
This study is to investigate the characteristics of polyvinyl alcohol (PVOH) added with different loading level of calcined fish bone (HAp). HAp was extracted from Chirocentrus nudus skeletal via thermal calcination; followed by solution casting method to produce PVOH composite films, consisting 0 phr to 5 phr calcined fish bone. In term of tensile strength, it decreases upon added with 1 phr calcined fish bone then increases at 2 phr loading level. The addition of 3 phr calcined fish bone was found to be the optimum loading level to exhibit reinforcement function; whereas for elongation at break, a decreasing trend with an increase in calcined fish bone loading level was found due to the continuity of chain mobility in PVOH being interrupted and the inducement of internal friction within PVOH matrix. For scanning electron microscopy, the wave-liked shrinkage, fibrils and agglomerates were reduced with increasing calcined fish bone loading level, indicating a high tensile strength and low elongation when increasing the loading level. In x-ray diffraction analysis, the peak broadening effect indicates a decrease in HAp crystallite size which has ruptured and well dispersed within PVOH matrix. For differential scanning calorimetry, the enthalpy of melting and crystallinity (in terms of qualitative and quantitative) possessed similar trend as the finding in tensile strength. In fourier transform infrared spectroscopy analysis, the unique peaks occurred approximately at 3263 cm-1 and 2923 cm-1, indicating the presence of O–H group bonding and C–H bonding respectively. Lastly, this study concluded that the 3 phr calcined fish bone was the optimum loading level to be employed in biomedical application such as bone scaffolding due to the high strength and compatibility.
Polymer/magnetic particle composites have high magnetic sensitivity, and thus have wide applications in biomedical fields. B. Tural et al. reported recently that if polymer containing a carboxyl group such as poly(methacrylic acid) was added to the coprecipitation solution during magnetite nanoparticle (MNP) synthesis, the polymer was immobilized on the magnetite surface immediately following particle generation. The method is one-step process that does not require complex and time-consuming manipulation.
However, so far, there are no reports on what kind of polymer (or functional group) can be used for this type of one step synthesis of polymer/MNP composite. Therefore, in this study, we tried one-step synthesis of polymer/MNP composite by using different polymers which contains different functional group to clarify 1) what kind of functional groups can be applicable for one step polymer/MNP synthesis, and 2) the effect of the polymer difference on the immobilized polymer amount.
In the experiment, the synthesis of polyacrylic acid (PAAc) / MNP was carried out using the following procedure; first, PAAc, iron (II) sulfate heptahydrate and iron (III) chloride hexahydrate were dissolved in deionized water. Then, sodium hydroxide was dropped into the solution at room temperature to proceed MNP synthesis reaction. Polyacrylamide (PAAm), polyethylene glycol (PEG), polyethylene imine (PEI) and polyvinyl alcohol (PVA) immobilized on MNP were also synthesized using the same procedure just by changing PAAc to the corresponding polymers. The results of XRD analysis showed that for all the polymers used in this study, formation of MNP was confirmed, and the fact indicated that the presence of the polymers did not affect on the synthesis of MNP. Thermogravimetric analysis (TGA) indicated that for all the polymers, polymer immobilized on MNP was successfully synthesized, and the amounts of the immobilized polymers were in the order of PEG < PEI < PAAm < PAAc.
To prevent aggregation and oxidation of magnetite nanoparticle (MNP), silica coating on MNP was proposed. Recently, Setyawan et al. successfully achieved simultaneous magnetite nanoparticle formation and the modification of its surface with silica layer by using the electrooxidation method. Advantage of the method is that the process is very simple and easy since it's a one-step process.
The purposes of this study are following; 1) to synthesize MNP with silica coating under various conditions by electrooxidation, and to examine the effect of the synthesis conditions on the characteristics of the resulting sample, 2) to immobilize thermoresponsive copolymer, poly(NIPAM-co-AA) on the MNP with silica coating, and to evaluate their immobilized copolymer amount and Cu adsorption property.
As a result of X-ray diffractometer (XRD) analysis and Fourier transform infrared spectroscopy (FT-IR) measurement, it was confirmed that MNP with silica coating was successfully synthesized using this method. When the current value was changed during electrooxidation synthesis, it was found that the minimum particle diameter(maximum specific surface area) was obtained at the current value of 0.60 A. The results of thermogravimetric analysis (TGA) showed that the maximum immobilized amount of the copolymer of 0.255 g/g-MNP was obtained when the sample synthesized at a current value of 0.60 A was used. The immobilized amount was 3 times larger than that of the sample prepared using MNP without silica coating. This is considered to be due to less aggregation of MNP during polymer immobilization. The results of Cu (II) adsorption experiment showed that although Cu (II) recovery amount through temperature swing was similar to those of conventional thermoresponsive “gel” type heavy metal adsorbents, time required for Cu(II) recycling process could be decreased to 1/10. These results showed the effectiveness of this method.
Graphene-based materials have been studied for prospective applications in many fields, including catalysts and electrode materials. Graphene-based materials can be made by two kinds of approaches. The first one is the bottom-up approach represented by a chemical vapor deposition method. The second one is the top-down approach via graphene oxides. Although the latter approach is suited for mass production, it is difficult to control the crystallinity and the amount of defects by this approach. Therefore, tailoring some properties depending on applications has remained a significant challenge. We reported that it is possible to control the crystallinity and the concentration of defects by microwave-assisted (MW-assisted) annealing for carbon materials by adjusting distances between carbon particles through changing the bulk density. However, it can be difficult to change the bulk density depending on some types of carbon materials. This study is aimed at controlling the crystallinity and the concentration of defects regardless of the kind of carbon materials through development of a new up-flow reactor for MW-assisted annealing. Crystallinity as well as the concentration of defects were investigated by multiple techniques including Raman spectroscopy and thermogravimetric analysis. During MW irradiation, we observed moderate fluidization of sample particles in an up-flow reactor. Therefore, distances between carbon particles were adjusted, through which the concentration of defects was controlled. Our results indicate that extend of changing of the concentration of defects depends on the sample particle size and the gas flow rate during MW irradiation. Moreover, samples annealed by the up-flow reactor exhibited high oxidation resistance in air in comparison with ones treated by a down-flow reactor.
