
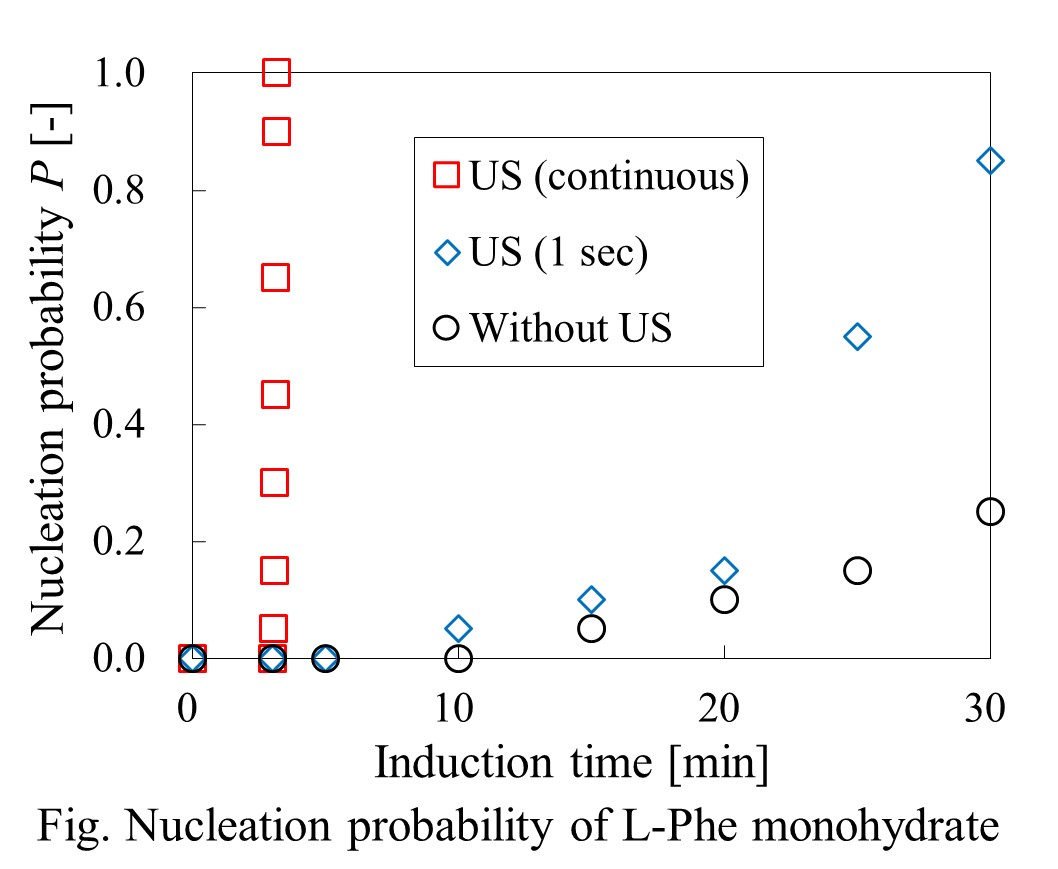
Amino acids are the components constituting proteins, and widely utilized as medical raw materials, food additives for seasonings and preservatives, and chelating agents and buffer agents of cosmetics. Most of the amino acids show polymorphism. Differences in the crystal structures affect the physical and chemical properties: solubility, stability, melting point, density, heat capacity, thermal conductivity, and optical activity. These differences influence the process acceptability, bioavailability, filtration, and tablet processes of pharmaceutical, food, and specialty materials. Therefore, an effective method for polymorph control is required in the crystallization process in order to improve the functionality of amino acid crystals and the process efficiency. As the method for polymorph control, sonocrystallization seems to have a great potential. The application of ultrasonic power causes localized mixing, heating and pressure oscillation that could contribute to control supersaturation, nucleation and crystal growth. During crystallization, sonication reduces the induction time, supersaturation, and metastable zone width. It is considerable that ultrasonic irradiation causes the nucleation directly, and sonocrystallization seems to be a simpler method to generate a desired polymorph. In this study, primary nucleation of L-Phenylalanine (L-Phe) in supersaturated solution by ultrasonic irradiation was investigated. Initially, predetermined amount of L-Phe was dissolved in purified water, and 2 mL of the L-Phe solution was added into a test tube and cooled in thermostat bath. Subsequently, in order to investigate the effects of ultrasonic irradiation, ultrasonic horn was inserted into the solution and ultrasonic wave of 20 kHz was irradiated. As a result, nucleation of L-Phe crystal was induced by ultrasonic irradiation, and nucleation probability was increased while the ultrasonic wave was irradiated. In addition, short time irradiation also caused the increase of nucleation probability. Moreover, in order to investigate the possibility of polymorph control, sonocrystallization experiment of each polymorph was performed.
Controlling of crystallization is a critical issue for chemical process in food and pharmaceuticals. In particular, nucleation behaviors of crystals are dominated by a variety of factors such as supersaturation degree, interfacial energy and solute characteristics. So far, we have performed droplet-based crystallization of lysozyme using a microfluidic device and found that nucleation frequency, N/N0, in monodisperse droplets would be a promising way to estimate the nucleation rate of solute. In this study, furthermore, the droplets with different sizes gives us a useful information for nucleation behaviors. From the results of droplet-size dependency in the nucleation frequencies of several solutes, nucleation in droplets was quite different from that in bulk solution.
