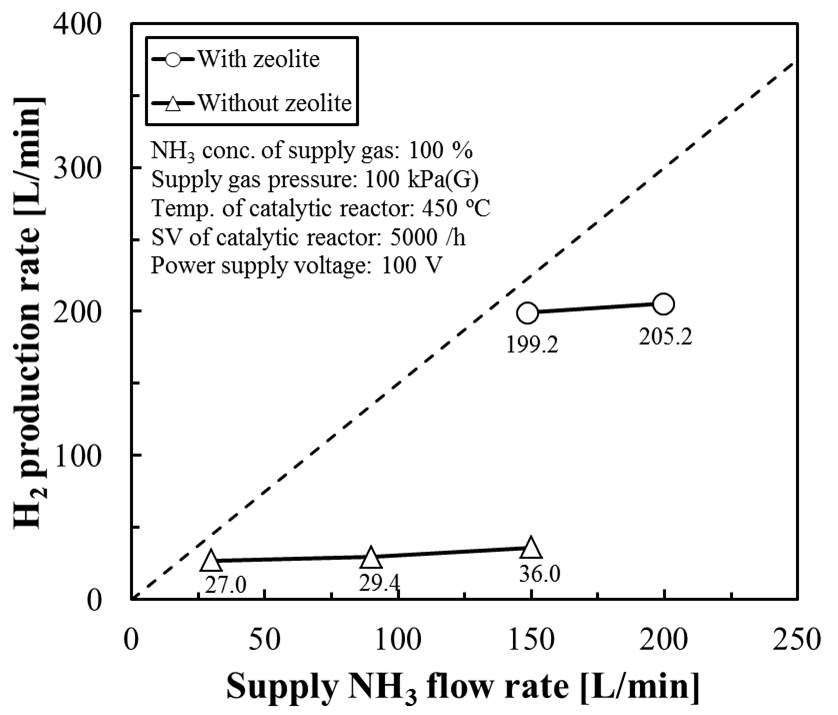
The bottleneck of realizing a hydrogen energy society is energy loss during hydrogen transport and storage. Ammonia is a promising raw material for hydrogen production because it may solve several problems related to hydrogen transport and storage. Ammonia has four advantages as an energy carrier: (1) Liquefaction is easy. (2) The transport and storage mechanisms have been established. (3) Carbon dioxide is not removed when ammonia is converted to hydrogen. (4) Ammonia has high weight-based and volume-based energy densities as fossil fuel. Hydrogen can be effectively produced from ammonia via catalytic thermal decomposition; however, the resulting residual ammonia negatively influences the fuel cells. Therefore, a high-purity hydrogen production system comprising a catalytic decomposition reactor and a plasma membrane reactor (PMR) has been developed. In this hydrogen production system, ammonia is quickly decomposed into hydrogen and nitrogen in a catalytic reactor, and hydrogen is separated from ammonia decomposition gas by PMR. However, the hydrogen yield of this hydrogen production system was low (16 %). The cause of the low yield was the low hydrogen separation capacity of the PMR with ununiformed plasma firing.
In this study, the state of plasma firing was improved by filling dielectric in the PMR. A columnar zeolite (Tosoh Corporation) was used as a dielectric. In the PMR filled with zeolite, plasma was uniformly fired. By packing the zeolite in the PMR, the hydrogen generation performance of the PMR was also improved. It was also found that the improvement of the production performance is affected by the properties of the zeolite to be filled. The hydrogen refining flow rate obtained 199 L-H2/h from 150L-NH3/h. The hydrogen yield of this hydrogen production system improved from 16 % to 88 %.
We investigated the reaction pathways for reversible formate (HCOO–) dehydrogenation and bicarbonate (HCO3–) hydrogenation on N-doped graphene-supported Pd nanoparticles such as Pd12NC-G, Pd12NC-N1G, Pd12NC-N2G, and Pd12NC-N3G using density functional theory (DFT) calculations, and proposed key factors for the enhancement of the reversible reaction efficiency. In order to achieve this, an anion environment caused by HCOO– and HCO3– was simulated by designing two-sided Pd12NC-G systems with extra electrons. The difficulty of conventional DFT calculations related to simulating the anion environment were overcome by using the two-sided systems. Using the systems, we analyzed reaction pathways for the HCOO– dehydrogenation and HCO3–hydrogenation, and demonstrated that the desorption strength of hydrogen was the potential limiting step for the HCOO– dehydrogenation with the energy barriers as 1.24 (1.49), 1.12 (1.27), 0.76 (0.96), and 1.35 (1.90) eV on Pd12NC-G, -N1G, -N2G, and -N3G, respectively (values for the HCO3– hydrogenation were indicated in parentheses). These results indicate that the adsorption energy of hydrogen can be utilized as a descriptor for the reversible reactions on other surface systems. In addition, we confirmed that the Pd12NC-N2G with the proper amount of N dopants showed optimal hydrogen adsorption strength depending on the smallest d-band center and spin density values, resulting in the lowest energy barriers for HCOO– dehydrogenation (0.76 eV) and HCO3– hydrogenation (0.96 eV). This showed that the appropriate number of nitrogen dopants can provide the optimal balance for the reversible reactions.
Ammonia is a hydrogen storage material that may solve several problems related to hydrogen transportation and storage in the hydrogen society. Catalytic thermal decomposition is a promising technique for producing hydrogen from ammonia. However, Catalytic thermal decomposition of ammonia needs long start-up time for heating reaction field. So, it is not suitable for power generation on site. Cylindrical plasma membrane reactors have been developed to produce pure hydrogen from ammonia. However, the gas flow is not uniform; therefore, the plasma state is unstable, resulting in a low ammonia decomposition rate in the cylindrical plasma reactor. A plasma membrane reactor with a flow channel was considered in order to address this issue, assuming that this configuration would simulate a fuel cell separator to create a uniform gas flow. A 1-mm-wide and 1-mm-deep flow channel was fabricated on the metal plate. 0.1% ammonia gas was supplied to the flow channel plasma reactor at a 0.1-L/min flow rate in an object to test the configuration. Stable plasma was observed; the ammonia decomposition rate reached 20.3 %, which represents a higher conversion efficiency than that measured in a cylindrical plasma membrane reactor with the same gas residence time. Because the plate plasma reactor can be laminated, it can be easily scaled up for large-scale hydrogen production. The effects of the applied voltage, gas flow rates, and ammonia concentration on the ammonia decomposition rate were investigated. The results showed that an increase in the applied voltage leads to a higher ammonia decomposition rate because the high plasma density causes greater dissociation of molecular ammonia by electron impacts. Further, increases in the ammonia gas flow rate or the ammonia concentration reduces the ammonia decomposition rate.
An efficient method for using pulsed plasma to produce hydrogen from ammonia was developed.
To keep the plasma steady under pressurized conditions, internals such as zeolite particles was available. Actually, hydrogen production rate from ammonia was increased by the packed bed plasma membrane reactor. This report discusses the cause of the increase of hydrogen. The V-Q lissajous and fundamental characterization of the particles was investigated for some kinds of zeolite particles. It seems that the dominant factor is H radical generation by the zeolite.
The technical issue of hydrogen, which is used for fuel cell electronic vehicle, is the storage and the transportation. Some chemicals, i.e., 2-propanol and methyl-cyclohexane, have potential as a carrier of hydrogen due to their advantages such as liquid phase at room temperature, lower toxicity, etc. The conventional hydrogen production process from these “hydrogen carrier” was complex and energy-wasting because the process consists of (1) dehydrogenation reactor (endothermic step), (2) condenser of an organic compound (exothermic step), and (3) mechanical compressor.
We propose a new electrochemical device which able to produce a proton, separate hydrogen, and compress hydrogen in one step. The setup of the device is similar to the polymer electrolyte fuel cell (PEFC). The proton forms at anode, hydrogen gas at the cathode is separated from the organic compounds by the polymer electrolyte membrane, and hydrogen is compressed by the conversion from electronic potential to chemical potential (Nernst equation).
In this work, we developed the electrochemical device to obtain pressurized hydrogen from 2-propanol. The one-pass conversion and obtained pressure were measured during constant voltage operation.
We prepared a PEFC-like device with Pt/C catalyst (for anode and cathode) and Nafion 117 membrane. A hydrogen carrier, 2 M 2-propanol, was supplied to the anode. The –1 V of voltage was applied to the device, the one-pass conversion was evaluated from the composition of anode effluent, and cathode gas pressure was measured by a pressure transducer.
The obtained one-pass conversion was ca. 8% at –1 V, and cathode gas pressure increased 0.9 bar during 1 h. The major component in cathode gas was hydrogen, in the meanwhile, some impurities, i.e., 2-propanol, acetone, and methane, were found. Methane is considered to be formed from acetone and hydrogen with Pt catalyst.
Plasma membrane reactor has been developed to produce hydrogen from ammonia. A cylindrical type reactor is available for hydrogen production; however, a plate-type reactor is desired for a mobility use. A plate type plasma membrane reactor has made by a flow channel plate and original electrode, which shows stable plasma firing by dielectric barrier discharge. Effect of membrane thickness on hydrogen permeation characteristics was examined, and its characteristics were compared with the cylindrical plasma membrane reactor.
The equilibrium diagrams for the decomposition of magnesium alanate (Mg(AlH4)2) nanoparticles were constructed as a function of particle size and temperature to better understand nanostructuring of the complex hydrides for hydrogen storage. Relatively smaller nanoparticles of Mg(AlH4)2, MgH2, and Al ranging from 1 to 2 nm were directly calculated by using density functional theory (DFT) calculations, and cluster expansion and Monte Carlo simulation methods were developed to predict the free energy contours of 1 ~ 50 nm nanoparticles. Our prediction demonstrates that bulk Mg(AlH4)2 can release hydrogen but its uptake reaction is unfavorable, and bulk Mg(AlH4)2 is metastable with respect to bulk MgH2. However, in the cases of nanoparticle systems, hydrogen release and its recharging may be possible by controlling the particle size and temperature, which may facilitate experimental studies to determine the thermodynamically favored reaction pathways for the dehydrogenation and hydrogenation processes of Mg(AlH4)2 nanoparticles. We also provide the equilibrium diagrams for Mg(AlH4)2 nanoparticle decomposition depending on a hydrogen partial pressure.
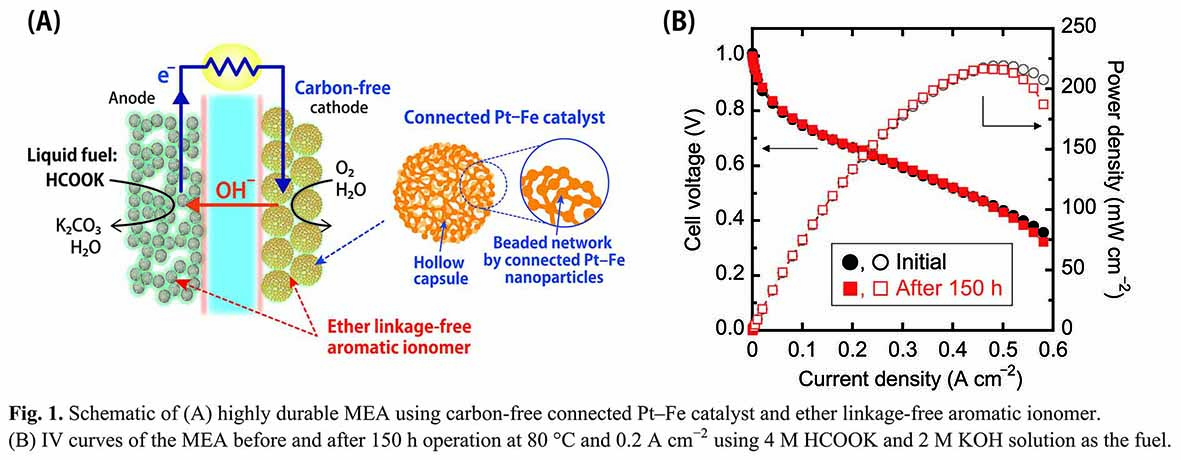
Solid alkaline fuel cells (SAFCs) have attracted much attention as next-generation energy conversion electrochemical devices. However, practical applications of these devices are limited because of the drastic decrease in the cell performance during the high temperature operation, under alkaline condition. This low performance of the device is mainly attributed to the severe degradation of membrane–electrode-assembly (MEA). Therefore, the development of a durable and stable MEA is critical to improve the cell performances and stabilities in this system.
Here we report the development of a highly durable MEA, as shown in Fig. 1A, using a carbon-free cathode catalyst [1,2] and an aromatic polyelectrolyte without ether linkage [3]. The carbon-free catalyst consists of a nanosized beaded network formed by the connection of Pt–Fe nanoparticles. The beaded metal network is electrically conductive, enabling the removal of carbon support from catalyst layers. Hence, carbon corrosion problems can be avoided, leading to high durability against the cyclic start-stop operation of the system.
The fabricated MEA, operating at a high temperature of 80 °C, exhibited high power density of 220 mW cm–2 and high open-circuit voltage of about 1.0 V (Fig. 1B), wherein, a mixture of aqueous solutions of 4 M HCOOK and 2 M KOH was used as the fuel. Further, more importantly, the high performance of the MEA was retained even after the operation at 80 °C and 0.2 A cm–2 for 150 h. Thus, for the first time, we demonstrated a highly durable and stable MEA in alkaline medium for the direct formate SAFCs.
[1] T. Tamaki, H. Kuroki, T. Yamaguchi et al., Energy Environ. Sci., 8, 3545–3549 (2015).
[2] H. Kuroki, T. Tamaki, T. Yamaguchi et al., ACS Appl. Energy Mater., 1(2), 324–330 (2018).
[3] S. Miyanishi and T. Yamaguchi, J. Mater. Chem. A, 7, 2219–2224 (2019).
Methanol synthesis from CO2 hydrogenation has been recently an object of study because CO2 is a potential feedstock based on the concept of a methanol-based economy. However, the commercial catalyst Cu/ZnO/Al2O3 does not show enough activity and selectivity of the CO2 hydrogenation. Hence, it is important to develop suitable catalysts for the CO2 hydrogenation. So far, we reported that Cu/ZrO2 catalysts hydrogenate CO2 selectively to methanol. In this study, we focused on flame spray pyrolysis (FSP) which are widely used for producing nanosized metals/metal oxides on an industrial scale. One of the advantages of FSP is the formation of small metal nanoparticles at extremely high metal loading on metal oxide particles. This time, we tried to prepare highly loaded Cu nanoparticles on ZrO2 (20-80 wt%_Cu) by the FSP technique and investigated the effect of Cu loading on activity and selectivity of CO2-to-methanol hydrogenation. When Cu loading was 60 wt%, the catalysts showed higher methanol yield and methanol selectivity than did a commercial catalyst Cu/ZnO/Al2O3. Its catalytic performance was derived from the unique structure: Cu nanoparticles (10-20 nm) were deposited on ZrO2 nanoparticles (5-10 nm). In addition, the Cu nanoparticles were strongly interacted with the ZrO2 nanoparticles, leading to electron transfer from Cu to ZrO2. The interaction might create specific active sites for the CO2 hydrogenation.
Direct Formate Fuel Cells (DFFCs) hold a key stake in the energy systems looked into for answers to the increasing futuristic energy demand, largely due to the offered potential practical energy generation capability along with the safety, low cost and environment friendliness compared to the counterpart alcohol fuels (DEFCs & DMFCs). Palladium has shown the most promising results in recent years for enhanced liquid-fuel oxidation kinetics that could effectively replace platinum in terms of performance and extensive application span, particularly in alkaline medium. In our study, we engineer alloys based on Pd and transition metal (Pd-Ni) /post-transition metal (Pd-Sn) over carbon support using an exclusive wet chemical technique. The formed alloy compositions showed overall enhanced formate oxidation half-cell performance and longer stability compared to Pd/C in alkaline medium. The performance analysis based on the structural and surface characteristics of these nanoalloys obtained over carbon support is elucidated and presented. The successfully developed catalyst compositions and technique employed paves way for a scalable strategy for realizing highly efficient anode catalysts for DFFCs.
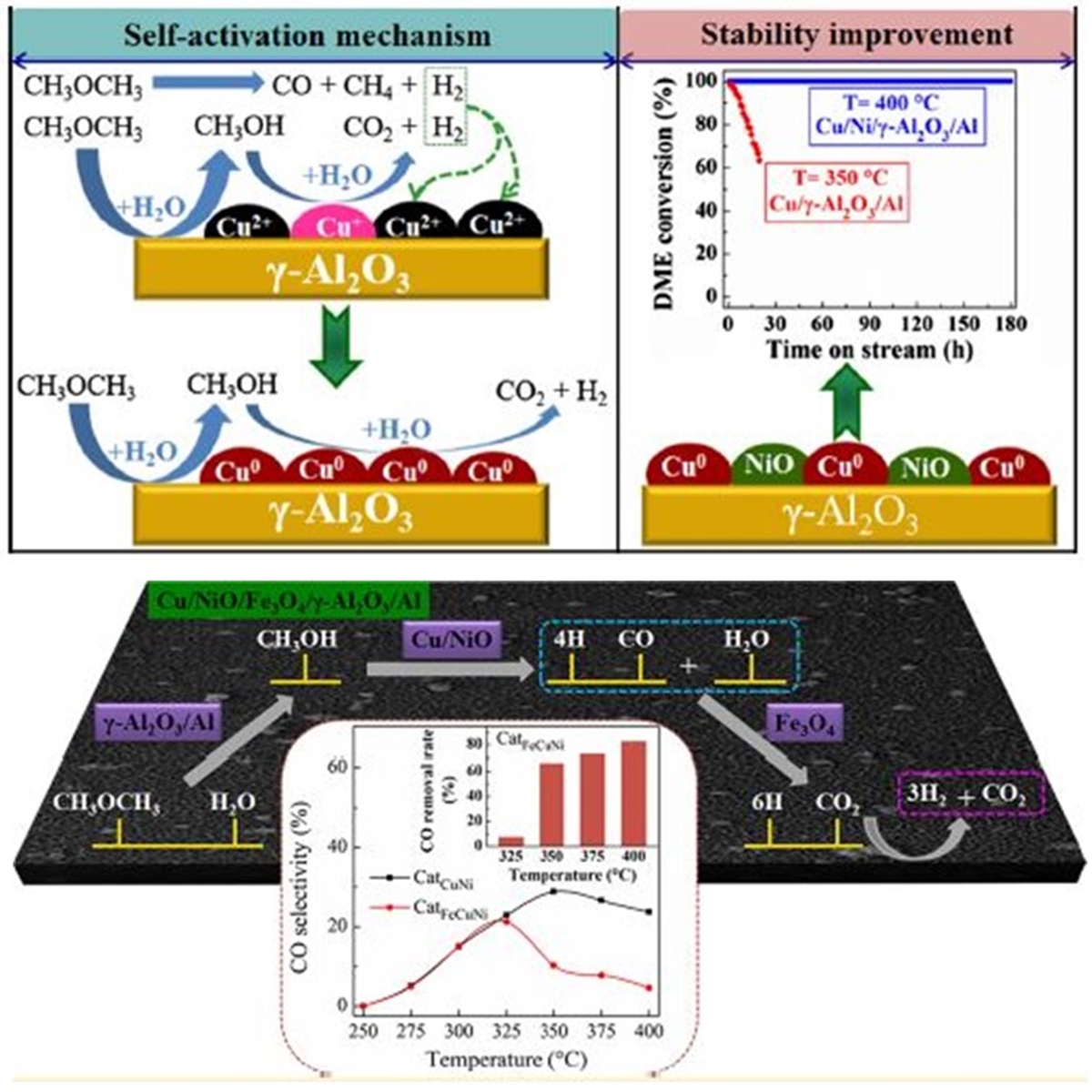
The depletion of fossil fuels and increasing energy demand has driven the research and exploration of new energy alternatives. Hydrogen is considered to be a clean source of energy and steam reforming of dimethyl ether (DME) is regarded as a promising hydrogen production process for fuel cell applications. A plate-type anodic alumina supported Cu composition catalyst was developed to investigate its catalytic performance in steam reforming of DME. It is found that the fresh Cu/γ-Al2O3/Al catalyst without H2 pre-reduction treatment exhibited the similar catalytic activity as the pre-reduced one. The XPS results show that Cu+ species exist in the surface of the fresh catalyst, which results in the self-activation reaction. However, the Cu/γ-Al2O3/Al catalyst showed a quick deactivation at 350 °C due to the sintering of copper. As an approach, a second component Ni was doped to the Cu-based catalyst. Furthermore, the effect of nickel loading and chemical state on the activity of catalyst was extensively investigated. The proper amount of nickel doping is helpful in improving the dispersion of copper species, and thus enhancing the catalytic activity. Finally, a 180 h stability evaluation was carried out and the results show that the optimized Cu/Ni/γ-Al2O3/Al catalyst has an excellent stability under critical conditions with 400 °C, and gives a 100% DME conversion. However, a high CO concentration (ca. 26%) was detected. As such, a multifunctional catalyst combined DME SR and high temperature water gas shift reaction (HT-WGSR) was developed. The CO reduction mechanism was proposed over the Fe-doped Cu-based multifunctional catalyst. Furthermore, the effects of iron loading on the physicochemical properties and performance of catalysts were extensively investigated. The results show that the proper amount of iron doping was helpful in improving the dispersion of Cu, and thus enhancing the catalytic performance and decreasing CO concentration. Finally, it is found that the optimized Cu/Ni/Fe/γ-Al2O3/Al multifunctional catalyst has excellent stability during a 200 h test, and gives a 100% DME conversion at 400 °C in both the microreactor and fixed-bed reactor. Furthermore, crossflow channels and parallel-flow channels were built respectively by mesh-type catalyst and plate-type catalyst in the microreactor. The gas/solid mass transfer limitations in both flow channels were investigated by Damköhler number (Da). The results showed that the Da values of cross flow were always < 0.01, while the values of parallel flow were > 0.01. It indicated that the diffusion over mesh-type catalyst was less influenced by temperature and reactor height, making it a more appropriate choice for the microreactor.
