
The challenge for applications of viruses, virus-like particles, or other extra cellular bionanoparticles in gene-therapy or in cancer therapy is the high dose with up to 1014 particles per dose. Downstream processing is one bottleneck in the production of these bionanoparticles. It has been often overlooked that a heterogenous population of bionanoparticles is present and they must be separated from the product. The heterogeneous populations of bionanoparticles are released from the cells, carrying different host cell proteins, DNA and RNA fragments. Additionally, cells produce extracellular vesicles (microvesicles and exosomes) with similar size and surface properties. The biological activity of these different particle populations is not fully understood. Particle characterization and biological activity assays require the separation of these populations with high purity. Here we compare the results obtained for the purification, as well as particle populations' separation, of HIV-1 gag VLPs produced in CHO cell culture using different downstream processing approaches. These approaches include polymer grafted anion exchangers, monoliths (anion exchange and hydrophobic interaction), a combination of flow-through and heparin affinity chromatography, and membrane adsorbers. Several analytical tools including Nanoparticle Tracking Analysis and Mass Spectrometry were used for VLP characterization. An outlook will be given how continuous ultracentrifuges will complement these purification approaches.
PEGylation is a well-known technique to develop biopharmaceuticals, such as proteins and DNAs. It can increase the hydrodynamic radius to reduce their kidney excretion and prolong their in vivo half-life. On the other hand, the control of PEGylation reaction is difficult because of the formation of heterogeneous mixtures of target PEGylated product, its isomers, unreacted proteins and PEG reagents. As the differences in the sizes and the surface properties such as charge and hydrophobicity of the isomers are sometimes small, several purification steps are always combined to obtain the target product from the reaction mixture. Among the purification method, ion exchange chromatography has an advantage for the separation of isomers on the basis of their difference in size and charge with a single operation. However the separation mechanism is not fully understood. In this study, synthetic PEGylated DNAs were used as a model of well-defined structure isomers to investigate the charge shielding effect of PEG on the charged biomolecules. Poly T possessing 9 to 95 thymine bases, an amine group for conjugation and middle base was reacted with NHS-activated PEG reagents. The retention factors on anionic exchange chromatography, Q sepharose HP and QA monolith, were determined by liner salt gradient experiment. Elution salt concentration of PEGylated DNA was decreased depending on the molecular weight of PEG. However, the decrease was not pronounced when the number of bases increased. On the other hand, the number of binding site was practically unaffected by the PEGylation which means that the conjugated PEG chain did not disturb the interaction of DNA with cationic ligand while the interaction was weakened by the conjugated chain.
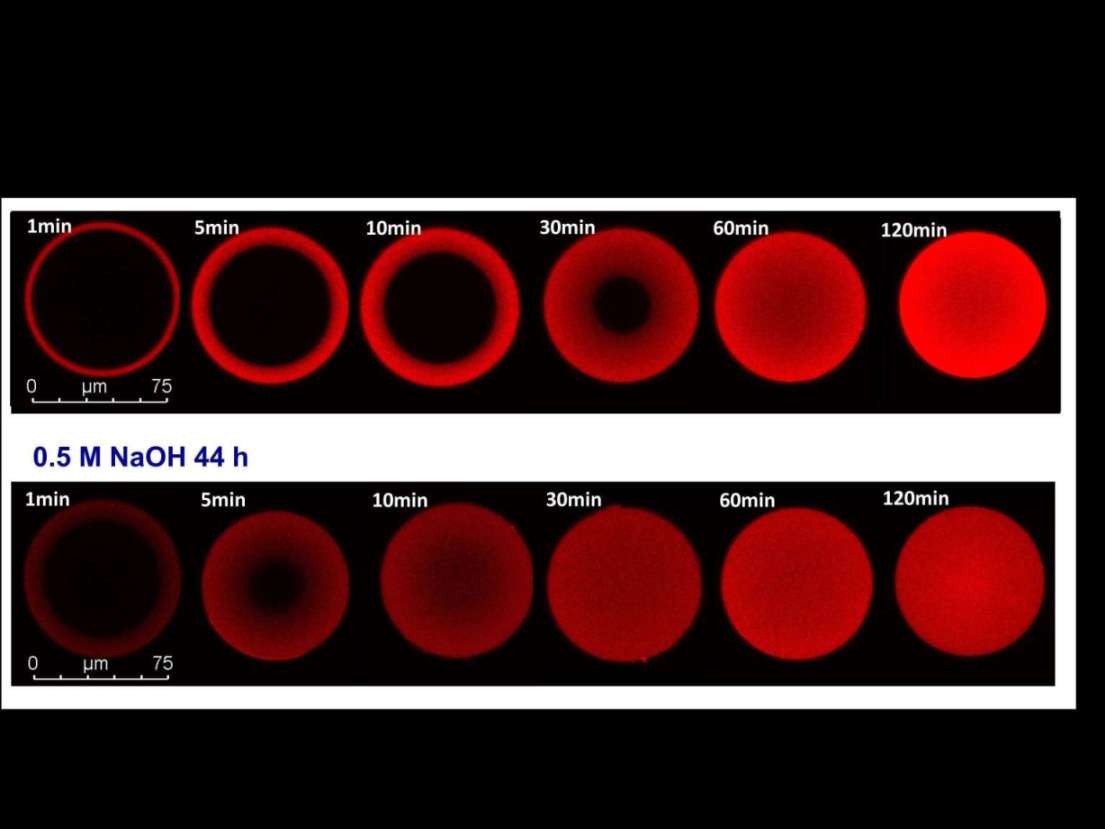
Protein A affinity chromatography is still the preferred option for antibody capture. In the past years, significant improvements have been made with respect to alkaline stability of the recombinant Protein A ligands, with resistance to 1 M NaOH over extended time periods as the latest milestone. We have investigated how exposure to alkaline conditions can alter antibody binding kinetics of Protein A resins. Break-through curves of fresh resins and resins after different time periods of alkaline exposure were compared using mAb and polyclonal IgG. As expected, equilibrium binding capacity dropped to some degree at long incubation times. In contrast, mass transfer of IgG was enhanced which was reflected by steeper break-through curves. Fitting of the break-through profiles with a pore diffusion model revealed effective pore diffusion coefficients which were increased by a factor of 2-3. Adsorption isotherm measurements showed that even after alkaline exposure, typical highly favorable isotherms were obtained, albeit with association constants that were reduced to some extent. Based on these altered binding properties we could identify a narrow window of specific operating conditions, where the alkaline treatment eventually led to an increase of the dynamic binding capacity. These conditions varied for different resins and strongly depended on the NaOH concentration and incubation time. Confocal laser scanning microscopy measurement showed that the accelerated mass transfer involved a transition from a shrinking core behavior with sharp fronts to a situation with more diffuse profiles typical for solid diffusion or pore diffusion with lower binding strength. Linear pH gradient elution studies showed that the desorption behavior was not changed significantly, as pH values at the peak maximum were almost the same as for fresh resin. The results of this study will contribute to a deeper understanding of phenomena associated with performance change of affinity media upon alkaline regeneration.