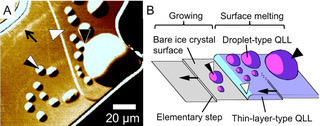
The phase transition of ice crystals governs a wide variety of phenomena on the earth. Hence, the molecular level understanding of the phase transition of ice is crucial to unlock the secrets of various phenomena. To visualize ice crystal surfaces at the molecular level, we and Olympus Corporation developed laser confocal microscopy combined with differential interference contrast microscopy (LCM-DIM) [1], which can visualize individual elementary steps (0.37 nm in height) on ice crystal surface [2]. By using LCM-DIM, we observed in-situ ice crystal surfaces under various conditions. In the conference, we will show the following two topics.
1) Thermodynamic origin of two-types of quasi-liquid layers (QLLs) with different morphologies (droplets and thin layers) [3]: with increasing water vapor pressure, the wettability between QLLs and ice crystal surfaces become significantly better, resulting in the changes in the wetting states from partial wetting (droplets) to pseudo-partial wetting (the coexistence of droplets and thin layers). The thickness of the thin layers agrees with the value predicted by the quantum theory calculation.
2) Novel uptake mechanism of HCl in ice crystals [4]: HCl gas, which triggers the depletion of the stratospheric ozone, is adsorbed on ice crystal surfaces. Then droplets of a HCl aqueous solution appear on the ice crystals surfaces. As the ice crystals grow, the HCl droplets are embedded in the ice crystals. This novel uptake mechanism allows ice crystals to store ten-times larger amount of HCl in ice crystals than the solubility of HCl in ice crystals.
[1] G. Sazaki et al., J. Cryst. Growth, 262, 536-542 (2004).
[2] G. Sazaki et al., Proc. Nat. Acad. Sci. USA., 107, 19702-19707 (2010).
[3] K. Murata et al., Proc. Nat. Acad. Sci. USA., 113, E6741-E6748 (2016).
[4] K. Nagashima et al., Cryst. Growth Des., 18, 4117-4122 (2018).
Reaction crystallization has been attracted attention because it can simultaneously perform synthesis, separation and purification process. To enhance productivity in the crystallization process, continuous flow system is used instead of conventional batch process. It is known that increasing of nucleation and growth rate is effective strategy for enhancement of the productivity in continuous flow production process. In particular, supersaturation is a main factor to achieve high productivity because nucleation and growth is promoted directly under high supersaturation condition. Unfortunately, agglomeration occurs when supersaturation increases, which affects purity of products and downstream process such as sieving or milling operation. Therefore, a novel process for enhancement of productivity without agglomeration is required.
According to the previous study, it is known that the nucleation is accelerated by shear stress. In addition, mass transfer promotes the growth rate. In this study, we focused on Taylor Vortex. Taylor Vortex is regular vortex ring and occurs continuously between rotating inner and stationary outer cylinders. Previous studies reported that Taylor Vortex shows high shear stress and fast mass transfer. This is expected that productivity can be enhanced without agglomeration by using Taylor Vortex flow. In addition, setting the various operating points is allowed because its mixing rate is extremely high compared with that of conventional batch system. Therefore, the novel crystallization process based on Taylor Vortex that is Reactallizer was proposed to obtain crystalline product with high quality in reaction crystallization.
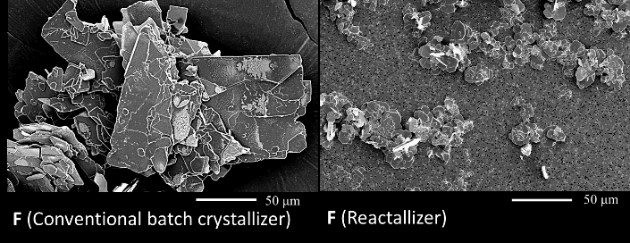
In this study, sodium L- aspartate - hydrochloric acid was used as feed. L-Aspartic acid is target product. From the result of SEM observation, it was showed that the size and agglomeration of obtained crystals using Reactallizer were improved comparing that of batch process (Figure 1). This means that our proposed crystallizer could provide fine crystals continuously without agglomeration in reaction crystallization.
Desired enantiomer crystals are obtained from a racemic solution using a preferential crystallization. However, undesired enantiomer is also crystallized from its solution, and consequently, the purity of product particles decreases. The different types of polymorphs indicate the different crystal structures although their molecular structures are the same. The solubilities differ by the types of polymorphs. If the different types of polymorphic crystals are deposited by the kinds of enantiomers, it is expected to be obtained the high purity products using a preferential crystallization. The purposes of this study are to examine the types of polymorphism crystallized from enantiomer aqueous solutions of glutamic acid (Glu) when phenylalanine (Phe) is added into their solution and to carry out a preferential crystallization of Glu.
When an L-Glu aqueous solution saturated at 333K was cooled to 323K and the a type of L-Glu seed crystals were added into its solution, the b type of L-Glu crystals was obtained. The b type of L-Glu crystallized from an L-Glu solution by addition of D-Phe. On the other hand, when L-Phe was dissolved into an L-Glu solution, the a type of L-Glu crystals was obtaned. Therefore, it is considered that L-Phe would inhibit the solvent-mediated polymorphic transition of L-Glu from an a type to an b one.
When an D-/L-Glu aqueous solution saturated at 323K was cooled to 313K and the a type of D-Glu crystals was seeded into its solution, the purity of product particles decreased with durations. On the contrary, since the L-Phe was dissoved in an D-/L-Glu solution and a preferential batch crystallization of D-Glu was carried out, the purity decreases seldom happened. Therefore, it is considered that the purity decrease for a preferential crystallization can be controlled by combination with polymorphism.