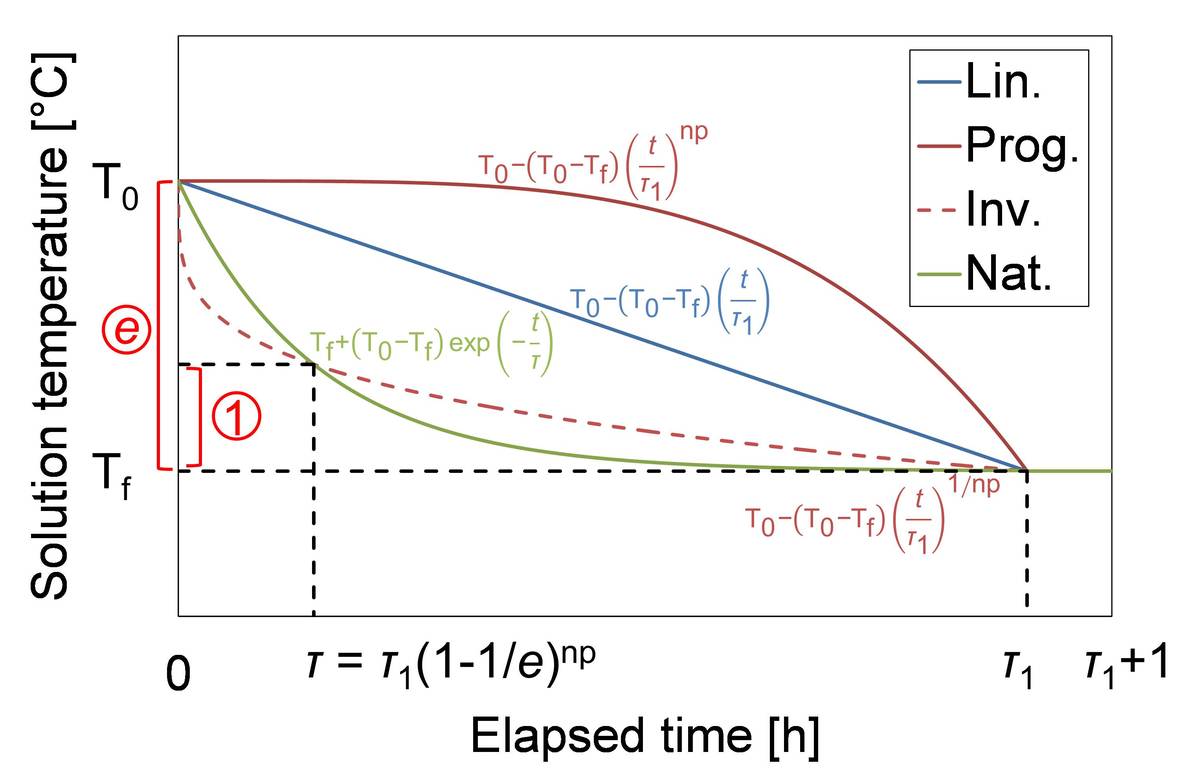
In the process of manufacturing crystal products, the crystal size distribution (CSD) of product crystals is required to be monodispersed and unimodal for improving productivity. Therefore, unintentional nucleation needs to be avoided in batch cooling crystallization because it leads to broad or bimodal CSD. Full seeding and partial seeding are well known as seeding policies for inhibiting or controlling nucleation. In full seeding, a large amount of seed crystals are added to inhibit secondary nucleation. On the other hand, in partial seeding, a small amount of seed crystals are added to trigger secondary nucleation and inhibit primary nucleation. In this study, partial seeding was mainly investigated. In the previous study, the optimum operating condition for partial seeding was successfully estimated based on coefficient of variation (CV) by utilizing computation results on inorganic model substance. Product CV was assumed to be small when seeding was effectively performed. Also in this study, the optimum operating condition and the resulting product properties were estimated for various cooling and seeding policies by utilizing computation results on inorganic model substance. Cooling policy was changed not only in the total batch time but also in the cooling mode, as seen in Figure. Here, linear cooling, programmed one, and natural one were performed. The order of programmed cooling np was set to 4 for small supersaturation in an early stage of crystallization. For improving symmetry of condition, the time constant of natural cooling was set to the same value as that of inverse programmed cooling the order of which was 1/np. Seeding policy was changed not only in the mean mass size but also in the CV. The population density of seed crystal was given by the convex quadratic function. Potassium sulfate was utilized as the inorganic model substance.
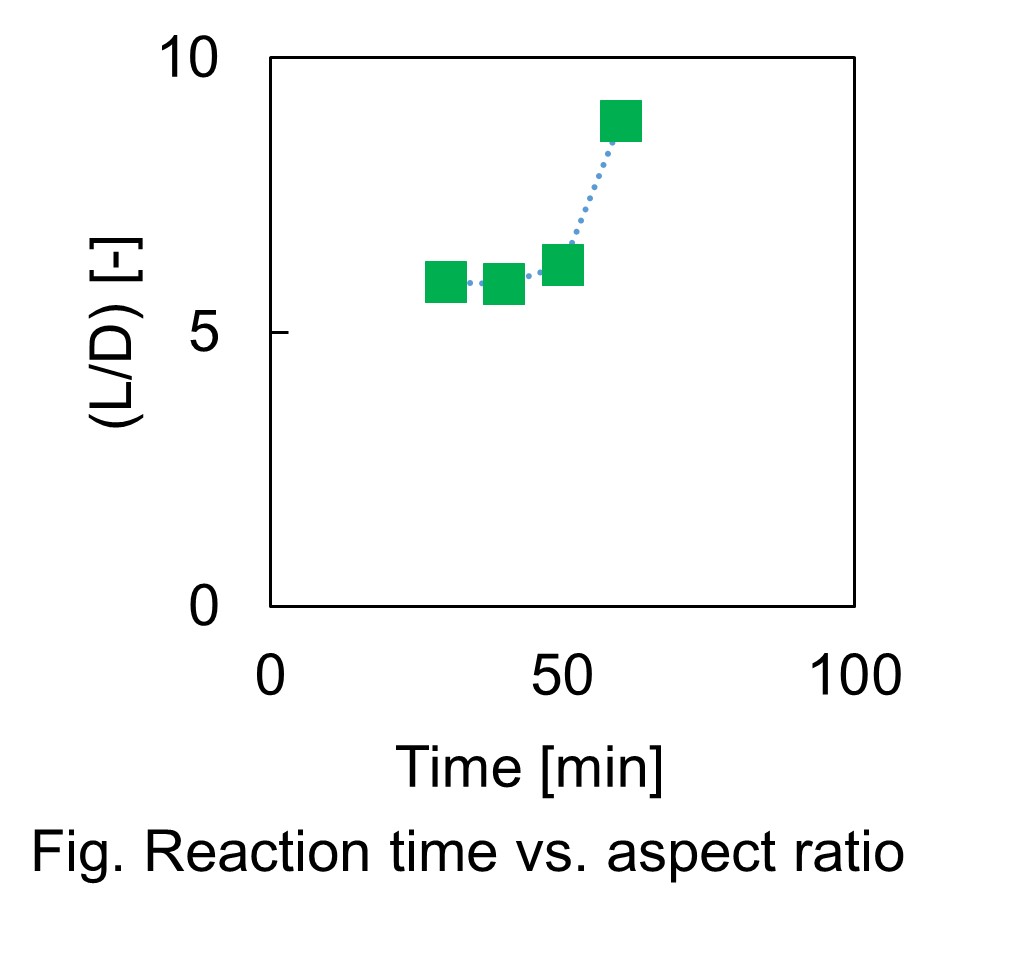
Transparent conductors are widely used for smart-phones, organic light emitting diodes and organic photovoltaics. Indium tin oxide (ITO) is the most common because of its high transmittance and conductivity. However, ITO have some defects. ITO is produced by a vapor phase synthesis and the rate is low, which makes ITO expensive. Second, indium is scarce. Finally, ITO is brittle. Therefore, the alternative transparent conductors have been studied eagerly. Among the studied transparent conductors, copper nanowires (Cu NWs) are promising. It is because copper is more inexpensive and abundant than indium. Moreover, Cu NWs are produced by a solution method, which is economical. Moreover, Cu NWs are flexible and this is advantageous for OLEDs and OPVs. Cu NWs are synthesized by a copper salt, a reducing agent and a capping agent, which determines the property, especially habit of crystals. The widely used capping agents for Cu NWs are amines. In this research, double-jet reactive reaction is applied for process of Cu NWs synthesis. In our previous study, it was difficult to synthesize Cu NWs of desired aspect ratio. On the other hand, double-jet process could control aspect ratio of Cu NWs by controlling reaction time, and to find out that concentration of reducing agent affects aspect ratio of Cu NWs.
