
The control of the interface of micro and nano-composite materials are essential for developing environmentally benign methods for manufacturing microcapsules for Drug Delivery Systems (DDS). However, toxic organic solvents and multiple steps were often used which were unsuitable for manufacturing microcapsules for DDS in conventional methods. Here, we will talk about new methods we developed to control the interface of micro- and nano-composite particles and bubbles using a high pressure techniques. These methods used pressure-induced phase separation using super critical carbon dioxide (scCO2) solution and ultrasound irradiation under high pressure conditions. Some medicine, such as Levofloxacin were used as core materials and polymers having pH-responsive functions such as Eudragit E100 or L100 were used as coating materials. The coating materials were dissolved in scCO2 solution containing a co-solvent. As the pressure of the high pressure cell slowly decreased into atmospheric pressure, microcapsules were formed in the cell. The structure of the microcapsules was observed by SEM equipped with an electron probe microanalyzer (EPMA) devices. In order to confirm the absence of co-solvent in the capsule, we used the FT-IR. In the later part of the talk, we will introduce a novel method for producing and quanitfing micro- and nano- bubbles. These micro- and nano- bubbles were produced in a specially designed high pressure cell equipped with an ultrasonication horn located in the vessel. This technique is unique as micro phase separation between the high pressure gas and liquid interphase can be achieved through direct sonication, leading to the enhancement of micro- and nano- sized bubble production. The characterization of the mico- and nano- bubbles as well as its comparison with other conventional methods and possible future applications will be discussed.
Gold nanomaterials are of technological importance in catalysis, photovoltaic conversion, sensing, and biomedicine. The attractiveness of gold nanomaterials lies in their unique surface plasmon resonance phenomenon, which arises from the collective oscillation of surface electrons when the materials interact with incident electromagnetic waves. An important application utilizing the phenomenon is the surface-enhanced Raman scattering (SERS) technology for the detection of trace amounts of chemicals such as heavy metals, pesticides, explosives, narcotics, contaminants in food and water, etc. In particular, the inertness and non-toxicity of gold makes it favorable for personal care as well as diagnostic purposes. The surface plasmon resonant property strongly depends on the particle size, particle shape, and aggregation states of the material, hence morphological engineering is an efficient strategy for developing new plasmonic gold materials. Great efforts have been made to prepare gold nanostructures with varying morphologies. To date, spherical, rod-like, flower-like, sheet-like, raspberry-like, polyhedral, branched, hollow, and chiral nanoparticles have been synthesized. Besides, satellite-like and other sophisticated morphologies have been prepared through self-assembly or multi-step synthesis schemes.
Here we report an unconventional gold nanostructure through facile one-pot synthesis in aqueous solution from chloroauric acid precursor. The architecture is featured by pomegranate-like interior structure and raspberry-like surface morphology. The pomegranate-like gold structure achieves tunable plasmonic resonance absorption within 580-730 nm and shows SERS activity. The SERS measurements were conducted with the paper-based SERS technique using thiophenol as the probe molecule and a portable Raman spectrometer. The results showed that the pomegranate-like gold structure achieved a detection limit of 10 ng thiophenol. The performance is comparable with commercial paper-based SERS substrates, indicating the potential of pomegranate-like gold nanostructure for practical applications.
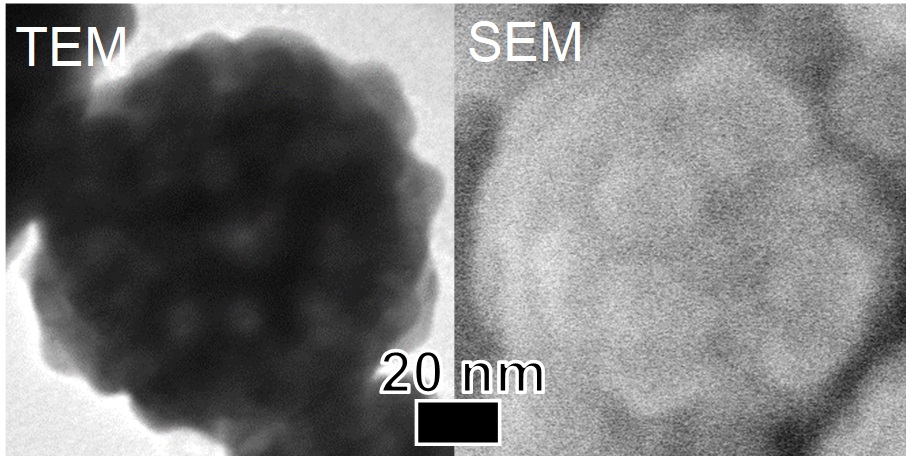
