
Natural gas reserves available worldwide includes varying amounts of CO2 ranging from CO2-free in Siberia to as high as 90% CO2 content in the Platong and Erawan fields in Thailand. The Natuna field in the Greater Sarawak Basin in Indonesia is the largest gas field in south east Asia, with estimated 46 trillion cubic feet of recoverable reserved. Unfortunately, it remains unexplored due to high CO2 content up to 71%. In Malaysia, CO2 content from natural gas fields varies up to 87%. Over 13 trillion cubic feet of natural gas reserves are undeveloped due to the presence of high CO2 content. The high CO2 content in natural gas is a major issue to monetizing the gas reserves. Without the removal of its CO2 content, natural gas cannot be further processed, liquefied, transported or commercially sold. However, the release of bulk CO2 into environment aggravates global warming and violates the requirement of Kyoto Protocol. Hence, there is a need for the development of technologies for the effective capture and subsequent utilization of CO2 for its conversion into value added products. In this paper, the recent advances on the potential technologies to manage the CO2 rich natural gas fields is deliberated. Challenges for gas treatment including capturing systems (from common technologies such as absorption, adsorption, cryogenic and membrane to novel technologies such as CUAS) and their applications towards a wide range of CO2 concentrations are presented. Additionally, the opportunities of separating huge amount of CO2 from such environments to produce added values products (bio fuel, synthetic-fuel, CO2-EOR, power production, etc.) are discussed.
In a well-known efficient CO2 absorption process, Rectisol, chilled and pressured methanol is used as the absorbent to capture of CO2 physically. Unfortunately, because of higher solubility of CO2 in methanol under lower temperature and higher pressure, this process requires strict conditions (-40 ~ -60 oC and 3~8 MPa) and this leads in high demand of both operating and equipment costs. Therefore, a rotating packed bed (RPB) was evaluated to capture CO2 with NaOH in aqueous methanol as well as pure methanol solution under ambient conditions to relax low-temperature and high-pressure constraints. The experimental results suggest that with the NaOH-methanol solution, the absorption could be almost completed (~100%) within one gas/liquid pass in an RPB at room temperature and atmospheric pressure. Furthermore, the apparent overall mass transfer coefficient could be elevated to almost 5 s-1, which is one of the highest values among the RPB-related chemical absorption processes. Under this intensified centrifugal field, it is clear that CO2 capture can be highly enhanced with this good combination of physical-chemical absorbent, NaOH-methanol solution.
High purity CO2 recovery with lower energy consumption is required for effective CO2 Capture and Utilization/Storage (CCUS), and membrane separation would be suitable for the CO2 separation. Our research group has investigated and developed amine-containing polymeric membranes for effective CO2 capture. For example, amine-immobilized poly(vinyl alcohol) (PVA) membranes show excellent CO2 separation properties even over smaller H2. It was found recently that alkanolamines, such as 2-(2-aminoethylamino)ethanol (AEAE), elevated the CO2 separation properties, and mechanism of the preferential CO2 transportation was elucidated. Under humidified conditions, carbamate formed by an interaction between CO2 and the secondary amino group of AEAE was hydrolyzed to give bicarbonate ion, which was the major migrating species through the membrane. The hydroxyl group of alkanolamines facilitate the interaction to enhance bicarbonate production upon hydrolysis of the carbamate.
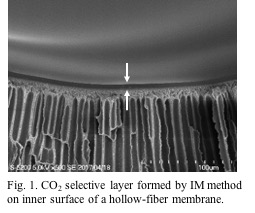
For pilot-scale demonstration, a hollow membrane module was prepared by in-situ modification (IM) method (Duan S., J. Membr. Sci., 2006). An aqueous solution of amines and PVA was circulated in a commercial hollow-fiber membrane module, and a CO2-selective layer was formed on the inner surface of hollow fiber membrane (Fig. 1). The thickness of the active layer was determined by circulation period, concentration of the solutes, and flow rate of the solution. The optimization of the IM method explained that 5 min circulation was enough to obtain higher CO2 permeability and CO2 selectivity. While the amines were physically immobilized in a polymer matrix, decrease in the CO2 separation performance was not found at all under 90 % relative humidity at 50 °C for about 300 h, which was triggered by the leakage of the amines. The IM method is preferable for mass production, and the scale-up is also feasible. The membrane modules developed would be suitable for biogas upgrading and air capture to obtain high purity CO2 in the permeate.
CO2 capture from dilute sources (e.g., flue gases, air) and subsequent storage or conversion will play a key role in the carbon-constrained future. The low driving force for CO2 capture from these dilute streams has positioned adsorbent materials and adsorption technology as a leading approach for addressing this global challenge. The creation of robust materials-enabled advanced adsorption separators and their manufacturing into low-cost, energy-efficient devices will be the focus of the talk. Engineering novel materials—such as solid-supported amines, metal-organic frameworks, and polymers of intrinsic microporosity—into structured contactor adsorption devices shows promise for these CO2 capture applications. Structured sorbent contactors can help manage kinetic and engineering factors associated with the separation, including pressure drop, sorption enthalpy effects, and external heat integration (for temperature swing adsorption). Monoliths, fiber sorbents, and 3D printed materials will be discussed as the three main classes of existing or emerging structured sorbent contactors. Recent developments in their manufacture; advantages and disadvantages of each structure relative to each other and to pellet packed beds; recent developments in system modeling; and finally, critical needs in this area of research will all be discussed.