Polyacrylonitrile (PAN) nanofibrous membrane was prepared by electro-spinning technique. After heat treatment, alkaline hydrolysis and neutralization reaction, the ion exchange membrane (namely P-COOH) was further chemically grafted with chitosan molecule. The obtained P-Chitosan membrane (namely P-CS) was then covalently immobilized with Procion orange MX-2R dye from simulated textile wastewater to be used as a dye-ligand membrane. Fiber diameter, porosity, pore size, immobilized dye-ligand concentration, and protein binding capacity were characterized. Furthermore, the membrane was applied to evaluate the binding capacity of lysozyme under various operating parameters (e.g., pH, chitosan mass per volume ratio, immobilized dye concentration, ionic strength, and temperature) in batch mode. The experimental results were directly applied to purify lysozyme from chicken egg white by newly designed membrane chromatography module. The results showed that the recovery yield and purification factor were 98.9 % and 56.9-fold, respectively, in a single step. The binding capacity remained consistent after five repeated cycles of adsorption-desorption operations. This work demonstrates that the dye-ligand nanofibrous membrane holds great potential for purification of lysozyme from real feedstock.
Protein precipitation by polyethylenglycol is a simple, robust and cost effective first capture step for purification. The resulting structure of the precipitate particles has a significant impact on process design and process performance like recovery and dissolution kinetics. Current technologies for visualizing and reconstructing 3D precipitate structures are either very time consuming and expensive (Cryo-TEM) or generate a single average value (a fractal dimension) for a population of precipitate particles (light scattering). We developed a new method using light microscopy to reconstruct the complete three dimensional structure of individual precipitate particles with a resolution of 0.1-0.2 μm. This methods was then used to reconstruct and characterize the three-dimensional structure of particles generated by batch precipitation by PEG as well as continuous precipitation in different shear stress environments. Both methods, batch and continuous precipitation, generate particle structures of very diverse nature, while the average fractal dimension is significantly different between the two (2.40 for batch in low shear environment and 2.52 for continuous in high shear environment). Besides the overall average, when investigating both populations in detail, the distributions of fractal dimensions of individual particles overlap significantly. We could also confirm that the generation of PEG-precipitated particles is diffusion limited, as simulations predict a fractal dimension of 2.4 for diffusion limited precipitation and 1.7 for reaction limited precipitation. A close inspection of the 3D structure of the precipitate also shows monofractality of the particles from micro to nano scale visualized by light microscopy. We showed that the fractal dimension and 3D reconstruction is a valuable tool for characterization of different shear stress conditions and mode of operation. The current switch from batch manufacturing to continuous manufacturing has to take the 3D structure and population of different protein precipitates into account in their design, engineering and scale up.
A preparative high-performance liquid chromatography is an extensively used technique for a purification of commercially important bio macromolecules, such as proteins and polynucleotides. Since process time is becoming more and more critical, fast and effective chromatographic methods are widely required. In this context, target molecule breakthrough point determination is of a great interest for optimization of downstream processing. Ideally, continuous analysis is preferred, such as UV absorbance or fluorescence monitoring, which in some cases however lack sufficient selectivity. An alternative is to perform fast analysis of column outlet fraction via chromatography providing equal or greater selectivity than purification step. This can be done using UPLC that however requires specialized equipment. On the other hand, fast analysis of macromolecules can also be achieved using convective chromatographic media at much lower pressure drop. This opens possibility to combine purification step and chromatographic analytics on a single chromatographic system.
In our case we implemented äKTA Explorer system to test feasibility of proposed approach. On-line analysis of preparative column outflow was performed by sequentially injecting outlet on convection based analytical column operating on the same chromatographic system where target molecules were analyzed. Cationic and/or anionic exchangers were used as chromatographic supports (along with selective protein A membrane), depending on feed sample and its characteristics. Three different case studies were tested: monoclonal antibodies purification, aggregate content and plasmid DNA (pDNA). To adjust limit of detection an algorithm varying number of injections was used. This enabled accurate monitoring of an early breakthrough for concentration below 1%. Due to its simplicity and flexibility such methodology can easily be adopted also in pharmaceutical environments.
