This study presents newly-developed simulation method of flow in twin screw extruder. This method based on the Hele-Shaw flow model and finite element method, and applicable wide range of screw geometry without empirical parameters. Moreover, the Hele-Shaw flow model reduced the number of unknown variables dramatically and calculation time. The simulation results of degree of fill, fiber attrition, and devolatilization phenomena were experimentally validated.
"Current energy and environmental issues, alongside the depletion in fossil fuels, have increased the demand for the use of sustainable energy carriers. Electrochemical water splitting can produce hydrogen from naturally abundant water and is a clean and cost-effective energy conversion process. However, the anodic oxygen evolution reaction (OER) in the water splitting process has a large overpotential; thus, there is a need to develop highly active and inexpensive electrocatalysts for OER to overcome this barrier. Perovskite-type metal oxides with the general formula ABO3 have attracted great interest due to their excellent OER activities, facile synthesis and environmental friendliness. Several Fe-based perovskite-type OER catalysts have been previously reported, but most of these studies focused on the choice of the A-site metals. In contrast, the effects of crystalline structures and elemental compositions of catalysts on OER activity have not yet been systematically investigated, but the knowledge is crucial for the efficient design of electroactive materials. Herein, we synthesize a variety of Fe-based oxides with the general formula AxFeyOz (A: Ca, Sr, Ba) (Figure 1A) and investigate them to find promising OER electrocatalysts and to elucidate the structural and compositional effects on the OER activity. Ca, Sr and Ba were selected as A-site metals. Figure 1B shows the OER specific activities of the Fe-based oxides and reveals that a Ca-containing oxide was found to possess the highest OER activity among other synthesized oxides, exhibiting one of the best performances observed when compared to previously reported Fe-based oxides. These findings not only present a prominent electrocatalyst for OER, but also provide new guidelines for the design of metal oxides-type OER electrocatalysts.
Acknowledgement: This paper is based on results obtained from a project
commissioned by the New Energy and Industrial Technology Development
Organization (NEDO).
For large scale use of renewable energy, hydrogen society is necessary to overcome supply and demand mismatch in time and space. Polymer-electrolyte fuel cells (PEFCs) represent a superior system that exhibits high-efficiency, offering better power generation, meeting the desired levels of demand. However, in order to facilitate widespread use of fuel cells, cost and lifetime problems must be resolved. Solid alkaline fuel cells (SAFCs) are another system that holds the potential to achieve high-energy conversion efficiency without Pt catalysts. Although most of metal catalysts can be used under alkaline environment, development of durable electrolyte membranes in alkaline media is the key for this technology. We are systematically designing and developing new materials from the molecular level to the device level. In the fuel cell systems, different components such as membrane, catalysts, and catalyst layer share significant functions and work in a well-coordinated manner, and hence, the total cell system must be optimized for the best performance. The systematic design and developing approaches concerning materials for PEFCs and SAFCs are proposing. Specifically, pore-filling electrolyte membranes, carbon free Pt alloy porous capsule catalysts and durable anion-exchange membranes and direct liquid fuel cells are developed based on the approach. Future energy system and water electrolysis materials are also presented.
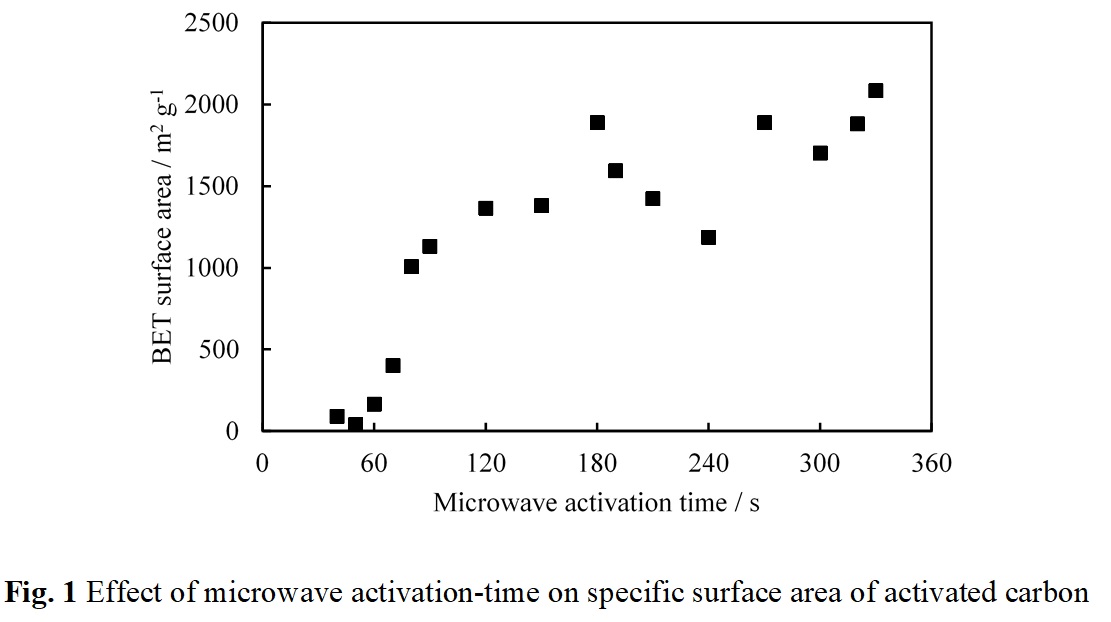
Several works reported that microwave heating can reduce production time required for activated carbon preparation. In this study, the mixture of carbonized carbon gel and KOH (mass ratio of carbon gel to KOH is 1:6) was heated by a modified microwave oven (2.45 GHz, 500 W) for 40-330 s under N2 atmosphere before being washed by distillation water. As presented in Fig. 1, BET surface area of resultant activated carbon tends to increase with an increase in microwave activation time. By using this proposed method, the large BET surface areas which are 1,007 and 2,084 m2g-1 can be achieved in the resultant activated carbon within 80 and 330 s, respectively. It has been reported that even when microwave heating is employed to reduce the activation time, the shortest record was 300 s. Such a short activation time as only 80 s to synthesize activated carbon with a large specific surface area above 1,000 m2g-1 by the proposed method is remarkable. It can be noted that bright plasma can be uniquely generated at the carbon-KOH mixture under microwave heating when the activated carbon with the large surface area is obtained. It may be considered that the carbonized carbon gel powders in contact with molten KOH may cause interfacial polarization under microwave heating, and this polarization may cause the observable plasma in the present condition, resulting in a high temperature. To date, the activation process for synthesizing activated carbon has been operated in batch system because of a long activation period. Once the mechanisms of the extremely fast activation method found in this study are clarified, a new concept of reactor design will be developed for operating in a continuous system.