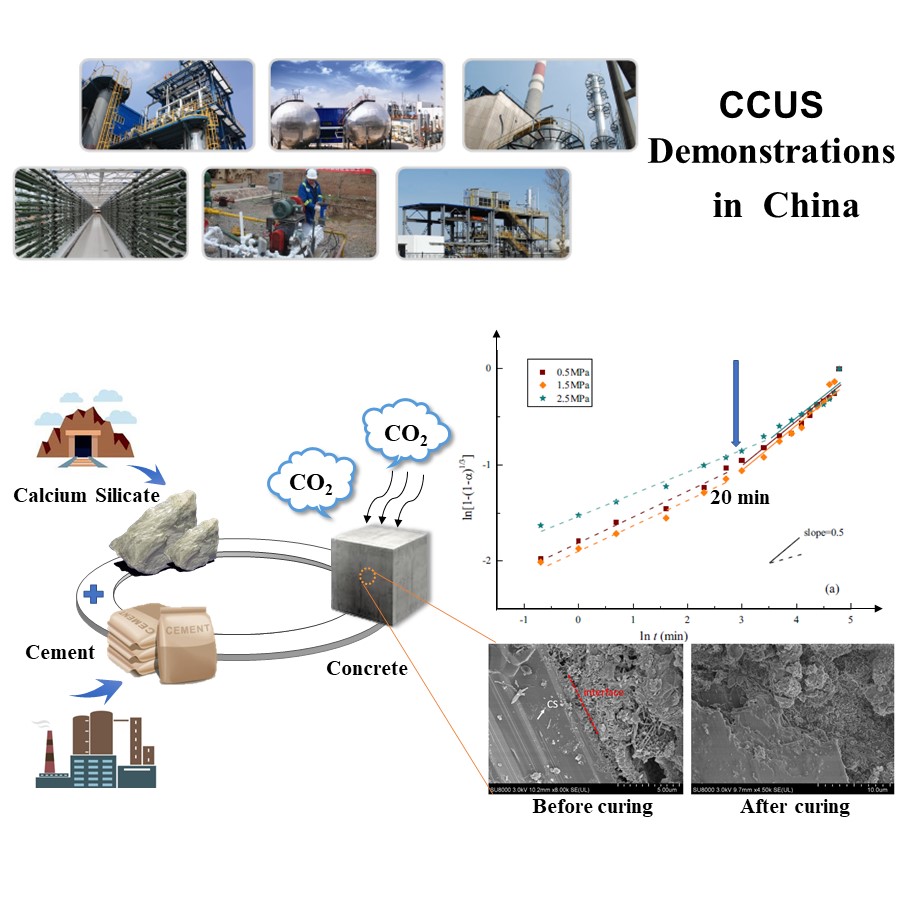
CCUS (Carbon Capture, Utilization and Storage) is an important choice for a sustainable low-carbon development in China. The Chinese government attaches great importance to guiding the development of CCUS technology and has taken a series of measures to reduce CO2 emission in the past decade. This speech provides an overview of CCUS technology development which contained specific details about supported program from National Key R&D Program of China. The speech will also introduce several leading large-scale CCUS industrial demonstrations in China, by summarizing the technical characteristics, reactivity, and procedural scheme of each demonstration. An outlook on future development will be provided according to the 13th Five-Year Plan of China (2016-2020), which is focused on promoting large-scale industrial demonstration in the coal-based industry and oil and gas exploration industry.
One of the promising CO2 utilization demonstration projects is carbonation curing of concrete. CO2 curing technology has two major benefits: (i) rapid strength gain of cementitious materials, and (ii) large-scale CO2 sequestration in concrete. The gas-solid reaction kinetics between CO2 and cement-based materials were studied. According to the fitting results of different kinetic models, the carbonation curing of concrete showed a progressive product-layer-diffusion limited kinetics despite different factors. Our group also evaluated the feasibility of blending calcium silicate (CS) as intensified addition, which was found to significantly enhance the reaction rate and CO2 uptake capacity. In terms of practical feasibility, carbonation curing also improved the compressive performance of cement paste with CS. In addition, three typical industrial solid wastes - fly ash, blast furnace slag, and red mud - were studied to replace part of the cement materials to reduce CO2 emissions. The effects of carbonation curing time, pressure, and water-to-solids ratio on the CO2 uptake and compressive strength were comprehensively studied.
As one of the most important technologies for Carbon dioxide (CO2) Capture, Utilization and Storage (CCUS), the practical application of CO2 enhanced oil recovery (CO2-EOR) has been growing rapidly. Since the profitability of CO2-EOR project can be improved by the re-use of CO2 contained in the associated gas, superior CO2 separation technology is essential. JGC Corporation (JGC) and NGK Insulators, Ltd. (NGK) have developed CO2 separation system with DDR-type zeolite membrane. In many cases, the associated gas also contains hydrocarbons in which predominant constituent is methane (CH4). Since DDR-type zeolite has subnano-size pores which are desirable for CO2/CH4 separation, this membrane achieves excellent CO2 permselectivity. In addition, this system can be operated under high CO2 partial pressure conditions (~ 8 MPa) which is suitable for CO2 recovery process from associated gas with high pressure and high concentration of CO2. Recently, JGC has launched the first field test with laboratory scale DDR-type zeolite membrane at a CO2-EOR oil field. The membrane was prepared onto the inner surface of 30 channels in a cylindrical monolithic alumina substrate (diameter: 30 mm, length: 160 mm). In this field test, it has been confirmed that this membrane performed well for CO2 separation from the associated gas. It's of note that the performance observed using the associated gas was substantially equivalent to that of obtained using a simulated gas consisting of CO2 and light hydrocarbons in laboratory tests. In this presentation, the features of the DDR-type zeolite membrane as well as the summary of the first field test at a CO2-EOR oil field are addressed. In addition, the outline of the ongoing demonstration project with commercial scale DDR-type zeolite membrane (diameter: 180 mm, length: 1,000 mm) is introduced.
Along with the growth of CO2 enhanced oil recovery (CO2-EOR) industries, demand of CO2 separation technologies has been increasing to recover CO2 from associated gas which contains not only CO2 but also hydrocarbons mainly CH4. DDR-type zeolite membrane has the subnano-size pores which are preferable for CO2/CH4 separation, resulting in the excellent CO2 permselectivity. In this study, CO2 permeance and selectivity of the DDR-type zeolite membrane were examined under high pressure conditions using CO2/CH4 binary gas mixture to evaluate the potential applicability for CO2 recovery process in CO2-EOR industries. The membrane was prepared onto the inner surface of multi channels in a cylindrical monolithic alumina substrate (diameter: 30 mm, length: 160 mm). The membrane properties are investigated using one channel of the substrate. The test pressure was increased up to 8 MPaG with the temperature range of 90 - 150 deg. C. The CO2 concentration in the gas mixtures were varied from 50 to 100 %. From these experiments, CO2 permeance decreased with increasing pressure and temperature. In addition, CO2 permeance and selectivity decreased with decreasing CO2 partial pressure in the experiments with CO2/CH4 binary gas mixture. CO2 permeance obtained in experiments was correlated by the surface diffusion model coupled with adsorption isotherm equations (Langmuir equation and Dubinin-Astakhov equation). The surface diffusion model could correlate CO2 permeances well against the wide range of CO2 partial pressure. The comparison of CO2 adsorption curves estimated by the correlation was implied that the competitive adsorption between CO2 and CH4 was occurred in this system and it affected to CO2 permeability of the DDR-type zeolite membrane. As mentioned above, the amount of adsorbed CO2 decreased as CH4 concentration in binary gas increased because of competitive adsorption. In contrast, gas diffusion constant and equilibrium constant were not changed.