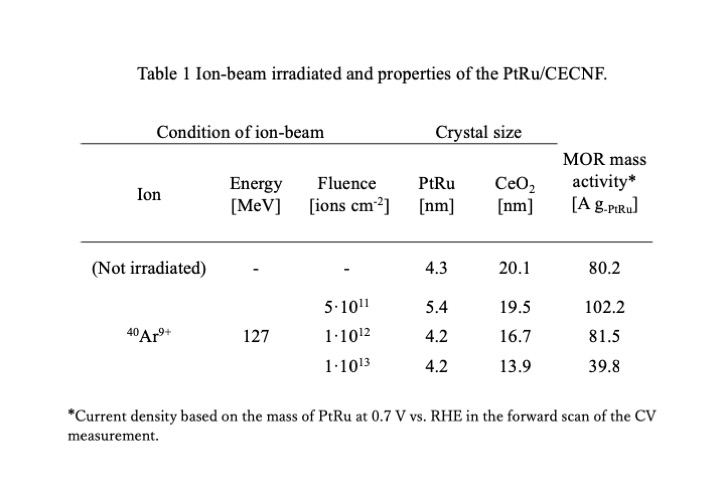
One of the issues for commercialization of direct methanol fuel cell (DMFC) is the slow kinetics of the anode reaction, and an alternative catalyst with high activity has been desired. PtRu nanoparticles supported on CeO2 nanoparticles embedded carbon nanofibers (CECNF), PtRu/CECNF, showed two to three times higher methanol oxidation reaction (MOR) activities due to the support effect of CECNF which efficiently develops the positive interaction between Pt and CeO2 by the CeO2 embedding fiber structure. The interaction can be explained by the reaction mechanism with an oxygen vacancy of the CeO2. In this study, the ion-beam irradiation was employed to introduce oxygen vacancies to the support material in order to increase the MOR activity of the catalyst.
A sheet of CECNF was prepared by using the electrospinning technique. To the CECNF sheet, irradiation of high-energy ion-beam, Ar9+ 127 MeV, was conducted at the different fluence (ion-beam density based on subjected area). After that, PtRu nanoparticles were deposited on the CECNF by a microwave polyol method. The catalytic activity for methanol oxidation reaction was evaluated by using a three -electrode cell with a glassy carbon electrode as a working electrode.
It was found that the MOR mass activity of the PtRu/CECNF increased, up to about 25%, with the increase of the fluence up to a certain value, then decreased with further increase of the fluence as shown in Table 1. The crystalline size of CeO2 in PtRu/CECNF, obtained from XRD profile using Scherrer's equation, decreased with the increase of fluence. EDX results elucidated a decrease of oxygen content in the catalyst due to the irradiation. These results suggested that the ion-beam irradiation destroyed a part of the crystal structure of CeO2, and it enhanced the MOR activity at the proper fluence.
Recent rapid decline in the price of renewable energy increases the importance of energy carriers. Formate solution is one of the envisaged candidate for liquid energy carriers, since formate can be electrochemically regenerated in high energy efficiency from carbon dioxide. For the use of formate solution as an energy carrier, formate needs to be converted to electricity with high energy efficiency.
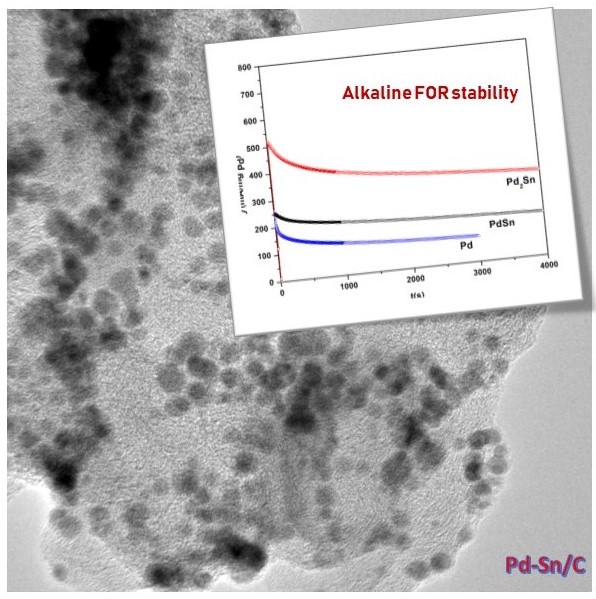
Our aim in this study is to clarify design strategy of electrocatalysts for formate oxidation reaction (FOR) in alkaline conditions. Palladium (Pd) is known to show better activity for FOR in alkaline solution than platinum. The reaction mechanism of FOR on Pd in alkaline condition remains controversial; although many groups suggested a reaction mechanism without any poisoning species (strongly bound species) such as carbon monoxide (CO), a few studies showed the presence of bound CO. If CO exists, the FOR activity of Pd can be improved by reducing CO adsorption on its surface, which is possible by incorporating oxophilic materials such as ceria (CeO2) and ruthenium (Ru).
Herein, we sequentially deposited CeO2 and Pd over carbon support (Pd/CeO2-C). This Pd/CeO2-C showed better activity for FOR in alkaline medium than Pd/C. The result of an in-situ x-ray absorption spectroscopy (XAS) measurement at Pd K-edge showed the formation of CO on Pd in the presence of formate. Also, the comparison of the spectra between Pd/CeO2-C and Pd/C showed a decrease in CO adsorption with the concomitant increase in OH adsorption on Pd/CeO2-C. We then synthesized Pd-Ru alloy to more effectively incorporate oxophilic materials with Pd. PdRu/C showed better activity than Pd-CeO2/C and Pd/C. These results suggest that the incorporation of oxophilic materials improves the FOR activity on Pd-based catalysts in alkaline medium by reducing the CO adsorption.
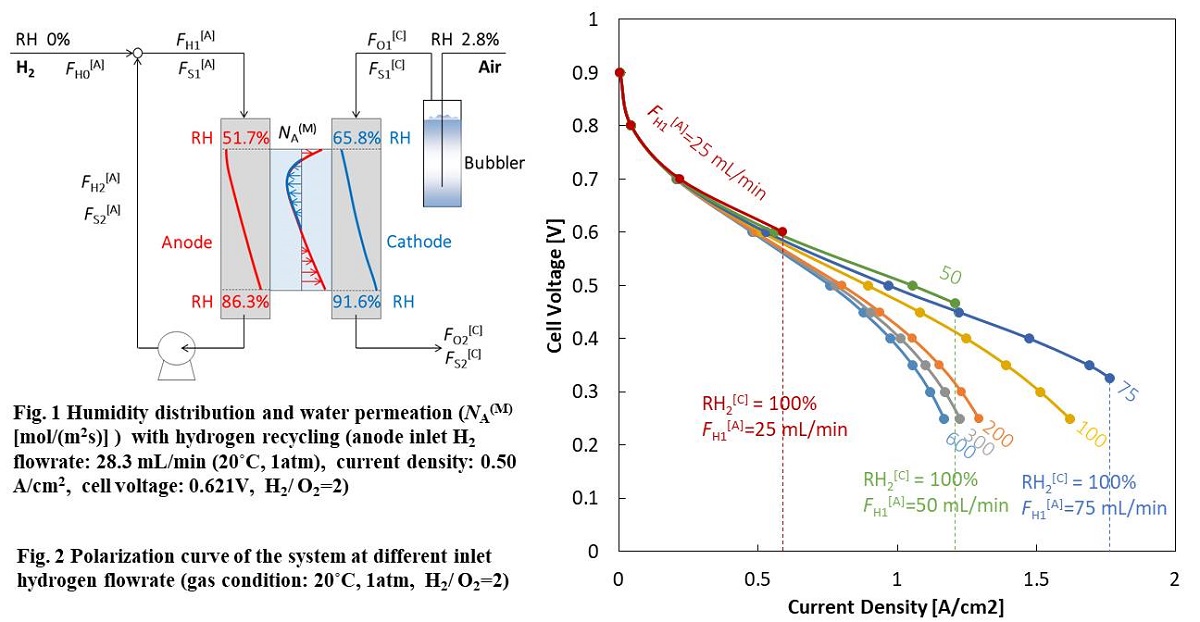
At present, polymer electrolyte fuel cell (PEFC) technology is supposed to be commercialized in a wide range. However, water management is an essential problem to achieve maximum performance. The relative humidity (RH) of gases should be controlled to prevent membrane dehydration and liquid water flooding to maintain a high performance level. Hydrogen recycling is one of methods to humidify the cell with water produced in redox reaction, while the overall hydrogen utilization can be 100%. In this study, water transport in a PEFC system with hydrogen recycling was analyzed by combining plug flow reactor model and dimensionless modulus model in the through-plane direction in steady state, when the dry hydrogen and humidified air are fed to an 80 °C isothermal cell. The gas channel was regarded as a straight line in the model. Although the water flux through proton exchange membrane (PEM) has different distribution at different current density, total water permeation rate is 0, as far as the outlet gas of anode recycles to the inlet entirely (Fig. 1). In the case of co-current which the gas flows have the same direction on the anode and cathode sides, current density as well as electroosmosis increases from inlet to outlet of gas channel in low current region, because of humidification by produced water, which enhances the proton conduction in PEM and cathode layer. Besides, the difference in RH of gas flow on two sides of PEM creates water diffusion. When the gas flowrate increases, decreasing proportion of produced water makes PEM dehydrated, and the performance deteriorated (Fig. 2). The RH in the cell increases with decrease in flowrate. At 1 A/cm2 and H2/O2=2, RH becomes 68% at the exit of the cathode in case of 300 mL/min oxygen and RH reaches 100% at 21 mL/min.
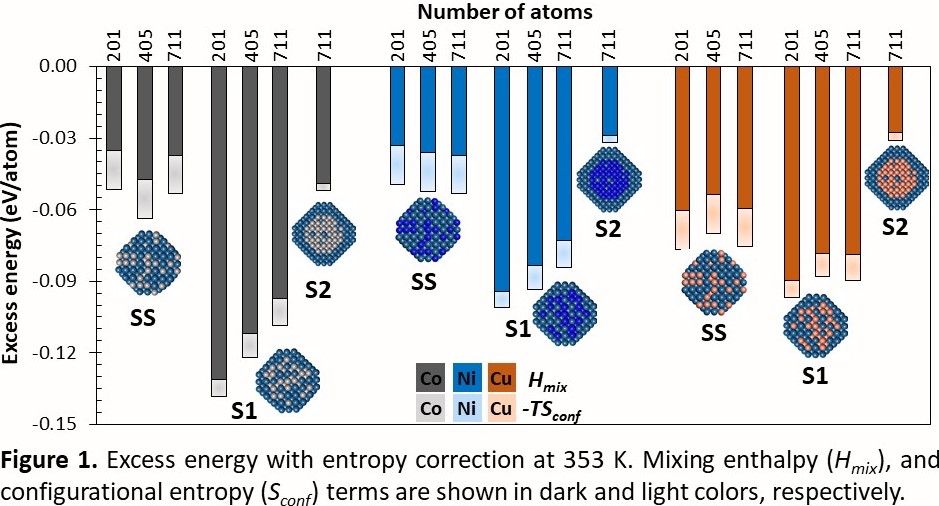
The use of bimetallic nanoparticles (NPs) reduces the noble metal usage without sacrificing the electrocatalytic activity. To theoretically investigate the influence of the NPs' properties, such as strain, stability, or electronic structures on the catalytic activity requires the use of a “close to real size” cluster models. In this study, the phase stability, its origin and electronic structure of Pt3M (M = Co, Ni, and Cu) are investigated using density functional theory (DFT) method. Bimetallic Pt3M-NPs containing 201 (Pt150M51), 405 (Pt303M102), and 711 (Pt533M178) atoms in total were modeled considering three different configurations; 1-skin layer (S1), 2-skin layers (S2), and solid solution (SS). The stability of the Pt3M-NPs was approximated by the excess energy, which contains the mixing enthalpy and configurational entropy. In all cases, the bimetallic NPs were more stable than the monometallic Pt-NPs. For the S1 systems, the mixing enthalpy is the dominant term, and the configurational entropy value is the smallest compared to SS, and S2 systems, as shown in Figure 1. Electronic equilibration in line with the work function difference between Pt and M led to charge transfer from the M to Pt, reducing the surface Pt atoms in the S1 configuration. Additionally, the presence of M of smaller atomic radius than Pt in the core of the nanoparticle made the surface atoms more relaxed compared to the monometallic system, thus decreasing the stress in the structure and helping to accommodate the extra electrons from the M in the subsurface. These changes in the electronic structure and geometrical features were used as descriptors of the Pt3M stability. For Pt3Co and Pt3Ni the stability is largely determined by the surface charge, while for Pt3Cu the dominant factor is the surface strain.
Polymer Electrolyte Fuel Cell (PEFC) is desired to be operated at high temperature such as 90 °C for stationary applications during the period from 2020 to 2025 in Japan. It can be expected thinner polymer electrolyte membrane (PEM) and gas diffusion layer (GDL) would promote the power generation performance of PEFC at this temperature. However, the current PEFC has Nafion membrane and is usually operated within the temperature range between 60 °C and 80 °C. The aim of this study is to clarify the impact of thicknesses of PEM and GDL on temperature distribution in single PEFC generated at high temperature such as 90 °C and to propose the optimum components combination.
As a result, from the investigation of impact of thickness of GDL on the power generation characteristics using the thickest PEM, Nafion 115, the impact of relative humidity of supply gas on the power generation characteristics was small for thin GDL. In addition, in-plane temperature distribution from the inlet to the outlet was flat by promotion of water transfer when using thin GDL. On the other hand, from the investigation of impact of thickness of PEM on the power generation characteristics using thin GDL, i.e., TGP-H-030, the impact of relative humidity of supply gas on the power generation characteristics was not acknowledged. However, the power generation performances using Nafion 212 and Nafion 211 were higher than that using Nafion 115. As to in-plane temperature distribution, it was flat when using Nafion 115 and Nafion 211, while it was increased from the inlet to the outlet using Nafion 212.
It was revealed that the thinnest combination of Nafion 211 and TGP-H-030 was the optimum for high temperature operation such as 90 °C from the viewpoint of the power generation performance as well as controlling in-plane temperature distribution.
Polymer electrolyte fuel cell (PEFC) has been attracting attention as a highly efficient power generation system. Since Pt used in a catalyst is scarce and expensive, it is important to develop a low-Pt catalyst. A core-shell catalyst, which consists of thin Pt shell on non-Pt metal core, has been developed to reduce the amount of Pt. Typically, Cu-UPD (underpotential deposition) followed by surface limited redox replacement (SLRR) is used to fabricate the core-shell catalysts. We have been developing a novel method suitable for mass production of membrane-electrode assembly (MEA) by initially fabricating a catalyst layer (CL) from the core material, and then perform Cu-UPD and SLRR on the CL to obtain a new CL of core-shell catalyst. Moreover, we focused on nanosheet (ns) catalysts. A general Pt-supported carbon catalyst has a problem in durability, since small Pt particles are easily aggregated and dissolved due to its large surface energy. It was reported that Ru@Pt-ns/C catalyst using Ru-ns as the core and Pt as the shell, exhibits high specific activity and high durability due to the low surface energy. In this study, a novel method to produce an electrode with low-Pt core-shell nanosheet catalyst was developed by using Cu-UPD/SLRR in the CL.
The effect of the ionomer ratio on the formation of core shell was investigated. Metallic luster was observed on the surface of the CL for I/C = 0.5 and I/C = 1, indicating abnormal deposition of Pt. However, it was not observed for I/C = 0.25, suggesting uniform core-shell formation. The influence of the number of times of Cu-UPD/SLRR was investigated. The Pt mass activity was low when 2 cycles of Cu-UPD/SLRR were applied but it improved when 4 cycles were applied. The Pt mass activity of Ru@Pt-ns/C prepared by the novel method was higher than Pt/C.
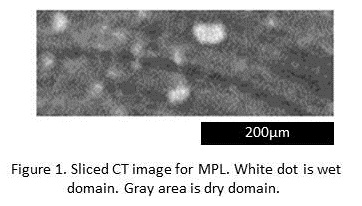
It is well known that laminating a microporous layer (MPL) to substrate such as carbon paper prevents from flooding in catalyst layer (CL) for polymer electrolyte fuel cell (PEFC). It is therefore important to visualize 3D water distribution in both the MPL and substrate to design the material. Visualization of 3D water distribution in the substrate was conventionally achieved by operando X-ray Computed Tomography (CT). However, 3D water distribution in the MPL has not been visualized due to the strong X-ray adsorption of the Pt in the CL. Here we present new method for visualizing 3D water distribution for the MPL. In the experiment, water vaper was diffused in the MPL, and its condensation process was measured by X-ray CT. This method does not need the CL. Conventional problem strong X-ray adsorption of the CL for Operando-CT can be avoided. Obtained sliced image of MPL is shown in Figure 1. Water in the MPL is observed as polka-dot patterns. This suggests that pore to water drain and pore to gas diffusion is separately exists in the MPL. In addition, water movement from wet domain from the MPL to substrate was visualized. These results show potential of the method to understand water condense and drain phenomena in the MPLs.
Optimization of the PEFC design is a crucial subject for its deployment and production expenses. However, a comprehensive understanding of its behaviour is needed, including its own structure, electrochemical reaction, reactants and products mass transfer limitations concerning proton and oxygen in principal. The mass transfer takes place through electrostatic drag for proton, and convection and diffusion of oxygen in the cathode catalyst layer (CCL). The authors introduced four dimensionless moduli which govern behavior of CCL.
Effectiveness factor is one under the reaction rate controlled regime, yet, is inversely proportional to Thiele modulus, as far as the convective flow effects are negligible and under oxygen transport control conditions. Under proton transport control conditions, the effectiveness factor is inversely proportional to the modulus that represents the ratio of the reaction rate constant to the effective proton conductivity.
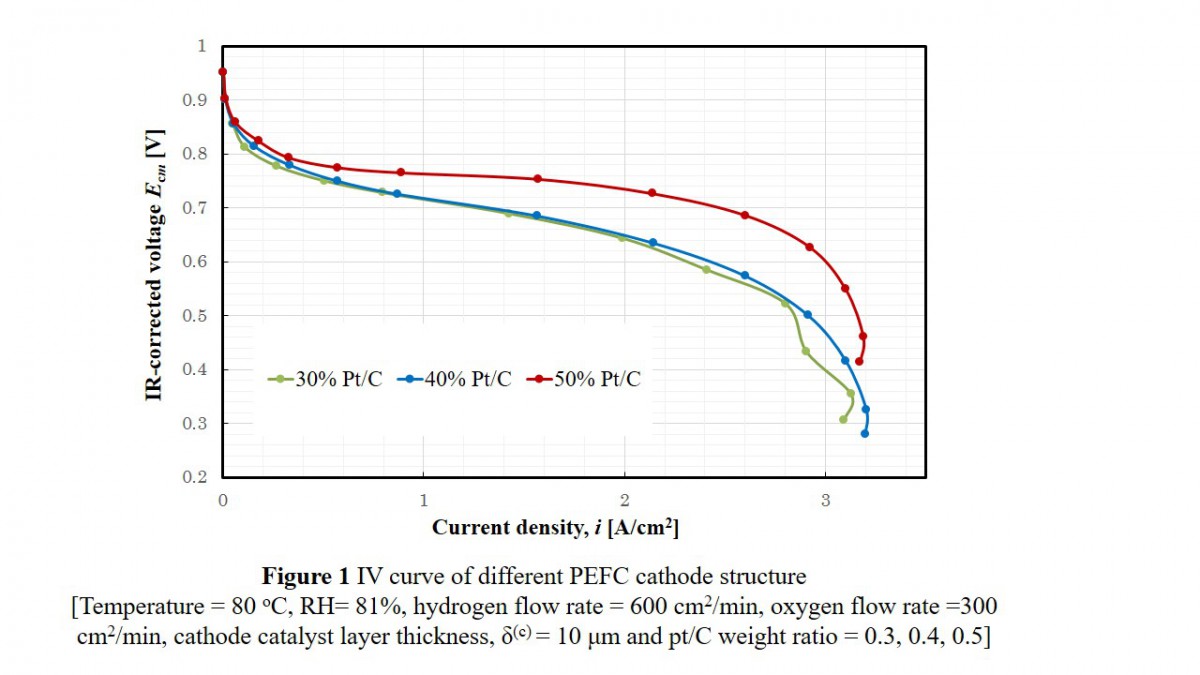
To determine the effectiveness factor and dimensionless moduli, experiments were done on NR-212 (50 μm) with Pt/C weight ratio of 0.3, 0.4, and 0.5. For each Pt/C weight ratio, cathode catalyst layer thickness was 10, 30, 50 μm, using electrochemical measurement system. The impedance spectra were measured in a range of frequency from 10 mHz to 100 kHz.
Figure 1 shows the relation between IR-corrected cell voltage and current density for 10 μm cathode catalyst layer having a Pt/C ratio of 0.3, 0.4 and 0.5. The overall mass transfer limitation between Pt/C = 0.3 and 0.4 was comparable. Oxygen and proton transfer resistances were estimated.
Solid Oxide Fuel Cells and Solid Oxide Electrolysis Cells (SOFCs / SOECs) have much attention because SOFCs have the advantage of being able to convert fossil fuels to electricity with the highest efficiency and SOECs have the advantage of requiring less power for electrolysis compared to PEMs due to high temperature operation. From these backgrounds, both have been considered for practical use.
In recent years, studies on reversible Solid Oxide Cells (R-SOCs) which means a combination of SOFCs and SOECs are in progress. R-SOCs can be regarded as an energy storage system similar to a battery, but have the drawback of a smaller conversion efficiency than that of a battery. In this work, we performed theoretical efficiency calculations of R-SOCs system using an oxide-ion conductor and a proton conductor to show the merit in comparison with a battery.
