed an improved adsorption performance: the capacity of SMG adsorption was 31.5 mgSMG g-1, corresponding to a decrease of SMG content of PO-B100 from 0.7 wt% to 0.35 wt%, when using 10 wt% adsorbent loading at 45 oC for 50 min. As a result, an increase in the cloud point of biodiesel blends was significantly retarded.
In this study, direct transesterification with a combination of methanol and a cosolvent was demon- strated to be promising for the production of biodiesel from black soldier fly larvae (BSFL) biomass. Of the solvents tested, n-hexane was identified as the most effective cosolvent for the reaction, resulting in a 14.5-fold increase in the biodiesel yield, compared with the reaction without a cosolvent. The direct transesterification using n-hexane as a cosolvent was then optimized to maximize the biodiesel yield. The highest biodiesel yield of 94.14% was achieved at an n-hexane:methanol volume ratio of 1:2 (v/v), a solvent dosage of 12 mL, a catalyst loading of 1.2 mL, a temperature of 120 °C, and a reaction time of 90 min. The properties of the BSFL biodiesel were also tested, and most—such as the biodiesel's density (875 kg/m3), water content (0.03 mg/kg), ester content (98.3%), acid value (< 0.8 mg KOH/g), viscosity (5.2 mm2/s), flash point (121 °C), and cetane index (50)— met the specifications of the European standard EN 14214. This study suggested that direct transesterification using n-hexane as a cosolvent could be a promising method for biodiesel production from BSFL and decrease production costs.
The main obstacles to the widespread use of lignocellulosic biomass include its high moisture content and low grindability. To overcome these problems, we have proposed a novel method, viz. the self-steam explosion (SE) method, which utilizes the moisture content of biomass to both reduce the particle size and enhance it properties. More specifically, the moisture content of the biomass sample is fully utilized as a steam resource to auto-hydrolyze the biomass component and yield fine particles with diameters < 2 mm through self-explosion. In this study, we demonstrated self-SE method effectively reduced the particle sizes of 8 various kinds of biomass, starting form woody biomass (hardwood, softwood, bark), bamboo, to agricultural waste biomass (rice husk, rice straw, EFB), into fine particles with high yields and quality. We calculated energy consumption of our proposed system which combines self-SE treatment and post-drying process to produce fine particles, by using aspen plus V9. It is found that our proposed system consumes 5.22 MJ/kg, which accounts for ~23% of the HHV of the solid products. However, the calculated energy consumption depends on the yield of fine particles and the moisture content of solid product after self-SE treatment.
Lignin-silica composites (LSC) has attracted much attention as a bio-renewable material. Currently, biomass is mainly used as an energy source for local power plants. However, this usage is uneconomical because of low thermal efficiency. Lignin and silica are the main components of rice straw and rice husk, and few of them are used for producing materials. Therefore LSC production was examined in this study. LSC is one of the organic-inorganic hybrid materials and considered to be applicable to a cathode material, an adsorbent for metal ions, and a polymer filler. The main problem in the application of lignin as a component of composites is the complex solubility due to the unclear structure. The structure strongly depends on the extraction process from the biomass resource. In addition, lignin has a low affinity with inorganic components. To synthesize homogeneous LSC, improvement of the affinity by chemical modification is required. In this study, we adopted kraft lignin and organosolv lignin as lignin materials and synthesized LSC by sol-gel method.
Lignin was chemically modified by reaction with formaldehyde and 3-aminopropyltriethoxysilane (APTES) in acidic 80 vol% 1,4-dioxane aqueous solution. Thus modified lignin was co-hydrolyzed with tetraethoxysilane. After the sol matured, the solution was dried to obtain LSC. FTIR was used to confirm the presence of Si in LSC. The more amount of APTES was used for modification, the more LSC was obtained. This implied the LSC contained more silica, thus modification by APTES was achieved. Organosolv LSC has more homogeneity than kraft LSC. Thus, it was concluded that the structure of the lignin greatly affected the properties of the obtained LSC.
Economic and environmental concerns have resulted in a great amount of research in the past couple of decades on renewable resources, particularly biomass, to replace fossil fuels. However, biomass is mainly used in an uneconomical way such as energy recovery by combustion. To establish the economical usage and promote the utilization of biomass, it is required to develop the scheme for producing the value-added products from biomass. To realize it, we propose a multi-step hydrolysis in hot compressed water for selective recovery of the component. This method takes advantage of the difference in the dissolving temperatures of biomass main components, cellulose, hemicellulose, lignin, and ash, enables selective recovery of each component in water by dissolving at sequential step optimal temperatures. This study aims to examine the optimal hydrolysis conditions for selective recovery of biomass main components.
At first, hydrothermal treatment was conducted at 130 °C. As a result, ash was recovered at the yield of 0.444 g/g-(ash in biomass), and this step avoided the other components decomposition, resulting in selective recovery of ash. Second, the residue was subsequently hydrolyzed at 180 °C. At this stage, hemicellulose was decomposed and xylo-oligosaccharide, known as high-value-added products in a wide range of areas, was recovered at the yield of 88.1%-(hemicellulose in biomass). At the second stage of hydrolysis, lignin was also recovered at the yield of 60.4%-(lignin in biomass). The remaining residue after hydrothermal treatment mainly consisted of cellulose, which can be saccharified by enzyme. Therefore, the proposed process can be envisaged as a great contribution to utilization of biomass.