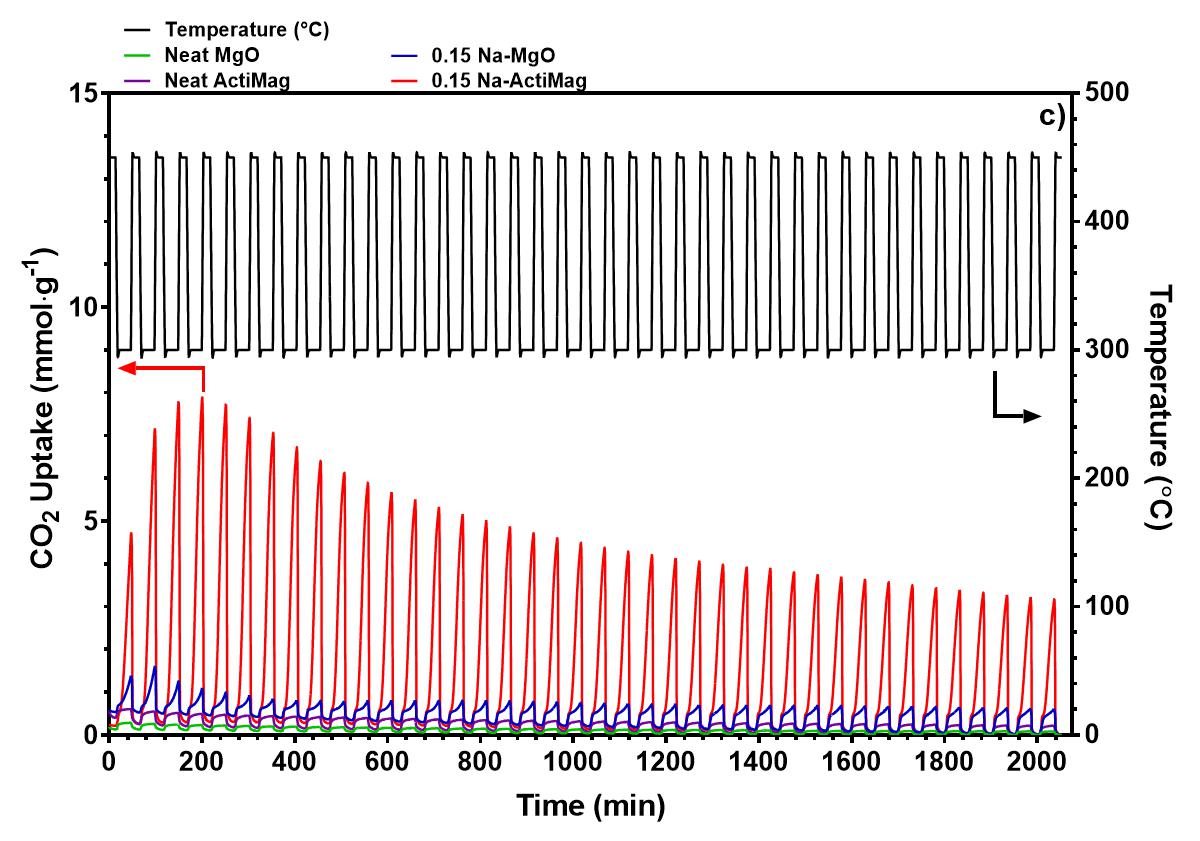
High surface area magnesium oxide (MgO) has garnered interest as an in-situ CO2 sorbent for the water-gas shift reaction (WGSR) for its low regeneration temperature and uptake of CO2 gas at intermediate temperatures of 300 °C upon modification with alkali nitrate salts. Semi-industrial scale production of these high surface area MgO sorbents (>200 m2·g-1) can be achieved via Calix's flash calcination process. Upon modification with NaNO3, the CO2 uptake of MgO in a pure CO2 environment after 1 h improved by a factor of 27 to 11.1 mmol CO2·g-1. The sorbent retained 60% of its CO2 capacity after 40 adsorption-desorption cycles, outperforming a commercial MgO control. The higher uptake of the NaNO3 modified high surface area MgO is attributed to a higher ratio of NaNO3 to MgO, resulting in larger and more similarly sized crystallites of MgO and NaNO3. This improves the extent of interphase boundaries between the molten salt and gaseous CO2, improving the rate of adsorption of CO2 into the sorbent. The molten salt also influences the sintering of MgO and NaNO3, which further increased the crystallite size and surface area in the first few cycles. When applied as a sorbent in the Cu catalysed WGSR, the NaNO3 modified MgO sorbent exhibited a CO conversion of 27% at 300 °C, while CO2 output remained relatively high. The high CO2 output is attributed to the loss of basic sites when the NaNO3-MgO sorbents are mixed with the Cu catalyst, which lowers the retention of CO2 at 300 °C, and impedes the effect of NaNO3 in capturing CO2 in the WGSR.