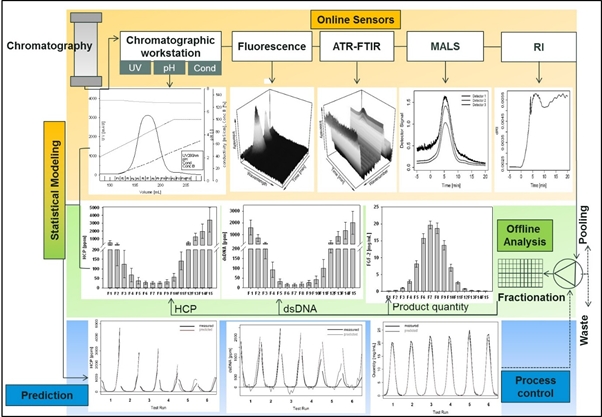
To enhance product quality and process robustness in biomanuacturing regulatory agencies have encouraged the implementation of process analytical technology (PAT). This combination of process understanding and control with real-time monitoring of quality and performance attributes also increases process efficiency and productivity. Currently, process performance is monitored by laborious and time-delayed offline analysis after each process step. This conventional approach increases hold times and overall process duration as well as the risk for batch failure. We have equipped a commercial chromatographic workstation with additional online sensors based on multi-angle light scattering, refractive index, attenuated total reflection Fourier-transform infrared, and fluorescence spectroscopy. The combination of these derived online data enables the prediction of product quantity and various purity attributes such as high molecular weight impurities, HCP and dsDNA content simultaneously during the elution phase of a chromatographic purification step. Such predictive models based solely on online data have been established for capture, intermediate and polishing steps of a monoclonal antibody produced in CHO and for fibroblast growth factor 2 overexpressed in E. coli. Online signals and corresponding offline data for product quantity and co-eluting impurities were analyzed by the statistical tools partial least square regression and boosted structured additive regression. Also chromatographic runs with varied process parameters were conducted to determine the predictive power of the models. The established methodology using complimentary online sensors enables the prediction of product quality and process performance attributes simultaneously in biopharmaceutical purification processes within a feedback time below 5 sec. The application of such models allows online pooling decisions and decreases the number of off-line analysis and hold times significantly. The costs for in-process analytics are lowered and the risk for batch failure is reduced. These findings are fundamental for the successful implementation of continuous manufacturing and real-time release in biomanufacturing.
Ion exchange chromatography is a widely used method for purification in all types of biomolecules in current biotechnological downstream processes. Knowledge on the binding behavior of proteins provides valuable insight for understanding the molecular mechanisms of protein interactions in a biological context. However, thermodynamic parameters such as enthalpy and entropy changes accompanied by protein adsorption and desorption are still unknown in ion exchange chromatography. This work relates the change in molar adsorption enthalpy, Δh0, with the salt concentration needed to elute a model protein with a gradient elution, IR. Bovine serum albumin (BSA) was adsorbed on four anion exchanger solid phases possessing grafted ligands (Toyopearl GigaCap Q-650M, Toyopearl SuperQ-650M, Toyopearl Q-600C AR, Q Sepharose XL). A linear relation was observed between the Δh0 and IR. From the highest Δh0 to the most negative: Toyopearl SuperQ > Q Sepharose XL > Toyopearl Gigacap Q-650M > Toyopearl Q-600C AR. The same experiments were also done with a non-grafted conventional solid phase, Q Sepharose FF. However, it did not fit in the same trend due to the ligand disposition. Δh0 was found to be endothermic for Q Sepharose FF and Toyopearl SuperQ-650M and exothermic for the other solid phases. Endothermic Δh0 indicate that entropic effects play a major role on the adsorption of BSA on these two solid phases.
Flexible adaptation to constantly changing market demand is getting increasingly important in the manufacturing workflow of biopharmaceuticals. Moreover, fast development times and reliable scale-up are required and are key to success.
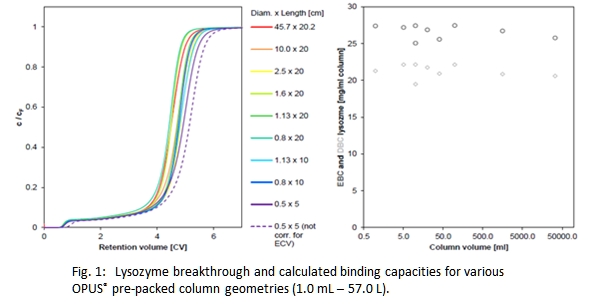
Pre-packed chromatography columns have been commonly adapted in industry during the recent years and allow to reach these requirements, however data showing scalability throughout various column sizes applied in early stage process development up to commercial manufacturing has been not available so far. This presentation confirms the scalability of column performance throughout Repligen's OPUS® pre-packed column line including MiniChrom, ValiChrom and OPUS® Process Scale columns covering a range of 0.5 to 60 cm inner diameter.
Scalability has been demonstrated for various resin types and functionalities and has been proven for both column packing performance and protein separation under isocratic conditions.
Experimental data on OPUS® packing performance has been compared to traditional self-packed columns and found at least equal if not better.
Furthermore a statistical data analysis using advanced software tools has been performed for more than 30.000 pre-packed columns supplied into global industry over a time span of 10 years.
Extra-column effects can have significant influence on separation performance. These effects are caused by dead-volumes, valves, column hardware and tubings. For small scale chromatography systems, and especially with very small columns, the extra-column band broadening can be dominant over the actual broadening of the column that is used for separation. This issue has long been recognized and has been investigated extensively by many researchers. However, though it is often overlooked, also for preparative and pilot scale chromatography extra-column effects can be an important factor. This is particularly valid, if the column size is relatively small. We have investigated the Aekta Pilot system volume contribution to band-broadening by non-binding tracer experiments on columns of various sizes using columns packed by axial compression to ensure consistent packing performance. Additionally, the influence of non-standard mixing behavior occurring in the bubble trap during step elution was investigated using a simplified model of interconnected reactors. Other flow parts of the chromatography system were modelled by dispersed plug flow reactors and CSTRs for dead zones, e.g. column in- and outlet and mixers. Essentially, the effective mixing volume of the bubble trap is affected by the flow rate and the density difference of the two buffers. As a consequence, the influence on the separation in the column varies with these parameters. Experimentally, the band broadening effect of the bubble trap on the chromatographic separation was investigated by performing linear gradient elution and step elution experiments using bovine serum albumin at analytical loads. There was no observable band broadening effect in linear gradient elution. However, during step elution the salt transition profile was affected leading to significant peak broadening of the BSA elution profile.
There is growing interest in mAb processing to improve individual unit operation performance and overall process train efficiency through linked processing. In the downstream process, several types of chromatography are typically performed to achieve the target of purity of therapeutic mAb. AEX chromatography is commonly operated under product flow-through conditions to bind negatively charged impurities such as HCP and virus. CEX chromatography focuses to separate the mAb related impurity. Ichihara et al., (mAbs J. 10-2, 325-334, 2018) reported the operating CEX chromatography in the flow-through mode for mAb aggregate removal. To intensified flow-through chromatography, controlling the feed concentration through single pass TFF (SPTFF) is one of the ideas to get the higher loadings from the isotherm binding condition, especially for AEX column. The use of SPTFF technology can help facilitate these process intensification efforts by modifying process intermediate volumes and concentrations without the need for recirculation. However, the impact of concentration change on other chromatography is not evaluated well on the fully combined in-series operation.