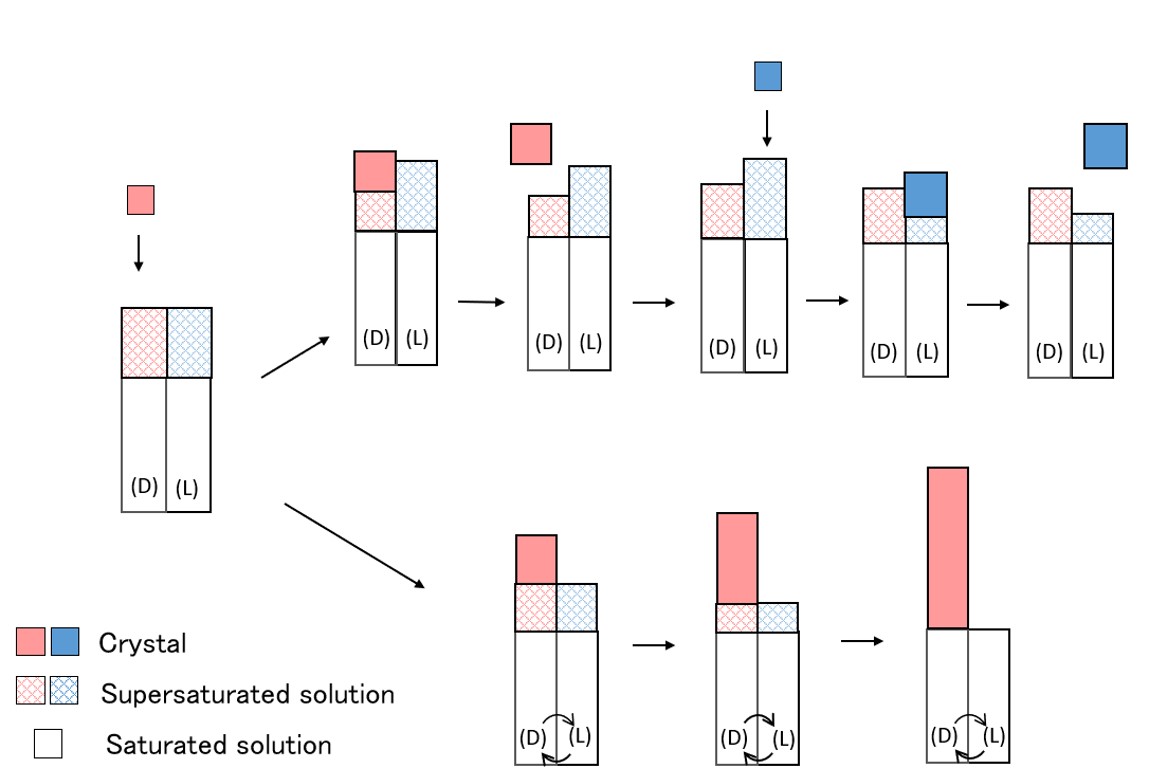
Many of the pharmaceutical compounds have asymmetric carbon. There is a need for techniques to reliably obtain one enantiomer. One of the methods of optical resolution is a preferential crystallization. In the preferential crystallization, crystals with high purity can be obtained by a simple operation. However, if chiral molecules do not racemize in solution, the maximum yield is 50%. By racemizing the chiral molecule in solution, other enantiomers can be converted to opposite enantiomer and crystallized as the desired product. So, the maximum yield would be 100%. Furthermore, repeated operations will be eliminated. In this study, the enzymatic reaction by racemase was examined as a method for racemization. We aimed to improve yield by performing preferential crystallization of DL-alanine combining it with racemization by alanine racemase. The alanine racemases gene from Streptococcus pneumoniae was artificially synthesized and expressed in E. coli shuffle T7. After several steps of purification, the enzyme of 35 kDa with electrophoretic homogeneity was obtained. The enzyme activity of alanine racemase was measured by monitoring the concentration of L-alanine with a circular dichroism dispersion meter. Specific activity for the conversion of L-alanine to D-alanine was 18.4 U/mg. Since alanine is crystallized as a racemic compound, it cannot be resolved by preferential crystallization. Thus, in order to crystallize as a racemic conglomerate, co-crystallization of alanine and p-chlorobenzene sulfonic acid (CBS) was performed. Optical resolution by preferential crystallization was performed. The alanine was dissolved at 40 °C and slowly cooled to 25 °C. When the solution reached to saturation temperature, seed crystals of D-Ala/CBS were added into the solution. After stirring for 1 hour at 25 °C, the crystals were recovered by filtration. The yield of crystals and the enantiomeric excess were 74% and 72%ee, respectively.
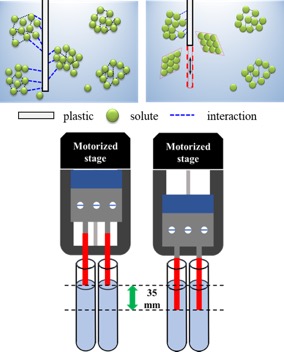
Since crystal nucleation directly affects the characteristics of the crystal, control of crystal nucleation is crucial in industrial crystallization. Previously, we reported that active diffusion of solute molecules in solution could be a trigger of nucleation. In this study, we investigated a new strategy to induce crystal nucleation. When immersing plastic pieces in solution, intermolecular interactions occur between the plastic surface and the solute molecules. When the plastic piece is moved vertically in this state, solute molecules which interact with the plastic also move together. The movement of the solute molecule reproduces unidirectional active diffusion of the solute molecule, and it is thought that crystal nucleation could be induced. In order to confirm this, we investigated the effect of the movement of plastic pieces on the crystal nucleation of L-alanine. Special experimental equipment and image processing software were developed. The plastic piece (polypropylene (PP)) was moved up and down vertically in alanine solution at a constant speed of 0.5, 2.0, and 5.0 mm/s. After that, the time when the first crystal was observed was recorded for at least 27 samples. Results were analyzed by cumulative distribution functions, and nucleation rate and average induction time were estimated. At all speeds, the nucleation rates were much higher than when the plastic was not in contact. In addition, it was also found that no crystals were generated when the plastic was immersed in the solution and left to stand. Therefore, it was suggested that the movement of plastic in solution induces nucleation of L-alanine. Comparing the nucleation rates at each speed, the nucleation rate was fastest at 2.0 mm/s. From these results, it was concluded that nucleation induction by the movement of plastic is caused through two processes, that is, the formation of intermolecular interaction and unidirectional movement of solute molecules.
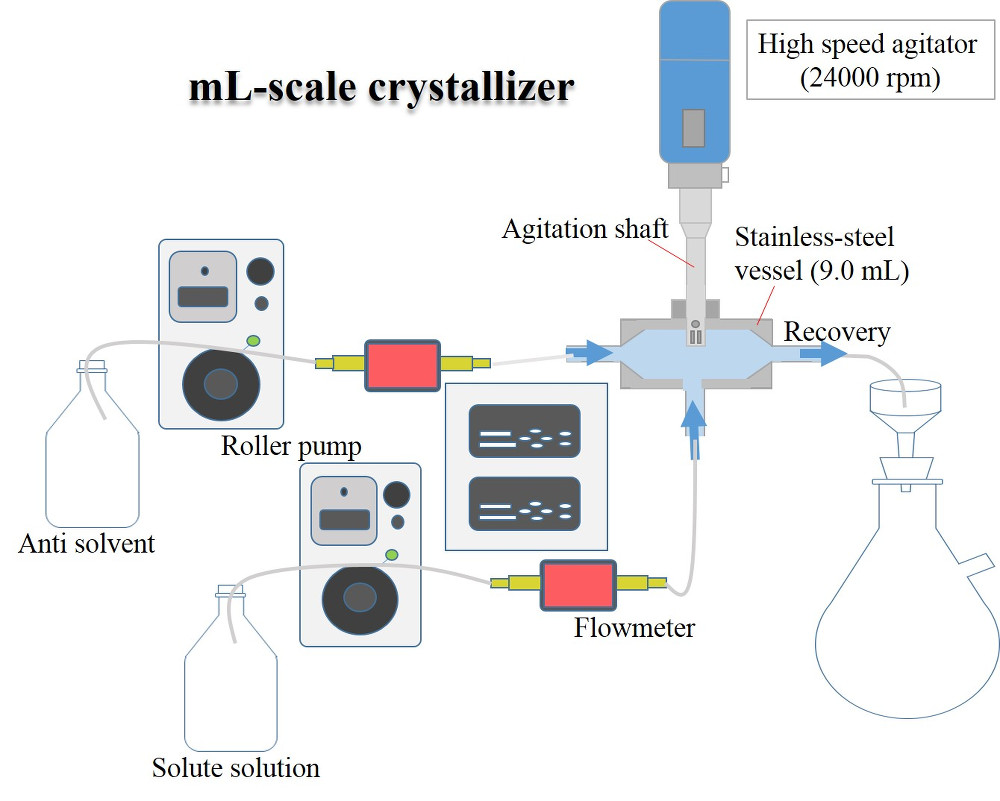
Novel small scale continuous crystallizer was developed to produce small uniform crystals. In the field of pharmaceutical crystallization, research on continuous crystallization is actively conducted. In particular, there is a need for a technique for continuously producing microcrystals without milling process. However, since the conventional continuous crystallizer has a large working volume, the residence time distribution becomes large, and it is difficult to control the crystal characteristics. Thus, we have developed a small continuous crystallizer to realize short residence time. The crystallizer is a 9 mL stainless steel device equipped with a high-speed agitator with a maximum stirring speed of 24000 rpm. Comparing with the large scale crystallizer, the average residence time is short, the distribution of residence times is narrow, and the concentration and temperature in the crystallizer are homogeneous. Therefore, small uniform crystals can be obtained. Anti-solvent crystallization of glycine was performed. Ethanol was used as a poor solvent. Almost 100% yield was attained even very short residence time (0.3-33 s). The shape and particle size of the obtained crystals were observed with a scanning electron microscope. More uniform crystals were observed compared to that obtained by the conventional crystallizer. Furthermore, it was also advantageous to polymorphic control. Due to the short residence time, the desired metastable crystals were recovered avoiding the solvent-mediated transition to the stable form.
Unseeded semi-batch cooling crystallization of potassium alum (AlK(SO4)12H2O, K-alum) was carried out to obtain uniform crystals with narrow size distribution. In this paper, influence of feeding condition on crystal size distribution is mainly discussed. Seeding policy studied by the research group of Iwate University is a powerful idea for the successful inhibition of secondary nucleation leading to monodisperse crystals. However, seeding methodology is often undesired particularly in the pharmaceutical and/or food industries because the possibility of containing impurity in the seed crystals is not always ignored. The internal seeding method (unseeded method) was reported by the above research group, however, the theoretical aspect of operation design has not been clarified. This study continues the internal seeding method to develop theoretical feed condition as unseeded crystallization policy. One of the strategy to obtain narrow size distribution is the division of cooling stage (feeding stage) for the separation of nucleation and growth period.