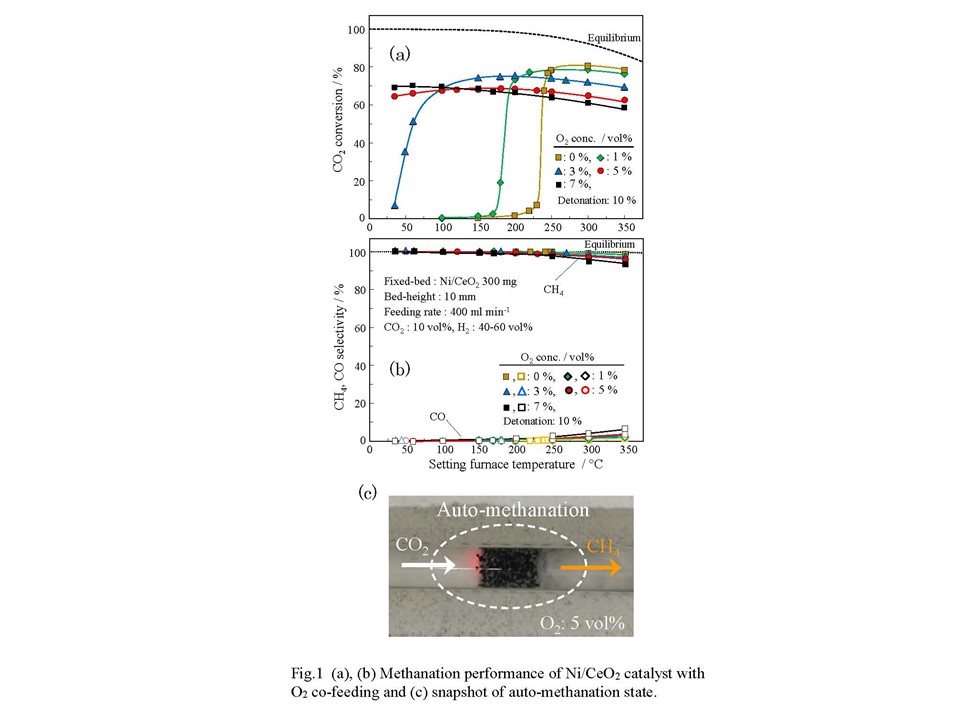
The CO2 methanation has received attention as a reaction converting CO2 into a useful resource such as methane (PtG technology). When assuming that methanation is used as a reaction to process CO2 emitted from an industrial process, the presence of oxygen may have a negative influence, such as an oxidation of the active site. In this study, to examine the influence of O2 on the catalytic function, the methanation performance of a Ni/CeO2 catalyst was evaluated using a raw material gas containing oxygen of 1–10 vol%. As a result, the methanation activity at a lower temperature was greatly improved, and the ability to produce a high methanation performance was realized even in a region at room temperature. We found a novel route of CO2 methanation that can be driven with no heating, even in ambient, which we named as auto-methanation. No one in the World has reported such transformation route yet.
A granular Ni/CeO2 catalyst was prepared using evaporation to dryness of Ni(NO3)2·6H2O on CeO2 support. The CO2 methanation was investigated using conventional flow-type reactor by introducing feed-gas at setting temperature of 350 °C down to room-temperature. The raw gas contained CO2, H2, O2, N2. The CO2 concentration was fixed at 10 vol% with 400 ml/min.
Figures 1(a) and 1(b) show CO2 conversion and product-selectivity. When supplying oxygen of 1 vol% and 3 vol%, the drastic change in conversion shifted to the lower side, which was near room temperature. At oxygen of 5 vol% and 7 vol%, about 65 and 70% conversions were obtained even at room temperature without external heating. Methane selectivity was almost 100% at lower temperature. Figure 1(c) is snapshot of the auto-methanation state. A combination of methanation and combustion might offer a novel CO2 transformation technique, which will open a new process.
The CO2 separation technology is considered to play a key role to reduce or control the CO2 emission from the fossil fuel, especially from coal in the world. Australia, rich in coal resources, is also facing “De-carbonization trend” and thus technical solutions are needed.
In Australia, we launched an internationally collaborative PCC (Post Combustion Capture) demonstration project in 2014. The name of the project is the abbreviation of PCC, and IHI (Japanese engineering company), CSIRO (Australian research institute), AGL (Australian power company), the partners of the project.
Prior to the project, we had developed IHI advanced CO2 captured system with 20 ton CO2/day scale pilot plant in Japan, which mainly consists of the IHI Solution No.162 (ISOL-162), the advanced packing material and the advanced process enabling to significantly reduce the solvent regeneration energy.
Through the long term operation at this demonstration project under the practical conditions, i.e., using the flue gas of an operating brown coal-fired power plant, we have evaluated our integrated PCC technologies. During the operation, 90 % CO2 capture ratio was achieved stably and other measured values were also kept stable. The results indicate that the PICA plant and the ISOL-162 solvent have sufficient stability and robustness to enable long term operation.
IHI has improved the design and refined operation to provide a robust and environmentally friendly PCC system based on the knowledge and technologies obtained through the ISOL-162 long-term operation using the PICA pilot plant and parallel research activities.
Over the long term operation, practical studies of the emission evaluation were carried out. The emissions of amine and its derivatives from the outlet of the washing tower were measured on various wash-type conditions. The result clearly demonstrated that the amine emission from the system was considerably reduced and minimized by the application of the washing technologies. The demonstration results are explained in detail in the presentation.
Indirect carbon sequestration via mineralization has been perceived as an alternative solution to address increasing anthropogenic emissions. Ex situ mineralization, in contrast with the in situ counterpart, has been the focus of recent studies as it provides a faster and more efficient carbon capture process due to the addition of a leaching step that releases the ions available for intimate contact with carbon dioxide. Mixed dump serpentenite samples from a Nickel mine site in Mindanao, Philippines were characterized through leaching tests to determine their viability in carbon sequestration. Ground samples of 75-150 microns particle size were leached with hydrochloric acid concentrations of 1 M, 2.5 M, and 4 M, at temperatures of 50C, 75C and 100C. The reaction time was also set at 1 hr, 2.5 hrs, and 4 hrs, respectively. The optimum set of conditions were then determined by analyzing the extraction efficiencies of Mg, Fe, Si, Ca, and Al ions from the aforementioned set of parameters, using the Face-Centered Cube model for Response Surface Methodology. Prior to leaching, XRD, XRF, and ICP-MS analyses were performed to determine the dominant minerals and elemental composition of the sample. The morphology and crystal structure of the optimum and non-optimum samples were also confirmed by BET and SEM analyses to establish the relationship of the crystal structure of the media to the ion extraction efficiencies.