In this work, SPTFF by Pellicon® 3 30kD is used prior to a flow-through chromatography polishing step to boost chromatography resin loading. Then, Eshmuno® CP-FT resin was challenged with various concentrations of mAb feed and evaluated the difference of impurity removal. And results compared the performance of previously reported SPTFF-AEX results (Merck Application Note, AN5364EN00 2017), and evaluate the impact of connected flow-through polishing on feed concentration.
The biopharmaceutical downstream process is designed to achieve high product yield with efficient impurity removal. “Process derived impurities” such as host cell proteins (HCPs), lipid, DNA and fermentation ingredients and “Product related impurities” like aggregates and fragments should be reduced to meet each specification of drugs for quality and safely.
Economical pressure on biopharmaceuticals, especially monoclonal antibodies (mAb), has motivated to develop high capacity chromatography resins and new purification technologies including periodic counter-current chromatography and multi cycle batch chromatography using smaller particle size resins with high flow rate. Thus, load amount onto chromatography resins are tend to increase.
Higher amount loading of not only target proteins but also impurities on chromatography resins affect selectivity and life time of chromatography resins. Increased risk of fouling on chromatography resins by accumulation of impurities should be mitigated as much as possible to minimize burdens on each step to develop stable process with lower COGs.
We developed new impurity reduction method using co-precipitation of impurities without loss of target proteins. The method reduced HCPs and DNA in harvested cell culture fluid expressing mAb one third and one thousandth respectively. We also evaluated the effect on the quality of eluate from protein A chromatography and other benefits on the process.
1. Introduction
There is increased interest in continuous processing for monoclonal antibody (mAb) purification in bind and elute mode. This is because such continuous chromatography offers higher productivity and cost effectiveness than conventional batch mode. JSR developed and conducted a DoE for continuous chromatography process of mAb in bind and elute mode using a high-capacity Protein A resin, Amsphere™ A3.
2. Experimental
The mAb breakthrough was monitored by HPLC. The concentration of Protein A purified mAb was determined from the measured absorbance at 280 nm. Host cell protein (HCP) was quantified by CHO HCP ELISA kit, 3G (Cygnus Technologies). A harvested cell culture fluid (HCCF) contains IgG1 subclass mAb.
A three factor and three level DoE was designed using Minitab (Minitab, Inc.) to determine the impact of purification conditions on productivity (g/L/hr) and HCP concentration. The tested factors were IgG-loading, residence time for washing step and washing volume.
3. Results and discussion
Productivity of the continuous process with Amsphere A3 was calculated on various purification conditions. The results showed that the process time for sample loading and washing steps have significant effect on the productivity. The total amount of HCP in the eluates showed a positive correlation with the sample loading amount, especially when the flow rate for the loading step was relatively faster. That is, washing conditions have smaller effect on the HCP clearance performance with shorter residence time of sample loading step.
4. Conclusions
Amsphere A3 showed high productivity with continuous process because of its high DBC even for high linear flow velocity. Also, we found that the HCP clearance performance is independent from the residence time for washing step. This indicates that higher productivity and good HCP clearance can be achieved together by increasing flow rate and decreasing process time of washing step.
The development of efficient downstream processing plays an importance in therapeutic antibodies (mAb) purification. As an example, a continuous multicolumn chromatography technology in ProteinA capturing step has been attracted attention from biopharmaceutical companies. Recently an integrated flow-through (FT) process is considered to be one of effective approaches for mAb purification. FT mode has an advantage to increase loading amount of mAb per column volume. Further an integrated FT-FT process technology is expected to achieve much less installed chromatography resins, buffer and tanks to use in comparison with a conventional polishing step consisting of bind/elute (B/E) mode and FT mode. This technology will promise pool-less manufacturing for economical mAb process in near future.
Cellulose based chromatography resins are well-known to be stable to caustic condition for cleaning and less nonspecific adsorption properties. These unique properties are suitable to apply an integrated FT-FT process technology for mAb purification. In the past few years we succeeded in developing a unique cellulose based mixed mode resin, Cellufine MAX IB, which can remove impurities effectively in FT mode for mAb purification under high salt condition. The resin is further tested to use integrated FT-FT mode for mAb purification by examining mAb recovery and impurities removal. In this poster, we report development of efficient mAb polishing process by integrated FT-FT mode which consists of a combination of Cellufine MAX IB resin with other cellulose based cation exchange or hydrophobic chromatography resins.
To accelerate process development and optimization of chromatography, it is essential to use mechanistic models to simulate and predict the implementation of such operation for certain products. The universal prerequisites for chromatography modeling involve adsorption isotherm and mass transfer mechanism, where users can choose the models based on the balance of predictability and calculation ability. Unfortunately, most software packages are still not user-friendly because of complexity of models and input parameters.
In the study, different mechanistic models for various simulation platforms that depend on the same isotherm and mass transfer coefficients were tested for linear gradient elution (LGE) of proteins. The model system was separation of basic proteins such as lysozyme and IgG by ion exchange chromatography (IEC). Our model (Yamamoto model) was used for determining the two parameters (A, B) needed for steric mass action (SMA) isotherm model from LGE experiments. As for mass transfer, lumped kinetic model and linear driving force model (LDF) were used as only one lumped mass transfer coefficient (km) is included, which reduces the computational burden in determining individual effect from axial dispersion and pore diffusion, and such. The km value was determined also from LGE experiments based on Yamamoto LGE-HETP model, which includes the compression factor for the peak sharpening effect in LGE.
By a set of LGE experiment, the major parameters (A, B, HETP) can be obtained, and thus the adsorption and mass transfer models for mechanistic modeling can be constructed. Different simulation platforms have given similar outputs in comparison, which demonstrates that modeling for protein chromatography can be achieved from limited LGE data in different computational tools, albeit the coefficients definition varies in the software.