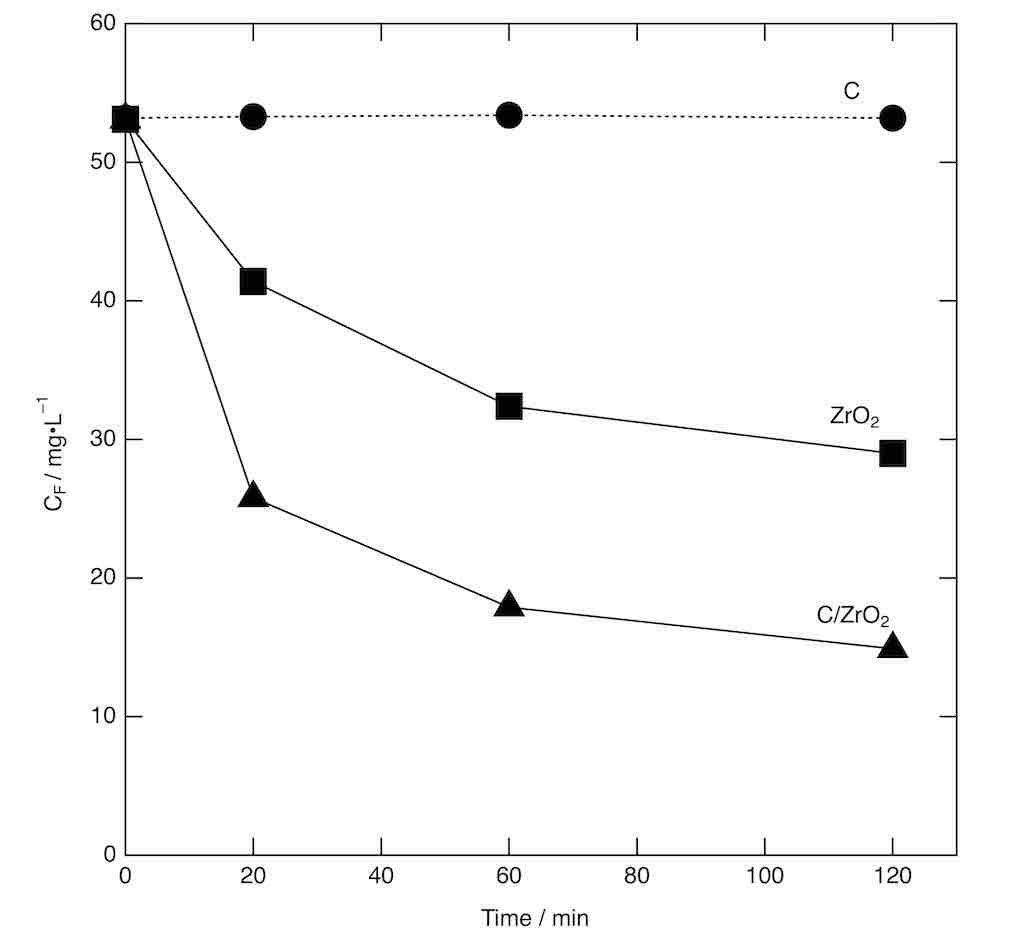
Magnesium oxide (MgO)-templated mesoporous carbons were modified with hydrated zirconium oxide (ZrO2·xH2O) synthesized through hydrolysis of zirconium chloride oxide (ZrOCl2) to prepare adsorbents for fluoride ion (F-) removal from water. Powder X-ray diffractometry (XRD) revealed that ZrO2·xH2O was successfully formed on the surface of mesoporous carbons. Adsorbed amount of F– was observed as a function of time by using pristine mesoporous carbons, modified mesoporous carbons, and ZrO2·xH2O prepared without carbon. The figure shows the F– concentration of solution (CF) as a function of contact time. The pristine carbon was found to adsorb little amount of F–. In contrast, the modified carbon and ZrO2·xH2O were capable of removing F– from water. The amount of F– removed with the modified carbons was larger than that observed using ZrO2·xH2O. This result indicates that the capability of ZrO2·xH2O to remove F– is improved by attaching it to the mesoporous carbon surface. The authors presume that this positive effect is probably provided by formation of ZrO2·xH2O on the carbon surface giving crystals that are smaller than crystals fomed without carbon. The F– removal was described by conventional adsorption isotherms. The other results such as effect of mesopore size and pH on the F– removal will be shown at the conference.
Porous carbon materials have attracted much attention because of their large surface area, large pore volume, chemical inertness, and electrical conducting properties. Mesoporous carbons with well-tailored pore system are essential for a number of applications, such as adsorbents, catalyst supports, and energy storage devices. Recently, a great deal of effort has been made to develop a method of synthesizing mesoporous carbons, including hard- and soft-templating techniques. Hard-templating techniques require preparation of inorganic template such as mesoporous silicas, impregnation of pores with appropriate carbon precursors, carbonization, and subsequent selective removal of inorganic template using HF
or NaOH. Another recently developed approach is based on the application of soft-template such as triblock copolymers access for simpler and facile synthesis of mesoporous carbons because of the avoidance of multiple step to prepare and remove the hard-template. However, the soft-templating techniques still suffer from long preparation durations and the excessive use of solvents for the formation of mesostructure in solutions through the self-assembly process, which causes low efficiency, high production cost, and environmental burden.