Polymorphism occurs when a compound can exist in more than one crystalline form. Because of the different properties of polymorphs, it is advantageous to choose the proper polymorph for the desired application. In the crystallization processes involving polymorphs, the formation of the desired polymorph has to be controlled and such a process should be robust and reproducible. In the polymorphic crystallization, relative nucleation rates, relative growth rates, and the transformation of crystallizing polymorphs should be controlled individually. Therefore, the determination of the crystallization (nucleation and growth) and dissolution kinetics, and thermodynamics are important for characterization of the crystallization behavior and transformation of the polymorphs. Thermodynamics determines the polymorphic forms and their properties such as solubility. The nucleation, growth and dissolution kinetics determine the kinetics of the crystallization and transformation of the polymorphs. In this work L-histidine (L-his) is the model substance; it is one of the essential amino acids and is significant in the food and feed industries. There are two known polymorphic forms, forms A and B. Form A is the stable form and form B is the metastable form. In the present study the crystal growth kinetics of the metastable polymorph B of L-his were performed and studied. The crystal growth rates were measured in aqueous solution of L-his. The crystal growth rates increased with increasing temperature, concentration of solute, and seed mass. The growth rates were correlated to the supersaturation with a power-low equation. The results indicated that the crystal growth process was controlled by surface integration mechanism at temperature of 10 and 25 degreeC and diffusion mechanism at 40 degreeC. Growth kinetics of the polymorphs of L-his will be used to begin characterization of the polymorphic transformations and the overall crystallization rate of L-histidine.
Crystallization is used widely in industrial processes for products such as chemicals and pharmaceuticals. Stirred-type crystallizers can produce various complex phenomena such as crystal growth, abrasion, and aggregation, which affect the total number of crystal particles in a vessel and their particle size distribution. Population balance analysis of the number of crystals coupled with mass balance of the solute material provides an important means of predicting and controlling the number of crystals in the crystallizer. In this study, particularly addressing the aggregation phenomenon, methods for measuring the degree of supersaturation in solution, the particle size distribution, and the amount of aggregation were developed. Relations among them were investigated for various seeding and cooling rate conditions.
Batch-type cooling crystallization of potash alum started with seeding was examined as a model case. The solution contains crystal particles in the vessel aspirated through the quartz observation cell every 10 min. A high-speed movie camera recorded the motion of crystals through the cell. The particle size distribution was calculated from the obtained image using the original MATLAB program. Moreover, the time evolution of solution concentration was estimated from measurements of the solution electrical conductivity and temperature. The solution conductivity is affected by the suspended crystal particles. Therefore, the effects of particle size and suspension concentration on conductivity were calibrated using unimodal glass particles.
Results show that the time evolution of the solution supersaturation degree and crystal aggregation were estimated successfully based on the combination of the solution conductivity, temperature, and particle size distribution measured during the crystallization operation. When the seed crystals were few, the crystal surface area was small during the initial stage of crystallization; then the mode diameter of crystals in the final stage became large.
Low aqueous solubility of crystalline drugs is the major issue in the pharmaceutical field. Normally, two-component amorphous solid dispersion is used as conventional strategy for improvement of their solubility. Unfortunately, it is known that amorphous phase shows poor stability. Therefore, development of novel strategy for improvement of solubility is strongly desired. On the other hand, our recent study using eutectic solid dispersion found that nucleation rate enhancement of second component encourages refining eutectic phase including target component. Normally, refining eutectic phase including target component is favorable structure for enhancement of dissolution rate. Therefore, it is suggested that enhancement of nucleation rate is probably effective strategy for improvement their solubility. In addition, it is expected that formation of eutectic having a refined microstructure occur with refining the eutectic phase, which affects their solubility. Therefore, the objective of this present study is development of newly strategy for improvement of dissolution rate by nucleation rate enhancement of second component based on eutectic melt system. Sorbitol (target component)-Xylitol (second component) was used in the experiment system. In this study, we focused on two nucleation factors that are shear force and supersaturation. Therefore, we investigated the influence of seed crystal mass and cooling rate on the nucleation of the second component and dissolution rate of target component. As a result, it is showed that dissolution rate of the crystal phase was influenced by the seed crystal mass and the cooling rate. Furthermore, it was found that the dissolution rate increased with increasing of seed crystal mass and cooling rate. This means that solubility could be improved by promoting the nucleation rate of the second component. Therefore, our novel concept based on nucleation rate enhancement of second component is probably effective strategy to improve the solubility of target component.
Crystallization operation is used as the final process in chemical products manufacturing. The production of crystals with high quality is required because the final product quality strongly depends on the crystal quality. Therefore, quality control in the crystallization operation is essential.
The crystal quality is determined by desired many kinds of characteristics. Generally, the average value of each characteristic of individual crystals is used as standard whether the desired quality is achieved or not. On the other hand, it is also important to evaluate the variation of their qualities constituting the crystalline particles. In particular, their qualities tend to vary when the agglomeration phenomenon occurs. Therefore, achievement of the desired quality and improvement of the variations in individual crystal quality is strongly needed to control in crystallization process. Our present work reported that homogeneity is a useful index to evaluate variations of crystalline particles. Thereby, the purpose of this research is to develop the newly method of crystallization operation for improvement of homogeneity for agglomerated crystalline particles in reaction crystallization. In this study, L-Aspartate sodium (L-AspNa) and hydrochloric acid (HCl) were used as reaction system. L-Aspartic acid is objective crystalline material.
As a result, we found that homogeneity could be improved by supersaturation modulation operation. Unfortunately, it was also showed that using only supersaturation as an operation variable is not enough to product crystals with various qualities because it directly influenced on their characteristics such as size and shape. For this reason, pH of the solution was used as additional operation variable for quality control of crystals because solubility of L-Asparatic acid depends on pH. Consequently, production of crystals having various qualities was succeeded with keeping high homogeneity. Therefore, it was suggested that crystalline particles with high homogeneity could be obtained by using the multiple operation variable.
Quality control in the crystallization operation is required to obtain desired products because its quality depends on the properties of crystalline particles. It is known that their properties such as morphology and shape are determined by nucleation and growth of crystal. Thereby, controlling the nucleation and growth process individually is important. Normally, nucleation and growth occur simultaneously in conventional crystallizers, which means that conventional crystallizer are not suitable to obtain uniform product. For this reason, development of novel crystallizer for controlling nucleation and growth individually is needed. On the other hand, designing a newly production process based on conventional crystallizer has some advantages because of low investment cost and standard operation. Therefore, it is suggested that a crystallization process specializing nucleation should be integrated to conventional process in serial to separate nucleation and growth. We focused on the Taylor Vortex. Previous study reported that Taylor Vortex shows high shear stress due to its specific vortex ring, which is favorable flow state for enhancement of secondary nucleation. However, the driving force of nucleation and growth is determined by a supersatutration of feed solution. This indicates that the balance of each processes is limited even if nucleation is separated from growth using two continuous crystallizers. On the contrary, we designed the novel cascade type crystallizer based on Taylor Vortex. The purpose of this study is investigation of operation strategy for designed cascade type crystallizer to produce crystalline particles with uniform quality. The crystal shapes obtained by conventional and novel cascade process were evaluated using SEM. As a result, the dispersion of crystal size became narrow, which means that quality of crystal could be improved by using our designed crystallizer. We expect that our proposed crystallizer is favorable continuous process to produce crystalline products with desired quality.