Reference Yoshimoto, Yamamoto, Simplified methods based on mechanistic models for understanding and designing chromatography processes-Yamamoto Models and Approach-, in Preparative chromatography for separation of proteins, 2017
The changes from stainless steel equipment to single-use solutions as well as switching from batch to continuous operations are currently strong discussed topics in the field of downstream processing. Besides the product yields and the purity grades, the footprint reduction and higher productivities result in decreased cost of goods (CoGs). With these developments, not only economic but also ecological goals will be achieved through buffer and materials savings. In the course of the present evaluation, we compared continuous and single use solutions to conventional platform processes and quantified their economic and ecological impact. In detail, we have compared a cell culture antibody process consisting of conventional broth clarification by centrifugation and batch capture by protein A affinity chromatography with a continuous process consisting of continuous clarification by depth flocculation and continuous precipitation and/or periodic counter current chromatography (PCC). Our results show potential cost saving strategies and how ecological goals and improved costs of goods can be combined. Possible savings of 30 % can be achieved by combining novel technologies and buffer savings of 60 % can be realized by the use of precipitation or PCC. The scenarios were evaluated for an existing facility with a bioreactor scale of 1,000 L as well as for two theoretical facilities scaled for either an orphan drug production of 50 kg/year or a blockbuster production of 1,000 kg/year.
The antibody manufacturing process is consisted of a cell culture process using animal cells and a purification process including several chromatography steps and filtration steps. The purification process is important to obtain high quality bio-pharmaceutical products by removing impurities, and measurement of antibody concentration is a fundamental and essential factor to control the manufacturing process and to confirm the yield of each purification step.
The concentration range in the purification process is wide, and over 100 mg/mL concentration can be observed for example in a final polishing step. In this study, optical rotation was applied for the first time to measure various concentrations of antibody and their accuracy was evaluated. Furthermore, the possibility of application to the process analytical technology was also discussed in the poster presentation.
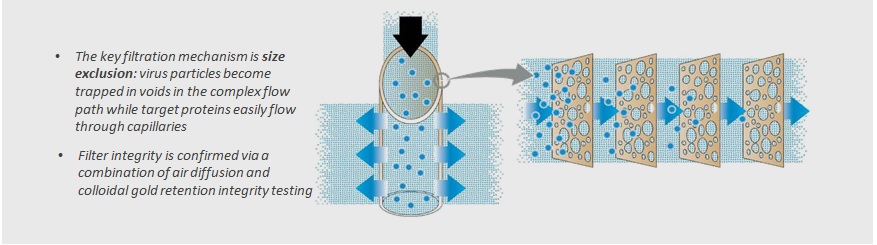
Virus filter is essential step to eliminate viruses in the Bio-pharmaceutical products. Continuous bioprocessing is of great interest for bioprocess manufacturers because of the possibilities of reducing production time, cost and footprint. One important element of continuous bioprocess is tankless connection between the adjacent steps, however virus filtration is mostly batch process, in which the target protein solution is pooled in a tank and then loaded to the virus filter with constant pressure. One reason of the preference of batch process is the concern of possible flux decay during the loading process Especially if chromatography process is connected directly to virus filter, flux decay by the sudden pressure rising is fatal for the manufacturing process. In this concern, virus filters with multi-layer structure as shown in the figure having high robustness in the continuous loading are necessary for the manufacturing process
Chromatography process is located just in front of the virus filter step and it affects the permeability of virus filter significantly. Especially ion exchange chromatography (IEX, AEX or CEX) step is usually just before the virus filter in the IgG purification process and impurity removability of IEX step is essential for the virus filter permeability.
In this study, the effect on the virus filter permeability of different types of IEX chromatography resins, i.e., normal IEX and mixed-mode IEX, are compared by using IgG solution with spiking aggregates. Mixed-mode AEX shows much higher aggregate removal than normal AEX. The virus filter permeability is tested both for constant pressure and constant flow rate loading. The dependencies of IgG concentration and flow rate are also tested. These results indicate that appropriate combination of IEX resin, column volume, virus filter membrane area and flow rate is very important for high protein recovery and the cost effectiveness process in the continuous bioprocessing.
Chromatography separation, which is essential in biopharmaceutical industry, is not widely used in food industry as food products are much less expensive than pharma products. However, as functional foods are highly anticipated, there is an increasing need for development of efficient and economically-feasible chromatography process for food separation.
In addition to the cost issues, food separations have many constraints and requirements, which make it difficult to use chromatography. As for the stationary phase (resins) and the mobile phase (liquid), they must be chosen based on the accepted materials by the regulatory agency. In addition, the resins must be cleaned and sanitized easily. Considering these requirements, polystyrene divinylbenzene (PS-DVB) resins-based chromatography with ethanol-water mobile phase is most attractive. The resins can be washed with sodium hydroxide. Ethanol is a very safe solvent, and can be recycled easily if needed.
In this study, a simple method for determining the optimum temperature for the above mentioned chromatography system was proposed based on the iso-resolution curve concept. The model samples were polyphenols (catechin and epigallocatechin gallate). PS-DVB resins were employed for the column stationary phase. The mobile phase was ethanol-water mixture. The distribution coefficient K was determined as a function of ethanol concentration,I , and temperature, T. A method for calculating the iso-resolution curve was developed with K, T, and mobile phase velocity, u. From the iso-resolution curve, the productivity was calculated as a function of I, T and u. It was found that there is an optimum T, where the highest productivity considering minimum mobile phase consumption.
From the industrial point of view, precise temperature control is costly. However, choosing the right temperature will result in more efficient separation processes.
Various different sizes of pre-packed chromatography columns are needed for process design, pre-clinical trials and production. It is therefore needed to evaluate the quality of pre-packed columns properly. HETP and asymmetrical factor As measured at non-binding conditions are commonly employed to check the packed bed quality. In this study, for capture chromatography (protein A) columns E = DBC/SBC vs. F0 plots [1] were used for the evaluation. DBC is the dynamic binding capacity and SBC is the static binding capacity. F0 is the dimensionless group, which is given by the square of particle diameter divided by the molecular diffusion coefficient and the residence time. For polishing chromatography columns (ion-exchange chromatography), another dimensionless group, Ym was used for the evaluation. Ym is composed of column length, velocity, particle diameter and gradient slope [2].