Efficient and economical CO2 capture from coal-fired power plants is a major challenge for the application of CCS concept as this step incurs primary cost and energy penalty. Oxy-fuel combustion and post-combustion CO2 capture using Amine process are few approaches but are energy intensive. Chemical Looping Combustion (CLC) technology where oxygen for combustion is supplied by metal oxygen carriers instead of air is finding application in coal fired power plants for its economical CO2 capture. The oxygen carrier is circulated between the two reactors, Fuel reactor and Air reactor which usually operate under fluidized bed conditions. The flue gases from Fuel reactor contain CO2 and H2O which can be easily separated leading to nearly pure CO2 and thus reducing the energy requirement for CO2 separation. Therefore, development of a robust, effective and economical metal oxygen carrier is of primary research interest. This study reports and compares the reduction-oxidation characteristics of several oxygen carriers. Basic reduction-oxidation experiments were carried out in a small fluidized bed reactor by switching gases. Effect of reduction temperature, gas residence time, and type of oxygen carrier on conversion efficiency was investigated. Results were discussed in terms of their physical characteristics, oxygen capacity, oxygen release rate and stability after repeated redox cycles. A first order reaction was applied to analyse the reduction kinetics. The study also reports long run performance results of a 100kW scale circulating fluidized bed bench scale CLC unit developed at AIST under NEDO project.
A novel process named a membrane flash process was studied to realize an energy-saving technology and to substitute it for a conventional process using steam for regeneration of the CO2 absorbent liquids in CCS. In this study, four different amine-based absorbents (MEA, DEA, MDEA, MDEA and piperazine (PZ)) were tested to find an absorbent favorable for the membrane flash process using an alumina microporous hollow fiber membrane.
CO2 rich solution of each absorbent was supplied into the tube side of the hollow fiber and the pressure on the shell side was reduced so that the pressure difference between the tube and shell sides was 45, 65, 85 and 95 kPa. The absorbent liquid was forced to permeate through the pores of the membrane and CO2 was released during and after permeation. MEA showed the highest liquid permeation rate while MDEA+PZ showed the lowest permeation rate. DEA and MDEA had the similar permeation rates. On the other hand, the highest CO2 desorption rate was obtained with MDEA+PZ and the second was obtained with MEA. As a result, the CO2 release ratio, which was defined as the ratio of actually desorbed CO2 amount to the potentially releasable CO2 amount, was highest with MDEA+PZ while MEA showed the lowest CO2 release ratio. The energy requirement for CO2 desorption consisting of the energy items required for liquid permeation, gas discharge and reaction heat was estimated based on the experimental results for each absorbent. The results indicated that the energy requirement was smallest in the case of MDEA+PZ. From these results, it has been found out that MDEA+PZ was most suitable for the membrane flash process.
In this research work, carbon nanofibers (CNFs) were initially synthesized and then post-coated on honeycomb monolith substrates using injection chemical vapor deposition (ICVD) technique. The synthesized CNF monolith was intended for CO2 adsorption study. The effect of various wash-coated materials and catalyst promoter on the growth rate of CNFs on monolith substrates were examined. The characteristics of the synthesized CNFs-coated monolith composites were examined using Raman spectroscopy, Brunauer–Emmett–Teller (BET), thermogravimetric analysis (TGA), field emission scanning electron microscopy (FE-SEM), and Transmission electron microscopy (TEM) techniques. According to the textural characterization study, the specific surface area and pore volume of CNFs-coated monolith composites were significantly improved as compared to bare monolith which might be attributed to the growth of highly pure and aligned CNFs over monolith substrate. Besides that, the synthesized CNFs-coated monolith possessed extremely well thermal stability up to the temperature of 550 °C which was corresponded to the strong attachment of highly graphitized CNFs over monolith substrates.
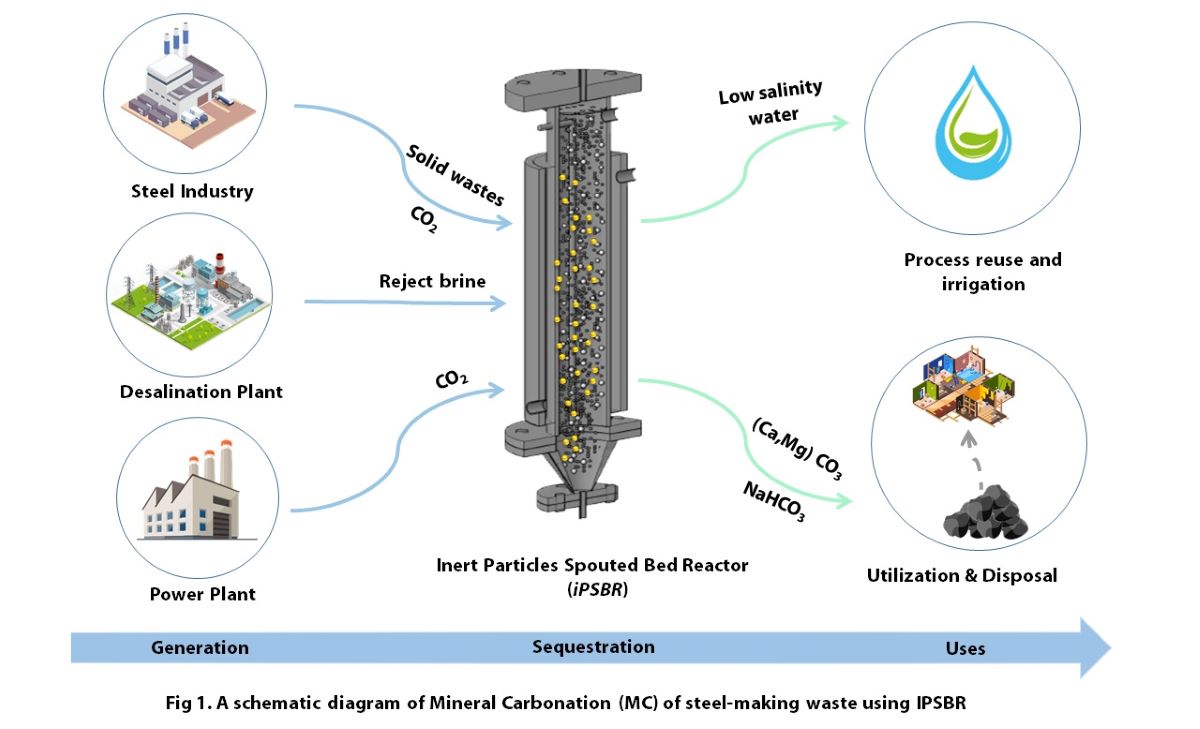
Mineral carbonation (MC) is the only recognized form of permanent CO2 storage options with no concerns regarding its long term stability. Industrial waste alkaline residues are utilized in mineral carbonation transforming them into useful products. The waste usually requires at least one pre-treatment step to make it more reactive. Pre-treatment is usually an energy-intensive process and involves the use of extraction agents which are not environmentally friendly. This translates into numerous environmental and economic concerns that hider the applicability of MC using industrial wastes. In this study, steel-making waste has been mineralized by CO2 to produce valuable calcium and magnesium carbonates and capture CO2 as illustrated in Figure 1. The study investigates an environmentally friendly and economical approach for MC by reacting electric arc furnace (EAF) baghouse dust (BHD) in a reject brine medium with CO2 in a novel reactor system, specially designed for contacting gases and liquids. This approach eliminates the need for pre-treatment chemicals and its associated cost which is estimated to be 0.4 €/kg of steel waste. Response surface methodology was used to optimize the MC process. At optimum operating parameters, the optimum CO2 uptake was 0.22 g CO2 /g BHD. A higher CO2 uptake performance of 0.98 g CO2/g BHD was achieved at ambient temperature and pressure of 5 bar. In addition, the concentration of the dissolved solids in the reject brine was reduced hence reducing its salinity. Thermal gravimetric analysis (TGA) of the solid products revealed that a variety of carbonate products being produced, particularly, calcium and magnesium carbonates.