Herein, we investigate a highly efficient and simple approach to synthesize mesoporous carbons using solid raw materials under solvent-free conditions. The solid–solid approach has potential advantages for large-scale production, low cost, environmental-friendly process. Mesoporous carbons were obtained by the solvent-free technique using various combinations of solid raw materials with adjustments of the molar ratio.
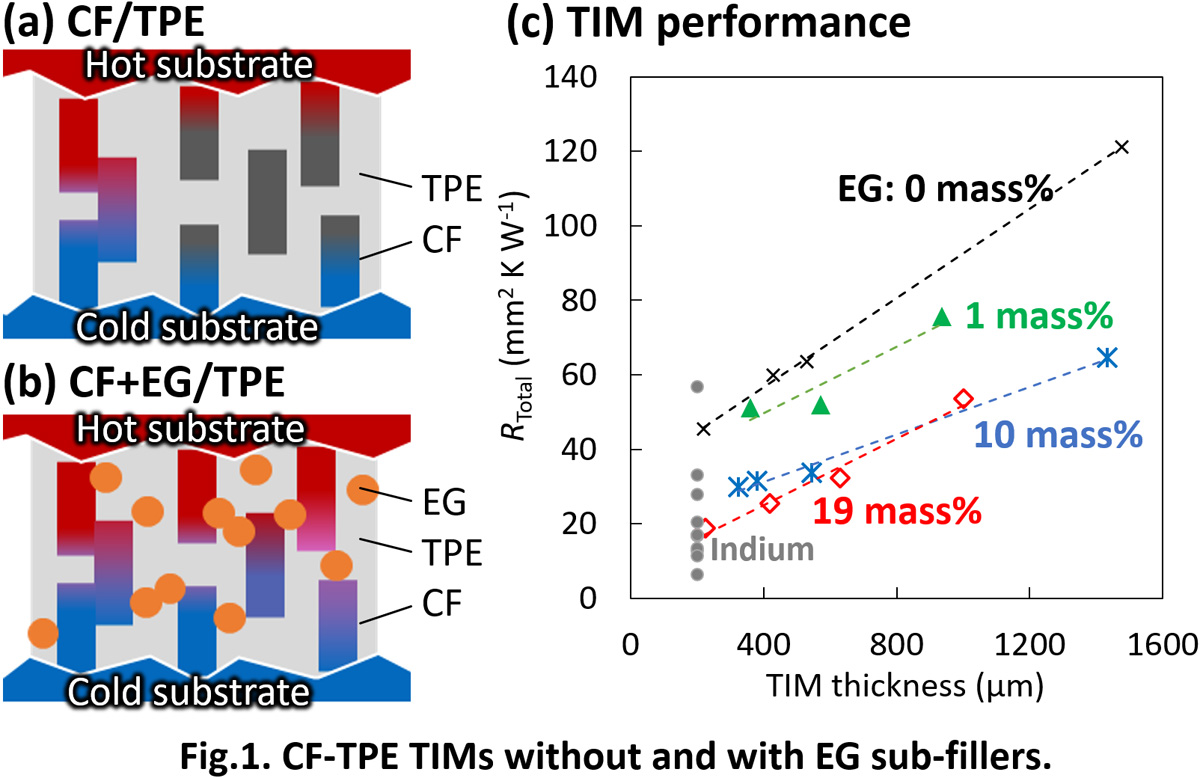
The demand for high-performance thermal interface materials (TIMs), which enhances releasing heat from the electronic devices, is increasing for further performance improvement and miniaturization of electronic devices. The performance of TIM is characterized by the total thermal resistance RTotal which is the sum of the bulk thermal resistance and the contact thermal resistance between the TIM and the opposing substrate. Therefore, it is required for the TIM to have high thermal conductivity for vertical direction κ⊥ and softness, thus conventional TIMs consist of filler particles having high thermal conductivity and soft polymer matrix. By using fillers with high aspect ratio and aligning them vertically, κ⊥ can be improved [1]. We previously fabricated TIM using carbon fiber (CF) having high thermal conductivity and high aspect ratio. By a simple process using shear force, CFs were filled in the thermoplastic elastomer (TPE) matrix with vertical alignment and high mass ratio of over 50 mass% (Fig. 1a) [2]. However, the total thermal resistance is as high as 100 mm2 K W-1 possibly because of the poor connections between the CFs and between the CF and the opposing substrate surface. This time, expanded graphite (EG) having high thermal conductivity and flexible sheet-like structure is added as a sub-filler (Fig. 1b). Thermal resistance was measured by the steady-state method and plotted against the TIM thickness (Fig. 1c). The thermal resistance decreased with the EG content, resulting in the significant reduction of the thermal resistance to RTotal = 19 mm2 K W-1 with 19 mass% EG. This value is similarly small as the high-performance TIM of an indium sheet. The improvement comes from both the improvement of the thermal conductivity (slope) and the reduction of contact thermal resistance (intercept).
[1] K. Uetani, et al., Adv. Mater., 26, 5857 (2014).
[2] R. Yamada et al., The 51st FNTG symposium, 2P-38 (2016).