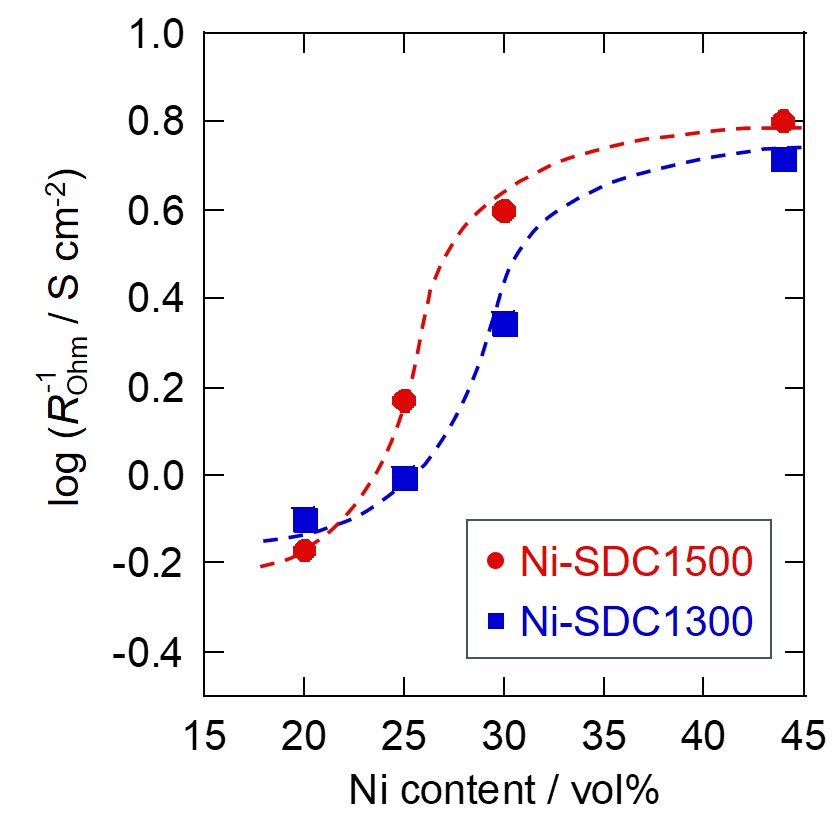
Ni-based cermets are typically used for anodes of solid oxide fuel cells. However, the Ni phase in such anodes can cause durability problems. One possible approach for mitigating the problems is to reduce the Ni content in the anode, although decreased Ni amount in turn reduces conductivity and electrochemical activity of the anode. In this study, Ni-SDC (samarium-doped ceria) cermet anodes were prepared with different SDC particle sizes and Ni contents, and it was investigated how particle size ratio of the SDC and Ni affected the performance at low Ni contents. Ni-SDC|YSZ|LSM cells were fabricated, then current-voltage characteristics and AC impedance spectra were measured. Impedance analyses with equivalent circuits revealed that ohmic resistance was the primary factor affecting the anode performance in the tested system, and it was found that large SDC particles were effective in reducing the ohmic resistance even at low Ni contents. To further investigate the effects of the particle size ratio, conductances of the Ni-SDC anodes with different SDC particle sizes were calculated from the measured ohmic resistances. The conductances decreased sharply at certain volume fractions of Ni, and the threshold volume fractions shifted toward lower Ni contents when large SDC particles were used. Independently, threshold Ni volume fractions were calculated according to percolation theory, using particle sizes of Ni and SDC estimated from SEM observations. The thresholds thus calculated were in good agreement with those obtained from the ohmic resistance, indicating that the electrical conductivity of the Ni-SDC anodes was governed by the formation of segregated and conductive Ni clusters.
Due to the expanding installation of renewable energy, to solve the gap between the unsteady power supply and the demand becomes a key issue, and the reversible solid oxide fuel cell/ electrolyzer (rSOFC/EC) are increased their attention as a large-scale energy storage. To control their unsteady operations, the quantitative understanding of the reversible reaction kinetics is required. A number of reaction kinetics models of SOFC on Ni/ yttria stabilized zirconia (YSZ) have been proposed [1-3]. The rate determining was described as a surface chemistry on triple phase boundary (TPB) under the local equilibrium between oxygen, O2- and electron [1, 2], or the charge transfer reaction of neutral absorbent, surface ion and electron between Ni and YSZ [3]. In both case, the reaction of SOFC/EC have not been successfully described as a unified kinetics.
We applied the existing reaction model of SOFC anode, which we previously developed [2], to the reversible SOFC/EC with the series of H2/H2O gas components to discuss the SOEC anode reaction. The model is described the kinetics by competitive adsorption reaction at TPB with oxygen activity (aO) calculated from anode potential. As a result, the current density of SOEC was much larger than the extrapolated value by SOFC data at any H2/H2O gas condition. By reinvestigating the local equilibrium of adsorbents, oxide ion and electron on Ni, we proposed a modified kinetics model by defining the electron activity ratio as another independent variable. The kinetics is discussed with the shift of local oxygen equilibrium by changing the charge balance.
Acknowledgement: a part of this work is supported by the New Energy and Industrial Technology Development Organization (NEDO)
[1] Mizusaki et al., Solid State Ionics, 70/71. 52 (1994).
[2] Ihara et al., J. Electrochem. Soc., 148(3), A209 (2001).
[3] Bieberle et al., J. Electrochem. Soc., 148(6), A646 (2001).
The triple phase boundary (TPB) of metal, oxide, and gas phases in the anode of solid oxide
fuel cells plays an important role in determining their performance. In this study, we explored the TPB structures and reaction at TPB combining two approaches: atomic-resolution microscopy observation based on HAADF-STEM (High-angle annular dark field scanning transmission electron microscopy) and reaction dynamics simulation based on reactive force field. From HAADF-STEM observations, two distinct structures are found with different contact angles of metal/oxide interfaces, metal surfaces, and pore opening sizes, which have never been adopted in theoretical simulations in literature. Chemical reaction dynamics simulations for the hydrogen oxidation reaction (HOR) at the TPB are performed using realistic models reconstructed from HAADF-STEM observations. In addition, extensive development of accurate reactive force field parameters was conducted to accurately trace the reaction pathways at TPB. As a result, the activity of different structures towards HOR is clarified, and a higher activity is obtained on the TPB with smaller pore opening size. Three HOR pathways are identified: two types of hydrogen diffusion processes, and one type of oxygen migration process which is a new pathway.
After the Great East Japan Earthquake, there is demand for transformation to a bidirectional system introducing distributed power sources, and a fuel cell cogeneration system with advanced energy utilization has been attracting attention. Even among them, solid oxide fuel cell (SOFC) are high efficiency power generation systems and expected as a promising power source. It is necessary to reduce the raw material cost by improving the power density for further penetration of SOFC. Electrochemical reactions occur only on an effective triple phase boundary (TPB) where each network path of the electron, the oxide ion and the fuel gas connects uninterruptedly. The power generation performance was affected by the effectiveness of TPB. This work aims to realize a microstructure expanded effective TPB between the anode and electrolyte by using a commercial ink-jet 3D printer. Anode and electrolyte inks suitable for material jetting were prepared by changing the viscosity and the particle size. The anode ink formed a porous structure by adding acrylic particles, which ensured the path of the fuel gas. The electrolyte ink formed a dense structure to avoid cross leakage. The microstructure was formed by laminating linear structures in which the porous anode and the dense electrolyte lines were alternately arranged in parallel and orthogonally stacked. The microstructure was inserted between the anode and electrolyte as the anode functional layer of which width and thickness of linear structure were approximately 100 and 1 microns, respectively. The single cell with the microstructure was tested at 600 °C being fueled by dry methane and showed a high performance.
Fuel cells are one of the electrochemical energy conversion devices which can directly convert the chemical energy into electric energy with a high efficiency. Conventional solid oxide fuel cells (SOFC) using oxide ion conducting electrolyte have various difficulties arising from high operating temperatures (700–1000 oC) such as degradation of materials and slow start up and cooldown. Development of electrolytes which can operate at intermediate temperature (500–700 oC) has been desired to overcome these problems.
In this situation, researchers have investigated proton conducting solid oxide fuel cells (PCFC) with thin-film proton-conducting electrolyte to realize intermediate temperature operation. Rare earth-doped BaZrO3 and BaCeO3 have been intensively studied as thin-film electrolytes for PCFC because of their high proton conductivity at intermediate temperatures. However, current leakage through the electrolyte layer occurs as the electrolyte thickness becomes thinner owning to a high hole conductivity in oxidative atmosphere at air side of the cell. Therefore, development of an approach to reduce the current leakage of proton-conducting electrolytes at oxidative atmosphere is required to realize intermediate temperature operation with a high power density.
In the SOFC with ceria-based oxide-ion-conducting electrolyte, formation of bilayer electrolyte consisting of a rare earth-doped ceria layer and a thin Y-doped zirconate layer at fuel side was reported to be effective in suppression of current leakage arising from reduction of ceria. In this research, we extended the concept of the bilayer electrolyte to PCFC in which oxidation of electrolytes at air side is an origin of the current leakage. Anode-supported PCFCs with bilayer electrolyte consisting of Y-doped SrZrO3 layer and Y-doped BaZrO3 layer were prepared by a pulsed-laser deposition (PLD) method and current-voltage measurements and electrochemical impedance spectroscopy measurements were carried out. We will discuss the effect of the bilayer electrolyte on the cell performance of anode-supported PCFC.
To introduce renewable energy or electric vehicles, hydrogen power to gas to power (PtoGtoP) or lithium air battery have been developed for increasing energy density of batteries. However, there remains issues that hydrogen needs to be compressed to several tens of MPa or to be below -250 °C for increasing the energy density, and reduction of Li2O2 needs large overvoltage. Therefore, we made an idea to apply the redox reaction of CO2 and carbon to secondary battery because of easy storage of CO2 and high energy density of carbon. Here we proposed carbon-air secondary battery (CASB) system.
The CASB system can be composed of solid oxide fuel cell (SOFC), stored liquid CO2 and solid carbon. The CASB system works as secondary battery by electrolysis of CO2 and power generation using carbon directly. Storage of CO2 can be easier and safer than storage of hydrogen because CO2 liquefies under 6 MPa. In addition, the theoretical conversion efficiency of the redox reaction C+O2⇄CO2 equals to 1, so that the CASB system is expected to work efficiently.
In this research, we demonstrated the redox reaction and evaluated potential of the CASB system as large-capacity energy storage by comparing the theoretical volumetric power density and energy density with existing and developing secondary batteries and hydrogen PtoGtoP.
We prepared a coin type SOFC supported by the electrolyte, and repeated electrolysis of CO2 and power generation using generated fuel at 800 °C, 100 mA/cm2. During charge operation, analysis of Nernst potential revealed that carbon deposited by Boudouard reaction 2CO→C+CO2 with increasing partial pressure of CO due to electrochemical reaction CO2+2e-→CO+O2-. During discharge operation, carbon or CO was used for power generation. A discussion about potential of the CASB system showed that it can have larger gravimetric and volumetric energy density than secondary batteries and hydrogen PtoGtoP.
Sustainability has emerged as a keyword in all aspects of life whether it is resources or technologies and products or processes. Besides, nearly a billion new consumers join the society in 13-15 years; and the growing demand for higher standards of living make the worldwide materials consumption continuously growing. Strategic solutions are therefore required not only for addressing the gaps but also for eliminating the undesirable environmental effects of supply chain to ensure quality and sustainable living. Energy storage is currently a multibillion dollar industry and is expected to be continuously growing because (i) electrification of products and services has been emerged as an efficient strategy to mitigate carbon footprints from major emitting sectors such as automobiles and (ii) internet of things, advanced communication devices and other modern electrical appliances such as drones and robots demand efficient electrical energy storage devices. Source of primary materials supply for this large industrial sector is therefore crucial; extensive use of earthborn materials as energy storage medium would not only lead to disasters but also would result in expensive devices. Functionalization of renewable materials such as biomass carbon, cellulose, oils etc. as components of energy storage devices would ensure a sustainable living. This lecture will focus on the current state of renewable materials as an energy storage medium, both in the lecturer's laboratory and elsewhere, and foreseeable initiatives required to build efficient energy storage devices using renewable materials.
Due to population and economic growth, further increases in energy demand are predicted globally. Therefore, the construction of a sustainable carbon cycle system is essential to address fossil fuel depletion. The conversion of carbon dioxide (CO2) to hydrocarbons is currently a major challenge.
Among available technologies, the electrochemical conversion of CO2 on copper (Cu) electrodes has gained popularity as a novel technique to realize a sustainable carbon cycle because of improvements in power generation using renewable energy sources, such as solar and wind energy. However, the electrochemical conversion of CO2 on Cu electrodes not only produces valuable hydrocarbon products but also emits byproducts such as carbon monoxide (CO) and hydrogen (H2) because the product selectivity of this conversion is sensitive to the Cu electrode's surface properties, such as atomic arrangement and morphology. Therefore, electrodes for a more selective electrochemical conversion of CO2, which promote hydrocarbon formation and suppress byproduct formation, are required for practical technology.
In this work, we report the selective electrochemical conversion of CO2 on nickel (Ni) / Cu binary electrodes. The formation of hydrocarbons on these electrodes was strongly dependent on both the crystal structure of the supporting Cu electrode and the amount of Ni deposition. This suggested that the adsorption of a carbonate intermediate on the surface of Ni/Cu binary electrodes, formed by the electrochemical conversion of CO2, improves to the selective electrochemical CO2 conversion for hydrocarbon formation compared with pristine Cu electrodes. Based on these results, we discuss the mechanism of the selective electrochemical CO2 conversion and the inhibition of H2 byproduct formation on Ni/Cu binary electrodes.
Formic acid or formate is considered as a perfect fuel for clean energy generation through fuel cells. Formate can be electrochemically generated from CO2/HCO3–. According to techno economic analysis, formic acid/formte is one of the most profitable products of electrochemical CO2 reduction reaction. Sn based electrocatalysts are known for the selective production of formate from CO2 with a faradaic efficiency >80%. But main drawback of these catalysts is high overpotential for the reaction. Various methods have been tried to reduce the overpotential and to improve the catalytic activity of Sn. In this work, we investigated the effect of Pd doping on the surface of Sn for the electrochemical generation formate from CO2 and HCO3–.
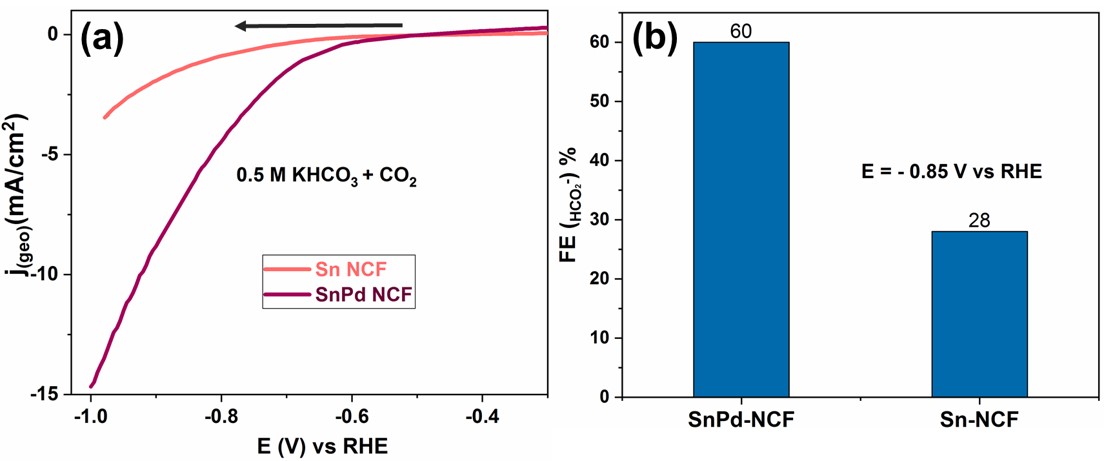
We prepared Sn and Pd nanoparticles decorated N doped carbon fibers (SnPd-NCF) from SnCl2, PdCl2 and polyacrylonitrile using electrospinning method. SEM and TEM analysis confirm the uniform distribution of Sn and Pd nanoparticles over N doped carbon fibers. XPS spectra confirmed nitrogen-doping on carbon fibers. We found that an addition nearly 1.5 wt % of Pd on Sn surface greatly enhance the faradaic efficiency of formate formation and reduce the overpotential nearly by 0.3 V compared to that of Sn nanoparticles decorated N doped carbon fibers (Sn-NCF).
Figure. (a) Voltammetric response of CO2 reduction on Sn-NCF and SnPd-NCF in 0.5 M KHCO3 solution saturated with CO2. (b) Faradic efficiency of formate produced on Sn-NCF and SnPd-NCF during CO2 reduction reaction at -0.85 V vs RHE.
Non-Faradaic electrochemical modification of catalytic activity (NEMCA) by impression of electric field (EF) is one of the methods to improve catalyst performance. It has been discussed that oxygen anions forced electrochemically to adsorb on catalyst surface alter the catalyst electric property. However, given EF also changes the catalyst electric property directly. Because it is difficult to divide down these effects with an experimental approach, we have tried to theoretically investigate the mechanism of NEMCA in CO2 methanation (CO2 + 4H2 → CH4 + 2H2O (1)) in solid oxide electrolysis cell (SOEC) using the density functional theory (DFT). In concreate, we have focused on the rate-determining steps (RDSs) of CO2 methanation proposed by our detailed reaction mechanism analysis. We have calculated the adsorption energies of hydrocarbon species related to RDSs on Ni(111) (CO2 → CO + O (2), CHO → CH + O (3), CH4 → CH3 + H (4)) with EF or co-adsorbed oxygen atoms. In our calculative setup, used is the model sandwiched between thin film condenser boards connected to the outlet electrode for direct EF impression calculations, While used is the Ni(111) surface with different number of oxygen atoms for co-adsorption calculations. Due to space limitation, just an example of our calculations is shown in this abstract. With external EF, the equilibriums in reactions (2), (3) and (4) are all leaned to products side in the EF from the gas phase to the Ni surface (negative values in Table 1), which means CO2 methanation is promoted in this EF direction. This tendency is caused by the destabilization of reactants (CH3 in reaction (3)) or the stabilization of products (O, CO in reactions (1), (2)) with an EF as shown in Table 1 (right side). Other results and detailed discussion will be reported at our presentation in this conference.
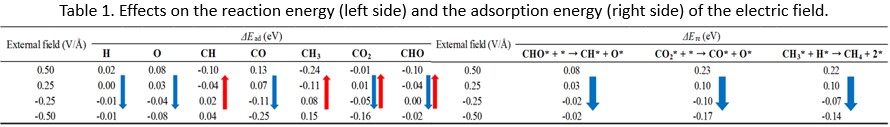
Electric double layer capacitor (EDLC) can store energy by an electric double layer at the interface between the electrode and the electrolyte, which is one of the emerging energy storing devices. High capacity, high durability and rapid charge-discharge capability are attractive, however, low energy density poses drawback to practical use. Specific surface area, surface functional group, and pore size distribution are important controlling factors to improve energy density. In this study, the relationship between the surface functional group and the pore size distribution, and the EDLC capacity was investigated while the specific surface area was nearly equal value as 1200±100 m2/g. In addition, the effect of particle was investigated.
We prepared activated carburized furfural resin particles (1 μm in diameter) with KOH activation for 0 to 0.5 h at 700 to 800 °C in N2 flow, then investigated the electrostatic characteristics of the capacitors in 6M KOH by using a two coin-shaped electrodes. Activated carbon (1 μm in diameter) treated by 750 °C-0 h or 800 °C-0 h had 2.5 times larger mesopore volume and 1.9 times larger mesopore ratio than the one by 700 °C-0.5 h. The fractional order of lactone increased from 700 °C-0.5 h (9.7%), 800 °C-0 h (10.6%) to 750 °C-0 h (24.5%). The fractional order of carboxyl groups increased from 750 °C-0 h (18.1%), 800 °C-0 h (23.0%) to 700 °C-0.5 h (31.4%). Activated carbon treated by 750 °C-0 h (227 F/g) and 800 °C-0 h (225 F/g) were higher than 700 °C-0.5 h (198 F/g). From these relationship, there would be no clear trend for specific capacity per weight under the situation having the nearly equal SBET. We assumed that mesopore ratio should be a main factor to obtain high specific capacity per weight at this moment.
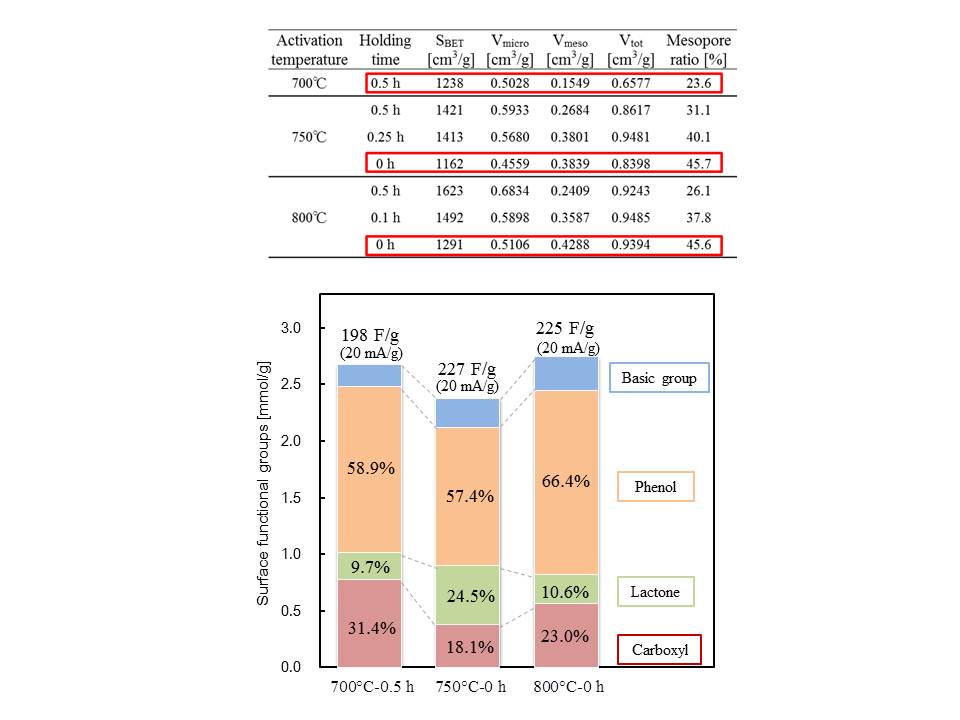
TiO2 is a promising material for the development of lithium-ion capacitors, because side reactions hardly occur at the charge/discharge potential of TiO2, compared to graphite. When using TiO2 as an electrode material, it is necessary to combine it with carbon at the nanometer level to improve its low electrical conductivity and low rate of reaction with Li+. However, preparation methods of reported TiO2/carbon nanocomposites are generally not cost effective, and their productivities are low. In this study, the vacuum liquid pulse chemical vapor deposition (VLP-CVD) technique was developed to easily prepare nanocomposites of TiO2 nanoparticles and commercially-available porous carbons. Using this technique, TiO2 nanoparticles with a diameter of ~4 nm could be homogeneously deposited inside pores of meso- or macroporous carbon. Because the deposited TiO2 nanoparticles connect to electrical conductive paths of the porous-carbon substrates, they showed a high discharge capacity of ~200 mAh/g-TiO2 (based on the TiO2 weight). In particular, the composite prepared from macroporous carbon showed extremely high rate performance, where 50% of the discharge capacity was retained at a current density of 15000 mA/g when compared to that measured at 50 mA/g. In addition, the composite also showed very high cyclability, where 80% of the discharge capacity was retained at the 10000th cycle. Because the VLP-CVD technique can be performed using simple apparatus and commercially available starting materials, it can be expected to apply this technique to industrial production of TiO2/porous-carbon nanocomposites for lithium-ion capacitors.