Reference
[1] N. Yoshimoto, T. Yada, S. Yamamoto; A simple method for predicting the adsorption performance of capture chromatography of proteins. Jpn. J. Food Eng., 17, 95–98 (2016).
[2] N. Yoshimoto, S.Yamamoto; Simplified methods based on mechanistic models for understanding and designing chromatography processes for proteins and other biological products-Yamamoto models and Yamamoto approach-, in Preparative chromatography for separation of proteins, Chap 4, Wiley, New York, 2017, pp. 111–157.
<Introduction>
In antibody drug manufacturing, Protein A affinity chromatography is performed as separation and purification steps using its specific binding ability to antibodies. In the manufacturing process, two different packing systems, Axial compression (AX) and Pack in Place (PIP), are mainly used to pack Protein A chromatography resins into large-scale columns.
KANEKA KanCapATM (KanCapA), which is one of our commercially available Protein A resins, can be appropriately packed into both AX and PIP system columns. With PIP system column, however, it is important to select conditions because PIP packing is susceptible to various parameters.
<Experiment and Result>
Some parameters for PIP packing were investigated with 80cm I.D. Chromaflow column (PIP system column of GE HealthCare) in this study. Higher slurry concentration and higher slurry flow rate led to better column performance. In addition, pump pulsation at the feeding slurry had an influence on the performance and installation of pulsation dampener reduced the influence. Consequently, good column performance was realized by setting slurry concentration and its flow rate appropriately with pulsation dampener.
<Conclusion>
It is feasible to realize good column performance by packing KanCapA into PIP system column with proper slurry concentration and its flow rate. Even with pumps generating large pulsations, good column performance is also realizable using pulsation dampener.
Continuous manufacturing is expected to be more efficient for production of biologic such as monoclonal antibodies (mAbs) compared with batch productions. Various unit operations are included in downstream processing (DSP) of mAbs such as chromatography and membrane separation. Virus inactivation is also required in DSP.
Tubular reactors are possible formats for continuous virus inactivation. It is important to know dispersion behavior in tubular reactors. For open tubular reactors, the dispersion is described by Taylor dispersion due to molecular diffusion. We carried out pulse response experiments to measure response curves (residence distribution curves). Various factors affecting the dispersion such as molecular diffusivity, tube diameter, tube diameter to coli diameter ratio, temperature and viscosity were examined.
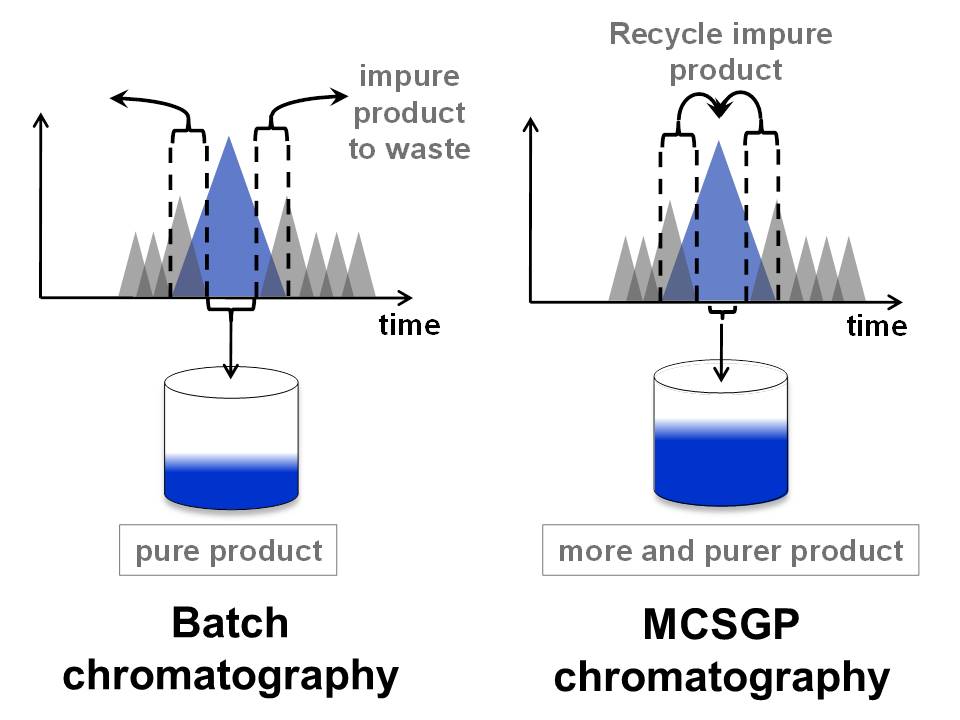
Continuous countercurrent chromatography is a valuable tool for the purification of peptides produced by chemical synthesis. Using a process comprising two reverse-phase columns and internal recycling (MCSGP), peptides are purified on a preparative scale with high yield and purity simultaneously while the generation of side-fractions and re-chromatography is avoided. The poster introduces the process concept, lab- and production scale equipment, and recent results of an economic evaluation of MCSGP in comparison with batch chromatography, showing the large upside of employing the two-column process.
Chromatography is considered as a critical part in antibody manufacturing process. To improve the productivity of the process, different strategies have been investigated. Flow-through chromatography (FTC) is being considered as one operation which can increase the efficiency in downstream process by adjusting the mobile phase environment that makes contaminants bind tighter to the column while the product elutes out as flow-through fraction.
A mechanistic model containing distribution coefficient and plate number to describe the start and the end of the protein elution fraction and contaminants is developed for FTC. From a set of linear gradient elution experiments in different gradients, the correlation between distribution coefficient and salt concentration can be determined. Combined with the particle diameter and diffusion coefficient, plate number under certain elution condition (e.g. pH, retention time, etc.) can be calculated. By setting the end of the product to be equal to the start of the contaminant, the optimal amount of sample loading and its productivity can be obtained.