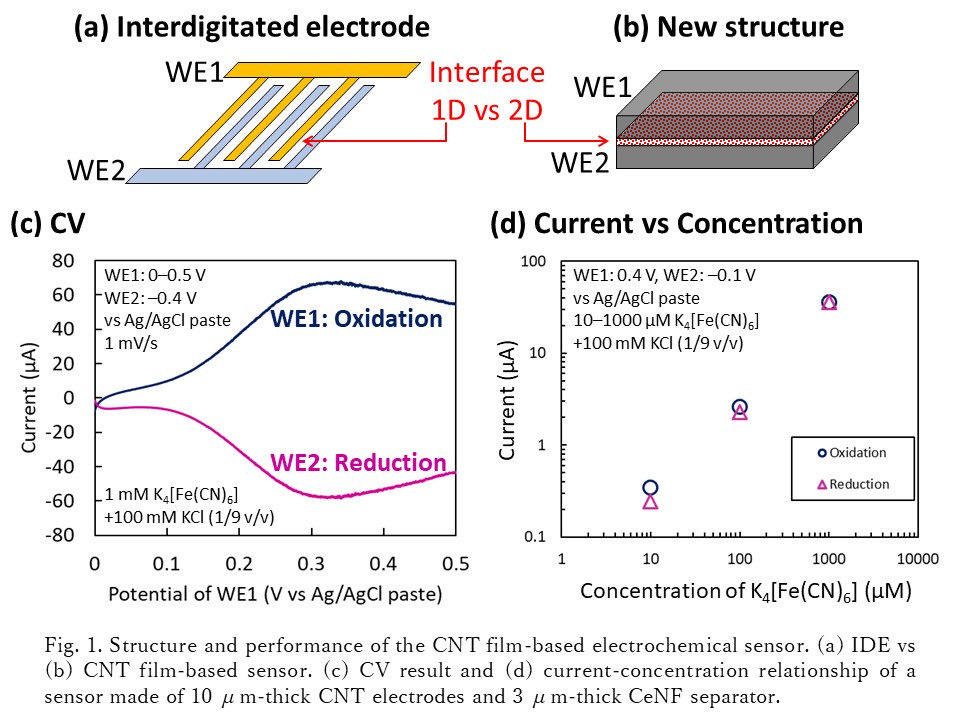
Electrochemical sensor based on redox cycle of repeated oxidation and reduction has advantages of compactness and high sensitivity. To promote the redox cycle, two working electrodes for oxidation and reduction should have large surface area, be placed closely, be insulated electronically while be conductive ionically each other. The typical example is the interdigitated electrode (IDE) (Fig. 1a), but the fabrication of the microstructure requires complex processes using lithography. In this work, we propose a new structure similar to electrochemical capacitor in which a separator made of cellulose nanofiber (CeNF) paper holds two working electrodes (WEs) made of carbon nanotube (CNT) paper (Fig. 1b). Cyclic voltammetry (CV) using K4[Fe(CN)6] aqueous solution shows the effectivity of this structure as a sensor (Fig. 1c).
Larger current was obtained with 3D electrodes of 10 μm-thick CNT papers and separator of 3 μm-thin CeNF paper, enabling detection of K4[Fe(CN)6] for concentrations down to 10 μM.
The conventional hydrogen storage materials such as metallic alloys are heavy and difficult to transport, and there is a problem that their costs are high. Therefore, in this research, we aim to develop hydrogen storage materials using carbon nanohorn (CNH), which is a lightweight and inexpensive carbon material.
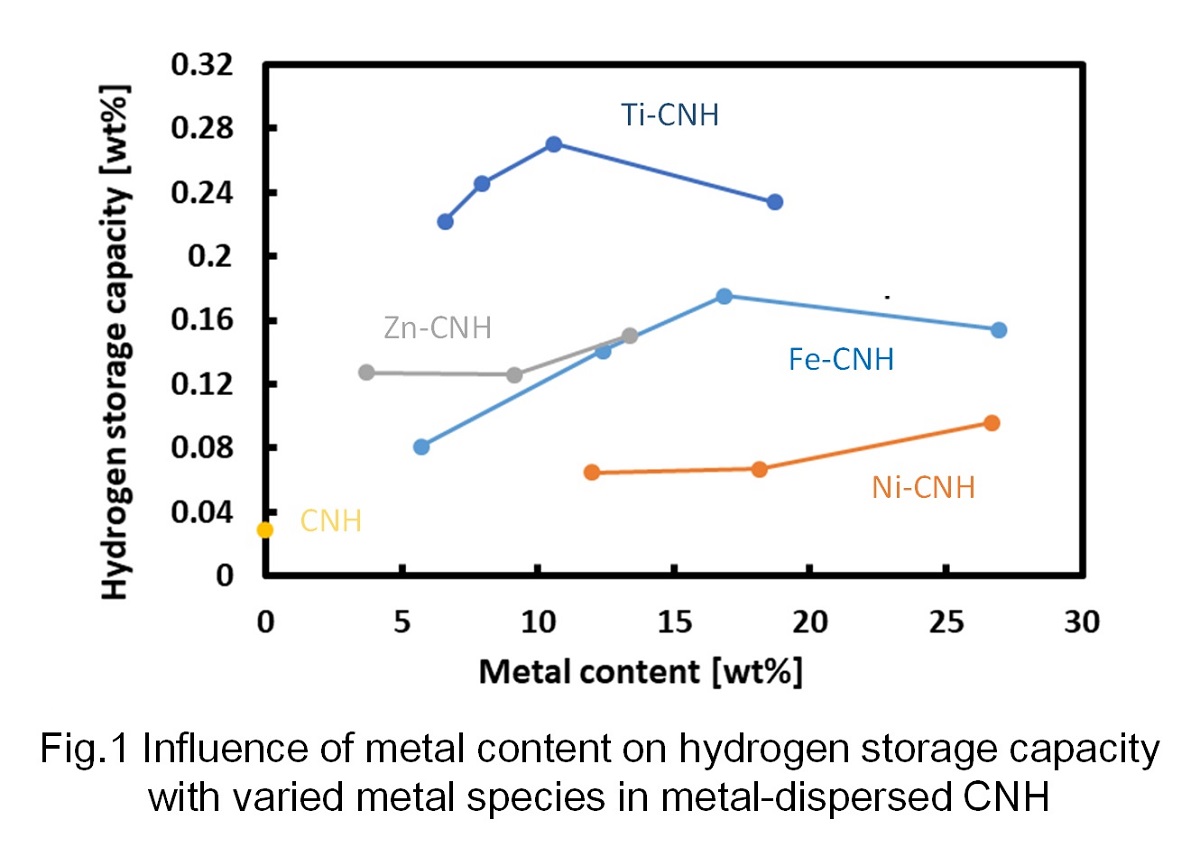
CNH dispersed with metal nanoparticles was prepared using arc-in-water method. After that, the amount of absorbed hydrogen was measured under the condition of 0.95 MPa and 25 °C, and the influence of the metal composition and the content on the amount of absorbed hydrogen was clarified. It can be seen in Fig. 1 that there is the optimum metal content to maximize the hydrogen storage capacity. The increase in the amount of hydrogen storage with the increase in the metal content in relatively low metal content range is considered to be due to the increase of hydrogen spillover effect (dissociative adorption of H2 on carbon surface close to metal nanoparticles). Further, the decrease in the storage amount with metal content in high metal content range is considered to be caused by the decrease in carbon surface per material. Also, it can be observed that the amount of hydrogen storage becomes the highest when using Ti to disperse in CNH among Fe, Ni, Zn and Ti.