The future energy system will be based on sustainable and renewable sources. However, the intermittent nature of solar and wind power will lead to the greater need for energy storage and flexibility of the energy infrastructure. The additional renewable energy facilities of 48.7 GW, which is composed of 63% (30.8GW) of solar power plants and 34% (16.5GW) of wind power plants will be constructed by 2030 in Korea. The power-to-gas (PtG) is a technology that enables flexible operation in the short and long term by storing renewable electricity energy into storable methane via electrolysis and subsequent methanation. Especially for power-to-methane (PtM) technology, Korea is the world's second liquefied natural gas (LNG) import country and the domestic supply rate reached over 80% in 2017. The total LNG line length in Korea is over 42,714 km; which means the existing gas distribution grid can be used and accessed easily. In KIER (Korea Institute of Energy Research), the alkaline electrolysis system with the production of 5 Nm3/h of H2 and the fluidized bed CO2 methanation reactor with the production of 1 Nm3/h of CH4 is designed and developed for the PtM technology. The operating range extended alkaline water electrolysis (AWE) technology to cope with power load fluctuation down to 10% is developed. The bubbling fluidized bed reactor was chosen for CO2 methanation reaction. The result shows that the stable operation is possible to cope with intermittent and fluctuating renewable surplus power.
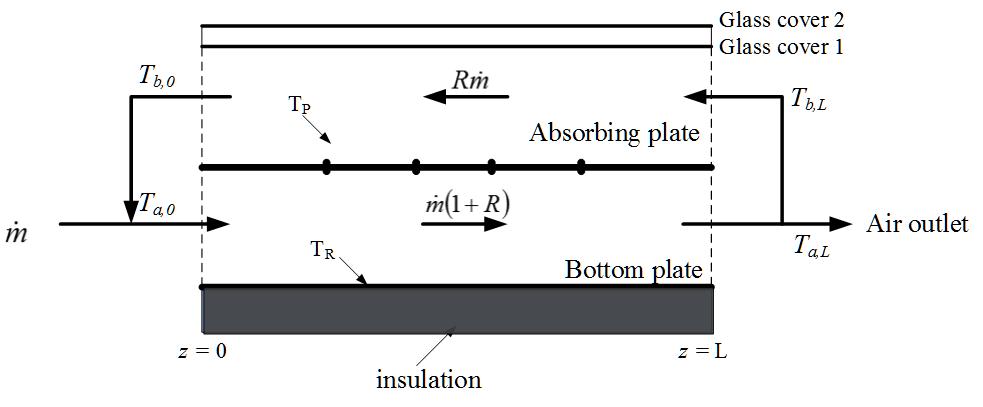
The device efficiency enhancement of the recycling double-pass solar air collectors with welding W-ribs on the absorber plate was investigated experimentally and theoretically. Operations of the solar air collector with welding W-ribs on both sides of the absorber plate were proposed to strengthen the convective heat-transfer coefficient due to the turbulent intensity increment. An economic consideration in terms of both heat transfer efficiency improvement and power consumption increment was studied to determine the optimal operating condition. A considerable device performance improvement on recycling W-ribs double-pass solar air collectors was obtained as compared to that of the flat-plate device under the same working dimensions. The good agreement between the experimental measurements and theoretical predictions was achieved within acceptable accuracy.
In recent years, most of the power supply on remote islands is by diesel power generation, and the generation of carbon dioxide accompanying the use of fuel is becoming a problem. Therefore, introduction of renewable energy such as solar power generation and wind power generation is being considered in remote islands in various places. In this study, we will construct an isolated island microgrid for the purpose of reducing carbon dioxide emissions of the Teuri island and Yagisiri island, which belong to Haboro-cho in northern Hokkaido.
At present, the power consumed by the Teuris Island and Yaishiri Island is supplied by a diesel generator already installed at the Yaishiri Island, and power is transmitted to the Teuri Island using a submarine cable. Therefore, if an abnormality occurs in the submarine cable due to a disaster or an accident, Teuri Island becomes unpowered. Therefore, in the proposed system, power is supplied by diesel power generation, solar power generation, and wind power generation. In addition, renewable energy will be installed on the Teuri Island and linked to a diesel generator to compensate for output fluctuations. In this paper, we create an analysis model using the above system using MATLAB / Simulink, analyze the introduction limit amount of renewable energy, and investigate the reduction amount of carbon dioxide emitted from the diesel generator.