A technology for converting CO2 recovered from a power plant into fuels or chemicals like methane or methanol is attracting attention as a CO2 effective utilization technology. We have been developing phase separation solvent for the purpose of energy saving of CO2 recovery. The absorbent consists of amine / ether / water and is characterized by low temperature regeneration. In this presentation, we will report on the prospect of significant energy savings by integrating CO2 recovery in the first stage and conversion in the second stage.
The following carbon capture, storage and investigation of potential CO2 storage sites projects are being conducted in Japan.
1. Tomakomai CCS Demonstration Project
Full-chain CCS demonstration Project
2. Investigation of Potential CO2 Storage Sites
Identify potential CO2 storage sites in waters surrounding Japan by 2021.
3. Sustainable CCS Project
Demonstration of capture facilities and comprehensive studies to introduce CCS
4. Osaki Coolgen Demonstration Project
Step 1: Oxygen-blown IGCC, Step 2: IGCC+CO2 Capture, Step 3: IGFC+CO2 Capture
The description of Tomakomai CCS Demonstration Project is as follows.
CO2 Capture Facility: The CO2 source is a hydrogen production unit (HPU) of an adjacent oil refinery, which supplies off gas containing approximately 50% CO2 from a Pressure Swing Adsorption (PSA) hydrogen purification unit. In the capture facility, gaseous CO2 of 99% purity is recovered by a commercially proven amine scrubbing process. A two-stage absorption system reduces the amine reboiler duty in the capture system.
CO2 Injection Wells: The CO2 is compressed and injected into shallow and deep offshore reservoirs by two separate deviated wells. The cumulative injected CO2 volume is 230,000 tonnes as of March 20, 2019.
Monitoring Facilities: An important objective of the project is to confirm the safety and stability of CO2 injection. As Japan is highly susceptible to earthquakes, natural earthquakes and micro-seismicity are also monitored to verify that natural earthquakes do not affect the stored CO2, and that CO2 injection does not cause any noticeable tremors. An extensive monitoring system comprising 3 observation wells with bottomhole temperature & pressure sensors and seismometers, 4 ocean bottom seismometers (OBSs), 1 ocean bottom cable (OBC) and 1 onshore seismometer was established. Monitoring was commenced one year prior to the start of CO2 injection and has been conducted continuously. In addition, seismic surveys are conducted to delineate the subsurface CO2 distribution.
In a standard post-combustion carbon capture (PCC) process, the regeneration energy of the CO2 lean solvent dominates the overall energy consumption. The energy reduction in the CO2 stripper can be achieved by either formulating new solvents or optimizing the process configurations. The energy reduction achieved by stripper modifications, which include the rich-split process, the interheating process, and the integration of both configurations, have been reported in the literature. In the rich-split process, the cold rich stream is split to recover the energy contained in the overhead vapor, which was directly fed into the condenser in the traditional stripper configuration; therefore the regeneration energy can be reduced. The interheating process draws the liquid flow from the middle of the stripper and exchanges heat with the high-temperature lean solvent from the reboiler; thereby, the overall column temperature can be raised that favors CO2 desorption along the column. Therefore, the combined process, which is the rich-split process integrated with inter-heaters (IHs), takes both advantages of above modifications that can further reduce the energy requirement. However, the present work shows that energy-saving effect of the combined process is not as promising as the literature claimed. Once the design parameters of the rich-split process are selected properly, the rich-split process without IHs can achieve the same energy-saving effect as that achieved by the process of integration with IHs.
Aqueous solutions of alkanolamines are used for absorbents of the post-combustion capture of CO2. Energy requirements for the currently available systems are very high. Thus, it is important to lower the regeneration energy (ΔHreg, kJ/mol-CO2), which consists of three parts: the heat of chemical reactions for CO2 release (ΔHrxn, kJ/mol-CO2); sensible heat (ΔHsen, kJ/mol-CO2); heat of vaporization (ΔHvap, kJ/mol-CO2). In most previous works, it is assumed that the heat of CO2 release is equal to the absorption heat of CO2. However, it is a rough assumption. Thus, we present a thermodynamic model for predicting energy performance of amine absorbents (TMPEA) in order to evaluate the regeneration energy based on the enthalpies of chemical composition changes in a chemical equilibrium analysis. Firstly, we obtained the concentrations of chemical species at a low temperature Ta with a partial pressure of CO2, Pa(CO2), and at a high temperature Tr with Pr(CO2). Thus, we obtained the changes of the concentrations of chemical species from the low temperature state to the high temperature state. Secondly, the changes were expressed with reacted quantities of elementary chemical reactions. We calculated the ΔHrxn value from the enthalpies of the elementary reactions using the Hess's law. Finally, we calculated the ΔHsen and ΔHvap values according to the previous methods. We obtained the ΔHreg value as the sum of the ΔHrxn, ΔHsen, and ΔHvap values. We calculated the ΔHreg values for representative amines such as MEA, AMP, DEA, and IPAE. We found that calculated regeneration energies for MEA depends on working conditions. We demonstrated that TMPEA is useful for evaluating the regeneration energy of CO2.