In addition, semi-empirical molecular orbital calculation was conducted in order to theoretically verify the hydrogen spillover phenomenon on the metal dispersed CNH. As a result, the hydrogen spillover effect is likely to occur at high pressure. It was shown that the hydrogen spillover effect can easily occur when H2 molecules present at the interface between Fe cluster and C atoms.
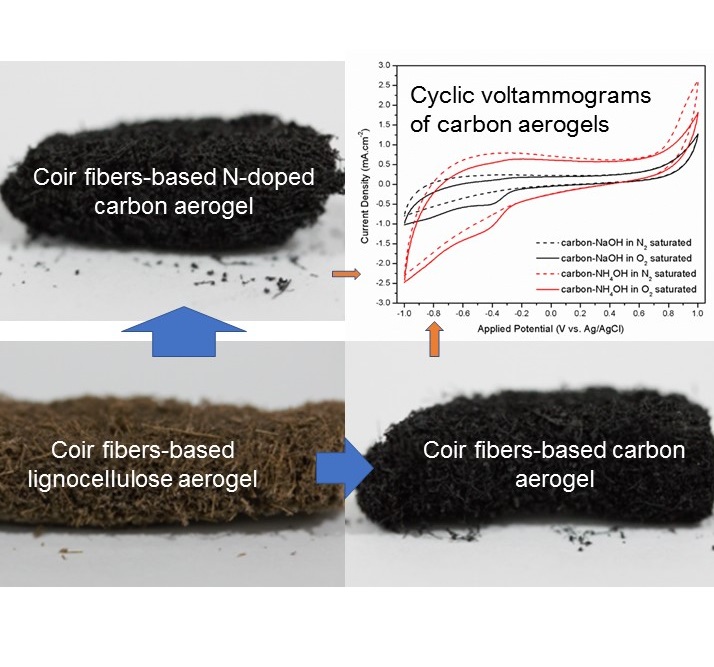
Metal-air battery and fuel cell are widely considered a promising candidate for next-generation renewable energy. One of the main problems of these technology is the catalyst for oxygen reduction reaction (ORR) that mainly depending on Pt, a very expensive material. The oxides of several transition metals have also been proposed for the purpose. Here, we propose nitrogen-doped carbon aerogel from coir fibers as an alternative metal-free electrocatalyst for the ORR. The nitrogen-doped carbon aerogel was prepared from coir fibers via simple ammonia–urea method followed by freeze drying and pyrolysis.Nitrogen molecules also have been well doped into the carbon structure, as confirmed by the existence of C=N functional group in FTIR spectra. Ammonia treatment could exfoliate the carbon structure causing defects and disorders, enhance the specific surface area, and maintain the morphology of carbon aerogel during carbonization. Kouteckỳ-Levich plots presented that the obtained nitrogen-doped carbon aerogel involved n ≈ 2 electrons towards ORR. The results suggest that the prepared nitrogen-doped carbon aerogel is a promising metal-free electrocatalyst toward ORR through 2e- pathways.
Proton-exchange membrane fuel cells, which can provide clean and efficient electricity, contain precious Pt electrocatalysts due to the sluggish kinetics of the oxygen reduction reaction (ORR). One of the major strategies to enhance the ORR catalytic activity is to control the morphology of the catalytic particles. Herein, a facile shape-directing mechanism was developed, and highly active Pt-based electrocatalysts were synthesized. Moreover, ammonium hexachroloplatinate(IV), which is known as recycling intermediate, was used as metal precursor of the synthesis experiments. It was revealed that NH4+ ions can behave as shape-directing agents in the presence of Cl– ions and O2. Based on the mechanism, Pt nanoparticles were synthesized in aqueous NH4Cl solution, forming a plate-like morphology that selectively exposes the {111} facets. Then, ammonium hexachroloplatinate(IV) and Cu salts were reduced together under the same synthesis condition, leading to Cu-doped Pt nanoplates. The Cu-doped Pt naonplates exhibited 3.7-times higher ORR catalytic activity than the commercial Pt catalysts. The present study, in which recycling intermediate has been used as metal precursor of highly active electrocatalysts, suggests a possibility to interconnect between material synthesis and recycling process.
Polymer electrolyte fuel cells (PEFCs) have received a great deal of attention for its utility in various applications, owing to their high energy conversion efficiency and clean emissions. However, a sluggish oxygen reduction reaction (ORR) rate and the low durability of conventional catalysts in PEFCs limits the wider commercialization prospects. To address these issues, our group had developed a carbon-free, connected platinum–iron (Pt–Fe) nanoparticle catalyst having porous hollow capsule structure. This catalyst exhibited an enhanced ORR activity as well as excellent durability against start-stop operations, due to the carbon-free structure. [1] Thus, the connected nanoparticle catalysts are identified promising, and further improvement in the ORR activity and durability can cater to the growing demands for PEFCs.