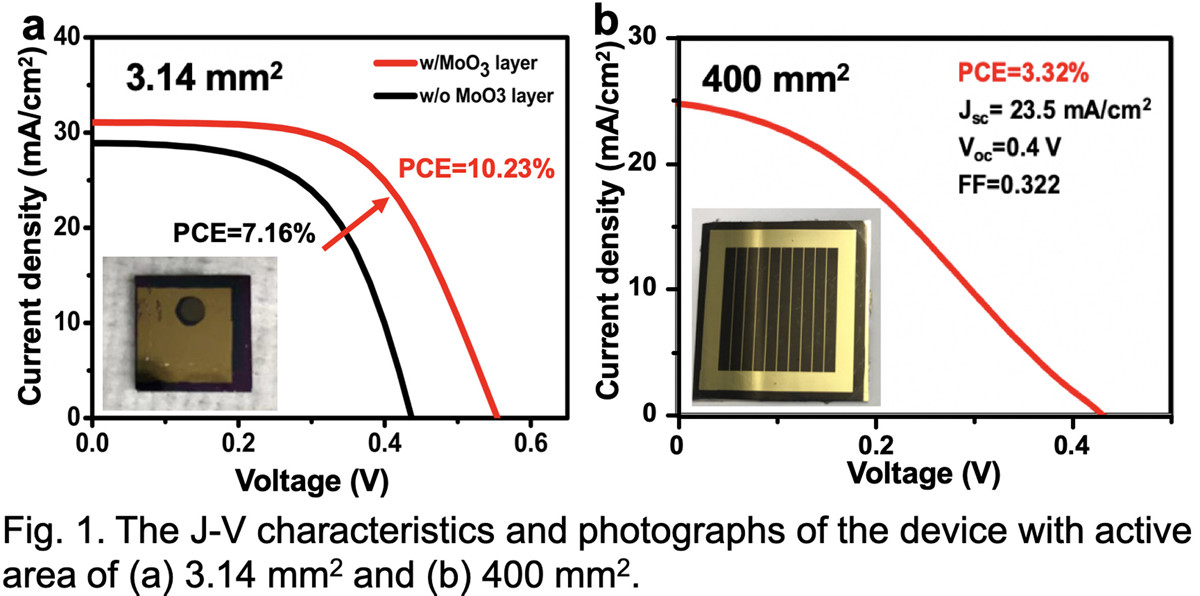
Carbon-based Si heterojunction solar cells, combining the transparent conductive carbon nanotube (CNT) films and crystalline Si wafers, have attracted increasing attention. The advantage is that the high-efficiency solar cell can be achieved in a cost-effective manner owing to the low-temperature solution process to fabricate the CNT/Si heterojunction. Since the first report of the CNT/Si heterojunction solar cell with the power conversion efficiency (PCE) of around 1.3 % by Wei et al. in 2007 [1], PCE has been improved drastically. Wang et al. reported the record-high efficiency over 17 % with an active area of 0.785 mm2 by applying a thermally evaporated MoO3 layer in 2015 [2]. However, it is essential to enlarge the active area toward practice application.
Herein, we applied the solution-processed MoO3 layer to the CNT/n-Si cells via hydration of the ammonium heptamolybdate ((NH4)6Mo7O24·4H2O) precursor [3]. Compared with the thermal evaporation of the MoO3 layer in high-vacuum, the low-cost and simple preparation process can be expected. The MoO3 layer enhanced the PCE significantly from 7.16 % to 10.23 % for the small cell (Fig. 1a). For the large cell (400 mm2), however, the efficiency remained at 3.32 % even the CNT film was doped with HNO3 (Fig. 1b). The Au bar electrodes were formed on the MoO3/CNT/n-Si structure to carry current for a large distance, and the uniformity of the MoO3 layer needs to be improved to prevent the short-circuit between Au and n-Si. It is noteworthy that the device with the solution-processed MoO3 layer showed considerable stability that remained the PCE at 80 % of its original value for more than 60 days in air without any protective layer.
The power generated from the Gibbs free energy of mixing through reverse electrodialysis (RED) is a promising renewable energy of great potential. In this study the performance of nanochannels having a pH-regulated surface in RED is examined, focusing on the influence of the bulk salt concentration, the nanochannel size, and its geometry. Taking account of the effect of osmotic flow, we consider two types of nanochannel: bullet-shaped nanochannel and trumpet-shaped nanochannel. The variations in the maximum power generation, the power efficiency, the diffusion potential, and the transference number under various conditions are investigated, and the underlying mechanisms discussed comprehensively. The results of numerical simulation reveal that the more a nanochannel is trumpet-shaped the better its ion selectivity. This is because the smaller the nanochannel space, the more significant the degree of electric double layer overlapping. For a bullet-shaped nanochannel, the maximum power generated has a local maximum as the ratio of (nanochannel length/curvature radius) varies. This can be explained by the associated diffusion potential and the electric current passing through the nanochannel. The results gathered provide desirable and necessary information for designing a RED system.
MoS2 has a layered structure and its electrocatalytic activity of hydrogen evolution reaction (HER) is strongly dependent on layer number of its nanosheets. For example, few-layered MoS2 is well known to show efficient HER activity, whereas multi-layered or bulk MoS2 show poor HER activity.Thus, a MoS2 synthesis process that allows controlling the layer number is required. We recently developed a synthesis method on few-layered MoS2 nanosheets using supercritical ethanol process and showed that synthesized materials possess a certain HER activity.
In this study, we propose a layer-number selective MoS2 symthesis process under supercritical hydrothermal reductive conditions using organic reducing agents. We systematically synthesized MoS2 nanosheets using various synthesis condition and precursors. Physical characterizations (such as XRD and TEM) and measurements of HER activity of synthesized MoS2 nanosheets are conducted. It was found that product (MoS2 or other Mo containing by-products), layer-number and HER activity of our synthesized materials are strongly dependent on synthesis condition and precursors. Detailed result of characterizations and formation mechanism of MoS2 nanosheets with different layer number are discussed in the poster session.
Water electrolysis seems to be well established technology with high energy conversion efficiency. The biggest problem is high costs of electrical energy, especially in Japan. The seawater electrolysis is one of the solutions for reduction of costs because seawater is infinite natural source. In seawater electrolysis for energy production, a huge amount of chlorine emission is not allowed. But, appropriate amount of chlorine production can be used to sterilization and industrial source. In this study, we examined to the clarify the relation between the hydrogen, oxygen and chlorine evolution behavior and electrolyte and supply conditions (pH, concentration, flow rate, etc.). The flow-type electrolysis cell was consisted that active area is 70cm2 and flow path thickness is 5 mm.