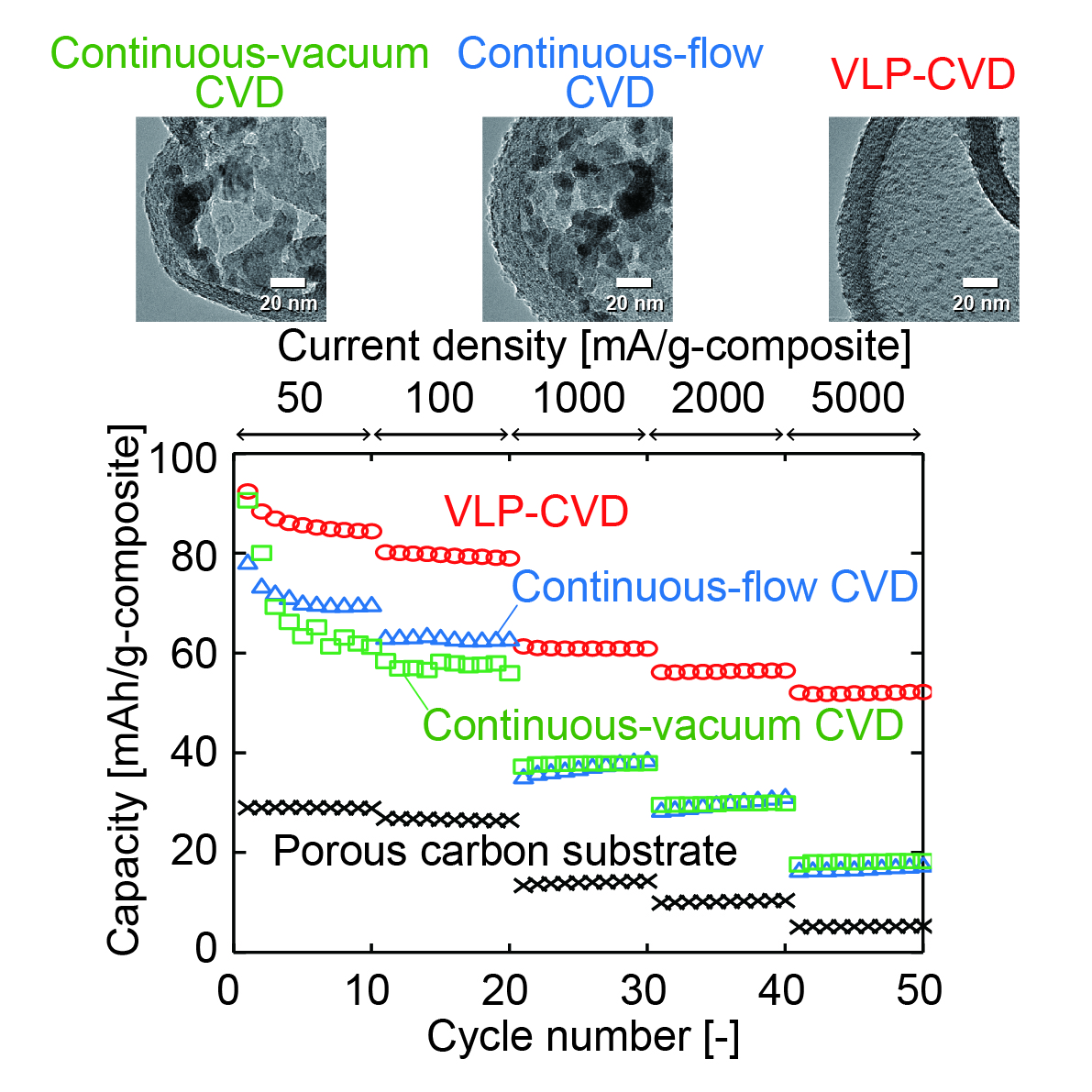
The utilization of crystalline silicon solar cell and organometal halide perovskite solar cell in tandem application has attracted a lot of attention for its high theoretical energy conversion efficiency (>35%). However, the approach of superposing these two solar cells raised issues that have to be clarified and rectified in order for this technology to reach its potential.
In a 2-terminal perovskite/Si solar cell configuration, the bottom silicon solar cell is superposed by perovskite based top cell using highly doped silicon layers as a tunnel junction. This approach comes with three main issues: 1. interface between silicon and TiO2, 2. quality of the perovskite thin layer, 3. transparency and conductivity of front electrode.
The main challenge in the Si/TiO2 interface is to reduce carrier recombination. In addition, constraints on fabrication techniques such as high temperature treatment in TiO2 deposition requires careful consideration to make sure a good surface passivating layer is fabricated properly. Secondly, as topological structure of substrate affects the structure of the thin film, the quality of perovskite layer when fabricated on silicon substrate and ITO covered glass has to be compared and analyzed. Thirdly, as light has to pass through the front electrode in order to reach perovskite absorbing layer, the transparency and conductivity of front electrode has to be optimized in order to achieve the maximum performance of the tandem solar cell.
In this study, we designed an experimental approach to understand these challenges by fabricating perovskite solar cell using highly doped n-type silicon wafer as substrate (Figure). We found that when typical TiO2 is used to passivate silicon wafer, the carrier lifetime decreased dramatically. To mitigate this phenomenon, we are currently modifying the structure of TiO2 with various annealing methods and analyzing the relationship between TiO2 structure and its properties as passivating layer.
Exploring earth-abundant, active and stable electrocatalysts to replace noble metal materials for hydrogen evolution reaction through alkaline water electrolysis system is key to the development of sustainable energy conversion technologies. Here, we report a novel hybrid electrocatalyst comprised of atomically dispersed Ni-Nx species anchored porous carbon matrix with embedded Ni nanoparticles. Benefiting from the high surface area and strong coupling interaction of the Ni NP and Ni-N-C, the achieved Ni NP Ni-N-C EG hybrid electrode displays excellent electrocatalytic activity for HER in basic condition with a low overpotential of 147 mV to reach current density of 10 mA cm-2. The overpotential for the Ni NP Ni-N-C EG is well comparable to the best reported value in literature for all existing heteroatom doped nanocarbon catalysts and even lower than those reported for other transition metal based compounds in basic media. Furthermore, the Ni NP Ni-N-C EG hybrid electrode exhibits outstanding catalytic activity for overall water splitting under alkaline condition, as reflected by delivering a current density of 10 mA cm 2 at 1.58 V, which surpasses that of the benchmark combination catalyst for sufficiently high overpotentials. Electrochemical results, coupled with the thiocyanate poisoning experiments, HAADF-STEM analyses, XPS analysis, as well as the EXAFS and XANES results, reveal that the hybridization of atomically dispersed Ni-Nx active centers with that of embedding Ni NP modulates the electronic structure and facilitates electron transfer at the constructed interface, which synergistically boost the HER performance of Ni NP Ni-N-C. Theoretical calculations manifest that the incorporation of Ni NP into atomically dispersed Ni-N-C frameworks can effectively promote initial water dissociation process and simultaneously optimize the OH adsorption free energy on the Ni NP Ni-N-C, resulting in the improved kinetics of the HER in alkaline solutions.
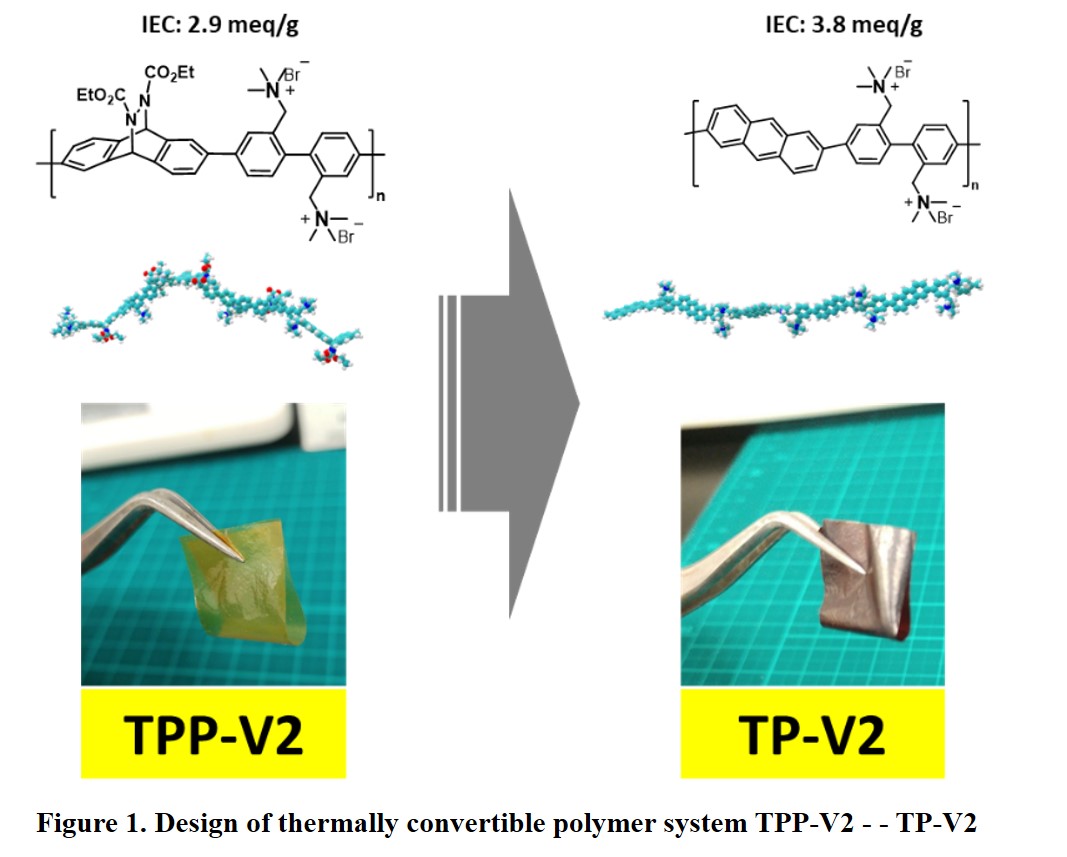
Hydrogen is a promising energy carrier to utilize renewable energy sources. It can be generated by using water electrolysis technology. Alkaline water electrolysis using anion exchange membranes (AEMs) draws a lot of attention. Besides being able to use non-noble metal catalysts, its performance is better than conventional alkaline water electrolysis thanks to lower internal cell resistance. However, the development of this technology is hindered by the lack of high performance and durable AEMs. Ether-free aromatic AEMs can be promising candidates to overcome the performance and durability issues. In this research, we proposed a unique AEMs molecular design using thermally convertible polymer system (Fig.1). Highly soluble precursor polymer is used to prepare a thin membrane. This precursor membrane then heated to obtain ether free backbone membrane.
We prepared the precursor polymer by Suzuki-Miyaura coupling of the monomers followed by bromination then quaternized to afford TPP-V2. Number average molecular weight (Mn), polydispersity (PDI) and ion exchange capacity (IEC) of TPP-V2 are Mn= 24,600 g/mol,, PDI=2.9 and IEC= 2.90 meq/g, respectively. Ultra-thin flexible membrane as thin as 8 μm can be cast from TPP-V2 solution in dimethylsulfoxide. TP-V2 membrane was obtained by heating TPP-V2 membrane at 180 oC for 1 h under vacuum. After conversion to TP-V2, IEC value increases to 3.78 meq/g. Despite higher IEC, water uptake of the TP-V2 is lower than it's precursor as π-π stacking appears. TP-V2 shows high alkaline and oxidative durability after exposed to 8M NaOH for 120 h at 80 oC (alkaline stability test) and 3% H2O2 & 3 ppm FeSO4 60 oC for 8 h (oxidative stability test). The ionic conductivity was almost the same even after exposure to such very harsh conditions. These results suggest our approach is promising for making high-performance AEM for alkaline water electrolysis application.
Acknowledgement: Part of this paper is based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO).
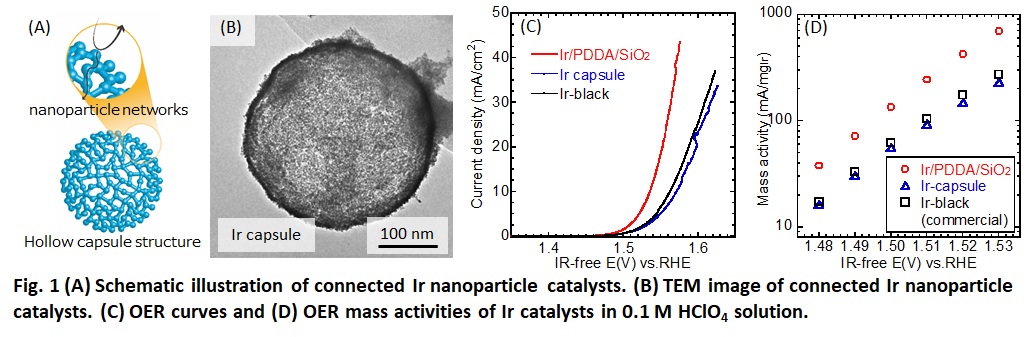
Oxygen evolution reaction (OER) catalysts for polymer electrolyte water electrolysis (PEWE) suffered from their low surface area because carbon support cannot be used for OER operating at high potential. Connecting metal nanoparticles enable catalysts to conduct electrons through nanoparticle networks without any support materials while keeping their high surface area. Thus, connected nanoparticle catalysts (Fig. 1 (A)) are promising for OER. Previously, we have developed connected Pt-Fe nanoparticle catalysts with hollow capsule structure for oxygen reduction reaction (ORR) in polymer electrolyte fuel cells (PEFCs).[1]
In this study, we proposed Ir nanoparticle catalysts for OER in water electrolysis because Ir has high OER activity. Ir nanoparticle catalysts were synthesized as follows. First, Ir nanoparticles were synthesized on silica template via polyol method using tetraethylene glycol as reducing agent and Ir(III) acetylacetonate as the metallic precursors (Ir/PDDA/SiO2). Then, these nanoparticles were coated with SiO2 using tetraethoxysilane in a mixed solvent of ethanol and NH3 solution. After coating, they treated in supercritical ethanol at 330 °C for 90 min. Dissolution of SiO2 in 3 M NaOH solution at 85 °C for 3 h made porous hollow structure (Ir capsule). OER performance were evaluated by cyclic voltammetry in 0.1 M HClO4 solution. A membrane electrode assembly (MEA) was fabricated by Ir/PDDA/SiO2 as an anode catalyst, and its water electrolysis performance was measured.
Fig. 1 (B) shows TEM image of Ir capsule. Capsule and networks structure of the catalyst were observed by TEM images. Fig. 1 (C) and (D) show OER curves and mass activities of Ir catalysts. Mass activity of Ir/PDDA/SiO2 was 2–3 times higher than Ir capsule and commercial Ir black. MEA using Ir/PDDA/SiO2 showed good performance.
[1] T. Tamaki, H. Kuroki, T. Yamaguchi et al., Energy Environ. Sci., 8, 3545-3549 (2015).
Acknowledgement: This paper is based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO).
Water electrolysis by using renewable energy such as wind and solar energy for hydrogen production is considered a promising way to storage the unstable electricity and realize low-carbon society. However, for large-scale application of water electrolysis, the key is to design low-cost electrocatalysts with high performance for the replacing of the noble metal based catalysts. Meanwhile, in the water electrolysis, the electrolyte includes acidic, alkaline, industrial waste water, and seawater with different pH values. It is expected to have the electrocatalysts which can work in a wide pH range, especially in the neutral solution so that it can be applied anywhere. Moreover, the solutions with the neutral pH value is harmless and environmentally friendly. In this study, nanostructured manganese-nickel mixed oxide with nanosheet array was fabricated on the Cu nanowires growning on the copper form <CF> by electrodeposition method, and then further phosphorized to MnNiP. The obtained Cu nanowire @ MnNiP/CF electrode was used for the hydrogen evolution reaction in water electrolysis. As a result, a low overpotential of 104 mV@10mAcm-2 with a small Tafel slope of 55.1 mV dec-1 in the neutral solution was achieved due to the enhanced charge transfer ability, improved surface active area and fast reaction kinetics.
Recent studies on polymer electrolyte fuel cell (PEFC) based energy generation have focused more on elevated temperature (above 100 °C), since high temperature PEFC (HT-PEFC) enhances tolerance to CO and prevents flooding as well as makes waste heat emission and its utilization easy. However, relative humidity (RH) management appears more difficult at elevated temperature. RH profile inside the cell should be well analyzed to ensure proton exchange membrane (PEM) is sufficiently hydrated to keep moisture content. In the current study, 1D model in the direction along the gas channel has been built for the straight co-current flow channels for analyzing water behavior at varied cell temperature and total pressure. Water permeation flux, moisture content, and proton conductivity of PEM were calculated. Employing straight channels, RH profile in the cell can be measured as change in RH at the cell outlet with varying the gas flow rate in experiments. Measured and calculated RH profiles through the gas channel were investigated at varied cell temperature. Total water flow rate at the outlet increases as the current increases. Partition of generated water to two sides is determined by the inlet RH and flow rate conditions. As the cell temperature is higher, the saturated vapor pressure is the higher. The more water generation is required to attain a certain RH through the cell at the higher cell temperature. This is the most important reason that water management is more difficult at elevated temperature.
The power grid supplies electric power while maintaining 1. voltage, 2. frequency, and 3. transient stability, three conditions for stable power supply from the electric grid. However, recent large-scale implementation of solar cells has caused difficulties in meeting these conditions because of the power supply-demand gap. To solve this problem “leveling power”, the ability to keep the balance of supply and demand of power, is required. Although power has been leveled by power grid, the leveling by distributed energy system is also required. Energy storage technology is one of the methods to introduce power leveling in the distributed energy system. Combination of energy storage technologies, such as battery or Power to Gas (P2G), which converts power to hydrogen, is expected to contribute to the stability of power supply. Actual data with the range of minute to second is required to investigate the feasibility of each technology as power leveling.
In this research, firstly we analyzed the current status of power leveling required for power-grid by using actual generation and power demand data published by power companies across Japan and the actual data at Ookayama campus in Tokyo Institute of Technology. Smart energy system “Ene-swallow” installed at Ookayama campus accumulates power demand data in a second and solar generation data in a minute. Secondly, we calculated total supply-demand gap in the assumption of large-scale implementation of solar cells by using actual annual data in a minute from “Ene-swallow” and then we designed required amount of P2G system and battery for the leveling. The analysis showed that the total system cost could be reduced by using the combined system of P2G and battery.
[Acknowledgements] A part of this study is supported by the New Energy and Industrial Technology Development Organization (NEDO) and MIRAI program of Japan Science and Technology Agency (MIRAI JST).
Recently, the implementation of renewable energy, especially photovoltaics (PVs), has been rapidly expanded due to its significant cost decline with technology and market development. However, the supply fluctuation of renewable power sources makes keeping the balance of electricity demand and supply difficult. In order to make the best use of renewable power sources, the electricity demand-supply gap has to be balanced using energy storage technologies. Power-to-Gas (P2G), which converts electricity to hydrogen, is a promising candidate for a large-scale, long-term energy storage technology.
In this research, we analyzed the economic efficiency of distributed P2G system considering not only horizontal but also vertical installation of PVs. This analysis was carried out based on actual data accumulated in smart energy system “Ene-Swallow”. We used electricity demand data from the Ookayama campus in Tokyo Institute of Technology, and power generation data of PVs installed at the Environmental Energy Innovation (EEI) building, where PVs are installed on the rooftop, the south and west walls as shown in figure 1. We modeled the P2G system to simulate power generation and consumption at every hour of a single year, and to evaluate the total cost including the fixed and variable cost.
This analysis shows that if the price of each device decreases due to technology and/or market development in the future, appropriate implementation of a distributed P2G system will lower the total cost compared to the case where all of the required power was purchased from the power grid. This study also shows that in Tokyo area, appropriate combination of horizontal and vertical PV installation can lower the total cost compared to the case where only horizontal PV is installed. In addition, to discuss the feasibility of this analysis results, we investigated the possible installation amount of PVs at the Ookayama campus.

Photovoltaic (PV) cells powered water electrolysis cells (WECs) are capable of converting and storing the solar energy in the form of hydrogen (H2), thereby facilitating the continuous usage as well as distributing of this intermittent and diffusive energy resource (1). However, precious metal based catalysts are required to catalyse the two half reactions in WEC, e.g. Pt and its alloys for hydrogen evolution reaction (HER), and oxides of Ru and Ir for oxygen evolution reaction (OER), which has constrained the widespread applications of PV-WEC systems. Thus, advanced electrode materials for WECs that are active and cost-effective are highly sought after.