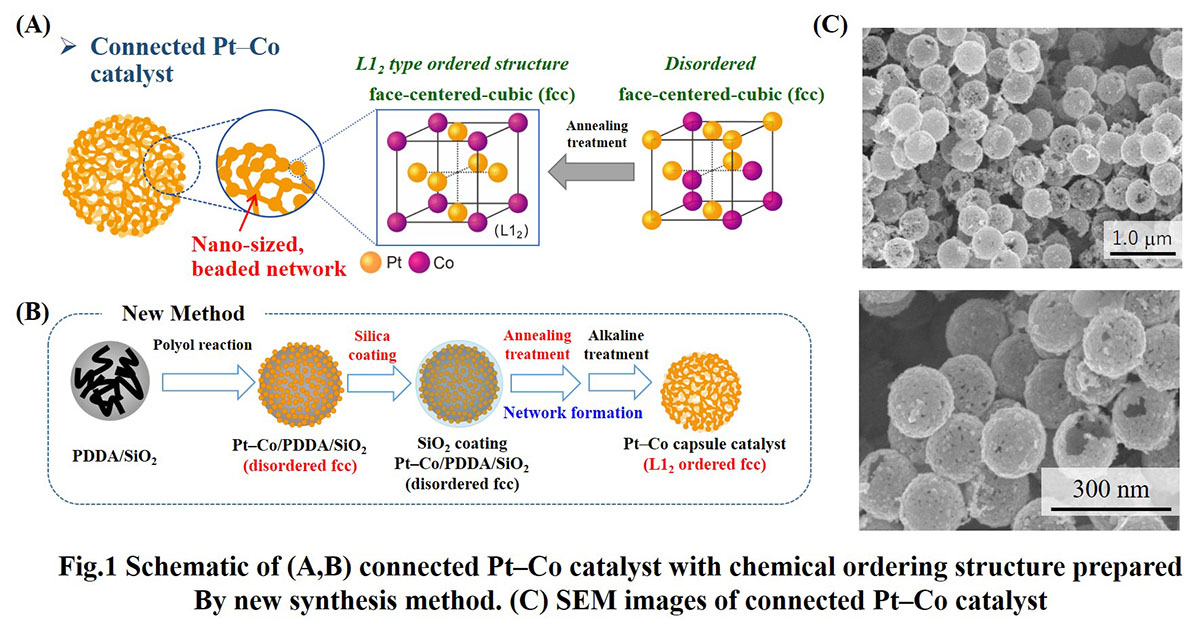
In this work, we developed a new connected catalyst, platinum–cobalt (Pt–Co) with a chemically ordered structure (FIG. 1A) having different catalyst atomic structures to that of connected Pt–Fe. Herein, we employed a new synthesis method for the catalyst processing, having the combination of silica coating and high temperature annealing as shown in Fig.1B. The SEM images (FIG. 1C) of the obtained Pt–Co catalysts showed a hollow capsule structure, indicating the formation of a connected Pt–Co network. The XRD patterns of the catalysts showed the peaks corresponding to an L12 type chemically ordered structure, when the annealing temperature was higher than 500 °C. Additionally, chemically ordered degree in the connected Pt–Co catalysts was successfully controlled by annealing temperature and time. Thus, we succeeded in the development and structural control of a connected Pt–Co catalyst composition for the first time. Investigating structural effects of connected Pt–Co catalysts on the ORR activity and durability is expected to provide useful guidelines to design an advanced connected nanoparticle catalyst for application in PEFCs.
References
[1] T. Tamaki et al., Energy Environ. Sci., 8, 2015, 3545–3549.
Polymer electrolyte fuel cells (PEFCs) have attracted much attention as promising energy conversion electrochemical devices. However, a conventional cathode catalyst of platinum nanoparticles supported on carbon black (Pt/C) exhibits a sluggish oxygen reduction reaction (ORR) rate and a low durability against start–stop and load cycle operations. In our group, a carbon-free, connected platinum–iron (Pt–Fe) nanoparticle catalyst with porous hollow capsule structure (FIG. 1A) has been successfully developed for an ORR. [1] This catalyst provides enhanced ORR activity and excellent durability during start-stop operations. However, there is a dissolution of alloyed metal occurring during load cycles, resulting in reduced ORR activity for the connected Pt–Fe catalyst.
In this study, we focused on a chemically ordered face-centered-tetragonal (fct) structure (FIG. 1A) to improve the load cycle durability of the Pt–Fe catalyst. The conventional synthesis method of connected Pt–Fe catalyst using a supercritical fluid treatment produces low fct degree (40–50%). Here, we propose a new and simple synthesis method using the combination of silica coating and high temperature annealing as shown in FIG. 1B. Using this method, we succeeded in preparing connected Pt–Fe catalysts having nano-sized network and a high fct degree (70–80%). The obtained catalyst exhibited a ten-times higher ORR specific activity, compared with that of the Pt/C. Furthermore, as showed in FIG. 1C, the connected Pt–Fe catalyst with the high fct degree (74%) exhibited a higher retention of ORR activity after 10,000 load cycles, compared to the low fct catalyst (44%). Thus, this study demonstrated that a highly ordered fct structure greatly contributes to the suppression of dissolution of metal, resulting in an improved load cycle durability of the connected Pt–Fe catalyst.
Reference
[1] T. Tamaki et al., Energy Environ. Sci., 8, 2015, 3545–3549.