Carbon dioxide capture, utilization, and storage (CCUS) is a key technology to decrease the emission of greenhouse gas, CO2. The benchmark process using a 30% monoethanolamine aqueous solvent for CO2 capture requires a large amount of energy, in particular, for the regeneration of absorbent at high temperature up to 120 °C. In a recent decade, a variety of absorbents have been investigated to reduce such high energy consumption. Phase separation solvents, which separate into immiscible CO2-rich and CO2-lean phases after CO2 absorption, have been proposed to improve the process efficiency for CO2 capture at atmospheric pressure [1]. In the present study, we attempt to extend the phase separation solvents for high-pressure CO2 capture processes. The p-V-T-x behaviors are studied for the systems of CO2 and phase separation solvents, composed of some amines and ethers at different compositions, over a wider range of temperature and pressure. The could point, inducing the phase separation, can be controlled by the basicity and concentration of amine, which are the predominant factors in the formation of ionic species. The phase separation and p-V-T-x behaviors are discussed in detail for the development of efficient CO2 capture processes at high pressure.
References.
[1] H. Machida, K. Oba, T. Tomikawa, T. Esaki, T. Yamaguchi, H. Horizoe, J. Chem. Thermodyn., 113 (2017) 64-70.
Carbone dioxide Capture, Utilization and Storage has attracted significant attention in the past two decades to reduce greenhouse gas (GHG) emissions and mitigate global warming. However, its capture cost should be further decreased to facilitate its commercial implementation. In fact, CO2 capture is considered as the most energy-intensive part.
In this research, an innovative process using chemical absorption by solid with a circulating fluidized bed has been proposed to reduce energy consumption of CO2 capture. In this process, reaction heat accompanying absorption is successfully supplied to thermal decomposition in regenerator through a heat pump, leading to circulation of whole process heat. To see the influence of the separation performance of the proposed process, some experiments have been conducted. An energy balance of the proposed process using experimental data is simulated and examined using a commercial process simulator (PRO/II, Ver. 9.1). The simulation results indicate that the proposed CO2 separation process has the large energy saving potential as compared with the conventional counterparts.
CCU (Carbon dioxide Capture and Utilization) technology, in which CO2 is captured from large-scale emission sources and the CO2 is converted into high-value added chemicals and fuels, is expected to improve the economical efficiency of CO2 separation and recovery and to enable its commercialization. Among the proposed CCU technologies, organic matter synthesis from water and CO2 under hydrothermal conditions has advantages such as no use of hydrogen and no use of precious metals as catalyst and reductant. Then, in CO2 chemical absorption method using potassium carbonate, we propose that the rich absorbent solution containing CO2 be directly subjected to hydrothermal treatment so as to convert CO2 into organic compounds simultaneously with the regeneration of the absorbent solution for the following absorption process. In this study, we aim to clarify the possibility of converting CO2 into organic compounds by hydrothermal treatment of KHCO3 as a CO2 source. Furthermore, another purpose of this study is to elucidate whether it is possible to regenerate CO2 absorbent solution during the hydrothermal treatment.
A stainless batch reactor was charged with specified amounts of KHCO3 or NaHCO3 as a CO2 source, Fe powders as a reductant, Ni powders as a catalyst and distilled water. The reactor was heated in a sand bath at 300 °C for 120 minutes. After cooling the reactor, the concentration of formic acid and inorganic carbon (IC) contained in the liquid sample were measured with HPLC and a TOC meter, respectively. The results confirmed the formation of formic acid from both KHCO3 and NaHCO3. The yield of formic acid was higher when KHCO3 was used. From the results of IC in the solution after the treatment, it was indicated that most of the introduced bicarbonate was converted to carbonate after the reaction and the absorbent solution could be regenerated.
A key factor for commercialization of CCUS (carbon dioxide capture, utilization, and sequestration) is to establish an efficient and effective CO2 capture process because the capture process possesses the 50 to 80 % of total CCUS operating expense. In this study, we suggests an energy efficient CO2 absorbent comprising the aqueous blend of potassium serinate (K-Ser) and piperazine (Pz). Especially, this CO2 absorbents have high surface tension which make them proper for use in polypropylene (PP) hollow fiber membrane contactor to reduce the membrane pore wetting. By fast solvent screening test, it is revealed that the cyclic capacity of the aqueous blend of K-Ser and Pz is 25 % higher than that of 30 wt% MEA (monoethanoleamine). Vapor-liquid equilibrium was measured to estimate the heat of reaction by calculation using Gibbs-Helmholtz equation. Heat capacity was measured to calculate the sensible heat. Finally, the solvent regeneration energy of the aqueous blend of K-Ser and Pz are estimated and compared with 30 wt% MEA by summation of heat of reaction, sensible heat and heat of vaporization. The long term CO2 removal experiment using Lab-scale membrane contactor will be our future work.
For the CO2 capture from high-volume and CO2-dilute gas such as combustion gas from power plants, chemical absorption with amine is expected to be one of the most feasible technologies. However, its application to the commercial plants for the explicit purpose of CO2 emission abatement still has been limited to a small number. Although there might be several reasons, one of the most critical reasons is its low profitability. Amine solvents require a lot of energy for regeneration, which results in high running cost for CO2 separation.
Our unique phase separation type amine/ether absorbent is expected to realize the CO2 separation process with low-energy consumption (target: less than 2.0 GJ/ton-CO2). This liquid shows a single uniform phase just by mixing the raw materials; amine, ether and water. And it is splitted into two phases consisting of an amine-rich phase and an ether-rich phase by absorbing CO2. Further, it returns to its original uniform phase after CO2 desorbing. During liquid regeneration the ether phase assists the CO2 desorption from the amine phase rich in CO2 by the extraction of CO2, which results in a reduction of energy input for liquid regeneration.
In this study, 500 hours degradation test on this absorbent under cyclic thermal load was conducted in order to clarify 1) the behavior of its performance declination for CO2 capture and 2) the behavior of amine loss. Its test condition was chosen based on two assumptions that a) high-oxidation atmosphere containing O2 and SOX which is destructive condition for the absorbent, and b) adopting practical operating temperature when absorbing or desorbing.