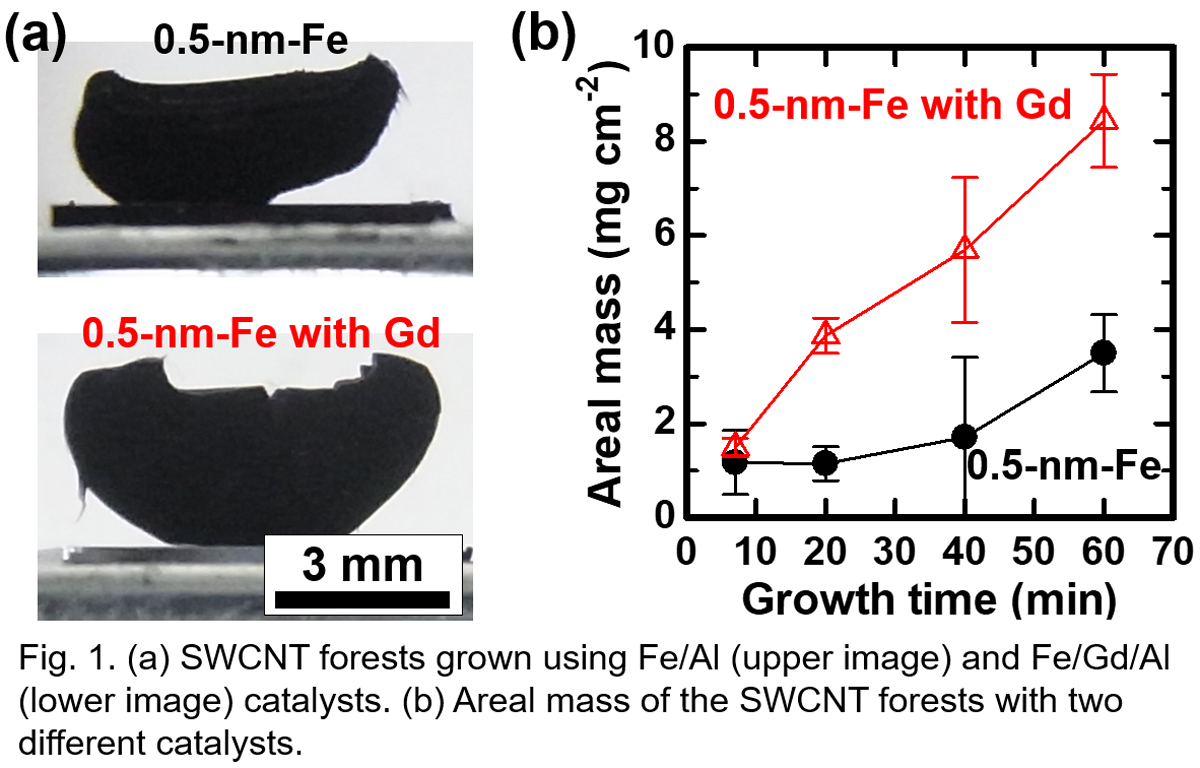
Growth of vertically-aligned single-wall carbon nanotube (VA-SWCNT) forests by the catalytic chemical vapor deposition (CVD) is an attractive method for making applications. However, the growth termination of the CNT forests is an obstacle, and the deactivation of the catalyst nanoparticles due to the structure change of the catalyst nanoparticles is thought to be one reason for the termination. In general, maintaining smaller catalyst nanoparticles which are necessary for the SWCNT growth is more difficult because the smaller nanoparticles are less stable. To realize the longer growth lifetime, engineering catalysts is crucially important. So far, Fe-Gd catalyst on Al2O3 layer was reported to realize the growth lifetime of 13 h and 22-mm-tall multi-walled CNT (MWCNT) forest at the growth temperature of 780 °C [1]. However, the growth rate is relatively low as ~0.5 μm s-1, and the possibility of using Gd for SWCNT growth was not discussed in detail.
In this work, we applied the Fe/Gd/Al catalyst to the growth of SWCNT forests, and systematically studied the mechanism behind the enhanced growth (Fig. 1a). By optimizing the catalyst condition, we achieved a high initial growth rate of ~2 μm s-1 and long catalyst lifetime of ~50 min at 800 °C. Correspondingly, the areal mass continued increasing up to ~8 mg cm-2 in 60 min (Fig. 1b). It was found that Gd layer with the thickness of less than 1 nm is effective when it is deposited between Fe and Al layers. The Raman spectra showed the radial breathing mode (RBM) peaks from the top to the bottom of the CNT forests, which suggests the continuous growth of SWCNTs.
References
[1] W. Cho et al., Carbon 72, 264 (2014).