The experimentally observed the potentials of oxygen and chlorine evolution reaction are similar potentials in 3.5wt% NaCl solution (pH 6.5). However, the current efficiency of chlorine calculated from the concentration in the exit flow (impressed current density; 7.14 mA/cm2, linear velocity; 1.78 cm/s). It caused by the decreasing of chloride ion on the electrode surface due to the difference of ionic mobility.
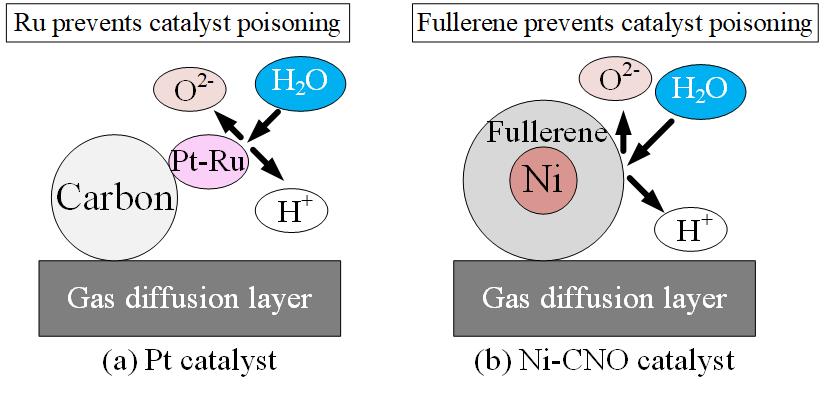
Currently, we aim for a clean energy society by introducing a large amount of renewable energy. As a method, hydrogen production using surplus power is attracting attention. In this research, we focused on PEM (Polymer Electrolyte Membrane) type water electrolyzer which electrolyzes distilled water. PEM-type water electrolyser have the advantage of not discharging carbon dioxide when producing hydrogen, but have the problem of using expensive Pt metal for the catalyst. Therefore, in this research, we used Ni-CNO (Nickel-Carbon nano Onion) coated with fullerene, consisting of a large number of carbon atoms in a closed shell cavity, which is less expensive than Pt metal. Figure 1 shows the difference in hydrogen generation mechanism between Ni-CNO and a general Pt catalyst. PEM-type water electrolyser is comprised by a separator and MEA (Membrane Electrolyte Assembly). MEA is a place where the water electrolysis reaction actually occurs, and it is a combination of a gas diffusion layer, a catalyst layer, and an ion exchange membrane. Although PEM-type water electrolyser is affected by the material as well, it is also greatly influenced by the MEA manufacturing conditions. In addition, conditions for producing MEA (Membrane Electrolyte Assembly) suitable for the Ni-CNO catalyst are unknown. Therefore, in this research, we investigated suitable MEA fabrication conditions and evaluated their performance. As the performance evaluation method, we used the energy conversion efficiency obtained by dividing heat quantity of generated hydrogen per second and the
power supplied. The best result was an energy conversion efficiency of approximately 80% compared to a common Pt catalyst. In addition, since the cost of the catalyst material is about 1/8, it is considered to be sufficient performance. As a future subject, it is necessary to investigate how the energy conversion efficiency changes when a voltage fluctuation simulating renewable energy is added.
Vanadium redox flow battery (VRFB) is one kind of energy storage system (ESS), which converts electricity to chemical energy by redox reaction in each electrolyte tank. Vanadium redox flow battery is recently an attractive energy storage system of renewable energy sources for its low cost and flexible design. For the operation of VRFB, ion exchange membrane plays important role. Ion exchange membrane provides ion transfer channel for charge carriers, and it prevents electrolyte of each electrode from cross-mixing. Commercially, Nafion membrane is used for ion exchange membrane for VRFB. Although Nafion has high proton conductivity and good chemical stability, high vanadium ion permeability, which is main factor of self-discharge reaction, is major defect to be used in VRFB.
In this study, the anion exchange membrane is synthesized from poly(arylene ether ketone) with 1-(3-aminopropyl) imidazole(API) as pendant groups for vanadium redox flow battery application. PAEK is one of the hydrocarbon based polymer which has good mechanical and chemical stability, and it can be modified easiliy by introducing functional groups. Imidazolium group is one kind of positively charged functional groups, which can reduce permeability of vanadium ion by Donnan exclusion phenomena. And due to the N-heterocyclic structure in functional groups, excellent chemical stability in electrolyte solution can be expected.
As the content of imidazolium group increases, the ion exchange capacity of membrane increases, showing higher value than Nafion 117 membranes. All of the synthesized membranes show significantly low vanadium ion permeability compared to Nafion 117 membranes. In addition, During the 100 cycling test, PAEK-API 2.0 membrane shows higher coulombic and energy efficiencies than Nafion 117 membrane without any degradation.