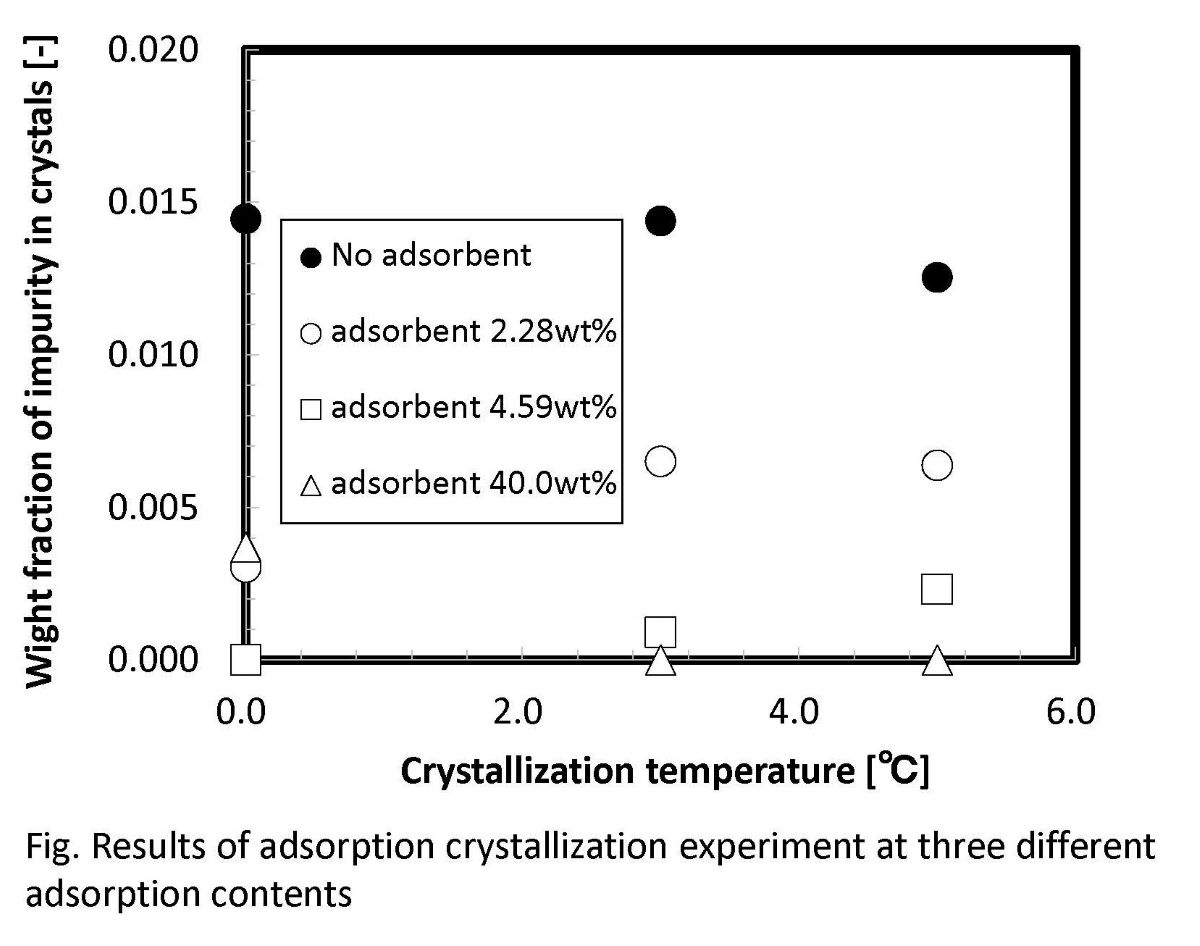
The adsorption experiments were first carried out to remove the impure acetic acid from the mixed acids (phosphoric acid + acetic acid) as raw model waste solutions. The adsorbent used in this study was very effective to adsorb acetic acid selectively. The adsorption capacity of adsorbent was about 5% for acetic acid. Moreover, new adsorption-assisted crystallization was performed for purification of phosphoric acid crystals from the mixed acids. The crystallization temperature and the additional ratio of the adsorbent was changed as the experimental conditions. It was found that crystals of purity more than 99% phosphoric acid were easily obtained. The yield of phosphoric acid crystals increased as the crystallization temperature was lowered. The purity of phosphoric acid crystals increased slightly when the additional ratio of adsorbent was increased in the mixed acids. It was clarified that high purity phosphoric acid crystals can be purified efficiently by the synergetic effect of the adsorbent.
Recovery of valuable resources from waste plating solution has been of great interest from the viewpoints of both environmental protection and resource management. In this study, we attempt reactive crystallization combined with ozone oxidation to recover phosphorus species, which are contained in a waste plating solution as the reducing agent for electroless nickel-phosphorus plating, as calcium phosphate. The effects of the experimental conditions such as pH, temperature, the amount of calcium chloride added to the solution on the recovery yield are examined.
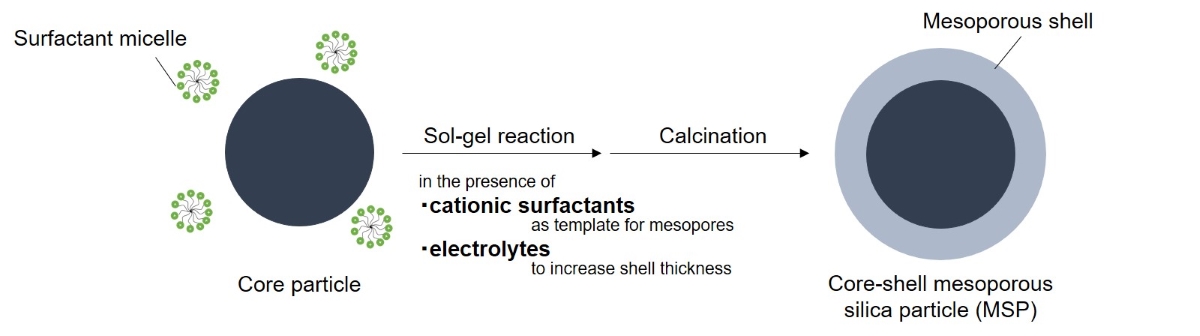
Core-shell mesoporous silica particles (MSPs) composed of a nonporous core and porous shell have attracted attentions due to higher monodispersity in particle sizes and more highly mechanical strength than mesoporous particles without a core. The core-shell MSPs can be used as packing materials to separate chemical substances in high performance liquid chromatography (HPLC) [1]. The performance of separation strongly depends on physical properties of MSPs, including particle sizes, pore sizes and shell thickness. The physical properties should be tuned to separate a variety of chemical substances.
Our group has been working on the synthesis of MSPs in sol-gel reaction where hydrolysis and condensation of silicon alkoxide are conducted in the presence of submicron-sized cores and cationic surfactants under basic conditions [2]. In conventional sol-gel reaction without cationic surfactants, sizes of silica particles formed are increased by electrolyte addition to the reaction system [3]. The increase in particle sizes by electrolyte addition has a potential of thickening mesoporous shell on the cores in the presence of cationic surfactants.
In this study, synthesis of core-shell MSPs was performed in the presence of electrolytes to increase the mesoporous shell thickness.
References
[1] Hayes R., Ahmed A., Edge T. and Zhang H., “Core–shell particles: Preparation, fundamentals and applications in high performance liquid chromatography”, J. Chromatogr. A, 1357, 36–52 (2014)
[2] Ishii H., Nara M., Hashimoto Y., Kanno A., Ishikawa S., Nagao D. and Konno M., “Uniform formation of mesoporous silica shell on micron-sized cores in the presence of hydrocarbon used as a swelling agent”, J. Sol-Gel Sci. Tech., 85, 539-545 (2018)
[3] Nagao D., Kon Y., Satoh T. and Konno M. “Electrostatic interactions in Formation of Particles from Tetraethyl Orthosilicate”, J. Chem. Eng. Japan. 33, 468-473 (2000)
Mesoporous silica are functional materials with high specific surface area caused by regularly ordered mesopores. Mesoporous silica have been applied to absorbents, catalyst supports and drug delivery carrier. In order to apply the mesoporous silica to various fields, particulate mesoporous silica is attracting a lot of attention. One of the important factor to be controlled is pore size distributions in the synthesis of mesoporous silica particles (MSPs). Micelles of surfactants can be a template for mesopores of silica particles formed in the sol-gel reaction [1, 2]. Addition of hydrophobic molecules as a swelling agent to the synthetic system could enlarge pores in the silica matrix [1].
Our group has been working on the synthesis of MSPs in sol-gel reactions in mixed solvents of water and ethanol in the presence of cationic surfactant used as template micelles [3]. The addition of swelling agents increased sizes of mesopores in MSPs [4], although mechanism on enlargement of mesopores in the formation of MSPs is not sufficiently clarified.
The present study examined the effects of solvent composition on spherical particle formation and pore size distribution. The affinity of swelling agents to the mixed solvent was examined using different hydrophobic molecules. Analysis by Hansen solubility parameter (HSP) could correlate swelling agents with pore size distribution.
[1] J. S. Beck et al., J. Am. Chem. Soc., 114, 10834–10843 (1992), [2] T. Yanagisawa et al., Bull. Chem.Soc.Jpn, 63, 988–992 (1990), [3] H. Ishii et al., Adv. Powder Technol., 27, 448–453 (2016), [4] H. Ishii et al., J. Sol-Gel Sci. Technol., 85, 539–545 (2018)
Characteristics of particles strongly depend on the particle shape. Synthesis of anisotropic particles has been extensively studied to functionalize particles in recent years. Location and orientation of the anisotropic particles can diversify colloidal assemblies of the anisotropic particles.
To fabricate the colloidal assemblies, several methods such as suspension drying and using chemical interaction between particles have been developed. Depletion interaction is one of the powerful approaches to assemble colloids into desired structures in solution. The attractive depletion interaction between particles is induced by osmotic pressure in the presence of depletants (e.g. non-absorbing polymer, surfactants and nanoparticles). The attractive interaction is proportional to both depletant concentration and overlapping between depletant layers to which depletants cannot access.
Our group previously reported depletion-interaction-driven assembly of golf ball-like particles for development of colloidal macromolecules [1]. Golf ball-like particles have a large number of dimples on their surface.
In the present work, we tried to synthesize golf ball-like particles having hemi-rod-shaped dimples and demonstrated an assembling of the golf ball-like particles and rod-shaped particles via depletion interaction. Anisotropic particles with rod-shapes are expected to be captured by the hemi-rod-shaped dimples due to depletion interaction. The capture of particles has a potential of new purification process in which targeting particles can be intentionally collected depending on their shapes and sizes.
[1] K. Watanabe, Y. Tajima, T. Shimura, H. Ishii, D. Nagao, Journal of Colloid and Interface Science, 534, 81-87 (2019).
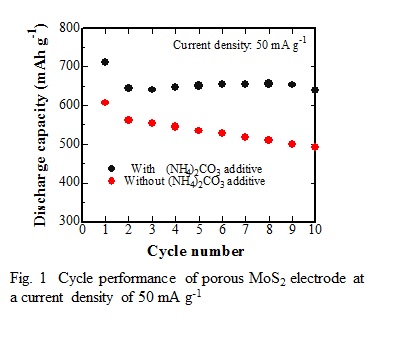
Recently, MoS2 is considered to be the most prospective candidate of anode materials for lithium ion batteries becuase of its high theoretical capacity (670 mAh g-1) and layered structure. However, it has usually suffered from structural deterioration, due to a large volume change on charge/discharge and low intrinsic electrical conductivity, which lead to a poor cycling performance. In this study, we have prepared porous MoS2 microspheres by spray pyrolysis with (NH4)2CO3 additive and investigated their pysical and electrochemical properties.