In the study, samples containing bovine serum albumin monomer and dimer were selected for separation by ion exchange chromatography. Based on the mechanistic model, productivities under different mobile phase condition including pH, particle size, and retention time were determined for 100L of material with 100 minutes of operation time. A small scale of FTC experiment was conducted to verify the model predictability. Based on the result, separation process for proteins can be developed and simulated by adjusting the parameters involved in the model to achieve higher productivity.
Diafiltration is a critical unit operation which is part of practically every downstream purification train of monoclonal antibody or bio therapeutic modality production. It is typically employed to provide an efficient and robust method for exchanging buffer matrix for the therapeutic modality and offers simultaneous clearance of small molecule and elemental impurities.
Continuous manufacturing and process intensification approaches has been an area of keen interest and various technologies are being investigated to make biotherapeutic manufacturing process more efficient in terms of facility utilization and capital cost reduction by reducing reliance on large scale facilities. Technologies like multi-column cycling, inline viral inactivation and single pass tangential flow filtration (SPTFF) for protein concentration have been developed in past few years, offering significant improvement over their existing batch equivalents. Ability to develop a similar technology for diafiltration suitable for GMP manufacturing has remain rather elusive.
Our approach here is based on a shift in perspective wherein the process design is based on membrane utilization and system footprint as opposed to flux and process time in a typical batch diafiltration process. This rather flexible technology offers 6-8-fold increased membrane utilization, up to 3-fold reduction in pump passes and system footprint can be substantially decreased. Buffer usage, extent of buffer exchange, and product yield are consistent with a constant volume diafiltration process.
In this presentation, we will present a system design for this concept and a 24-hour experimental data demonstrating operational robustness. Implementation into a fully continuous, or intensified process train and flexibility of the system to accommodate complex diafiltation schemes for challenging molecules will also be discussed.
Monoclonal antibodies conjugated with drugs (ADC) have received much attention as pharmaceutical agents for treating serious diseases such as cancer. However, it is difficult to separate them on the basis of the number of conjugated drugs. Hydrophobic chromatography (HIC) is commonly used for the analysis of the drug to antibody ratio, DAR. The retention of ADCs on HIC can be controlled by the hydrophobic nature of ADCs, depending on the mobile phase conditions. They are sometimes performed at the restricted conditions where the solubility is too low and thus one cannot archive high capacity. Ion exchange chromatography (IEC) using electrostatic interaction is an orthogonal method to HIC. IEC is widely used because of its higher capacity than HIC. We investigated the retention behavior of the protein conjugated surrogate drugs on IEC. The surrogate drugs employed are 7-diethylamino-3-(4'-maleimidylhenyl) 4-methylcoumarin (CPM), N-(1-pyrenyl)maleimide (NPM) and Cyanine5 mono NHS Ester (Cy5-NHS). Bovine serum albumin (BSA) and human immunoglobulin G (h-IgG) were used as a model protein. The molar ratio (CPM or NPM or Cy5-NHS to protein) was set to 3. The maleimide group of CPM and NPM reacts with the thiol group of the proteins, and the NHS ester group of Cy5-NHS reacts with the amino group of the protein. On the linear gradient elution experiments, the elution salt concentrations of the conjugated and non-conjugated proteins were measured to obtain chromatographic parameter of the number of binding site, B.
The graft-type ion exchange chromatography is expected to have a high binding capacity. However, in some cases, the steric hindrance needs to be considered in the pore fulfilled by the grafted ligand. It is difficult to visualize the structure in the pore, and it is not known how the adsorption reaction is progressing. In this study, the heat of adsorption reaction was measured by using isothermal titration calorimeter (ITC), the enthalpy and entropy changes associated with protein adsorption to the graft-type ligand were analyzed, and the relationship with the capacity and structure of pore was discussed. SuperQ 650M, GigaCapQ 650M and Q-600C AR were used as the resins with graft-type ligands, and bovine serum albumin (BSA) monomer was used as the model protein. The binding isotherm was obtained by varying the concentration of BSA from 0mg/mL to 20mg/mL incubated with 50% resin slurry. The equilibrated binding capacity of Super Q was only about 1/4 of that of Q-AR. For the measured enthalpies by ITC, GigaCapQ and Q-AR were negative and SuperQ was positive. From these, it can be seen that adsorption is exothermic and endothermic, respectively. Also, ΔS was calculated by ΔG from the adsoprtion isotherm and ΔH from ITC. As the result, it turned out that enthalpy contribution was large in GigaCapQ and Q-AR, entropy contribution was large in SuperQ. This indicates SuperQ needs the release of water and counter ions when electrostatic interaction occurs. There is less steric hindrance in GigaCapQ and Q-AR (=there is little entropy contribution), the adsorption mechanism is driven by electrostatic interactions.