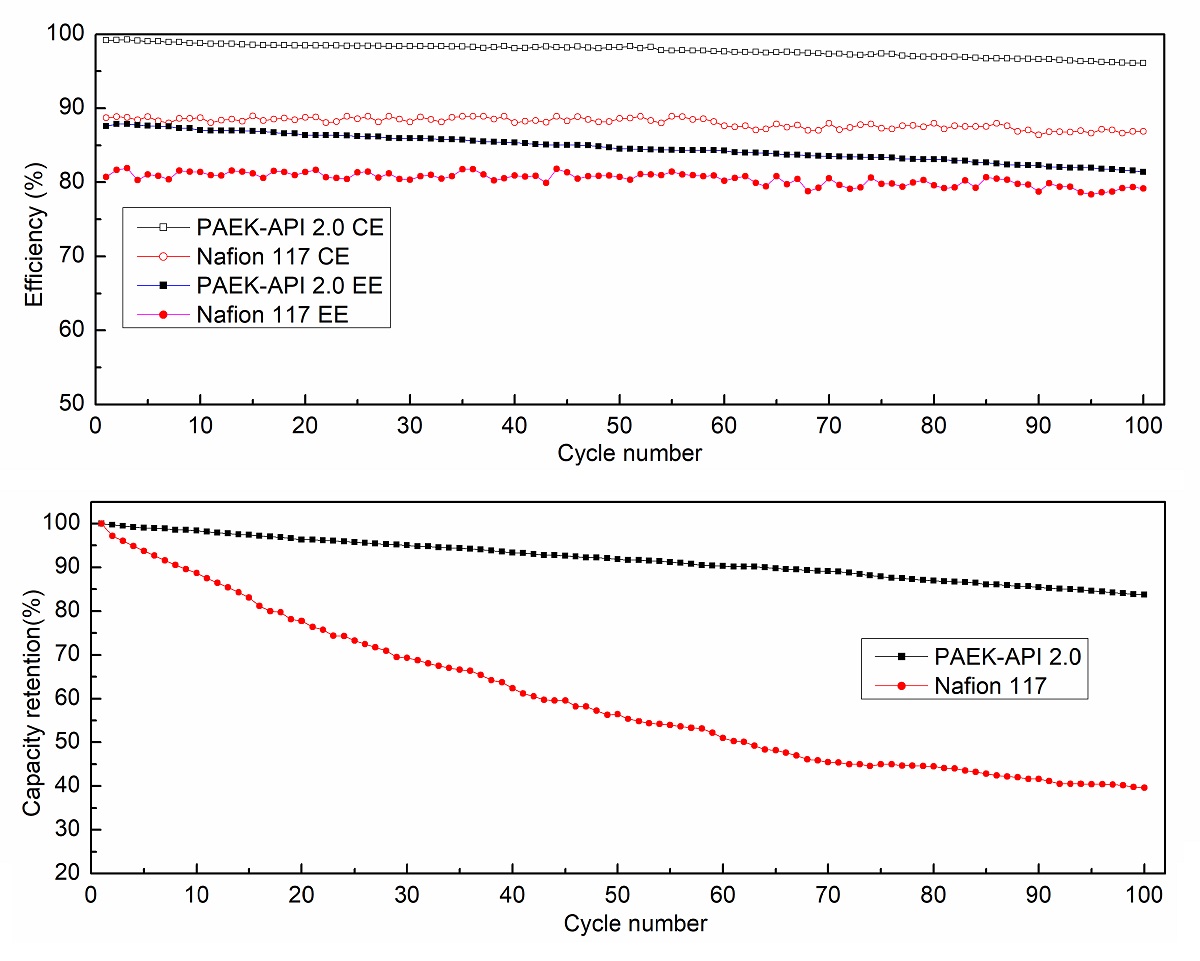
There has been a considerable interest in one-dimensional nanostructures owing to their remarkable characteristics in particular these electronic properties which have been considered to significantly improve the electron transport time and reduce the recombination rate. The range of research area of one-dimensional nanostructures covers meanwhile a wide range of transition metal oxides. Among them, TiO2 is the most widely studied material because of its promising applications in solar-cells, photoelectrochemical hydrogen production, and CO2 reduction etc. Because, highly aligned TiO2 nanostructures have been suitable semiconductive properties with ∼3 eV and optimum band-edges to water, fast electron path way, and chemical stability.
Recently, noble metal decorated TiO2 nanotubes has been considered a key materials for photocatalytic hydrogen production. In the present presentation, we show that when growing anodic self-organized TiO2 nanotubes from Ti-noble metal alloy (Ti-Au, Ti-Pt) with at low noble metal contents, after formation the resulting oxide nanotubes show a regular self-decoration with Au or Pt nanoparticles of ∼5 nm in diameter. The average spacing is probably adjustable by the anodization conditions (in the range of ∼60 nm). The type of decoration the co-catalyst on TiO2 nanotubes leads to a very high activity for photocatalytic H2 production under UV or visible light conditions.
Anode recirculation in the solid oxide fuel cell (SOFC) increases the system fuel utilization so that the electrical efficiency of the system would be improved. However, large amounts of water (steam) existing in the recirculation loop not only decreases the cell open circuit voltage but also aggravates the concentration polarization, thereby limiting the enhancement of electrical efficiency. Therefore, proposed systems consisting of water separators could be a solution of this problem. In this study, intermediate temperature SOFC (IT-SOFC) CHP systems with anode recirculation and water separators are investigated by using Aspen Plus software to evaluate their performances. Several system configurations with various steam separator layouts are considered and the effects of anode recirculation ratio on the system performance are analyzed. Simulation results indicates that electrical and thermal efficiency of the conventional system (without water separator) could be significantly improved by adding a water separator at the outlet of the anode. On the other hand, the system adding a water separator at the inlet of the afterburner exhibits superior thermal efficiency and total efficiency at a high recirculation ratio.