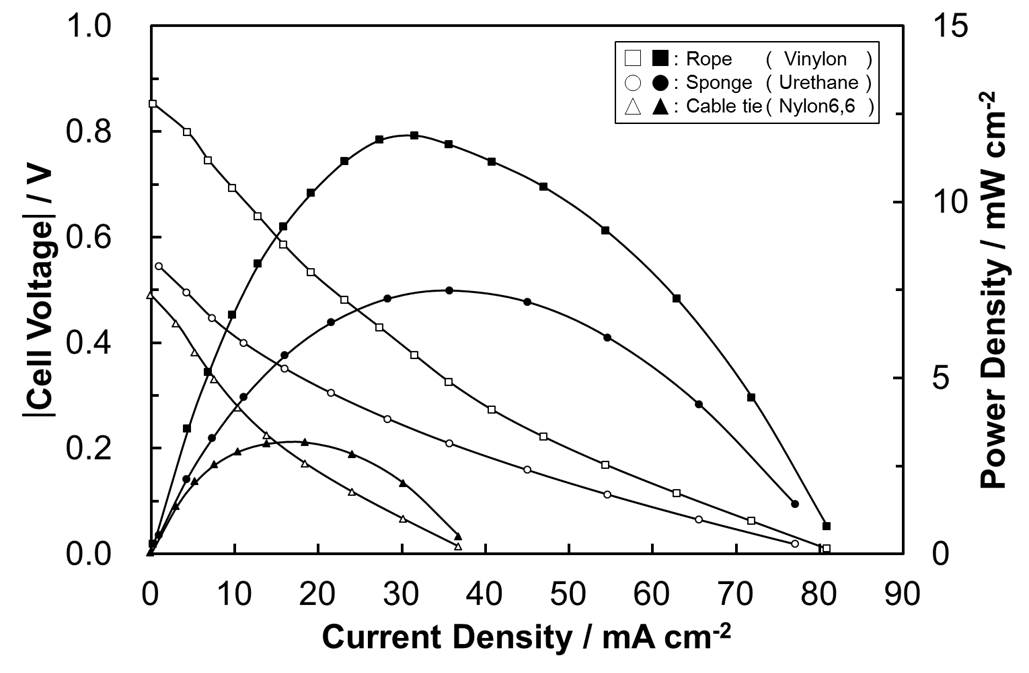
We present an electrochemical reaction system that enables the generation of electricity, employing waste plastics as raw materials. These plastics can include polyvinyl alcohol, polyurethane, nylon, vinylon and polyethylene terephthalate. In the proposed process, the polymers are partially or completely dissolved in phosphoric acid (H3PO4) at temperatures of 100 °C or higher. When a physical mixture of plastic solid and H3PO4 solution was supplied to the anode in a temperature-controlled fuel cell, the in situ dissolved plastic component was oxidized to CO2, protons and electrons as a result of anodic polarization. This oxidation reaction at the anode was optimized by designing a catalyst support with an enlarged, ordered pore structure. An active oxygen species, believed to be the OH· radical, also plays a key role in the plastic oxidation reaction. The fuel cell demonstrated in this work functioned continuously to generate power densities on the order of mW cm-2(Fig.1). Conventional plastic disposal systems based on combustion emit toxic pollutants. The present study demonstrates a process that overcomes this issue by allowing the treatment temperature to be significantly decreased.
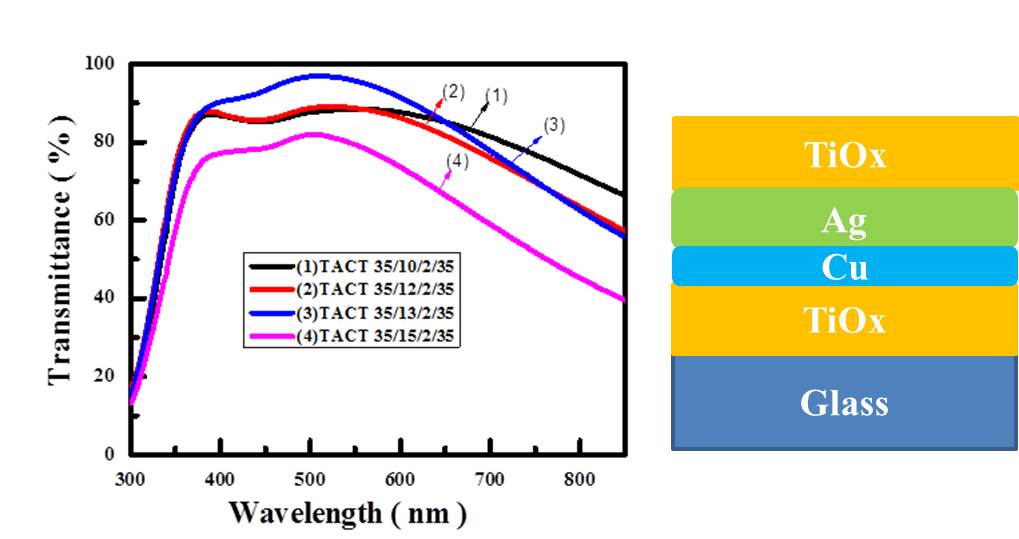
Transparent conductive oxides (TCO) with high visible transmittance and low resistance have been used in applications of optoelectronics. Dielectric/Ag/dielectric (DAD) multilayer was proposed to meet the requirements of low resistance and enough transparency [1]. In this work, a Cu-Ag bi-layer was proposed to serve as the mid-layer in oxides, including TiOx and WO3. All the films were prepared through E-beam evaporation at room temperature. The optical and structure properties of the D/Ag/Cu/D were studied by X-ray diffraction, UV-Vis, SEM, TEM and XPS. The surface morphology of Ag will be flattened by the Cu buffer layer, which can reduce the critical thickness of Ag layer. The thickness effects of Ag and Cu film on the optical properties of the D1/Ag/Cu/D2 were also investigated. According to the UV/VIS transmission spectra, the TiOx (35 nm)/Ag (12 nm)/Cu (3 nm)/TiOx (35 nm) exhibited the highest average visible light transmittance of 88%. The figure of merit (FOM) of TACT stacking is ~ 126 mホゥ-1 at the wavelength of 550 nm. Using WO3 with an thickness of 40 nm as the capping layer above Ag/Cu/WO3 (WACW) shows a FOM of 73 mホゥ-1. Use of Ag/Cu bilayer can widen the transmittance of the D1/Ag/Cu/D2 with an appropriate Ag and/or Cu thickness. The experimental results were also compared with the optical simulation to figure out the role of the ultra-thin Cu buried layer.