Herein, I will present some of our recent research progress regarding the development of advanced electrodes for WECs. These electrodes are mainly comprised of earth abundant elements, such as first row transition metals (Ni, Co, Mn, Fe) and/or carbon. For instance, by depositing mesoporous NiFe nanosheets onto the skeleton of macroporous nickel foam (NiF), a highly efficient, freestanding oxygen evolution electrode is prepared, showing hierarchical micro- to nanoscale porosities, and is among the most active in bases (2). Besides that, a Mn doped NiO/Ni heterostructured electrode (Mn-NiO-Ni/NiF) is also successfully synthesized. The prepared material is highly active for HER in both neutral electrolytes and natural seawater, exhibiting a Pt-like catalytic activity (3). A customised WEC was assembled based on the NiFe/Ni-F anode and the Mn–NiO–Ni/Ni-F cathode, which is highly active in splitting water, reaching a high current of 0.2 A at 1.8 V. Moreover, the WEC can be directly powered by PV cells tol enable the production of renewable hydrogen (Figure 1).
Reference
(1) X. Lu, et. al, J. Am. Chem. Soc. 2015, 137, 2901-2907
(2) X. Lu, et. al, Nat. Commun. 2015, 6, 6616
(3) X. Lu, et. al, Energy Environ. Sci. 2018, 11, 1898-1910

Automobile sales in China decreased by 2.76% to 28 million vehicles in 2018, that is the first decrease in the past 28 years. Market developments in New Energy Vehicle (NEV) including hybrid and pure electrical vehicles (BEV), as well as fuel cell vehicles (FCV) are inevitable and the number of NEV will break through up to 1.5 million vehicles in 2019. However, LIB with high energy density used in BEV is facing many safety risks with various faults and life issues due to less development accumulation in material process and manufacture. Meanwhile FCV is difficult to form into a mature commercial market, where many relative core materials and components are lower in quality and less reliable as compared with overseas products and most of them are depended on imports.
Shanghai AI NEV Innovative Platform upholds a critical function position for supporting industrial chain innovation, supporting R&D transformation to key products and servicing entrepreneurship in automotive field, by focusing on promoting the opening and coordination of innovative resources to reduce the cost of starting a new business. One of the major functions is battery material analytical service to give key solutions to safety, energy density and life of on-board LIB and to support developing high-performance FC stacks with low lost materials.
In this presentation, China's NEV policy, regulation, EV evaluation, material analysis situation will be explained and the function position of Shanghai AI NEV Innovative Platform will be introduced. Some LIB estimation methods and corresponding material analytical results relative to battery capacity (energy density), degradation (life), failure (warning) and exothermic (safety) items are given.
Lithium ion batteries (LiBs) are used in electric vehicles (EVs), hybrid electric vehicles (HEVs), compact devices and so on. However, both more power density and energy density is required, so active materials, binders, electrolytes, and separators are enthusiastically developed. Moreover, the ability of LiB determines not only materials but also the electrode structure. In the case of high output density cell, in other words, in the case of rapid charge or discharge condition, ionic conduction in porous electrode layer is dominant resistance, and it strongly depends on the heterogeneous porous structure. Recently, some researchers have been trying to understand the relationship between this porous structure and the effective ionic conductivity. In our previous research, the relative ionic conductivity was simulated in different porous electrode structure which consists of active material and binder, and the effect of morphology was discussed with electrochemical experiment, direct observation and numerical simulation. However, most of these research focused on only single component which is anode, cathode and separator. In order to increase the cell performance that is capacity, power density and durability, the effect of combination of these porous component have to be considered. In this study, various porous structure is modeled with direct observation results obtained by FIB-SEM and X-ray CT, and the charge and discharge simulation is carried out with some combination of these porous component. From this result, the morphology and the anisotropy of porous structure are evaluated to check the not only the internal resistance but also the ion flux distribution which cause to generate the Li dendrite. In addition, the effect of micro protective layer on ion flux distribution and conductive resistance is considered.
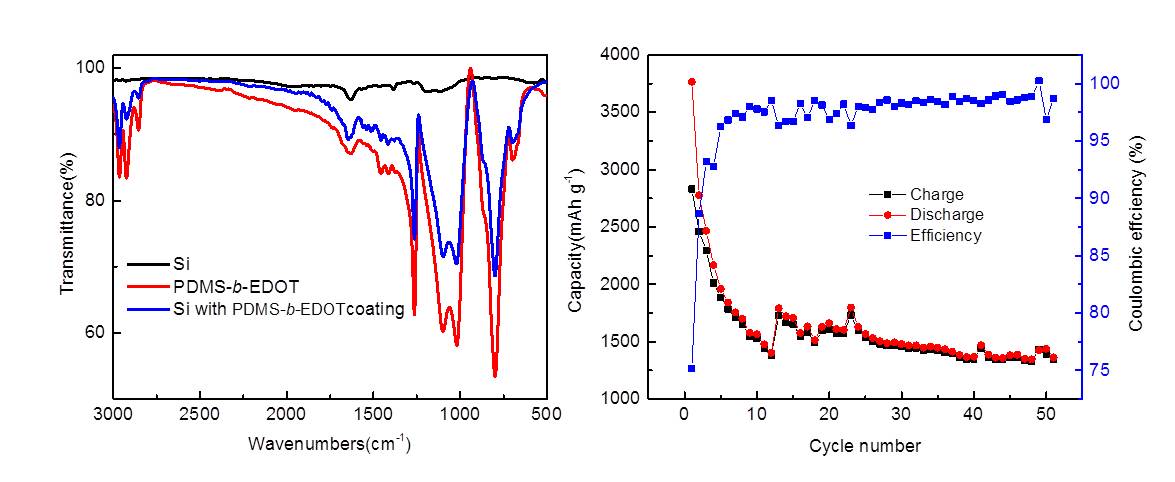
Silicon anode material has been considered as one of the most promising candidates for next-generation Li-ion batteries due to its high specific capacity. Its low conductivity and large volume change during charge-discharge process still hinder its practical applications. In this work, a conducting and flexible polymer is employed as a protective coating layer on the surface of commercially available silicon nanoparticles. The polymer can be prepared through hydrosilylation between hydride terminated poly(dimethylsiloxane)s (h2PDMS) and 3,4-Ethylenedioxythiophene (EDOT). The PDMS-b-EDOT was found successfully coated on the Si nanoparticles. The coated Si was found to show a much improved performance when it is used in making the anode for lithium ion battery. The cell assembled with a conventional PVDF binder shows reversible capacity of 1400 mAh g-1 after 50 cycles at the rate of 0.1C.
Acknowledgments: This work was supported by the Area of Excellence Grant from HKPolyU (1-ZE30), and the Science and Technology Foundation of Shenzhen (Grant No. JCYJ20170307150808594).
Lithium metal batteries <LMBs> are considered promising energy storage systems because of their high energy density and low redox potential. However, safety issue associated with the dendrite growth hinders their practical applications. One of the effective ways to suppress the dendrite growth and extend the cycle life is addition of the additive in the electrolyte. In this study, a novel low-cost electrolyte additive named as THP-Li salt was synthesized with a handy method for the first time, and introduced into both the solid-state electrolyte and the liquid electrolyte. For the solid-state electrolyte, the impedance value of solid polymer electrolyte <SPE> membrane was decreased apparently, and meanwhile, the all-solid-state LMBs with it delivered the excellent discharge capacity at high current density with good cycling performance <the capacity retention 78.7 % after 200 cycles> and higher coulombic efficiency. For the liquid ester-based and ether-based electrolytes, the addition of it greatly enhanced the overall cell performance of LMBs and particularly resulted in a higher discharge capacity, and remarkable improvement of the capacity retention <the capacity retention 68.4 % after 1000 cycles>. It is found that this novel additive can participate in the formation of stable solid electrolyte interphase <SEI> layer, preventing the side reaction between the electrolyte and electrode, suppressing the Li dendrites growth, which finally improved the safety and stability of LMBs.
A novel configuration by pressing two electrodes containing electrocatalysts for the oxygen reduction and evolution reactions (ORR and OER) into a bi-functional air electrode is designed for rechargeable Zn-air batteries. MOC/25BC carbon paper (MOC consisting of a-MnO2 and XC-72 carbon black) and Fe0.1Ni0.9CO2O4/Ti mesh on this air electrode mainly serve as the cathode of the ORR and the anode of the OER, respectively. Electrochemical studies include linear sweep voltammetry (LSV), rotating ring-disk electrode (RRDE) voltammetry, and the full-cell charge-discharge-cycling test. The discharge peak power density of a Zn-air battery with this unique air electrode reaches 88.8 mW cm-2 at 133.6 mA cm-2 and 0.66 V in the alkaline electrolyte under the ambient condition. After 100 discharge-charge cycles at 10 mA cm-2, an increase in 0.3 V between charge and discharge cell voltages is found. The long-time discharge-charge-cycling curve (10 h in each step) shows that the cell voltages of discharge (1.3 V) and charge (1.97 V) keep constant during the entire process. The performances of this rechargeable Zn-air battery are superior to most reports in recent literature.
Carbons in various forms are also chosen as substrates for uniform dispersion of a-MnO2 to form air electrode catalysts to evaluate the influences of carbon types on the catalytic activities of the ORR and OER (oxygen evolution reaction). The morphology and physicochemical properties of various a-MnO2/carbon composites are characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray diffraction (XRD). Electrochemical studies include rotating ring-disk electrode (RRDE) voltammetry of catalysts, linear sweep voltammetry (LSV) of air electrodes, and the charge-discharge-cycling test of full cells. The discharge peak power density of Zn-air batteries varies from 66.3 (a-MnO2/carbon nanotubes with diameter ca. 10 nm, denoted as a-MnO2/CNT10) to 40.5 mW cm-2 (a-MnO2/super fine mesophase graphite powder) in 6 M KOH under ambient condition. The rechargeable Zn-air battery with the air electrode containing a-MnO2/CNT10 is stably operated for 100 cycles at 10 mA cm-2, which shows that an increase in 0.09 V between charge (decayed ca. 0.05 V) and discharge (decayed ca. 0.04 V) cell voltages.
For the practical use of Lithium-air batteries (LABs), their cycle performance must be improved. In this system, oxygen supplied from the atmosphere reacts with Li+ and Li2O2 is deposited in the cathode during discharging. To obtain a large capacity and a high rate performance, the cathodes need to have a high surface area and a high electrical conductivity. Therefore, porous carbons are generally used for the cathodes.
To improve cycle performance, side reactions, which involve the decomposition of the electrolyte and result in irreversible capacity, need to be suppressed. It has been reported that oxygen functional groups existing on carbon materials, promote such side reactions. On the other hand, it has been also reported that amorphous Li2O2 tend to be formed on carbon materials having oxygen functional groups more than pristine carbon materials. Such amorphous Li2O2 easily decomposes during subsequent charging, and the charge over potential is relatively low. These facts indicate that there is a suitable amount of oxygen functional groups to increase cycle performance, which has not been clarified.
In this study, the quantitative effect of oxygen functional groups on cycle performance was investigated using carbon gels (CGs) as a model carbon material. CGs have a large mesopore volume which can contribute to the capacity of LABs, and their surface properties can also be tuned. While CGs originally have many oxygen functional groups, they were reduced by heat treatment at various temperatures. Then, the oxygen functional groups were quantitatively evaluated by temperature programed desorption analysis (TPD) performed under vacuum. The results obtained using CGs heated at 2200 °C showed that most of the functional groups were removed at this temperature and the resulting carbon can maintain a discharge capacity of 500 mAh g-1 for 21 cycles, while CGs heated at 1000 °C could maintain this capacity only for 8 cycles.
Lithium-air batteries are expected to become a secondary battery of the next generation owing to their high theoretical energy density. Carbon paper combined with a porous carbon are generally used as the cathode in this system because the surface area of a typical carbon paper is too low to be used independently. In contrast, carbon nanofibers (CNFs) with a fiber diameter of several tens of nanometers have a sufficient surface area for this purpose. In this study, sheet electrodes were developed using CNFs produced through the liquid pulse injection (LPI) technique. The CNFs can be efficiently synthesized and easily casted into a sheet electrode only by vacuum filtration. Cathode performance of the sheet electrodes for lithium-air batteries was evaluated and optimal conditions to obtain higher capacities were explored.
LPI-CNFs with a diameter around 20 nm were dispersed into ethylene glycol. The resulting dispersion was filtered under vacuum to form a sheet. Coin cells of 2032-type were assembled using the sheet and a solution of CF3SO4Li in tetraglyme (1:4, molar ratio) as the cathode and electrolyte, respectively, and the cells were galvanostatically discharged and charged in an oxygen atmosphere.
The LPI-CNF sheets had a mechanical strength similar to that of a commercial carbon paper and much higher surface area than that of them, suggesting that the LPI-CNF sheets can be used independently as cathodes.
From results of discharge/charge measurements, the LPI-CNF sheets showed about a 3 times larger discharge capacity than that of an electrode prepared from a carbon paper and porous carbon.
It has been reported that usable depth of electrodes is limited because of the influence of oxygen diffusion. Thus, the structure of the LPI-CNF cathodes such as porosity and thickness were optimized to increase discharge capacity.
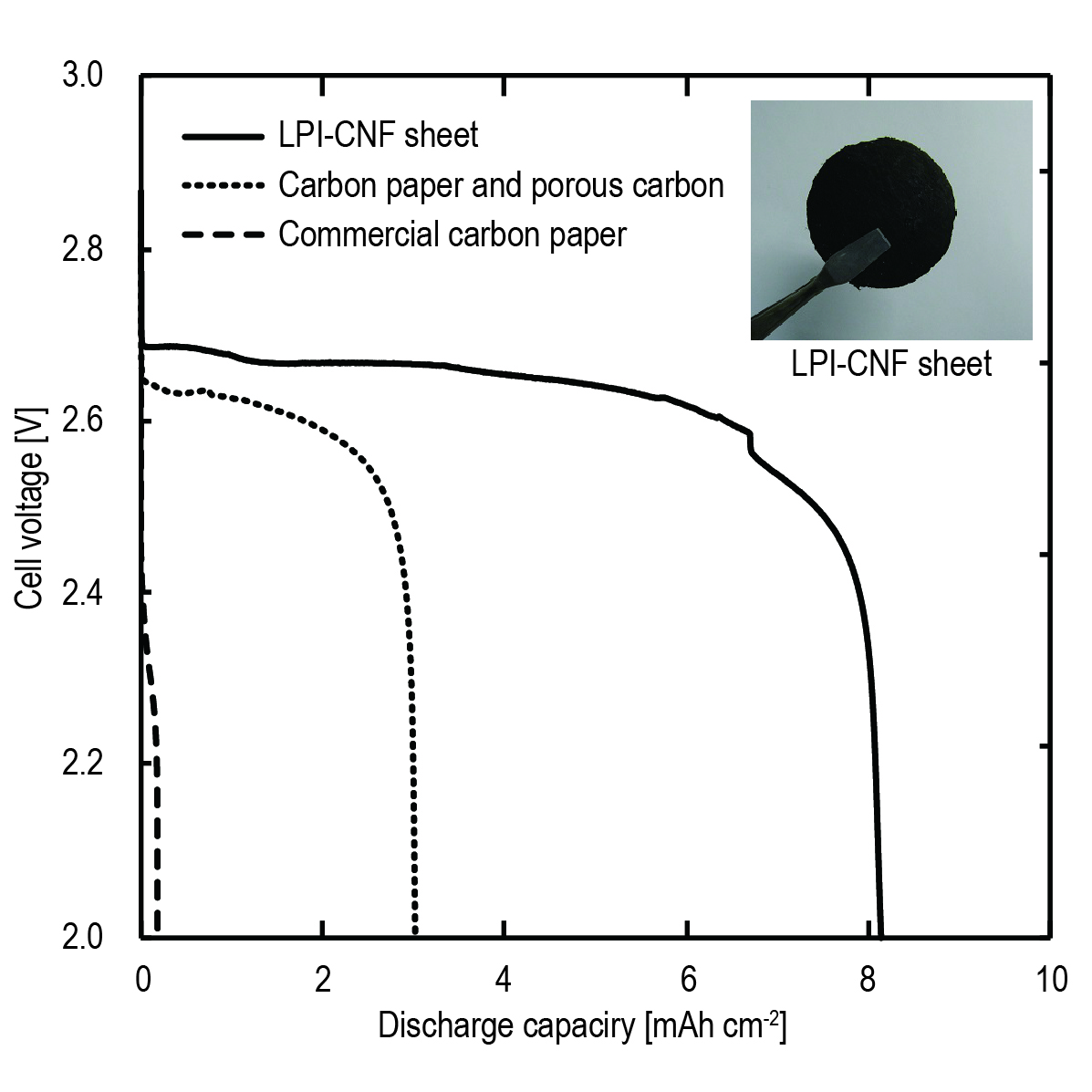
Lithium ion storage devices are indispensable for modern society because of their high energy densities, low self-discharge, no memory effects, and applications in many high-tech industries such as electronic devices, military, and battery powered vehicles. However, most commercial lithium ion storage devices use carbon electrodes, which have low theoretical capacity (≈ 372 mAh g-1) and are not expected to meet the future needs of the energy storage industry. Consequently, silicon, possessing high theoretical capacity (≈ 4200 mAh g-1), being abundant in nature, and exhibiting low discharge potentials, has become a promising anode material. Despite these advantages, the use of Si anode is hindered by its low electrical conductivity and large volume changes during the lithiation/de-lithiation cycles leading to subsequent pulverization. Herein we designed a porous composite nanostructure of silicon with N-doped carbon (NDC) from mesoporous silica sphere and dopamine, which can offer space to accommodate the volume changes and improve the electrical conductivity of the electrode for performance enhancements. Indeed, the as-synthesized Si/NDC composite delivered a high capacity of 2,518 mAh g-1 at a current density of 200 mA g-1 and maintained a high capacity of 980 mAh g-1 at a high current density of 5,000 mA g-1.
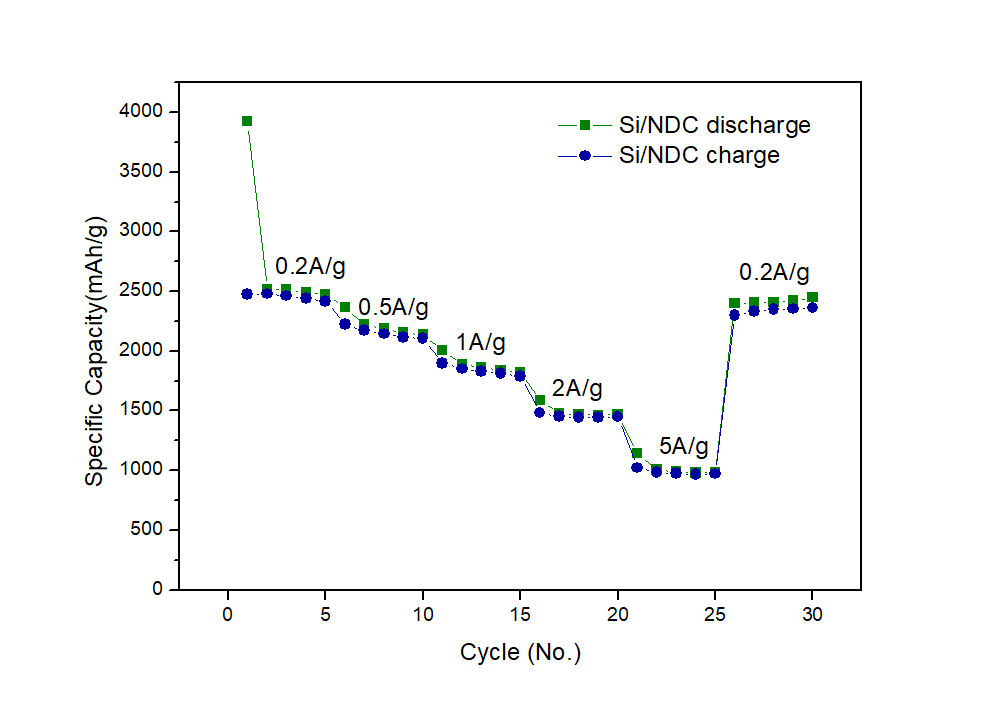
Side reactions on high-voltage cathode for lithium ion batteries would be influenced by physicochemical properties of carbon black (CB) conductor used in it. We have prepared various CB conductors with different surface characteristics and crystallinity, and have applied them to a 5V cathode, LiNi0.5Mn1.5O4 (LNMO) as conductors. By electrochemical evaluation some samples were found to be suitable for the 5V cathode. The reaction mechanism would be reported.
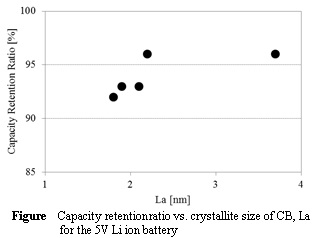
At the present time, ternary cathode materials, NCM, have attracted many researchers to study due to their excellent electrochemical properties, such as high specific capacity and high charge-discharge voltage. By tuning the portions of nickel, cobalt and manganese, NCM can obtain different electrode characteristic. However, their poor stability when worked at high operating voltage have limited their applications in industry. In this study, polyimide (PI) was covered on the surface of NCM523 cathode material by a simple liquid phase modification. At first, 2,2-Bis(3-amino-4- hydroxyphenyl)hexafluoropropane (BisAPAF) and 2,3,3,4-oxydiphthalic anhydride (ODPA) were mixed in the solvent, N-Methyl-2-Pyrrolidone (NMP), at room temperature to synthesize the polyamic acid (PAA). Then, a desired amount of NCM523 powder were added and dispersed in PAA solution by stirring for 4h to obtain NCM523/PAA material. Finally, the NCM523/PAA material was converted into NCM523/PI by multistep heating process. XRD analysis showed that the addition of PI did not affect the original layered structure of NCM523 cathode material. The energy dispersive spectroscopy (EDS) analysis showed that polyimide could be evenly distributed on the surface of NCM523 particles. TGA analysis also confirmed a slight weight percentage of PI was added to the NCM523 material. The effect of PI addition on the electrochemical properties of NCM523 cathode material is still under investigation, and it is hoped that the present polyimide (PI) coating can effectively prevent the undesired interfacial reaction between the electrode material and the liquid electrolyte.