In this study polybenzimidazole (PBI) nanocomposite membranes using sulfophenylated titanium oxide (s-TiO2) nanoparticles was synthesized for application in high temperature polymer electrolyte membrane fuel cell (HT-PEMFC). The neat TiO2 was surface modified via two-step synthesis to improve proton conductivity and cell performance of PBI, Different concentrations of inorganic fillers were incorporated to test their effect. The PBI nanocomposite membranes were doped with phosphoric acid (PA) for performance evaluation. Properties relating to fuel cell performance such as PA doping level, proton conductivity, PA retention and cell performance were evaluated and compared to neat PBI. Increase in performance was observed for certain concentrations while excess caused decline in performance.
Low temperature heat below 473 K is produced massively. It is useful to convert low temperature heat into electricity. In carbon dioxide (CO2) capturing systems, amine solutions absorb CO2 at 313 K and the CO2 partial pressure (PCO2) of 0.01 - 10 kPa and desorb CO2 at 393 K and PCO2 of 100-200 kPa. We consider the possibility of the low temperature cycle whose turbine is driven by the desorbed CO2 and H2O. The power cycle is as follows. An amine - CO2 - H2O mixture is cooled to 313 K in a cooler. The cooled amine - CO2 - H2O solution is pressurized in a pump and heated in a recuperator. The preheated fluid is heated at a heat exchanger by hot fluid. The CO2 - H2O gas mixture desorbed from the preheated fliud and the residue are separated by a gas - liquid separator. The CO2 - H2O gas mixture drives a turbine and expanded adiabatically. The residue heats the pressurized solution. The expanded CO2 - H2O gas mixture and the cooled residue are mixed in a mixer. The basic configuration is the same as the water - ammonia based Kalina cycle. However, it is a feature of this cycle that it contains CO2 and a large amount of water vapor in the generated gas. In our thermodynamic study, it was expected that this cycle can obtain the performance equal to or higher than that of the current ORC. Therefore, a principle verification test was carried out. The test showed ten per cent of thermal efficiency at 393 K and CO2 loading of 20 NL/L. We are planning a 10 kW amine-CO2 cycle test.
In a low temperature power cycle using amine – CO2 fluid, an amine solution absorbs CO2 at 313 K in an absorber/condenser, the CO2 absorbed amine solution is pressurized by a pump, heated by a recuperator and a boiler, and desorbs CO2 – H2O gas mixture, which drives a turbine. In our thermodynamic study, it was expected that low temperature power cycle using amine-CO2 fluid can obtain the performance equal to or higher than that of the current ORC. Therefore, a principle verification test was carried out. The test showed ten per cent of thermal efficiency at 393 K and CO2 loading of 20 NL/L. We are designing a 10 kW test equipment. We set high temperature heat source is hot water at the temperature of 363 K, which is used as a coolant of a gas engine. The heat and mass balance of the equipment was calculated thermodynamically. The result showed the flow rate of the hot water flow and amine – CO2 fluid, the turbine expansion ratio, the power, and the system efficiency are 8,200 kg/h, 1,000 kg/h, 4.8, 10.5 kW, and 7.3 per cent, respectively. The preliminary estimation shows as follows. The diameter and rotational speed of the turbine blade are 0.115 m and 54,400 min-1, respectively. The numbers of tubes in the boiler, the recuperator, and the absorber/condenser, which are shell and tube heat exchangers, are 4400, 910, 1020, respectively. The length and diameter of the tube are 1 m and 6 mm, respectively.
In the background of global warming and scarce resources depletion, Japan depends on the fossil fuel for most of energy resources. So utilization expanding of renewable energy and thorough energy conservation are necessary for stable utilization of energies and climate change measures in the future. Since Hokkaido prefecture that is a northern island of Japan has the abundant renewable energy, such as solar, wind, small and medium-sized hydropower, biomass, and geothermal energy, it will show the highest feasibility for renewable energy throughout Japan, toward the utilization of new energy. Then use concept of various energies has been proposed as an opportunity of the local production for local consumption of energy or feed-in tariffs. In this study, optimum mix and dispersed models of energy in Hokkaido prefecture were investigated to develop the technology support system utilizing energy resource dispersed in wide regions, like renewable energy and to construct the appropriate energy demand and supply system increasing the regional development or activation and the energy self-sufficiency rate. Many methods estimating substantial, available and demand amounts of regional energy were evaluated. Integrated geographic information system (GIS) supporting local governments or independent workers was designed by combining the collected and calculated amounts of regional energy. The GIS is divided into two types of Web site and stand-alone. The Web site type can be viewed for anyone in Hokkaido by accessing required data to computer. The stand-alone one may be efficient for planning and coordinating energy policies in each of local governments. Moreover, various element technologies, such as combustible waste fuel, combustion system and energy best mix for facilities group were developed to improve the regional energy balance and creation of new industry. Regarding Furano region as a typical local model, dispersed utilization models for the energy suitable to regional and industrial characteristics were proposed.