The precursor solutions were prepared by dissolving the required amount of (NH4)2MoS4 and (NH4)2CO3 in distilled water. Spray pyrolysis synthesis was conducted at different synthesis temperatures and concentrations of precursor solutions at a gas (3%H2+97%Ar) flow rate of 2 L min-1. The resulting samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS). N2 adsorption–desorption measurements were performed at a liquid N2 temperature of 77K. Pore volume and size distribution were estimated from the desorption branches of the isotherms by the Barrett–Joyner–Halenda model. Their electrochemical properties have been investigated using CR2032 coin-type cells.
The XRD peaks of the sample prepared at 800°C from the precursor solution of 0.05 mol L-1 (NH4)2MoS4 and 0.50 mol L-1 (NH4)2CO3 could be indexed well to the hexagonal phase of MoS2 (ICSD No. 31067) without any other phases. The desired Mo-related bond features of the sample were confirmed by XPS analysis. The pore structure analysis based on N2 adsorption–desorption isotherm led that the MoS2 microspheres with pore size less than 200 nm could be prepared and the (NH4)2CO3 additive in precursor solution could enlarge the porosity in MoS2 microspheres, especially the pore size between 20 to 100 nm. As a result, the porous MoS2 microspheres improved its cycle performance, as shown in Fig. 1.
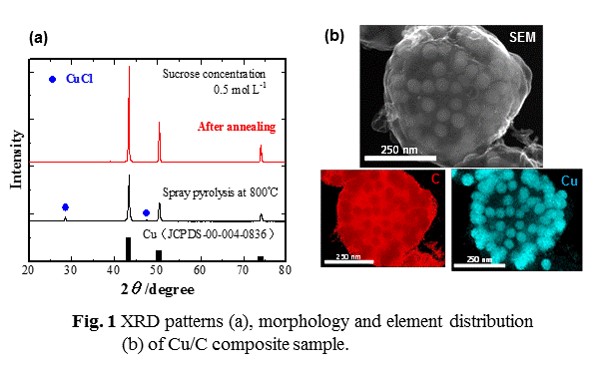
Porous carbon microspheres impregnated with transition metal-based nanoparticles have been considered as next generation materials for energy and environmental applications. In this study, we have prepared cupper (Cu) nanoparticles impregnated porous carbon microspheres by spray pyrolysis with heat treatment from the precursor solutions which appropriate amount of sucrose (C12H22O11) and cupper chloride dihydrate( CuCl2 2H2O) with citric acid (C6H8O7) additive were dissolved in distilled water, and investigated their pysical properties.
The precursor solutions were prepared by dissolving the required amount of C12H22O11,CuCl2 2H2O and C6H8O7 in distilled water. Spray pyrolysis synthesis was conducted for different synthesis temperatures and concentrations of precursor solutions at a gas (N2) flow rate of 2 L min-1. The as-prepared samples were further annealed at 800 °C for 2 h in a 3%H2/97%N2 mixture gas. The resulting samples were characterized by X-ray diffraction (XRD), field emission-scanning electron microscopy (FE-SEM) with an energy dispersive spectroscopy (EDS). Nitrogen (N2) adsorption–desorption measurements were performed at a liquid N2 temperature of 77 K The carbon content of the samples was estimated using an element analyzer (CHNS, Elementar, Vario Micro Cube).
Fig. 1a shows the XRD patterns of the sample prepared at 800 °C from the precursor solution by spray pyrolysis and then annealed at 800 °C for 2 h in a 3%H2/97%N2 mixture gas. It can be clearly seen from the XRD pattern of as-annealed sample that crystallized Cu could be obtained from the present preparation route. The N2 adsorption-disorption isotherm of the as-annealed sample indicated that it had mesopore structure, and the content of carbon was 28 wt% by CHNS analysis. Furthermore, it could be verified from the SEM-EDS analysis that the porous carbon microspheres impregnated with cupper nanoparticles could be sythesised by the present preparation route (Fig. 1b)
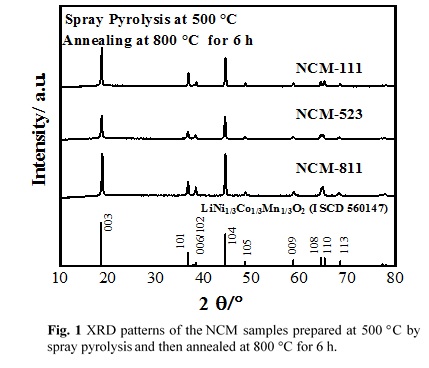
LiNixCoyMnzO2 (x≥0.33, x+y+z=1) (NCM) have a great potential as cathode materials for Lithium-ion batteries due to their abundant sources, high specific capacities and outstanding thermal stability. With the increasing content of nickel, NCM shows higher reversible capacity together with lower cost and toxicity. In this study, spherical nanostructured NCM particles were synthesized directly from homogeneous precursor solutions by spray pyrolysis followed by annealing in air atmosphere. The effect of process parameters on the physical and electrochemical properties of NCM was investigated.
LiNO3, Ni(NO3)2·6H2O, Mn(NO3)3·6H2O and Co(NO3)2·6H2O were used as the starting materials. These materials were dissolved in distilled water with the stoichiometric ratios of NMC. The starting solutions were atomized by an ultrasonic nebulizer at a frequency of 1.7 MHz. Then the generated droplets were carried to a spray pyrolysis reactor heated at different temperatures (400 -600 °C ) by the air gas with a flow rate of 2 L min-1. The as-prepared samples were further annealed at different temperatures in air atmosphere.
Crystal structures of the synthesized materials were determined by X-ray diffraction (XRD) analysis using Cu-Kα radiation. Fig. 1 shows the XRD patterns of the LiNi1/3Co1/3Mn1/3O2 (NCM-111), LiNi0.5Co0.2Mn0.3O2 (NCM-523) and LiNi0.8Co0.1Mn0.1O2 (NCM-811) samples synthesized at 500 °C by spray pyrolysis followed by annealing at 800 °C for 6 h. It can be seen that the well-crystallized NCM samples are formed with α-NaFeO2 hexagonal structure.
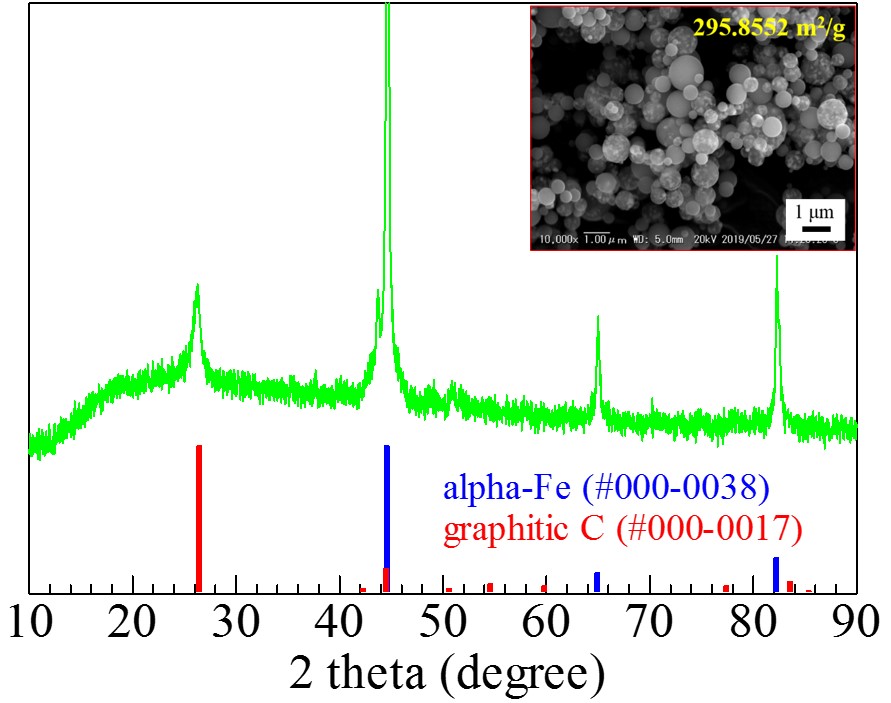
Integration of renewable energy sources with energy storage systems appears to be an encouraging approach to reach sustainability in green energy supply. It is expected that iron particles dispersed carbon microspheres (Fe@C) will find a broad application in many future generation energy storage systems and environmental issues. In this work, we have synthesized Fe@C using ultrasonic spray pyrolysis (USP) followed by annealing and studied synthesis parameters.
The precursor solution was prepared by dissolving an appropriate amount of sucrose, ammonium nitrate and iron nitrate in distilled water. Spray pyrolysis synthesis of Fe@C was carried out at 700 °C in an inert atmosphere (pure N2). Post-annealing of the samples was conducted at 700 °C for 4 hours in a reducing atmosphere (3%H2/97%N2). The obtained samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectra, and Brunauer-Emmett-Teller (BET).
Fig.1 shows the XRD pattern of the Fe@C prepared at 700 °C spray pyrolysis temperature followed by annealing at 700 °C for 4 hours. Three sharp peaks at approximately 45 °, 65 ° and 82 ° correspond to zero valent Fe while a peak at about 27 ° indicates the formation of graphitic carbon. SEM observation of the obtained sample shows that zero valent Fe particles evenly dispersed in the carbon microspheres (Fig.1 inset).
Gallium oxide (Ga2O3) is known to have five polymorphs, that are, α-, β-, γ-, ホエ-, and ε- Ga2O3. Among them, β-Ga2O3 is the most stable crystal structure and attracted recent attention as the material for power electronics, solar cells, and sensors. The conventional synthetic method of β-Ga2O3 nanoparticles is the so-called two-step method where hydrothermally synthesized GaOOH is calcinated at temperatures around 700 °C or above. In this presentation, we report an alternative one-step method to synthesize of β-Ga2O3 nanoparticles under solvothermal condition. In this method, gallium nitrate was dissolved in alcohol and then heated at 300 °C or above in a high-pressure reactor. The solid products were purified and analyzed by an X-ray diffractometer and a scanning electron microscope. Results showed that the crystal structure of the products gradually changed from γ-Ga2O3 to β-Ga2O3 as the reaction time increased. The average size of the produced nanoparticles was about 50 to 100 nm, which is much smaller than the products from the two-step method. This might be because our one-step method does not require a calcination process that allows the aggregation of particles during annealing.