All-solid state batteries (ASSBs) are expected as next-generation battery for electric vehicles (EVs), hybrid electric vehicles (HEVs) because it is safer than usual liquid-type Lithium-ion battery. Recently, the innovative solid electrolyte have been developed, and it is comparable to organic liquid electrolyte in ionic conductivity. However, it is desired to attain more power density and more energy density. In order to improve the performance of ASSBs, it is required to optimize an electrode structure as well as material characteristics such as active materials and electrolytes. Therefore, monitoring the state in electrode layer during charge and discharge using the numerical computation is a critical measure to understand the phenomena in a cell. In the relatively micro-scale system such as the electrode layer, even a slight difference in structure remarkably affects the battery performance. However, usual simulations employ the empirical overall characteristics such as the reactive interface area and the tortuosity factor, so the phenomena resulting from minute structure of electrode layer which notably affect the cell performance might be overlooked. Hence, in this study, the numerical computation technique that can reflect the micro-characteristics of electrode structure directly by building a multi-element network model based on porous structures was developed. And the effect of heterogeneous electrode structure on cell performance was considered.
In this study, 0.5 M nickel hydroxide, cobalt nitrate, and manganese nitrate were used as the metal sources with molar ratio of Ni:Co:Mn=1:1:1 and sodium hydroxide as the precipitant to prepare the NCM(OH)2 precursor by co-precipitation for application in the synthesis of NCM ternary cathode materials. The volume flow ratio of metal nitrate and NaOH was 1:2 and the pH value of the precipitation reaction was controlled at 10-12. After the reactants were mixed, the mixture was stirred at 50 °C for 12 h under nitrogen atmosphere. It was found that the color of the precipitate gradually changed from grey to brown in the experiment. It was speculated that the color change was resulted from the formation of manganese oxide-hydroxide. It was known that the manganese hydroxide could be reacted easily with trace oxygen even in a nitrogen atmosphere. SEM images showed that the precursor powder obtained by the preparation was irregular granular and had the obvious aggregation phenomenon. Besides, the XRD analysis showed that the precursor powder of the ternary positive electrode material was successfully prepared in this study and the precursor was composed of nickel cobalt manganese-hydroxide NCM(OH)2 and nickel cobalt manganese-oxygen-hydroxide NCMO(OH)2.
Vanadium redox flow batteries (VRFB), which use vanadium species as the active materials, have advantages such as the flexible design between power and capacity. Thus, VRFB is expected to store renewable energy. However, the current density of a single cell is small due to the higher internal resistance such as reaction resistances. Therefore, researches have been conducted to reduce the reaction resistance by addition of surface oxygen functional groups by heat treatment in air or liquid phase oxidation to the carbon electrode material. However, the conventional treatment methods have issues such as longer treatment time and necessity of waste liquid treatment. In consequence, in this research, we focused on electron beam irradiation as the effective oxidation method. It is considered that the surface oxygen groups can be added by the reaction between surface carbon and radicals and/or ozone from the water or the oxygen formed by the irradiation. This processing method can be performed in a short time of 60 minutes or less at room temperature.
In this study, Electron beam irradiation was performed on the carbon cloth, then, characterization and electrochemical measurement were performed. Two-sheet of Carbon cloth (CC) was used as the electrode material. The electron beam with acceleration voltage of 2.0 MV was irradiated to the CC under air atmosphere at Takasaki Advanced Radiation Research Institute. To evaluate the cell performance, current-voltage curves were obtained using a single cell with interdigitated flow channel. The current density of the irradiated electrode under air was improved compared with the non-irradiated electrode. X-ray photoelectron spectroscopy (XPS) measurements were performed to analyze surface oxygen groups. As a result, the surface oxygen, i.e., C-O, COO, etc., of electrode material increased by electron beam irradiation within 60 minutes.
Supercapacitors are one of the most promising energy storage devices because of their long cycle life, high power densities, and high discharge efficiencies. The power and energy densities of supercapacitors depend critically on the electrode materials. In recent years, metal-organic frameworks (MOF) have attracted much research attention as electrode materials for supercapacitors, because of their unique properties such as high porosities, large surface areas, and tunable morphologies, which are beneficial to the capacitive performances of supercapacitors. In this work, we synthesized Mn-based and Mn-Fe based MOFs via a simple solvothermal method, using 1,4-benzenedicarboxylate as the organic linker. The incorporation of Fe into the Mn-based MOF significantly enhanced the capacitive performances. The specific capacitances of Mn-based MOF and Mn-Fe based MOF were 21 and 75 F/g, respectively in 2M KOH at a scan rate of 5 mV/s. The enhancement is attributable to the synergistic effect between Mn and Fe.
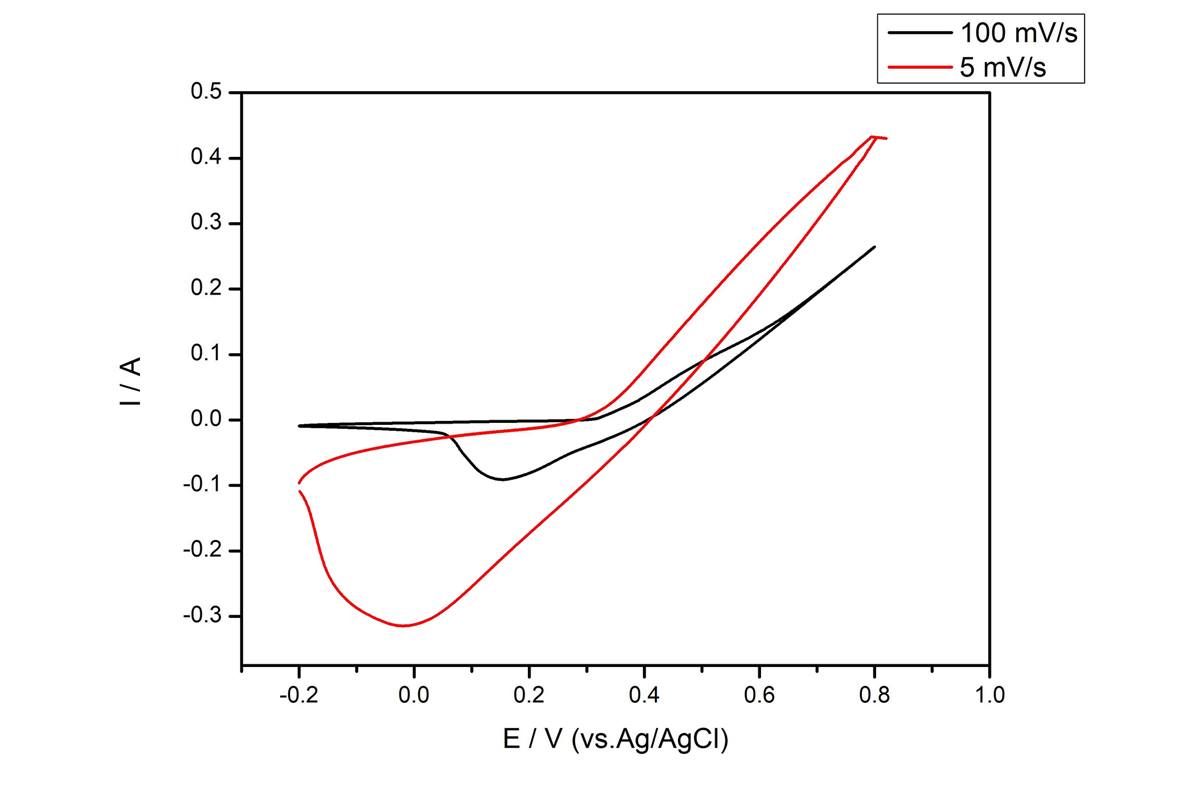
Recently, Microwave is an efficient, green and selective heating method, it has been employed to reduce graphene oxide (GO) in solvent or under dry condition. Firstly, we used modifification of Hummers method to fabricate graphene oxide (GO), then graphene oxide (GO)/Titanium oxide (TiO2) nanocomposites were synthesized with different wt/wt % of GO/TiO2 (1:1; 1:2; 1:3; 1:4 and 1:5), finally the nanocomposites were reduced by microwave method. To assess the properties of materials for use in supercapacitors, cyclic voltammetry and galvanostatic charging–discharging measurements were performed. And the results show that the microwave method synthesis of reduced graphene oxide (GO)/Titanium oxide composites with wt/wt % of GO/TiO2 (1:4) has the highest specific capacitance of 454 F/g at a scan rate of 10 mV/s. What's more, as shown in Figure 1, it has a high energy density of E = 24.2 Wh/kg and high power density of P = 500.1 kW/kg. As a result, graphene-based material obtained by MW-assisted reduction has a porous and loose structure and relatively high special surface area, making it perfectly suitable for supercapacitor devices.
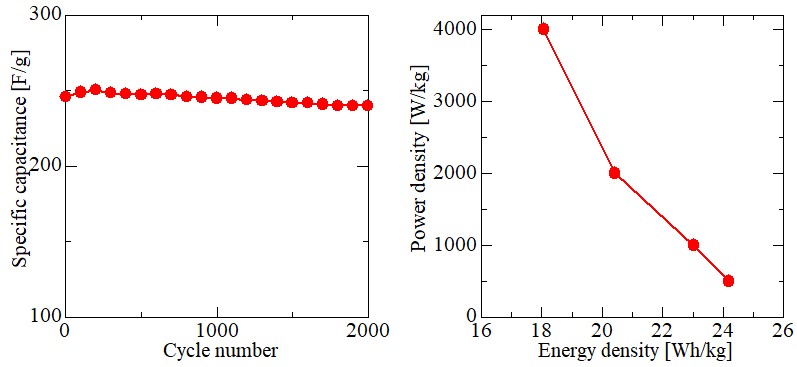
For widespread use of renewable energy, effective utilization of excess energy from the renewable energy is a key issue. Electrolysis of hydrogen carrier, especially ammonia, is a possible solution because ammonia possesses some advantages including stable chemical property, high hydrogen density, easier storage and non-explosion. Unfortunately, during electrochemical reaction of ammonia synthesis with metal catalysts, hydrogen evolution reaction also occurs, and thus the faraday efficiency of ammonia electrosynthesis becomes low. In this study, the effect of addition of tungsten into iron on improving electrochemical reaction of ammonia synthesis and suppressing hydrogen evolution reaction were investigated using 90%N2-10%H2, W-Fe-BaCe0.9Y0.1O3 (BCY)|BCY|Pt, 3%H2O-77%Ar-20%H2. W-Fe-BCY catalyst cathode was fabricated by the incipient wetness co-impregnation method. After addition of W into Fe catalyst, the current density decreased because of larger energy of tungsten hydride formation which suppresses hydrogen evolution reaction. Impedance spectroscopic analysis also suggested the same result of suppression of hydrogen evolution reaction. Furthermore, the ammonia formation rate became higher at lower operating temperatures than those of pure Fe catalyst. This was probably caused by lower coverage of hydrogen adatoms on W-Fe catalyst surface and retaining active sites for N2 dissociation. In addition, with an increase in ammonia formation rate and a decrease in current density, the faraday efficiency also increased by around two times with the addition of W into Fe catalyst.
Recently, Global warming has become a serious problem. Therefore, Japan is aiming at a hydrogen society and conducting demonstration experiments of hydrogen supply chains in various places. Fuel cells are the most popular source of hydrogen energy. However, the load followability of fuel cells has not been studied. In this study, we will construct a power generator using the SOFC single cell which is the highest temperature operation and the highest efficiency among fuel cells, and investigate the load following ability. Currently, it is desirable to make SOFC operable at low temperatures. Therefore, in this experiment, the operating temperature is 700 degrees, and the flow rate of each gas is 200 mL/min. First, the I-V characteristic of a single cell is measured in order to investigate whether it is a suitable experimental device. From the experiment, the power per unit area at peak was 0.1 W/cm2. Since this power value is equal to the nominal value, it can be said that an appropriate experimental device has been produced. Next, based on this value, a variable load is created using the programmable load function of the DC electronic load. The loading rate is repeated at 20% and 100% every 10 seconds from 40 seconds to 80 seconds. The single cell output when the created variable load is input to the single cell is shown in the figure. From the figure, it can be seen that in any case, tracking follows less than 0.6 seconds. From the experimental results described above, it is found that the SOFC single cell has high load followability. In the future, CO2 is expected to be reduced by replacing the thermal power generator with a SOFC generator for the peak load power supply.
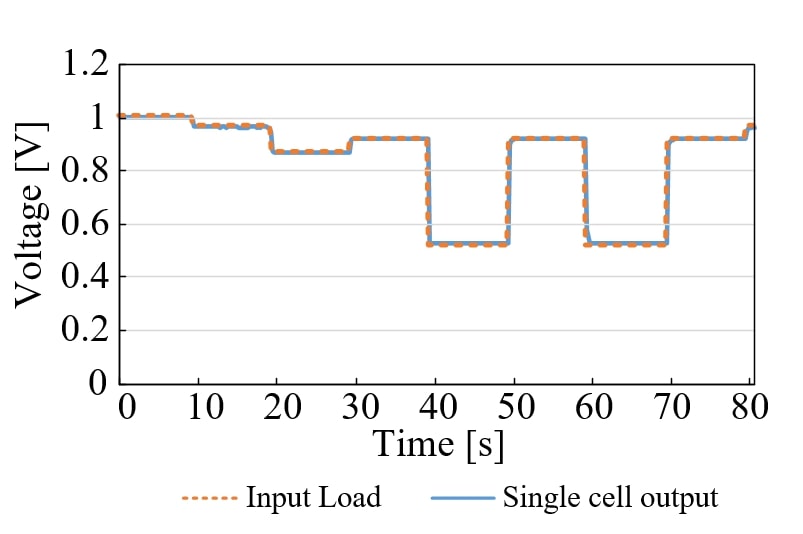
Anode materials based on transition metal phosphides were investigated for intermediate temperature fuel cells (ITFCs). Metal phosphides are world-widely investigated as highly-active and selective catalysts for hydrodesulfurization, hydrodenitrogenation, and hydrodeoxygenation. These reactions require catalytic ability for dissociation of H-H bond as well as C-S, C-N, or C-O bond. In addition to the catalytic activity, metal phosphides are known to be thermally and chemically stable and to possess electric conductivity comparable to metals and hardness similar to ceramics. Because of these features, metal phosphides are expected to function as electrodes in electrochemical cells. We have reported that molybdenum phosphide and tungsten phosphide as the anode of ITFCs demonstrated high power generation characteristics per electrochemical surface area. Accordingly, this study aimed to increase electrochemical surface area by supporting metal phosphides on carbon materials. Also, to increase the porosity of the anodes for ITFCs, carbon black (CB) and carbon nanofiber (CNF) were mixed with commercial platinum carbon (Pt/C) catalysts. Power generation and cyclic voltammetry were conducted to evaluate the catalysts' electrochemical activities. From the results of power generation and CV, molybdenum phosphide loaded on CB shows higher power generation characteristics per electrochemical surface area than Pt/C. It is suggested that phosphides loaded on carbon were well dispersed and many active sites were produced. Power generation measurements revealed that Pt/C and CB with the weight ratio 1: 1 and Pt/C and CNF with the weight ratio 1: 1 exhibited good cell performances. It indicates that increased porosity in the electrode gave rise to large triple-phase boundary.
In the proton exchange membrane fuel cell, the durability is recently the critical issue in its operation. As the Ce+3 cerium ion (Ce) is a potential scavenger for ·OH and ·OOH radicals, the main causes of the chemical degradation of the membrane, aminomethyl-15-crown-5-ether (CRE) and dual sulfonated 3,3-diphenylpropylamine (DSDPA) were grafted onto the poly(arylene ether ketone) (PAEK) to maintain high proton conductivity and chemical stability by alleviating the migration of Ce from the membrane. The chemical and physical structures of the synthesized CRE grafted dual sulfonated PAEK membrane (DSPAEK-CRE) along with the coordination complex of CRE and Ce ions were investigated using FT-IR, 1H-NMR and SAXs, and XPS spectroscopy. The Fenton's test showed huge improvement of anti-oxidation stability by the presence of CRE. While the CRE/Ce coordinated membrane showed higher proton conductivity and better chemical stability than the Ce+3 dispersed one, other properties such as water uptake, swelling ratio, and mechanical strength were not significantly affected by CRE.
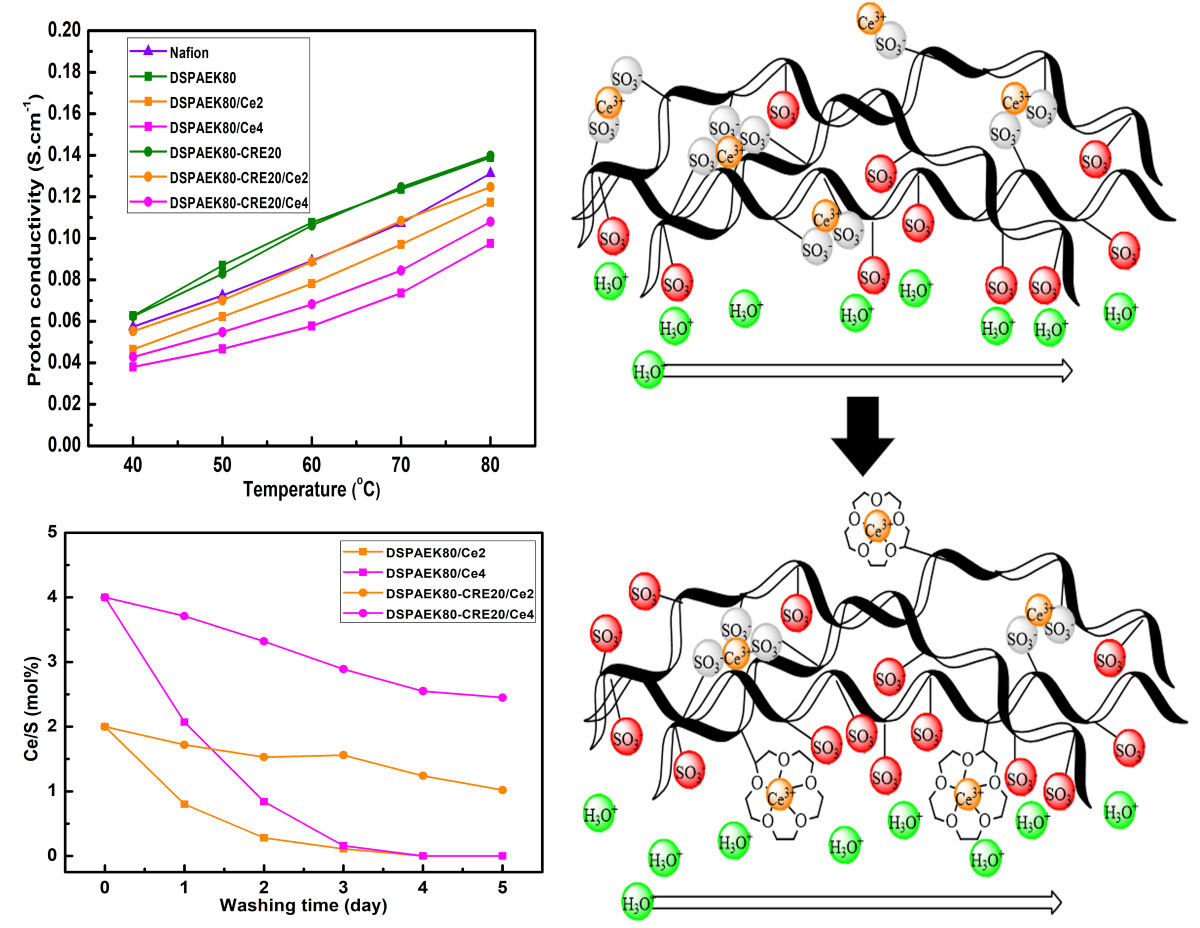
Recently, solid-state alkaline fuel cells (SAFCs) with anion-exchange membranes (AEMs) have attracted considerable attention as next generation fuel cells, because they can use non-noble catalysts and various fuels. The SAFCs show complex water behaviors; water is generated at the anode and is reacted at the cathode. Hence, SAFCs using gas as the fuel show low cell performance due to anode flooding and membrane drying. Although control of water behavior is crucial to improve cell performance, there are many parameters to be considered such as AEM properties and operating conditions. In such situation, modeling approach is effective because of less time-consuming, inexpensive, and more versatile than experimental approaches.
In this study, we make a mass balance model for SAFCs considering water transport in AEM and clarify guidelines to suppress anode flooding and membrane drying. Pore-filling anion conducting membrane[1] is used as AEM, and parameters needed for the calculation such as ion conductivity and water content of AEM were experimentally obtained. To make the AEM model, two mass balances of whole cell and AEM are considered. Then, outlet RHs and membrane IR loss were calculated by solving the two mass balances simultaneously under specific operating conditions and AEM thickness.
Fig. 1(a) and 1(b) show current density dependence of calculated outlet anode RHs and membrane IR losses with different AEM thickness, respectively. With thinner AEM, an increase in current density was obtained at 100% RH, which indicate the flooding is suppressed. Further, the suppression of membrane drying in the thinner AEM was confirmed by the low membrane IR loss. These results show that thinner AEM is effective for suppressing both anode flooding and membrane drying.