A microgrid is a localized group of electricity sources and loads typically connected to and synchronous with the traditional centralized grid (macrogrid), but it can disconnect and maintain operation autonomously. This study utilise analysis of correlation to determine the impact factors which is used to predict the demand of electricity, renewable energy sources (RES) and prices. Power dispatch of distributed generators (DG) and optimization of microgrid is designed to minimize the consumption and cost of fuel and emissions of exhaust. It is essential for power system security to set an adequate operating reserve (OR) power to compensate the unpredicted imbalance between RES generation and consumption. Matlab is used to create the prediction model and GAMS is utilised to optimize the unit commitment problem (UCP).
Keywords: Microgrid; Prediction; Neural Network; Power Dispatch; Optimization.
A chemical heat storage is advantageous in terms long periods of time storage and the high storage density are possible as compared to the direct storage methods. Normally, a reversible single reaction is utilized, then the heat storage temperature is limited. There were several MeO/H2O systems such as MgO/H2O and CaO/H2O as chemical heat storage material. However, in order to store industrial waste ranging widely in the temperatures, it's necessary to develop a new material which allows heat storage in a stepwise manner in response to temperature changes. Hydrotalcite [Mg6Al2(OH)16(CO3)4H2O] which is a kind of Layered double hydroxide (LDH), have possibility to get multi-step chemical heat storage reaction using dehydration – hydration reaction of Mg-OH, Al-OH and interlayer water. However, in order to occur the above hydration reaction, the supply of the interlayer anion of CO2 is essential, which is a major problem for the reaction rate and cycleability for chemical heat storage. Then, although it is necessary to design the kind of interlayer anions and metal ion, there have been few studies focusing on reaction reversibility of LDH. In this study, we have investigated the effect of interlayer anion on the dehydration-hydration reaction of Hydrotalcite and the application for chemical heat storage material. In addition, the potentials of the multistep chemical heat storage property have been investigated.
A CO2 hydrate power generation system for cold regions has been proposed, in which carbon dioxide hydrate is formed by cold heat from the outside air, and a generator is driven by high pressure gas obtained by dissociating the hydrate by supply of low temperature waste heat. The purpose of this study is to clarify the effect of formation reaction promoters on the improvement of carbon dioxide hydrate formation rate and increase of formation temperature upper limit. Cyclopentane, which shifts the phase equilibrium pressure curve to higher temperatures and lower pressures, was used as a formation reaction promoter in this study. This shift is expected to have the desirable effect of improving the formation rate, but adding the formation reaction promoter has the negative effect of reducing the amount of formation by reducing the amount of net water. In the experiment, we filled a 100 ml stainless steel reaction vessel with 50 ml of pure water or 50 ml of a mixed solution of pure water and cyclopentane at a mixing ratio of 1:1. After enough initial dissolution was performed at a desired pressure, thermal cycles of formation and dissociation for 6 hours were repeated by alternately flowing 273 K and 293 K heat media around the periphery of the reaction vessel. To calculate the formation rate, the measured values of pressure and temperature in the sealed reaction vessel were used, and the compression coefficient of CO2 was considered using the physical property database REFPROP. As a result, we were able to confirm that cyclopentane increased the formation rate and amount under the conditions without power stirring. This result suggests the possibility of improving the energy storage rate and the energy storage capacity per unit water volume of the proposed power generation system using cyclopentane.
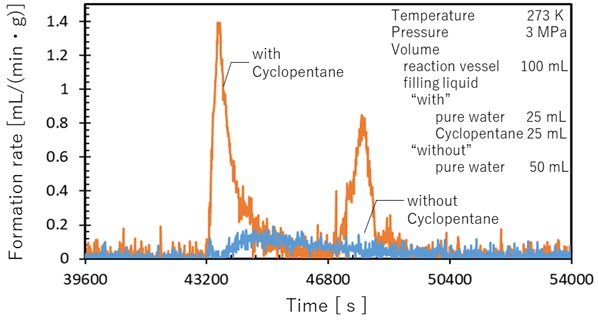
Recently, there is an increase in energy consumption of air conditioning for a comfortable life in the residential sector. Desiccant humidity control system has been gaining an attention to improve energy utilization efficiency of air conditioning. The system can be driven by low-temperature heat and control latent heat loads easily, independent of sensible heat loads. However, dehumidification performance is getting low by lowering regeneration temperature, because effective water adsorptivity, which is a difference in the amount of adsorbed water between dehumidification and regeneration, greatly decreases at lower temperatures. To solve this problem, we have focused on thermosensitive polymer gel as a novel adsorbent for desiccant humidity control system. The thermosensitive polymer gel is well-known to switch hydrophilic and hydrophobic natures in water at a lower critical solution temperature (LCST). We expected that thermosensitive gel adsorbs water vapor below the LCST and desorbs a large amount of adsorbed water over the LCST, resulting that it can achieve a high effective water adsorptivity even by a small temperature difference between dehumidification and regeneration. In this study, N-isopropylacrylamide, 2-(Dimethylamino)ethyl methacrylate, and N-isopropylmethacrylamide were employed as thermosensitive monomers which have different LCSTs in water. They were polymerized using a radical polymerization method with N,N′-methylenebisacrylamide (MBAA) as a crosslinker at various MBAA concentrations and polymerization temperatures. Water vapor adsorption behavior of the prepared gels were measured at several adsorption temperatures and relative humidities. Finally, the applicability of the gels toward a desiccant humidity control system was evaluated from the viewpoint of effective water adsorptivity in the operation range of the system.