Metal nanoparticles play an important role in catalysis, sensing, and imaging applications. Bimetallic systems are attractive for improving the physical and chemical properties by varying the elemental combination, composition ratio, and architectures of nanoparticles. However, designing the bimetallic nanoparticles are difficult because of many factors influences the nucleation and growth processes. In this study, we focused on the reduction rate of the metal precursors during the polyol synthesis of Ag-Rh nanoparticles. A microflow reactor was employed for obtaining reliable kinetic information by enhanced heat transfer performance. Reaction at elevated temperature in ethylene glycol was successfully examined by rapid heating and cooling with a microflow reactor. Ag precursor and Rh precursor showed strong interaction on the reduction rate. Reduction of Ag+ in ethylene glycol got slower with the higher reaction temperature, which contradicts to the Arrhenius law. The concentration profile of rhodium acetate would cause complex behavior due to its catalytic effect on the reduction. Elemental mapping of synthesized particles implied that the solid-state diffusion played a crucial role in the formation of alloyed Ag-Rh nanostructures.
When a small amount of water is added to a suspension in which particles are dispersed in oil, the number water between particles is formed. Such a substance is called a capillary suspension, it having fluidity change to flow characteristics like gel. However, detailed mechanisms and characteristics of capillary suspension have not been studied. In this study, the particle / oil / water capillary suspension was prepared using various particles and evaluated the rheological properties.
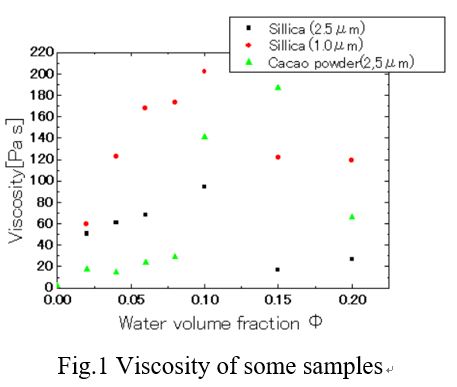
Firstly the particles and the oil were mixed with a dissolver stirrer to prepare a suspension. Next, capillary suspension was prepared by adding small amount of water to the suspension and stirring for 10min. Viscosity and rheology of the product was measured with a rotary rheometer. Fig.1 shows the effect of water volume fraction on the viscosity of capillary suspensions prepared by using three types of particles (Sillica(2.5μm),sillica(1.0μm),Cacao powder(2.5μm). The viscosity profile shows the maximum value at the certein water volume fraction, which we called the optimum crosslinked water content (OCWC) . From the results, the samples using particles of the same material showed the same OCWC. However, samples using different particle with same particle size showed different OCWC. When the three-phase contact angle of cocoa powder is lower than that of sillica. It is considered that the OCWC became high because more hydrophilic characteritics. It is thought that the rheological property value of the capillary suspension can be estimated by the three-phase contact angle, interfacial tension, particle size and particle concentration. By measuring the yield stress, it is possible to calculate the capillary force acting between particles of each sample. It was found that the three-phase contact angle, which varies depending on the material of the particles, is closely related to the rheological property.
We focused on the interface between the polypropylene (PP) resin and the polyamide 6(PA6) resin, which was generated when the PA6 resin was located at the center of the carbon fiber reinforced polypropylene (CFRPP) to enhance the strengthen of the CFRPP. By adsorbing the polymer particles on the surface of the carbon fiber through the electrodeposition, the particles played a important roles to improve the interfacial adhesion between the PP and the PA6 resins. And also, the interface between CFRPP and metal was investigated to incorporate CFRPP to multi-material structures. polymer particles with the same component as the resin were adsorbed on the metal surface, and the particles were impregnated to fine defects on the metal surface, thereby improving the interfacial adhesion between CFRPP and the metal. Therefore, we examined the application of polymer particles to fabricate CFRPP for practical use.
A microlens array is the optical element which has many lenses with a diameter of several to hundreds micrometers on its surface. At present, the microlens arrays are prepared by the top-down processes such as photolithography and etching, where the productivity of the arrays is not very high. Moreover, it is difficult to obtain the arrays with smaller lenses through the top-down processes. To prepare the smaller microlens arrays efficiently, the bottom-up processes such as self-assembly are required. In the present study, we have tried to prepare the mesolens array with a diameter of hundreds nanometers from the non-close-packed (NCP) colloidal monolayers, which are prepared by the convective self-assembly (CSA) using a glass substrate and the silica particles grafted with cationic polyelectrolyte, poly(vinylbenzyl trimethylammonium chloride) (PVBTA). When the NCP colloidal monolayers are annealed, PVBTA would be removed and a part of the silica particles are thought to be melted. It is therefore expected that the silica particles and the glass substrate could fuse together to form the mesolens array, because they are mainly composed of silicon dioxide.
PVBTA were grafted by free radical polymerization on the surface of the monodisperse silica particles, and then the PVBTA-grafted silica particles and the glass substrate were applied to the CSA process to prepare the NCP monolayers. The obtained monolayers were annealed at 973 K in a muffle furnace for several hours. The annealed particle monolayers were observed by the atomic force microscope (AFM) and the scanning electron microscope (SEM). As a result, the height of the silica particles were found to decrease with an increase in annealing time, which probably indicates that partial fusion of the silica particle and the grass substrate proceeded as the annealing time increases.
Polymeric microcapsules are micron-sized particles with a liquid core and a polymer shell. The shell of the microcapsules can isolate the encapsulated substance from the external environment. Microcapsules exhibit various functions by combining different core substances with shell materials. In particular, microcapsules containing a phase change material have advantages such as an increase in heat exchange efficiency due to an increase in specific surface area and prevention of the encapsulated phase change material from leakage, which can be served as efficient heat storage materials for energy saving applications. Most of the microcapsules used as heat storage materials are made of melamine-formaldehyde materials (MFM). MFM have excellent mechanical strength and heat resistance. However, it has been reported that the resultant microcapsules contain unreacted formaldehyde which has high carcinogenic to human. There is therefore an increasing demand for reducing or removing formaldehyde from the products. In this study, we developed a process to prepare formaldehyde-free melamine microcapsules by using melamine, urea, glyoxal and 2,2-dimethoxyacetaldehyde (DME) [1] as the shell materials, based on the previous study [1] and optimized the preparation conditions for controlling the shell thickness. Specifically, we prepared a prepolymer solution and mixed the solution to oil-in-water (O/W) emulsion composed of Isopar G and aqueous solution containing poly(E-MA), as the oil and water phases, respectively [2]. We discuss the effect of glyoxal/DME molar ratio and NH2/CHO molar ratio on the formation of the microcapsules as well as the thickness of the microcapsules.
[1] G. Leon et al., RSC Adv., 7, 18962-18975 (2017)
[2] Eiji Kamio et al., Langmuir., 24, 23, 12387-13298(2008)
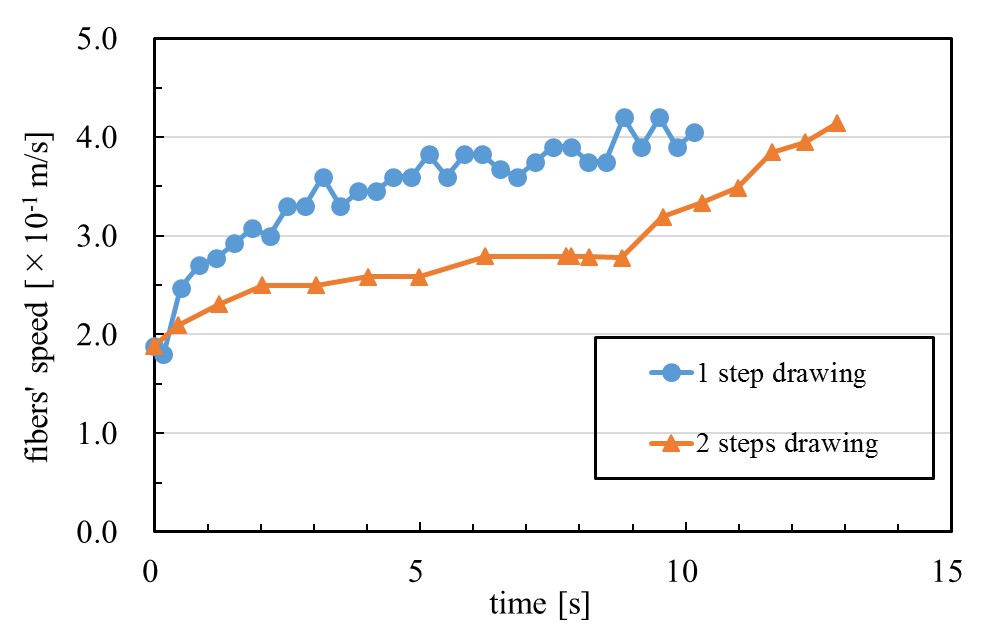
The behavior of drawing process was investigated by focusing on fiber stretching speed (spinning acceleration) to improve fibers' luster quality. Wet spinning method often has a problem of low luster level caused in higher spinning speed. We found that, (1) luster quality could be evaluated by fibers' arithmetic average (RΔa), which indicated fibers' roughness and (2) fibers' roughness was related to spinning acceleration by analyzing RΔa. Spinning acceleration was measured by chasing markers which was used to tie fibers. Regardless of length of spinning bath, fibers were stretched mostly during the first stretching stage. So we tried to stepwise drawing method. In case drawing ratio is 220 percent by one step, RΔa was 10.1 degree but by stepwise drawing (1st drawing ratio was 148.3 percent and 2nd drawing ratio was 148.3 percent) RΔa was decreased to 8.3 degree. We have cleared that stepwise drawing method has enabled to decrease fibers' roughness by preventing sudden change of fiber stress. In addition, we have cleared that higher temperature also improved fibers' roughness. In higher temperature, roughness was decreased in spite of higher acceleration because that fiber was easier to stretch than in lower temperature.