Fluorescent polymer particles immobilized with biological molecules on their surface are widely used as fluorescent probes and labels for biomedical applications. From this viewpoint, the present work proposes a simple method for fabricating such particles, which is one-pot formation in water medium. Submicron-sized monodisperse polymer particles not only surface-immobilized with sugar chain having a specific ability to be adsorbed to viruses and proteins but also incorporating fluorescent dye were fabricated with the proposed method. This method used octyl-β-D-glucopyranoside (octyl-β-D-glc), in which an octyl group is glycoside bound to a glucose skeleton as a sugar, and coumarin 7 as a fluorescent dye. During polymethylmetacrylate (PMMA) particle formation by soap-free emulsion polymerization, it is expected that the octyl group enter the PMMA particles due to its hydrophobicity and the glucose is in contact with the water medium due to its hydrophilicity. For the simplicity of the preparation procedure, both octyl-β-D-glc and coumarin 7 were added to the reaction solution at the beginning of the polymerization. The figure shows a SEM image of PMMA particles containing octyl-β-D-glc and coumarin 7. The monodisperse particles were obtained, and their average particle size was approximately 250 nm. Immobilization of octyl-β-D-glc on the particle surfaces was evaluated using concanavalin A (ConA) as a lectin, which has the selective affinity with glucose. The amount of Con A adsorbed onto the surfaces of particles fabricated with octyl-β-D-glc was twice larger than that with no octyl-β-D-glc, which confirmed successful octyl-β-D-glc-immobilization. The PMMA particles containing octyl-β-D-glc and coumarin 7 emitted fluorescence with a peak around 503 nm originated from coumarin 7. The spontaneous incorporation of coumarin 7 into the PMMA particles as well as surface-immobilization of octyl-β-D-glc on the particle surfaces took place during the particle growth in the proposed one-pot soap-free emulsion polymerization.
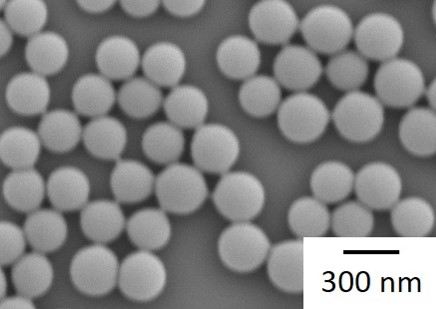
Flow microreactors have been widely studied for synthesizing monodispersed nanoparticles by utilizing their enhanced mixing performance owing to the shortened diffusion lengths. Mixing time is used exclusively to discuss the performance of mixing, however, more detailed parameters such as concentration profiles in the mixing space can affect the particle characters. In this study, we examined various nanoparticle synthesis processes with changing mixer structures and operating conditions. Various model materials were studied; calcium carbonate as inorganic crystal, Pt as a noble metal nanoparticle, ELM-12 as a metal-organic framework, and polystyrene as polymer latex. Two types of micromixers, tee mixers and micro-jet mixers, were employed with varying the inner diameters while keeping the Reynolds numbers. In this manner, factors involved in the nanoparticle synthesis processes with rapid mixing were separately analyzed and discussed. We found that change of the feed orientation with respect to the inlets of the micro-jet mixers can dramatically change the particle characters, implying the important role of detailed mixing behavior besides mixing time. The results showed that non-classical theory on the nucleation and growth processes should be considered for discussing the process precisely.
Microparticles of extracted compounds from seaweeds have been carried out by electrospraying method. The extracted compounds were obtained from hydrothermal extraction of Eucheuma cottonii (E. cottonii) and Gracilaria sp. The hydrothermal extraction was conducted in a semibatch extractor at temperature of 160oC, pressure of 7 MPa, and water flow rate of 1 mL/min. Subsequently, the extracted compounds were combined with polyvinylpyrrolidone (PVP) at various concentrations of 4, 6 and 8% w/v. The solution was sprayed via nozzle of 0.5 mm from positive electrode to negative collector. The distance between spinneret and collector was varied at 6, 8 and 10 cm. The applied voltage was 12, 14, and 16 kV. Electrospraying was carried out for 4 h with solution flow rate of 0.05 mL/h. The particles formed were analysed by SEM and FTIR to determine the particle morphology and their functional groups, respectively. In order to understand the change of materials by temperature, the particles formed were examined by thermal gravimetry analysis (TGA) method. Moreover, the antioxidant activity of particles was also measured by DPPH assay. Based on the experimental result, the highest mass production of particles was obtained at PVP concentration of 6% with applied voltage of 16 kV and distance of 6 cm. The highest mass production of particles from E. cottonii and Gracilaria sp were 19.97 mg and 20.02 mg, respectively. The mass production of particles increased with an increasing PVP concentration and voltage. The morphology of particles was spherical with particle size less than 3 μm. The increasing applied voltage and distance decreased the particle size. The antioxidant activity of particles was relatively high with the highest antioxidant efficiency for E. cottonii and Gracilaria sp of 0.182 min-1 and 0.106 min-1, respectively. The results indicated that electrospraying is promising method for production of pharmaceutical particles.