The improvement of chiller cycle performance has been investigated. We suggested the thermal and electric hybrid absorption refrigerator cycle. In this cycle, we selected the refrigerant and absorbent as HFC-134a (1,1,1,2-Tetrafluoroethane) and 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [BMIM][Tf2N] (Ionic Liquid), respectively. In this study, The Coefficient of Performance (COP) based on input heat energy for regeneration absorbent and electric power for compressor was calculated using a static analysis based on a state equilibrium in order to evaluate the effect of a compressor work on COP and regeneration temperature. As the results from analysis, the hybrid cycle could be generated cold heat below 0 °C, and driven below regeneration temperature 60 °C by assist of compressor. In addition, the absorption pressure working compressor was increased as the amount of absorption in Ionic liquid and COPsystem were obtained high value. The COPsystem was affected on the regeneration temperature, which the input sensible heat and amount of absorption in Ionic Liquid was depended on. For heat source in the temperature range of 60-90 °C, the hybrid cycle is superior in terms of COPelectricity compared to the mechanical cycle. COPelectricity was affected by liquid pump efficiency. The COPelectricity of hybrid cycle was higher than the COPelectricity of mechanical type when the liquid pump efficiency was over 0.15.
Vegetables cannot grow outside in cold regions because of snow in winter, so we focused on the plant factory which can provide a stable supply of crops. However, the plant factory consume a lot of power because they use air conditioning and lighting to grow plants. Therefore, in this research, we will consider the energy system of a clean plant factory with low power consumption. The vegetables grown in the plant factory are spinach. Spinach increase sugar content when exposed to low temperatures. This is called savoy-spinach. Therefore, in this paper, we propose a plant factory system that assumes low temperature processing of spinach. The plant factory install photovoltaics and a solid oxide fuel cell (SOFC cogeneration) with high power generation efficiency. Furthermore, in order to utilize the exhaust heat of SOFC cogeneration, an absorption refrigerator is installed to supply cold heat to the plant factory. The room temperature of the plant factory is cooled to 7 degrees Celsius, because the ambient temperature needs to be 7 degrees Celsius or less in order to perform the low temperature processing of spinach. Because the daily maximum temperature of from December to March of Kitami is less than 7 degrees Celsius, the room temperature of the plant factory is air cooled by a ventilation fan from December to March and cooled by an absorption refrigerator from April to November. The above system can be expected to reduce the power consumption of the air conditioning equipment because it effectively uses unused energy such as exhaust heat of SOFC cogeneration and outside air. Also, by introducing photovoltaics, CO2 emissions can be reduced by up to 8.45×106 kg-CO2. This corresponds to a reduction of fuel (natural gas) consumption of 3.91×106 kg, and it can be expected to reduce environmental burden.
Plant factories have attracted attention because the decrease in the number of employed farmers in Japan has become a problem. However, energy costs affect profitability, as plant factories use artificial light. Therefore, we will consider the profitability of a cold region plant factory from the energy efficiency (= calorific value of plant / energy input) of Corchorus olitorius, Japanese peppermint, Potherb mustard, Japanese mustard spinach, Bok choy and Crown daisy which have vegetation in the Okhotsk region. In order to investigate the calorific value of a plant, cultivation was performed using LED, and the calorific value of the plant was measured using DSC (Differential Scanning Calorimeter). In addition, in order to investigate the input energy, the heat demand and the power demand were investigated for the cold region plant factory installed at the University. As a result of investigation, the relationship between the energy efficiency of each plant and the number of cultivation days is as shown in Fig 1. Assuming that the energy efficiency is Y and the number of cultivation days is X, an approximate expression Y=0.0275X+1 can be obtained. Furthermore, as a result of investigating profitability from the running cost at the time of cultivation at a plant factory, it was found that Corchorus olitorius and Japanese peppermint have no profit, and the other 4 types have profit. Since the lower part of the approximation line is Corchorus olitorius and the Japanese peppermint, and the upper part is the other four types, it is considered that if the energy efficiency of the plant becomes larger than the approximation formula, it may be possible to be profitable. As future issues, it is considered necessary to increase the number of surveys due to lack of data on plants that can be harvested within 30 days.
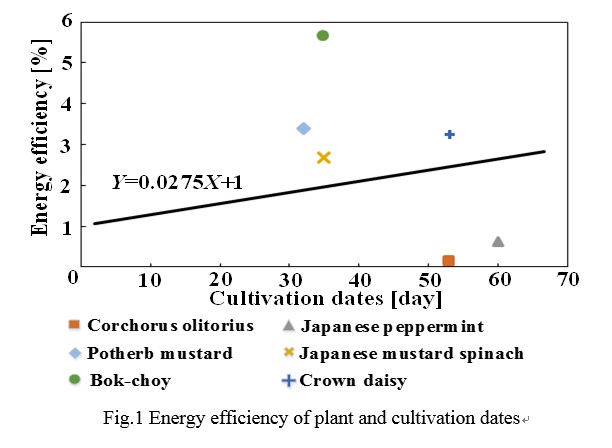
Based on the elementary reaction database on the steam reforming of methane on the Ni catalyst, an elementary reaction simulation of CO2 hydrogenation was performed. DETCHEM was used to calculate a one-dimensional reaction assuming a fixed bed reactor. The obtained results were compared with not only the methanation reaction test using commercial catalysts but also the actual measurement data obtained from the literature. The identification of the rate limiting step and the correlation between the reaction characteristics and the catalyst physical properties were examined. The temperature profile in the catalyst bed was obtained and The measured values of CO2 conversion and the reaction simulation predictions agree well.