Paper-based devices have attracted a lot of attention as simple analytical platforms for bioassays. However, paper-based biosensors have still many problems, especially nonspecific protein adsorption. Detection sensitivity and selectivity of such sensors are impaired by nonspecific protein adsorption because of the drifting and denaturing of the detection ligands, the loss of target analyte during transport to the detection zone, and background noise from nonspecific protein adsorption. Typically, this problem in a sensor device is addressed by surface modification with hydrophilic polymers. For example, poly(ethylene glycol) (PEG) provides protein-repellent properties on wide variety of surfaces.
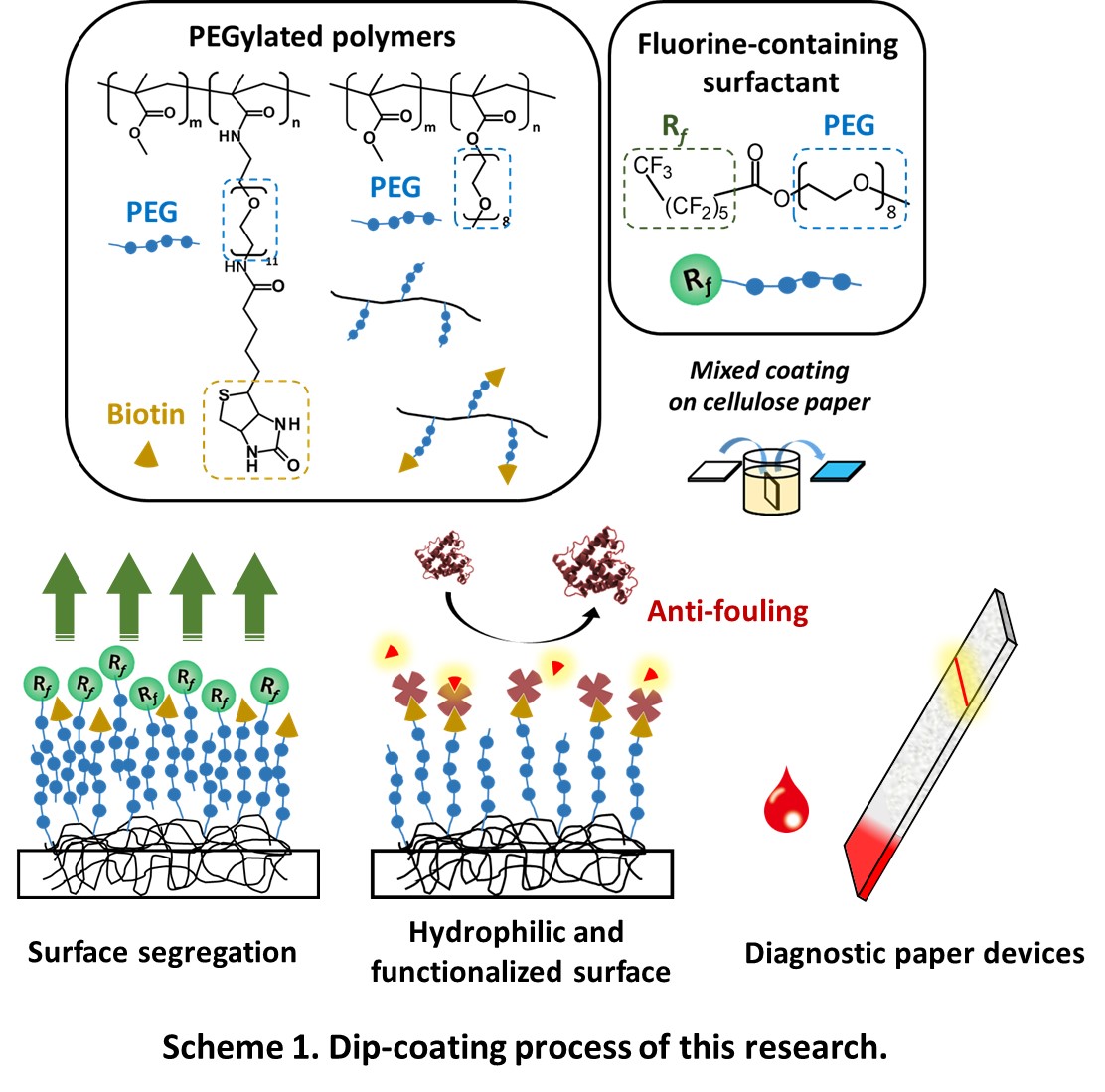
Herein we demonstrated surface modification of cellulose paper with a functional PEGylated polymer via simple dip-coating. This functional PEGylated polymer has biotin as a reactive site on the end PEG chains. In general, dip-coating with a PEGylated polymer followed by drying generates a hydrophobic surface because the hydrophobic moieties of the polymer are preferentially segregated at the air-polymer interface to minimize the surface energy. To display the hydrophilic moieties (functional parts) of a polymer on an outermost surface in dip-coating, we previously proposed that the surface segregation in the dip-coating of a PEGylated polymer can be controlled using a fluorine-containing surfactant. Based on the previous study, we here attempted to impart antifouling property and reactivity with other molecules such as antibody, DNA to the paper surface.
We synthesized PEGylated copolymers (Scheme 1) and a fluorine-containing surfactant (Rf-PEG). Cellulose paper was dip-coated with these copolymers in the presence of Rf-PEG. This coted paper had water absorbency in comparison with PMMA-coated paper and a paper coated PEGylated copolymers in the absence of Rf-PEG. Moreover, this coated paper had less nonspecific protein adsorption than a raw paper and a paper coated PEGylated copolymers in the absence of Rf-PEG. We expect that this coted paper will be applied to platforms for bioassays.
The excited electrons and holes generated upon by irradiating UV ray to TiO2 have powerful redox power. They produce reactive oxygen species (ROS) under aerobic conditions at the surface. These species rapidly decompose organic compounds to converts them into CO2 and H2O via carbon-carbon bond cleavage. TiO2 has been used as a powder to increase the catalytic effect. However, the use of particles involves the need of incorporating extra stages in the purification process, such as separation and catalyst recycling. We focused on a composite of TiO2 nanoparticles and polymer nanofibers. Polymer nanofibers have a large surface area. Polymer nanofibers are fabricated by electrospinning as follows. An electric field is used to create a charged jet of polymer solution. As this jet travels in air, the solvent evaporates leaving behind a charged fiber that can be collected on a metal plate. The purpose of this study is to develop novel composite polymer nanofibers with TiO2 nanoparticles and clarify its applicability as photocatalyst for degradation of organic compounds. N-isoprorylacrylamide-co-N-methylol acrylamide (NIPA-co-NMA) polymer was synthesized by free radical polymerization. NIPA polymer is a thermosensitive polymer, and NMA can be used as a crosslinking agent. The composite nanofibers were fabricated by electrospraying methanol containing NIPA-co-NMA polymer and TiO2 nanoparticles onto stainless-steel plate through a nozzle with application of high voltage. The composite nanofibers catalyzed decomposition reaction of methylene blue (MB) successfully.
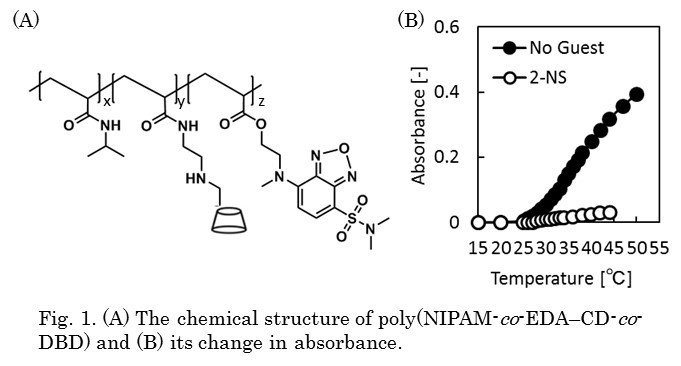
Development of novel materials which mimic the excellent molecular recognition function of biomembranes has recently got attention. Copolymer of N-isopropylacrylamide (NIPAM) bearing β-cyclodextrin (CD) (poly(NIPAM-co-CD)) exhibits molecular recognition function, which controls its volume phase transition behavior by the inclusion of guest molecules[1]. However, the mechanism of the molecular recognition of poly(NIPAM-co-CD) has not been fully explained. N,N-dimethylaminosulfonyl-2,3,3-benzoxadiazole (DBD) is a fluorophore and changes its fluorescence intensity in response to hydrophilicity around the molecule[2]. If DBD is introduced into the poly(NIPAM) chain, changes in polarity around PNIPAM accompanying phase transition can be detected.Herein, DBD is copolymerized with poly(NIPAM-co-CD) to directly analyze the change in hydrophilicity of the poly(NIPAM-co-CD) chains by inclusion of guest molecules (Fig. 1A). We discuss the correlation between the phase transition behavior and the hydration state around NIPAM, which is controlled by molecular recognition of CD in polymer chains. The change in phase transition and hydrophilicity around the polymer chains caused by inclusion of a guest molecule, sodium 2-naphthalenesulfonate (2-NS), was investigated by cloud point measurement and fluorescence intensity measurement. Fig. 1B. shows the results of the cloud point measurement. When 2-NS were added, aggregation of the polymer was suppressed. The fluorescence intensity measurement exhibited that there was no difference in the change in fluorescence intensity by addition of 2-NS, suggesting that inclusion of 2-NS in CD did not affect the hydrophilicity around NIPAM. Further, the cloud point measurement in NaCl solution did not display suppression of aggregation even the presence of 2-NS, resulting from electrostatic shielding by Na+ around SO3– group of 2-NS which was included in CD. It implies that the suppression of aggregation in poly(NIPAM-co-EDA-CD-co-DBD) by addition of 2-NS was caused by the electrostatic repulsion of 2-NS included in CD.