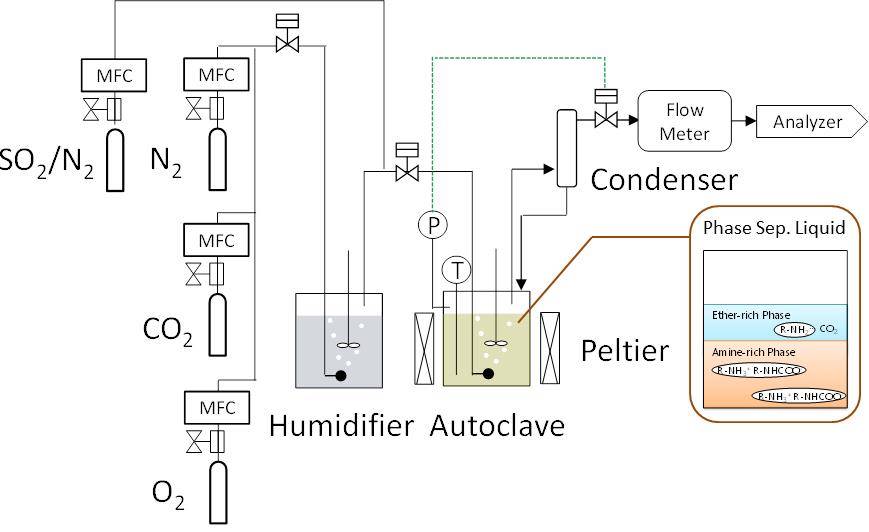
As measures against global warming, CO2 capture and storage technology (CCS) and capture and utilization technology (CCU) has drawn attention of researchers all over the world. So far, our laboratory has developed a new phase separation type CO2 absorbent to improve energy problem in CO2 separation and recovery technology. The CO2·H2 mixed gas recovered from the top of the column can be supplied as it is to the conversion process to methane, methanol and the like. In this process, H2 a component of the source gas, was applied to the bottom of the regeneration tower and reacted with CO2 freed from regeneration process to create methane, methanol and others, which makes it a CCU process. Moreover, without purifying CO2 concentration, this process reduces the energy requirement as compared to the conventional method. In this study, experimental data on stripping regeneration characteristics of phase separated absorbents were acquired. From that, CO2 separation / recovery energy was calculated.
Carbon capture and storage plays an important role in greenhouse gas reduction. Monoethanolamine(MEA) is now widely applied in industry. To overcome the energy penalty of this methods, phase separation solvent was suggested by Machida et al. Since the phase-separation solvent under developing composes of multiple components and separates into two liquid phases after CO2 absorption, it takes time to determine the composition.
Fourier transform infrared spectrometer (FTIR) can simultaneously collect spectra of chemicals within a short time. In order to establish an in-situ method to analyze the components of solution, process FTIR (reactIR) was applied during CO2 absorption. Calibration samples of amines, ethers and CO2 absorbed samples were used to build the model. TOC was used to verify the accuracy of method and model. From FTIR spectra, not only concentrations of components can be determined, phase separation was also observed. Therefore, this method has a prospective application in liquid composition monitoring in plant step.
As a countermeasure against the global warming, early introduction of CCS to large scale CO2 emission facilities, such as thermal power stations, is expected. In general, since CO2 capture process using amine-based solution requires a large quantity of heat for stripping CO2 and regeneration of rich loading solution, process improvement as well as exploring novel solutions is a key to reduction of energy penalty. High concentration 2-Amino-2-methyl-1-propanol (AMP) solution precipitates carbonate with absorption of CO2 and can be expected to reduce sensible heat of regeneration energy by sending the separated carbonate to the stripper. The solution of AMP50wt% promoted by adding Piperazine (PZ), has been tested and successfully operated with solid-liquid separation by a centrifuge. However, the CO2 recovery rate was up to 65% since PZ regeneration reaction did not occurred and PZ was circulated without regeneration. In this study, as a result of searching for new promoter, N-Methyl-1,3-diaminopropane (MAPA) was selected. MAPA primary-carbamate can form an eight-member ring which is easily regenerated, so it is expected to improve CO2 absorption rate in the low CO2 loading range without decrease in the CO2 loading capacity. The operating condition of the small-scale CO2 recovery test was updated by testing AMP/MAPA solution at various temperatures and CO2 loadings. No compounds derived from MAPA were detected in the precipitation solid by results of Raman spectroscopy and GC-MS. Precipitation characteristics of AMP carbonate was empirically obtained by batch tests to determine a stable condition of precipitation process, and cooling temperature was determined 53.4 °C. The injection of semi-lean liquid, which is separated from AMP carbonate by the centrifuge, to the middle inlet seems to be effective to keep the absorption rate even at the top of absorber where the CO2 concentration decreases. The rate-based 10 stages model of absorber using AMP/MAPA solution was built on Aspen Plus and CO2 recovery rate associated with precipitation rate was simulated to determine the position of middle inlet. Injecting the semi-lean liquid to the fifth stage showed the highest CO2 recovery rate. Aqueous solution of AMP 40 wt% + MAPA 5 wt% was evaluated by small-scale apparatus of CO2 capture and recovery with the middle injection process. As a result, the regeneration energy decreased, and CO2 recovery rate reached 90 %.
Global warming and its harmful implications are a major concern for the government and scientific communities worldwide. CO2 being a greenhouse gas is considered to be a major contributor of the global warming. To reduce the anthropogenic emissions of CO2, capturing CO2 from the industrial processes is a must. The solvent based CO2 capture processes is considered to be the most mature and promising technology to capture CO2 from the exhaust streams. Alkanol amines are widely used as a solvent in these processes. However, they are energy intensive and there are still need for development of novel solvents that can make the CO2 capture technology more energy efficient. Potassium salts of amino acids have recently emerged as potential candidate to be used both as a single solvent or as a promoter in the CO2 capture technology. In this work, the potentials of Potassium Salts of Glycine (KGly) as promoter is explored via a kinetics study of reactions of CO2 with different blends of N-Methyldiethanolamine (MDEA) and KGly. Stopped-flow technique was used to carry out the investigations. The experiments were performed over a temperature range of 298 to 313 K and solution concentration of 0.2 and 0.8 mole/l in different MDEA/KGly proportions. Obtained kinetics data were interpreted using zwitterion and termolecular mechanisms for the potassium salts of glycine. The individual rate constants of the participating reactions were regressed and their corresponding activation energies were estimated