[1]Y. Oshiba et al., Journal of Power Sources , 345, 221-226 (2017)
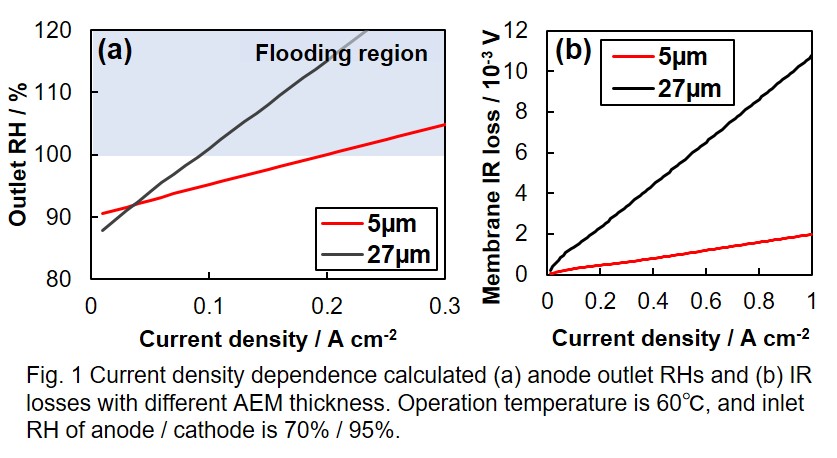
Direct methanol fuel cell (DMFC) has attracted attention as an alternative power source because of high energy density of liquid methanol. One of the main issues for DMFC is the sluggish kinetics of methanol oxidation reaction (MOR) at the anode. Therefore, we focused on developing a highly active anode catalyst by using reduced graphene oxide (RGO) having a high specific surface-area and a high electric conductivity as a catalyst support. Although many studies using RGO have been conducted so far, the effect of the particle size of RGO on the catalytic activity has not been sufficiently investigated. In this study, the catalyst was prepared using RGO with different average particle size, i.e., 5 μm (RGOL) and 0.5 μm (RGOS) and the effect of particle size of RGO on the catalytic activity was investigated.
PtRu catalyst supported on RGOL or RGOS was prepared from a GO slurry by using a high-power ultrasonic crusher followed by adding polydopamine (PDA) as a dispersant and PtRu deposition by a chemical reduction method using NaBH4.
For the prepared catalysts, PtRu/RGOL and PtRu/RGOS, characterization was carried out using TEM, FE-SEM, XRD and EDX. Catalytic activity of the catalyst was evaluated by cyclic voltammetry in an aqueous solution with 0.5 M CH3OH and 0.5 M H2SO4. Electrochemical surface area (ECSA) for the catalyst was also evaluated by CO stripping method.
PtRu/RGOS showed about 1.6 times higher ECSA and about 2.1 times higher MOR mass activity compared to that of PtRu/RGOL. The higher performance of PtRu/RGOS was attributed to the superior structure of the catalyst layer for facilitated mass transfer and ease of fuel access. This result suggested that RGO with the small particle size is proper for the support of PtRu nanoparticles to increase the MOR activity of PtRu/RGO in DMFC.
The efficient conversion of energy is one of the urgent problems because of the sharp increase in current energy consumption. To meet the electricity demand, the development of fuel cells (FCs) have attracted great interest by their sustainable, renewable, efficient and eco-friendly electrochemical conversion of energy. For widespread commercialization of FCs, the main challenge is to reduce the cost of catalysts although expensive platinum-based catalysts have been applied. However, it is still challenging to develop an inexpensive and effective alternative catalyst, due to its sluggish reaction towards the oxygen reduction reaction (ORR).
As the alternative catalysts, transition metal and heteroatoms co-doped carbon material have been attracted by their low-cost and high performance. In particular, the carbon materials containing the bonding of cobalt and nitrogen (CoNx) acted as high-performance catalysts for ORR. However, the fine design of CoNx is difficult, due to the aggregation of metal. Herein, we present a new synthesis method of graphene (G) with highly-dispersed CoNx species as an excellent electrocatalysts, by using sequential treatments of mixing with sulfuric acid, Co2+ ion-exchanging and 2-methylimidazole coordination. And we also report its excellent electrocatalytic performance.
The highly dispersed cobalt species was observed on the graphene by TEM images and STEM images. The results of ORR measurements were shown in Table 1. The samples after the treatment of Co2+ ion-exchanging and 2-methylimidazole coordination were carbonized under N2 atmosphere and denoted as Co/GS and Co-N/GS, respectively. Obviously, G and Co/GS performed sluggish ORR, while Co-N/GS showed both excellent onset potential and limiting current density. In particular, the limiting current density is comparable to commercial platinum catalysts (Pt/C). Moreover, Co-N/GS exhibits a high current density, and the number of electrons (n) in the ORR are also higher than other samples.
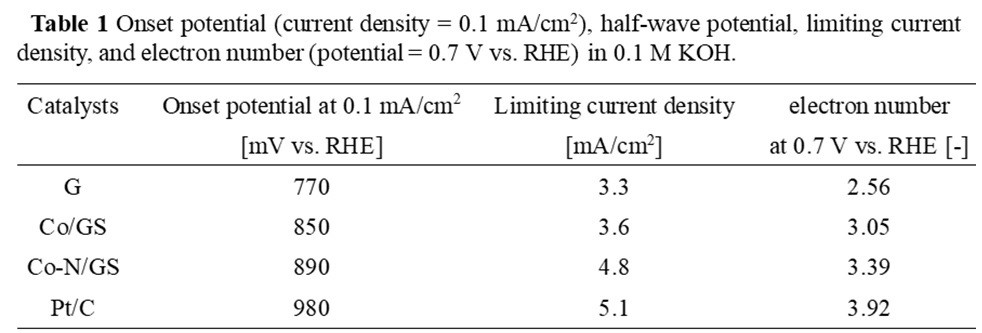
Polymer electrolyte fuel cell (PEFC) is beginning to be put into practical use as residential and transportation power generation system. However, the cost of PEFC is still high, since expensive platinum (Pt) is used as the catalyst. Therefore, development of non-Pt catalyst is required. We have been studying silk-derived activated carbon (SAC) as non-Pt catalyst, which is inexpensive but fairly active compared to the conventional Pt catalyst. The structure of the electrodes fabricated from such catalyst should be designed with a different concept. In this study, we investigated the effect of catalyst layer thickness on the performance of PEFC using non-Pt catalyst.
Membrane electrode assembly (MEA) was prepared using SAC as cathode catalyst. Catalyst layers with different thicknesses were fabricated on a carbon paper and was hot pressed to a Nafion membrane. Terminal voltage vs. current density and electrochemical impedance spectra of each MEA were measured at 80 °C.
The iR-free polarization curves of each MEA were deconvoluted by fitting the activation overpotential. As the amount of catalyst was increased, the activation overpotential decreased and the remaining overpotential increased. It was assumed that this residual overpotential was not concentration overpotential, since the shape of the curve was not convex downward. The applied voltage dependence of the impedance spectrum was observed. As the applied voltage was increased, the interfacial resistance initially decreased and then became constant. Interfacial resistance independent of the applied voltage was assumed to be related to the proton conduction in the catalyst layer. The interfacial resistance related to the proton conduction in the catalyst layer increased as the amount of catalyst increased. Therefore, it is considered that the increase of the residual overpotential was due to the increase of the proton conduction resistance in the catalyst layer.
Graphene oxide (GO) membrane is expected to be an alternative electrolyte membrane having high proton-conductivity and less fuel-crossover for a fuel cell application. However, the proton-conductivity of GO membrane is still not enough. In this study, effect of an additional oxidation of GO and an addition of sulfonic acid group to GO on the proton conductivity were investigated.
A commercial GO slurry with 1.0 wt% GO was subsequently treated with NaNO3, KMNO4 and H2O2 to obtain the additionally oxidized GO (GOh). By adding vinyl sulfonic acid (VS) to the GO and GOh slurries, VS treated GO (GOVS) and VS treated GOh (GOhVS) membranes were prepared after filtering and drying. Membrane electrode assembly (MEA) was fabricated by sandwiching the membrane by carbon papers with Pt/C catalyst on both sides. Current-voltage performance of the MEA as a H2-O2 fuel cell was evaluated, and the proton conductivity of the membrane was calculated from the ohmic resistance assuming that the cell resistance was governed by that of the membrane.
The proton conductivity of the membrane was higher in the order of GOhVS > GOVS > GOh > GO and found that each of the additional oxidation of GO and the VS treatment increased the proton conductivity as shown in Table 1. GOhVS membrane of which GO was additionally oxidized and also treated with VS showed the highest conductivity with 118 mW cm-2 fuel cell power-density. It was more than one order of magnitude higher than that of GO. It was found that the additional oxidation tof GO and the VS treatment are effective in improving the conductivity of GO membrane.
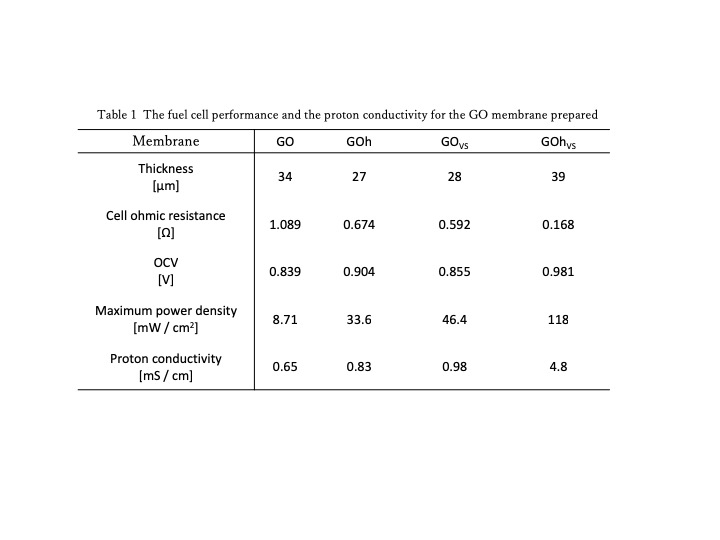
Polymer electrolyte water electrolysis (PEWE) is a key technology for the production of hydrogen in the power-to-gas process. As polymer electrolyte membranes for PEWE, perfluorosulfonic acid (PFSA) polymer membranes such as Nafion® are commonly used. In PEWE, although the produced hydrogen needs to be stored in pressurized gas tanks for transportation, the cell performance drops by high hydrogen crossover under the pressured condition. On the other hand, hydrocarbon-based polymer membranes including poly (arylene ether sulfone) (SPES) are expected to have a lower gas permeation property than the PFSA polymer membranes. However, the swelling of these hydrocarbon membranes is higher than the PFSA polymer membranes, which is a severe problem in fully humidified operating conditions of PEWE. The membrane with reduced swelling could be fabricated by a pore-filling technique, where the polymer electrolyte is filled inside pores of the porous substrate [1].
In our study, we prepared a pore-filling polymer electrolyte membrane using SPES for PEWE (Fig. 1A). This pore-filling membrane was prepared by drop casting 3 wt% SPES (IEC: 2.1 meq/g) solution over a 24 μm polyethylene porous substrate at 80 °C. The properties of the SPES pore-filling membrane was compared with that of SPES cast membrane and commercial Nafion 211.
The hydrogen permeability coefficient of the SPES cast membrane was found lower than Nafion 211, and that of the SPES pore-filling membrane is comparable with that of the SPES cast membrane (Fig. 1B). Moreover, the pore-filling membrane showed a lower swelling ratio between dry and wet conditions compared to the cast membrane (Fig. 1C). The results indicate that the SPES pore-filling membrane has both reduced hydrogen permeation and lowered swelling properties, which is suitable for PEWE applications.
[1] Nishimura, H. and T. Yamaguchi, Electrochemical and solid-state letters, 2004. 7(11): p. A385-A388.
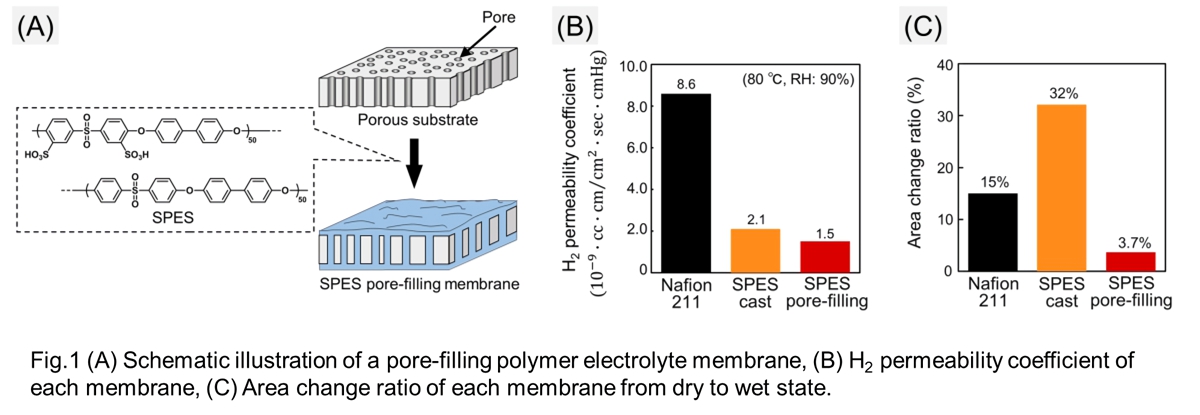
Fuel cells combined with carbon-free hydrogen productions, such as water electrolysis, are the most promising energy strategy to mitigate the global warming and climate changes. Since the reactions for fuel cells (oxygen reduction reaction, ORR and hydrogen oxidation reaction, HOR) and water electrolysis (oxygen evolution reaction, OER and hydrogen evolution reaction, HER) are heterogeneous electrochemical reactions, they require catalysts to reduce the activation barriers during the electrochemical reactions. In addition, since the ordinary catalysts used in the systems are noble metal such as Pt, Ru, Ir, etc., development of cheap catalysts is another key issue in the fields. In this presentation, alloys of both noble and transition metals are fabricated by electrodeposition method. The composition and shape of the catalysts were controlled through the modification of the electrolyte composition and the deposition potential. For the ORR of fuel cells, PtM alloys were electrodeposited and their ORR characteristics through the modification of the electronic structures were studied. In the case of hydrogen-involving reactions such as HOR and HER, the relatively fast kinetics enables the possible use of cheap Ni-based alloys as the catalysts materials. Addition of small amounts of noble metals to Ni or control of the electrochemical surface area as well as the crystalline structures enhance the reaction kinetics close to those by Pt-based materials. A variety of combinations of Ni-based alloys was fabricated by electrodeposition and their characteristics on HOR and HER were investigated.
Energy issue has become a considerable topic because of the rapid population growth and industrialization of the world. To reduce carbon emission and environmental pollution from burning fossil fuels, the development of alternative energies is urgent and essential nowadays. Hydrogen has been regarded as one of the most promising energy carriers because of its high gravimetric energy density and clean byproduct, water, when in use as an energy source. Electrolytic water splitting driven by renewable energies such solar and wind energies is considered a most promising environmentally friendly hydrogen production technology, which splits water into hydrogen and oxygen without hazardous byproducts. The development of high-efficiency and noble-metal-free electrocatalysts is the most crucial and challenging part of electrolytic water splitting. In this study, we successfully grown Ni-Fe based bi-metallic metal-organic frameworks (MOF) on nickel foam, denoted as (Ni, Fe)(BDC-NH2)(DMF,F)/NF, for both oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) involved in alkaline electrolytic water splitting. The as prepared MOF loaded nickel foam exhibited outstanding electrolytic activities, achieving overpotentials of 286 and 348 mV for the OER and 250 and 353 mV for the HER at current densities of 50 and 500 mA cm-2, respectively in 1.0 M KOH, showing substantial activity enhancements as compared to pristine nickel foam. The OER efficiency enhancement may be attributed to the synergistic effects between Ni and Fe and the presence of fluoride, which is a strong electron withdrawing ion. As for the HER, the presence of the electron donating group, –NH2, in the organic linker is beneficial to the reduction power of the catalyst.
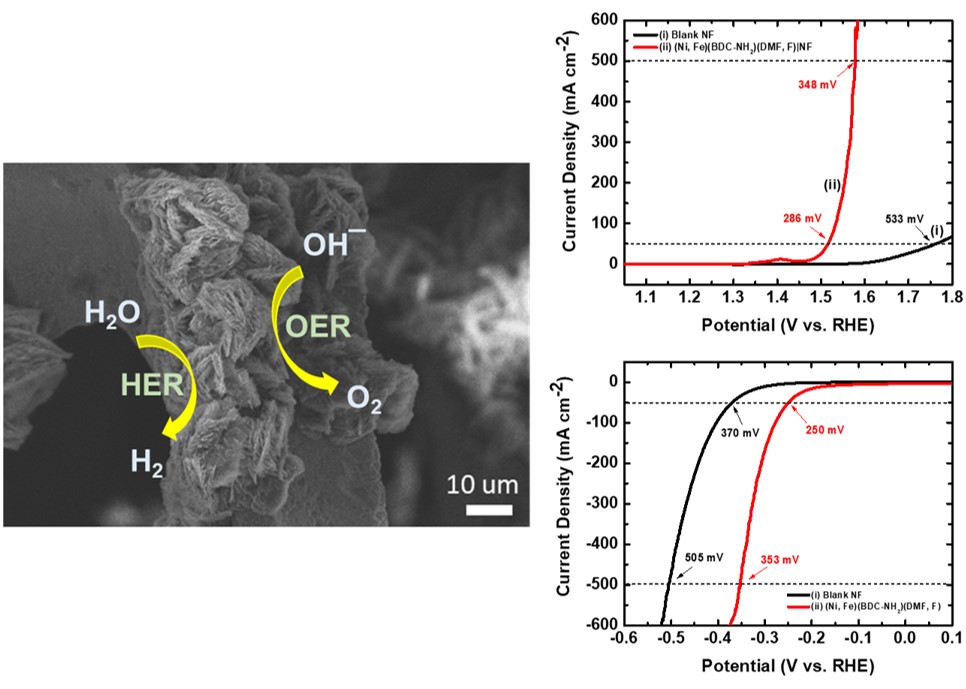
Efficient use of renewable energy resources is crucial to meet our future energy demand. Alkaline water splitting can produce hydrogen using electricity generated from intermittent natural energies, such as solar and wind. The oxygen evolution reaction (OER) is often considered as the bottleneck in water splitting because the OER still has large overpotential. RuO2 and IrO2 have high activities for OER, but these catalysts based on precious metals are very costly. In addition, high operating potential in OER region triggers corrosion of carbon supports of catalysts, leading their low durability.
Perovskite oxides with nonprecious metals have been recently attracting much interest because they are inexpensive and show high electrocatalytic activity. For electrochemical reaction, conductive supports need to be combined since most perovskite oxides have insufficient electrical conductivity. However, carbon supports are not suitable due to carbon corrosion.
Herein, we develop a composite electrocatalyst composed of perovskite oxide as catalyst and conductive metal oxide as support as shown in Fig. 1(a), and we have chosen LaCoO3 and SnO2, respectively. LaCoO3 is a model perovskite compound, which is well-known as OER catalyst. SnO2 is electrical conductive oxide and possesses high thermal stability and alkaline stability, suggesting that SnO2 can be stable during the synthesis of LaCoO3 and measurements in alkaline solution. Fig. 1(b) shows OER activities of LaCoO3, LaCoO3/SnO2 and physically-mixed LaCoO3+SnO2 and demonstrates that LaCoO3/SnO2 exhibited higher current than others. It implies a good interfacial contact in LaCoO3/SnO2. Moreover, Fig. 1(c) proves higher durability of LaCoO3/SnO2 than that of LaCoO3/CNT which employed carbon as support. These findings indicated that the composite electrocatalyst based on perovskite and conductive metal oxide support is a promising platform for development of highly durable OER catalysts.
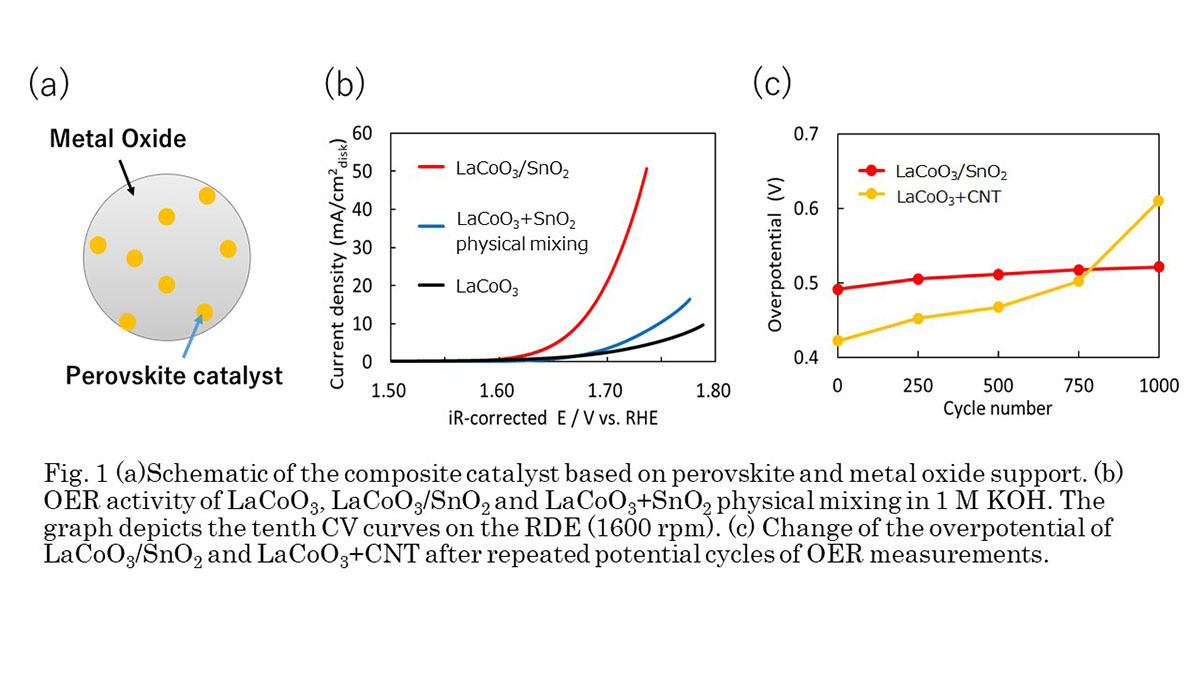
The development of green energy are getting increasing research attention. Hydrogen is an excellent energy carrier and is also considered an energy source suitable to serve as an intermediate energy carrier for solar and wind energies. The development of highly efficient, stable, and low-cost electrocatalysts to reduce the reaction potential of electrolytic water splitting can facilitate conversion of green energies into hydrogen energy. In this study, we successfully grew Ni-Fe-Mo based nanorod arrays on nickel foam, denoted as (Ni, Fe)MoO4@NF, as bifunctional electrocatalysts for overall water splitting. The product catalysts exhibited excellent electrocatalytic activities toward the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in 1.0 M KOH, achieving overpotentials of 128, 226, and 305 mV at 10, 50, and 500 mA cm-2, respectively for the HER and overpotentials of 227 and 333 mV at 50 and 500 mA cm-2, respectively for the OER. The enhancement in OER efficiencies can be mainly attributed to the synergistic effects between Ni and Fe. And the enhancement in HER efficiencies can be mainly attributed to the synergistic effects between Ni and Mo. Furthermore, the nanorod array structure on nickel foam enlarges the reaction surface areas and thus the active site number for the water splitting reaction.