[1] Macromolecules, 45, 9742-9750, (2012)
[2] Anal. Chem.,, 75, 5926-5935, (2003)
To investigate the effect of the surface properties and polymer properties on surface modification via electrostatic adsorption, the electrostatic adsorption behavior of zwitterionic copolymer on negatively charged surface was studied. A series of positively charged zwitterionic copolymer and a series of negatively charged surfaces including porous substrates and dense films were respectively fabricated. The electrostatic adsorption behavior of zwitterionic copolymer on negatively charged porous substrates were confirmed by contact angles and fluorescently labelled protein adsorption experiment. And the adsorption behavior of zwitterionic copolymer on negatively charged dense films were confirmed by quartz crystal microbalance (QCM) determination and fluorescently labelled protein adsorption experiment. The results indicate that the lower charge density on the zwitterionic copolymer bring to the higher adsorption on the charged surface, while the extremely low charge density results in the lower adsorption on the charged surface, due to the low interaction. The high density of film surface charge is beneficial for surface adsorption, while the extremely high density of the film surface charge leads to the lower surface adsorption, due to the steric hindrance of the negatively charged sites. This work provide the insight into the best strategy for surface modification via electrostatic adsorption.
Gating membrane is a porous membrane that opens and closes pores responding to heat[1] and specific ions[2]. Recently sensor system using photo-responsive material attracts attention because of several advantages such as simple operation and uninfluential nature to the substance. In this research, we aim to develop a photo-responsive gating membrane that opens up the possibility of conventional membranes.
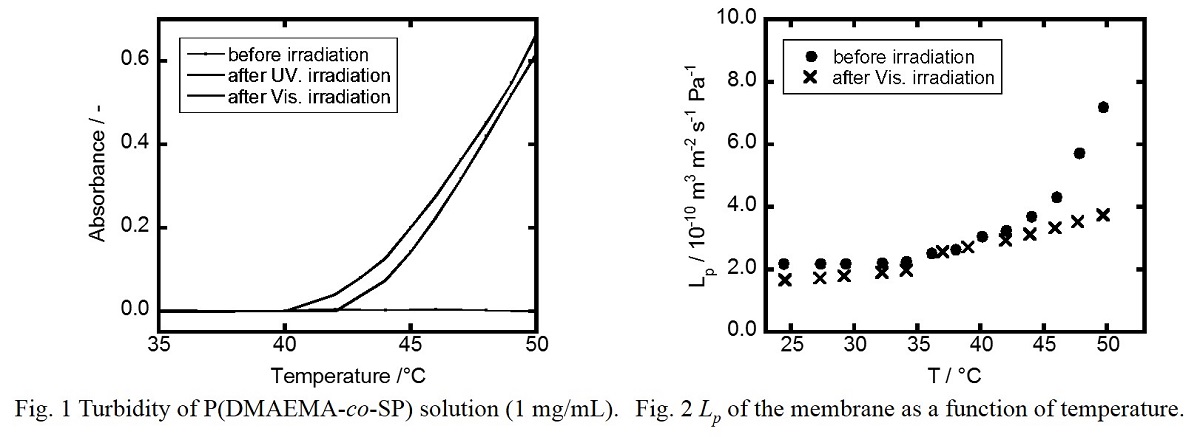
Photo-responsive polymer composed of poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) actuator and photo-sensing spiropyran (SP) was synthesized by free radical polymerization (molecular weight : Mn =1.49 × 104 , Mw / Mn = 2.07 and SP content: 0.81 mol% ). Lower critical solution temperature (LCST) of 0.1 wt% aqueous solution is 42 °C before visible light (Vis) irradiation and 40 °C after Vis-irradiation while no LCST was observed after ultraviolet light (UV) irradiation.
The copolymer was immobilized to the polyethylene (PE) porous membrane by plasma-induced graft polymerization (Graft ratio: 5.6% and SP content: 0.96 mol% ). Permeation coefficient (Lp) of the prepared membrane increases as water temperature rises before light irradiation (Fig 2). This is because the copolymer shrinks above LCST and opens the pore of the membrane. Just after UV-irradiation, Lp of the membrane decreases as we expected, but increases then. The latter behavior may be due to elimination of the copolymer from PE substrate by UV-irradiation, as indicated by FT-IR spectral change. After Vis-irradiation, Lp of the membrane did not increase even though linear polymer shows similar LCST behavior before and after Vis-irradiation (Fig 1). This is probably because LCST behavior of grafted polymer in the pore and linear polymer in diluted solution is different.
In conclusion, we have successfully fabricated visible light-responsive gating membrane.
1) H. Kuroki et al., J. Membr. Sci. 2010, 352, 22-31.
2) T. Yamaguchi et al., JACS, 1999, 121, 4078-4079
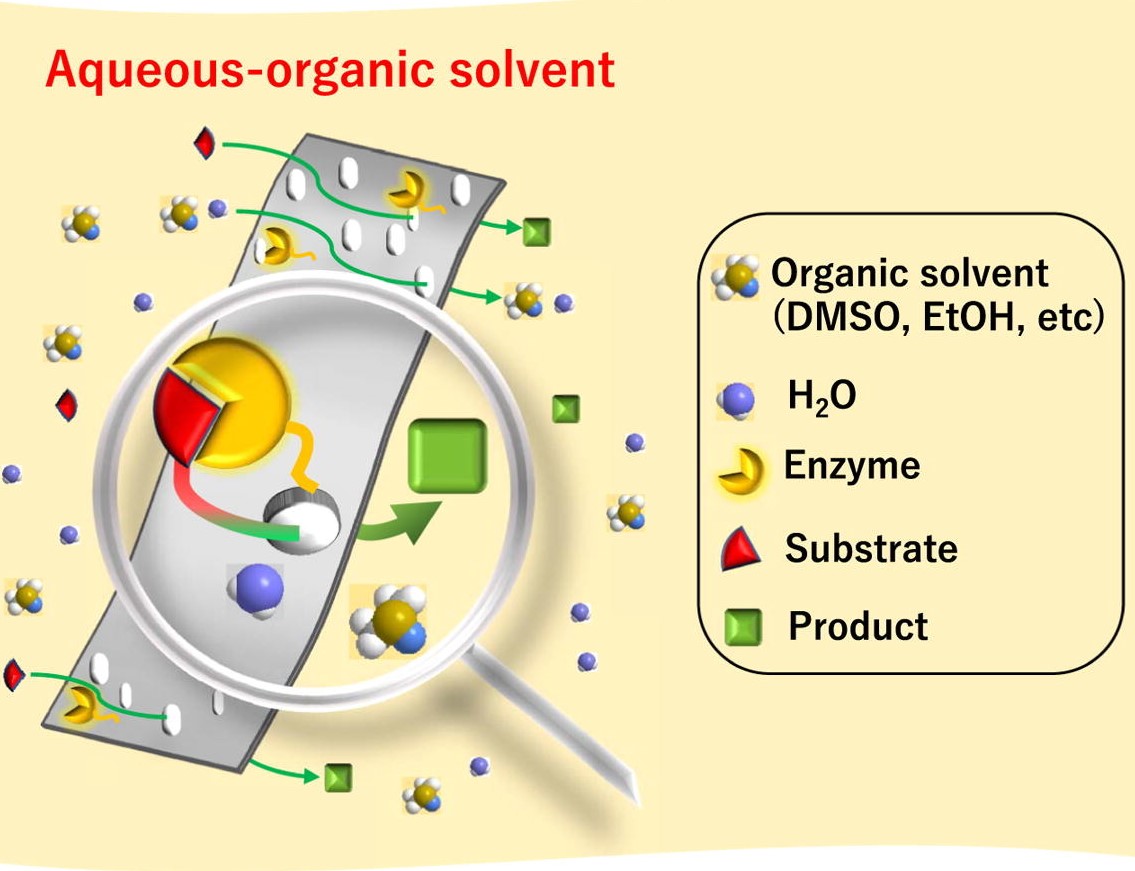
Enzymes play vital roles in biological transformations due to incomparable selectivity. Enzymatic membrane reactors (EMRs) combine enzymes with membranes, and many researchers have studied the synergistic effect of EMRs exerting on enzyme performance. Before the utility of EMRs can expand from natural aqueous media to organic solvents, robust membranes must be developed to promote enzyme protection from hostile forms of media. For this study, laccase was immobilized on an organic-solvent-resistant hydroxylated polyketone (PK-OH) membrane via covalent bonds and served as a model enzyme. Ketone groups facilitated the immobilization via hydrogen bonds, leading to a high immobilization density of 462 μg/cm2. In homogeneous aqueous-organic solvents, the activity of immobilized laccase was up to 3.5 times greater than that of free laccase towards 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid). In addition, the results also showed improved activity towards highly concentrated 2,4,6-trichlorophenol and bisphenol A (1.4-g/L). Furthermore, the activity in filtration mode showed a 240% increase over that in batch mode. The immobilized laccase maintained its activity after 40-days of storage, 10 reuse cycles, and 50 h of continuous reaction. These results show that robust polyketone based membrane support will create opportunities for the application of EMRs in aqueous-organic solvents.