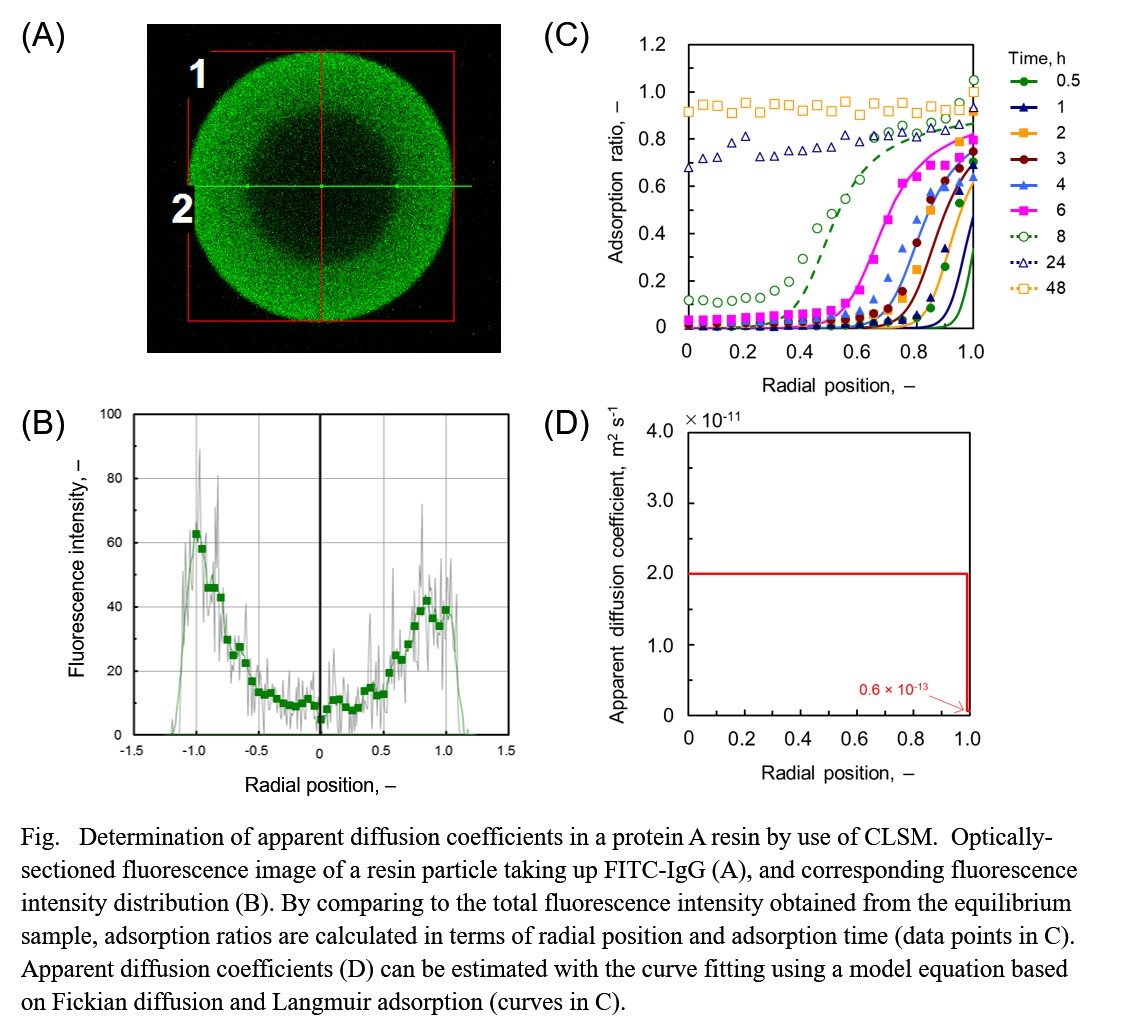
In the present work, we studied the adsorption behavior of immunoglobulin G (IgG) in commercially available protein A resins by use of confocal laser scanning microscopy (CLSM) together with the fluorescein isothiocyanate (FITC) conjugate, combining a newly proposed calibration method. CLSM provides the capability to collect serial optical sections from thick specimens, and thus applying to protein A resins taking up FITC-IgG it enables us to pursue the progress of adsorption front within a particle and furthermore to determine apparent diffusion coefficients through a model-based analysis. Although CLSM could count as powerful method to understand intraparticle mass transfer, there seems no availability of appropriate data without taking account of the optical hindrance inside a porous resin particle. The laser light penetrating a resin particle is likely to be attenuated with the scattering by particle matrix, absorption by fluorescent label, or both, and furthermore the fluorescent light emitted from the fluorescent label of adsorbed proteins could undergo these interactions, too. Therefore, the light attenuation of exciting and emitted light is necessary to be calibrated in determination of the distribution of protein amount adsorbed. For this purpose, we propose to adopt the attenuation ratio, determined from a negative image of the particle taken by use of the fluorescein solution with a concentration at which the solution itself can emit fluorescence with the laser light used. Though found a large difference in the light attenuation depending on resin, we concluded from the dependence of the attenuation ratio on the dye concentration that the scattering by particle matrix mainly contributed to the light attenuation but the absorption by fluorescent label did less. By combining this calibration method with CLSM it makes possible to compare the profile of protein amount adsorbed within a particle among different types of resins.
Antibody drugs have specificity and act only on the target site, so they have few side effects and are expected as therapeutic agents for cancer etc. In chromatography used for separation, purification, etc. of antibodies, there is graft type ligand chromatography where ligand is increased by introducing a graft chain for the purpose of increasing adsorption amount.
However, it is also feared that pore blockage may cause diffusion inhibition. Therefore, we used some grafted chromatography to investigate the actual adsorption mechanism.
In this study, two columns, graft type Super Q and Q Giga Cap, were used. The pore size was calculated using DNA. Q Giga Cap was 3.0 nm and Super Q was 4.6 nm. It was found that when the diffusion coefficient at that time was determined, the diffusion resistance increased as the pore size decreased. In particular, in the case of small pore size Q Giga Cap, the amount of decrease was large.
In addition, as a result of measuring the adsorption amount of BSA, Q Giga Cap showed higher DBC than Super Q. This is because the space volume of the graft chain layer is large.
From the above results, it was found that the introduction of the graft chain increases the diffusion resistance but increases the adsorption amount.
Antibody-Drug Conjugation (ADC) is studied actively as future Drug. ADC has parameter called Drug-to-Antibody Ratio (DAR). DAR is number of drugs on antibody. Now, DAR is controlled by genetically modified cell, bad this is not simply. To control the DAR of ADC from normal antibodies is difficult by reaction of antibody and drug. DAR should be controlled without complicated procedure. Therefore, it is necessary to separate precisely. In this work, for the development of separation technology of ADC by Ion Exchange Chromatography (IEC), we analyze the ADC's adsorption system. There are many studies of separation by Hydrophobicity Interaction Chromatography (HIC). However, HIC is complicated by column selection. IEC is more simply procedure and higher recovery rate than HIC.
Result of analysis of the number of adsorption site B, model ADC A from Lysozyme is lower number than native Lysozyme. However, model ADC B from Bovine Serum Albumin (BSA) is higher number than native BSA. Therefore, ADC B is considered to be aggregated with the conjugated BSA as a core. Also, ADC conjugated charged drug is higher amount of change than ADC conjugated hydrophobicity drug. Therefore, ADC conjugated charged drug is higher effect than ADC conjugated hydrophobicity drug.