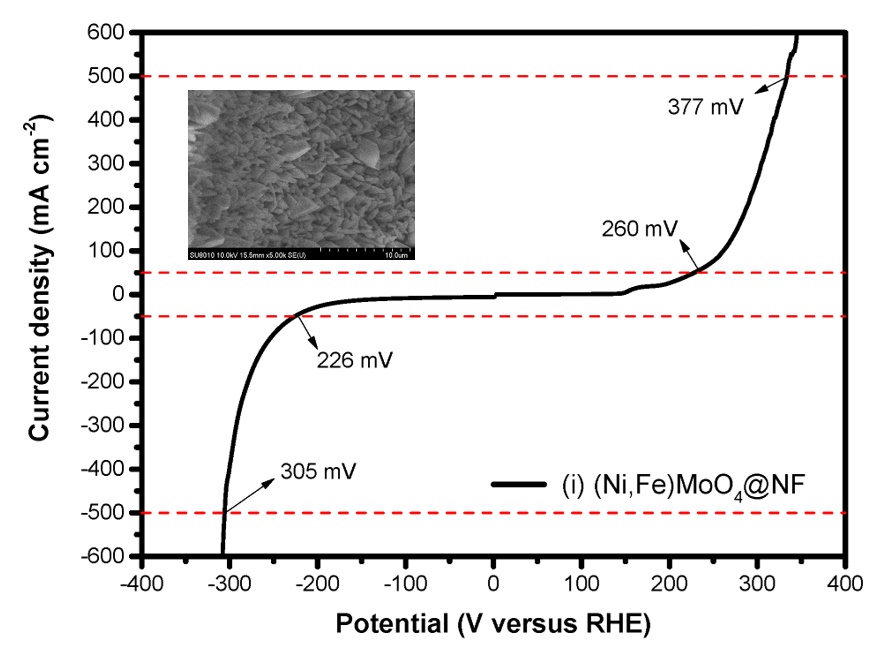
Hydrogen energy, an eco-friendly energy source for replacing fossil fuels, is widely regarded as a next-generation energy source with highly versatile application. However, there are technical limitations on hydrogen storage and production that should be satisfied for aspects of economy and efficiency. Among many technologies for solving the problems, liquid organic hydrogen carriers (LOHC) technology has been receiving much attention since it can be stored for a long time and safely transported to a destination using existing gasoline infrastructure. Recently, it has been experimentally reported that when biphenyl was mixed with diphenylmethane, it showed a reversible hydrogen storage and production capacity of 6.9 wt% H2 and 60 gH2/L, existing in a liquid state at room temperature. To understand this mechanism in detail, density functional theory (DFT) calculations were conducted using Gaussian 16 software package. We analyzed the reversible dehydrogenation reaction mechanisms based on the dehydrogenation enthalpy and free energy calculations. Furthermore, the same calculations were conducted with a various functional groups to propose higher efficient LOHC materials, which would make a great contribution to the development of optimal LOHC materials for the reversible hydrogen storage and production system.
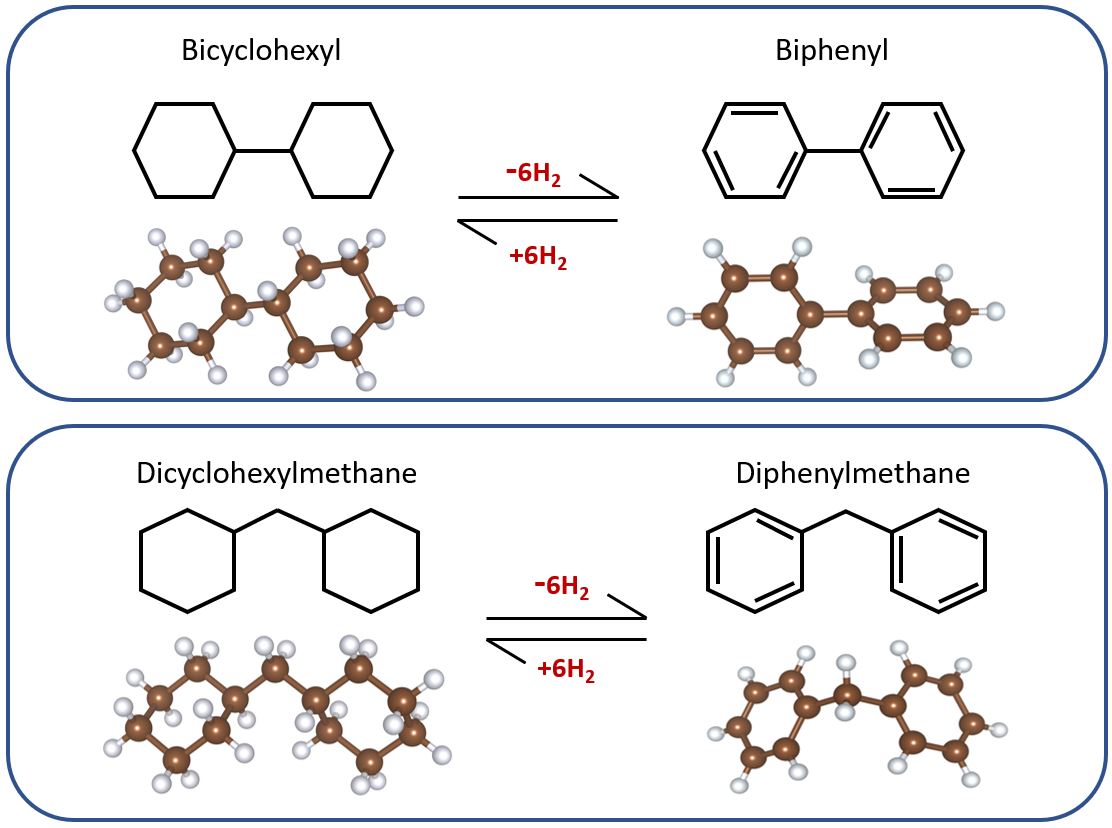
Hydrogen is a secondary energy source and can generate electricity by fuel cells without CO2 emission. Ammonia is considered for one of the promising hydrogen storage media. Ammonia has high gravimetric and volumetric hydrogen capacities (17.8 mass% and 107.3 kgH2/m3). And ammonia is easily liquefied at 1.0 MPa at room temperature. Hydrogen can be obtained by electrolysis of liquid ammonia. The theoretical standard voltage is 0.077 V, however the actual operation voltage is much higher due to the large overpotential for the anode reaction. In the anode reaction of N2 formation from NH2-, it is considered that either NH2- adsorption or N2 desorption can be the limiting step. Therefore, the catalyst effect on anode reaction was investigated by focusing on the metal nitride formation enthalpy ΔHfMNx which corresponds to the strength of metal-nitrogen bond. The electrolysis of liquid ammonia was performed using a three-electrode system with a counter electrode of a Pt plate, a reference electrode of a Pt wire (-2.59 V vs. SHE) and a working electrode of a catalyst plate (Ti, Ta, Fe, Co, Ni, Ru, Ir, Pt, Au, or Ag). The cyclic voltammetry (CV) of mono-component catalysts showed different activities for different metals (Fig. 1a). By plotting the current density at 0.3 V against the ΔHfMNx, the volcano plot was obtained (Fig. 1b). Next, to increase the catalytic performance, binary metal catalyst of Fe and Pt (Fe-Pt) having different bond strengths with nitrogen was prepared. Fe-Pt showed higher current density than the mono-Fe and mono-Pt catalysts at a low potential of 0.3 V.
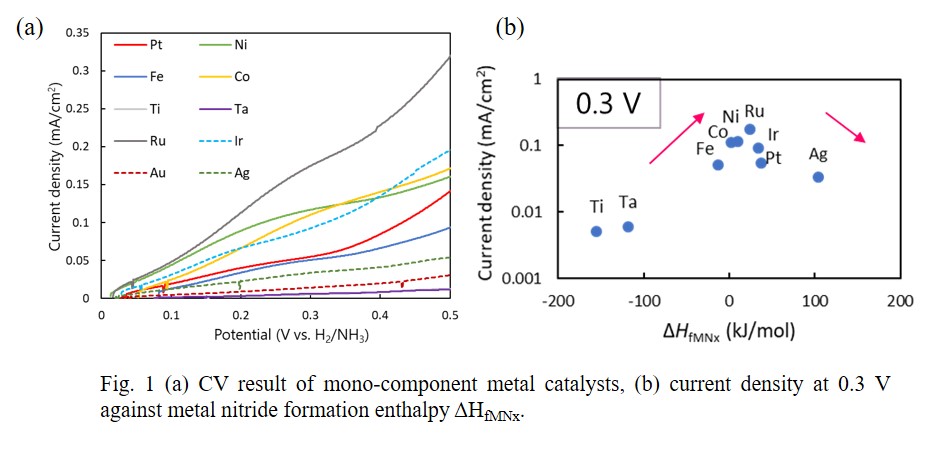
NH3 is one of the key chemicals in the world. For example, NH3-based chemicals occupy the vital position of a fertilizer, which directly affects the world's food supply. Besides, NH3 is considered a promising H2 carrier because of its high energy density, easy liquefaction, and no CO2 emission during H2 evolution reaction. Today, NH3 is synthesized from syngas and N2 at high pressure (15-30 MPa) and at high temperature (500 °C), which is known as a Haber-Bosch process. If this synthesis is achieved without using fossil fuel, we will obtain carbon-free NH3. We focused on electrochemical synthesis of NH3 which can be operated at atmospheric pressure by electricity from renewable energy resources. We chose a solid phosphate electrolyte (a CsH2PO4/SiP2O7 composite) which conducts protons at around 200 °C, based on the studies of intermediate-temperature fuel cells. Both electrodes consisted of Pt on carbon with 1 mg_Pt cm-2. Ideally, the electrochemical synthesis proceeds as follows. At an anode side, H2 is separated to protons and electrons (H2 → 2H+ + 2e-). At a cathode side, N2 reacts with protons and electrons (N2 + 6H+ + 6e- → 2NH3). Using the electrolyte and electrodes, however, NH3 was detected not only from cathode but also from anode probably because of the NH3 leakage from the cathode to the anode and desorption/decomposition of NH3-like contaminations. Thus, we struggled with changes in experimental conditions and improvement in the apparatus and experimental procedure. Ag-Pd membrane was found to be effective for preventing the gas leak, and by inserting Ag-Pd membrane between anode and electrolyte, NH3 detection in the anode side was limited, and electrochemical NH3 synthesis was confirmed in the cathode side.
Ammonia has been paid attention as a hydrogen medium for hydrogen energy. The aqueous ammonia solution has a high hydrogen mass density (6.1 mass%) and a vapor pressure (0.08 Pa) lower than atmospheric pressure at 20 °C. Electrolysis is one of the methods of generating hydrogen from aqueous ammonia solution. Hydrogen and nitrogen are generated at the anode and cathode, respectively, and the theoretical decomposition voltage is 0.06 V. Platinum is often used for the electrode catalyst but has a problem of the large overvoltage of the anode.
In this study, an electrode with platinum nanoparticles supported on carbon nanotube (Pt-CNT) was fabricated. CNT is a carbon material possessing a high specific surface area, that forms a sponge-like film by a dispersion-filtration process. The Pt-CNT electrode was adapted to electrolysis of ammonia aqueous solution to reduce the anode overpotential by increasing the Pt surface area.
Pt nanoparticles were deposited on CNT by polyol method at various temperatures of 100-180 °C. The electrochemical surface area of Pt was calculated from the amount of hydrogen adsorption and desorption in 0.5 M H2SO4 aq. The largest platinum surface area, 268 cm2 per 1 cm2 of electrode, was obtained for the Pt-CNT with Pt deposited at 160 °C. The ammonia electrolysis results at the anode with 1 M NH3 + 1 M KOH aqueous solution are shown in Fig. 1. The Pt-CNT electrode prepared at 160 °C and having the largest platinum surface area showed the highest peak current density and the lowest potential at 10 mA cm-2.
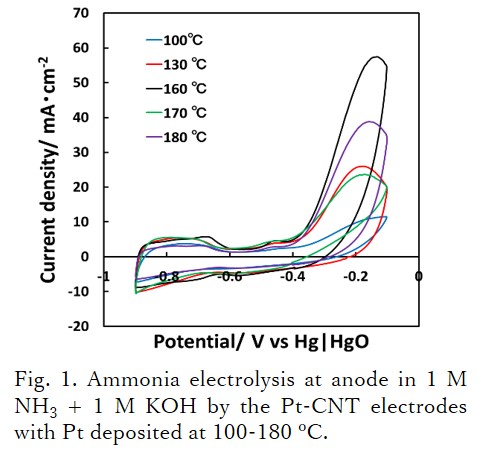
Among many eco-friendly energy sources, hydrogen energy has been attracting attention due to a higher versatility, but there is a difficulty in the practical use due to technical limitations in storing and transporting hydrogen. Liquid organic hydrogen carriers (LOHC) technology is one of the most promising alternative methods, storing hydrogen in a liquid state compound, thereby enabling stable and high-density hydrogen storage. Previou studies have reported various LOHCs such as metylcyclohexane, dibenzyl toluene, N ethylcarbazole, ammonia borane and bis-BN cyclohexane. Among them, it has been reported that bis-BN cyclohexane, a derivative of AB, can overcome the disadvantage of ammonia borane (i.e., AB is rapidly decomposed below 150 °C), because it is thermodynamically stable at 150 °C and has a hydrogen storage capacity of 4.7 wt%. In the current study, we conducted an ab-initio study to develop hydrogen storage materials with higher efficiency and reversibility using Gaussian 16 software package. The dehydrogenation mechanism of bis-BN cyclohexane was investigated by calculating the reaction enthalpies and free energies, and then highly efficient materials were screened by conducting the same calculation depending on various functional groups such as F, Cl, and CH3. When F was replaced in one of the H atom of bis-BN cyclohexane, the enthalpy and free energy for the dehydrogenation were 26 kcal/mol and 0.1 kcal/mol, respectively, which proved to be enhanced reversibility of bis-BN cyclohexane-based LOHC.
Electronic power generation in Japan contributed by thermal power generation in terms of economics, environmental compatibility, and stable supply. However, there is a problem that the environmental negative influence is high, such as the formation of greenhouse gases emitted when burning fossil fuels. Therefore, hydrogen has recently been attracted attention as a power source. Hydrogen has an advantage compared to the other energy medias, i.e., hydrogen can use be produced from various primary energies including renewable energies, and electricity is obtained by using a fuel cell. However, there is also an issue that the cost is high for transportation and storage due to the use of a high pressure tank because of the low volumetric energy density. Therefore, we propose an electrochemical device for production of hydrogen from methylcyclohexane, which is an energy carrier. The reaction, separation, and compression processes are performed by one device, the cell structure is similar to a PEFC, and the reaction principle is the electrolysis of methylcyclohexane by an external voltage.The formation of proton from methylcyclohexane carries out at an anode catalyst (reaction process), separation between hydrogen and organic compound by the Nafion membrane (separation process), and electrochemical compression based on the Nernst equation (compression process). In this study, hydrogen was obtained from reaction, separation, and compression from pure methylcyclohexane, and the concentrations of hydrogen and impurities were evaluated by gas chromatography. As a result, a current density was more than 50 mA cm–2 at a cell voltage of –2.0 V.
As an eco-friendly energy source to replace fossil fuels, hydrogen energy is seen as a next-generation energy source with its high general purpose applicable. However, there are technical limitations to stable hydrogen storage and release for economic feasibility and efficiency. Liquid organic hydrogen carriers (LOHCs) can be one of the ways to overcome this. In previous studies, it was demonstrated experimentally that biphenyl exists in a liquid state at room temperature when mixed with diphenylmethane, and has a hydrogen storage capacity of 6.9 %wtH2 and 60 g/L. Our preliminary study demonstrated that the dehydrogenation efficiency of biphenyl has increased when carbons were replaced with boron (B) and nitrogen (N) in the molecule. Based on the findings, the current study investigated the reversible dehydrogenation and hydrogenation mechanisms of BN-biphenyl and BN-diphenylmethane by predicting reaction enthalpies ($Delta;H) and free energies ($Delta;G) based on density function theory (DFT) calculations. In addition, alternative materials with higher efficiency are to be screened by adding functional groups (F, Cl, NH2, etc.) in the molecules. This will contribute to the development of an optimal hydrogen storage and release of highly efficient LOHCs.
Hydrogen, which is clean and storable fuel, is an attractive energy carrier for energy and environmental issues. In addition, hydrogen plays a vital role in chemical industry as a reactant. Nowadays, hydrogen is mainly produced from fossil fuels by steam reforming with CO2 emissions, so new hydrogen production process which is eco-friendly has been desired. Thermocatalytic decomposition of hydrocarbon is attracting attention because there is no emission of greenhouse gases such as CO2 during hydrogen production. Several researchers have investigated various catalysts for this reaction, but there is a problem of carbon deposition on the surface which blocks active sites of the catalyst. Based on the situation, “chemical looping methane cracking system” has been proposed. This system is composed of two reactors. In a first reactor, methane is cracked into H2 and carbon, and in a second reactor, deposited carbon is oxidized by CO2 into CO. The system has advantages in terms of separated syngas production and CO2 utilization. As an oxygen carrier, Fe/BaZr0.9Y0.1O3 composite was reported in our previous study, which exhibited high reaction kinetics for methane cracking because of high proton conductivity of BaZr0.9Y0.1O3
In this study, we synthesized Fe-doped BaZr0.9Y0.1O3 and investigated the reaction activity of these catalysts by thermal gravimetric analysis and gas analysis. Fe-supporting method, i.e., Fe doping, and the amount of Fe influenced the states of deposited carbon in methane cracking. For example, direction of growth and diameter of carbon fiber, and reaction kinetics also changed by the oxygen carriers. On the other hand, the carbon oxidation reaction with CO2 completed in a relatively short time for each catalyst. Besides, cyclic stability was investigated. Through the kinetic measurements, we discussed the effect of the feature of prepared catalysts on reaction kinetics and the efficiency of the system.
Zeolite templated carbons (ZTCs) composed of curved and single-layer graphene frameworks have uniform micropores (ca. 1.2 nm) and high surface areas (~4000 m2 g-1). Due to their outstanding properties originating from the porous structures, ZTCs have been utilized in many applications, e.g. hydrogen storage, CO2 capture, catalysts and electrochemical capacitors. To expand the utilization of ZTCs, metal atoms or heteroatoms have been doped to ZTCs. However, it is still challenging to fabricate single atomic transition metals coordinated with heteroatoms in ZTCs. In this work, we synthesized Co/N doped ZTC (Co/N-ZTC) by complexing Co ion with 2-methylimidazole in Y zeolite to expand the further utilization of ZTCs as shown in Figure 1. The first step is to anchor Co/2-methylimizole complex into HY zeolite as a precursor of Co/N-ZTC by complexing Co ion with 2-methylimidazole in HY zeolite. The next step is chemical vapor deposition (CVD) using methanol as a carbon source, followed by carbonization process. The last step is base and acid treatments to remove HY zeolite and aggregated Co particles. The obtained Co/N-ZTC has a high surface area (ca. 2000 m2 g-1) and single atomic Co species in CoNx moieties, which actually contributes to its excellent catalytic performance on oxygen reduction reaction. This synthetic concept is beneficial to fabricate single atomic transition metals coordinated with heteroatoms in ZTCs.
Hydrogen is paid attention as clean energy because it does not emit CO2 when converted to electricity. MgH2 is one of the promising hydrogen storage materials because of its high volumetric and gravimetric hydrogen densities and abundance. However, Mg expands and shrinks during hydrogen absorption and desorption, respectively. MgH2 powder pulverizes during hydrogen absorption and desorption cycles. In the tank filled with MgH2, pulverized powder drops and the density of packed bed at the bottom increases. This causes a problem of tank deformation during cycles. In this research, in order to absorb volume change of MgH2 and fix pulverized powder during cycles, MgH2 particles were retained in carbon nanotubes film. MgH2 and carbon nanotubes were co-dispersed in N-methyl-2-pyrrolidone. The MgH2-CNT composite was synthesized by vacuum filtration of the dispersion. The structure of MgH2-CNT composite (MgH2-CNT w/o press) was maintained after hydrogen ab/desorption (Fig.1 a), however, the hydrogen desorption rate was lower than that of MgH2 powder (Fig.1 b). In order to improve desorption rate, the MgH2-CNT composite was pressed to decrease its porosity. As the result, the desorption rate of pressed MgH2-CNT composite (MgH2-CNT w/ press) was improved compared to MgH2-CNT w/o press, but the film structure was broken into pieces. The MgH2-CNT composite was wrapped in stainless mesh and pressed (MgH2-CNT w/ mesh, press) to support the film structure. The composite achieved improved desorption rate and maintained film structure during hydrogen ab/desorption cycles.