As chemical industries have grown gradually, strong alkaline chemicals are commonly used in almost all industries worldwide. Despite their usefulness, alkaline chemicals can be very dangerous as they can cause severe intoxication. In particular, gaseous alkaline chemicals require more precautions because they spread very quickly without being noticed in case of leakage. Therefore, it is important to detect strong alkaline chemical leaks promptly and easily. In this study, to detect gaseous alkalis immediately with naked eyes, we fabricated colorimetric textile sensors based on halochromic dyes. Three halochromic dyes with superior pH sensitivity were synthesized and applied to textile surface through screen printing method. The sensitivity to gaseous alkaline chemicals of the fabricated textile sensors was investigated. Ammonia was used as a gaseous alkaline chemical and the test was conducted at a concentration of 1 to 100 ppm. All of the fabricated sensors showed a high reaction rate and distinctive color change under alkaline condition due to the high pH sensitivity of the dyes. These results indicate that the fabricated textile sensors can be a promising candidate for practical sensor applications.
Recently, nanosheets (NSs) have attracted attention because of their various fascinating properties. We have developed the synthesis method of NSs inside bilayers of hyperswollen lyotropic lamellar phases of amphiphile solutions; the distance of the bilayers with several nm thickness is kept several hundred nm. We named the synthesis methods "Two-dimensional Reactors in Amphiphilic Phases" (TRAP) method. We have already successfully synthesized NSs of polystyrene and metal organic frameworks (MOF) in the thin hydrophobic sites inside the bilayers of aqueous amphiphilic solutions. To extend the range of the application of the TRAP method, we focused on aluminosilicate that is commonly synthesized in the hydrophilic reaction fields.
Aluminosilicate can be a precursor of zeolites with molecular-sized pores (0.3-1.2 nm-sized pores), which lead to the unique functions of zeolites: catalysts, adsorbents, ion-exchangers and membranes. CHA-type zeolites (CZs) are preferable catalysts for methanol to olefins (MTO) processes, which are important for the production of ethylene and propylene requied for polymer industry. CZs, however, have some problems; in particular, the pores are quickly clogged with by-products, and the resulting restriction of the reaction site and the diffusion induces the catalyst deactivation. One of the most promising solutions of the problem is the downsizing of the catalysts. Because the downsizing effect is based on the decrease of diffusion path length, CZ-NSs should have a potential as catalysts with one of the ideal shapes for the MTO processes.
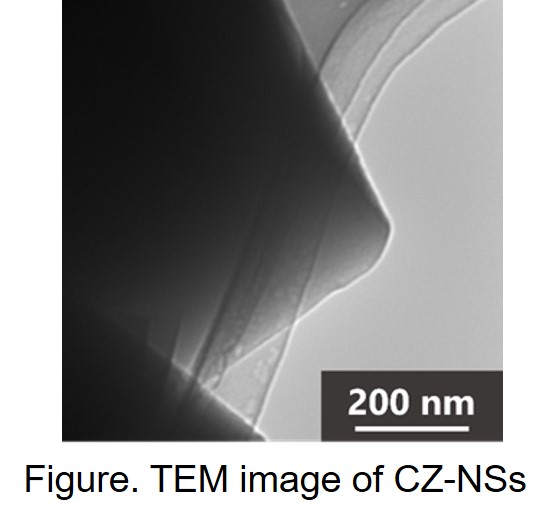
Here, we report the preparation of the suspension of aluminosilicate NSs as a precursor to synthesize CZ-NSs using the TRAP method. Atomic force microscopy (AFM) and transmission electron microscopy (TEM) of the obtained CZ-NSs indicate that their thickness and horizontal width are a few nm and several hundreds of nm, respectively (Figure). This is the first example of CZ-NSs with a few nm thickness.
Seawater is currently used for desalination and salt production, and then the bittern is discharged by the operation. The bittern contains various resources, but most of them are unutilized. It is important to consider the resource recovery process from the bittern in Japan. Therefore, we focused on synthesis of layered double hydroxides (LDH) from the components of the bittern. The LDH is a clay mineral and has anion exchange ability. In this study, the anion exchange property of LDH from the bittern was investigated in comparison with that from ingredients of LDH stoichiometric concentration without components of Na, K, etc which include in the bittern but not in the basic LDH.
The simulated bittern was adjusted based on the actual composition of the bittern and was mixed with Al solution. The precipitate of LDH obtained by dropping into sodium hydroxide solution with coprecipitation method at a high pH region and the powder samples were obtained by filtration from the slurry. The structure, size, shape and ion concentration and anion exchange property of the LDH were analyzed by XRD, SEM and ICP.
The precipitated LDH from the bittern was Mg-type hydrotalcite as results of the XRD measurements. Then, the synthesis of Ca-type hydrocalumite can be also synthesized at another higher pH region. In the syntheses, all LDHs had Chloride ions as charged intercalants for keeping charge neutrality in the layer structures. The anion exchange property was investigated between Cl- and PO43-. The exchange structures were checked by interlayer distance variation before and after the exchange operation. The anion exchange capacity of the hydrotalcite form the bittern showed lower than that from the artificial ingredients.
Zeolites are crystalline microporous aluminosilicates that are widely used as adsorbents, ion-exchangers, and catalysts owing to their large micropore volumes with well-defined pore structures, ion-exchange abilities, and solid acidities. However, the pore sizes of zeolites are usually smaller than 0.7 nm, and the diffusion of molecules in the micropores is often a rate-determining step. Therefore, nano-sized zeolites attract much attention. Proper supports should be used for practical use of nanosized zeolites to prevent their aggregation, and porous inorganic submicron fibers are desirable supports for nano-sized zeolites due to strength, high formability and high surface areas. We prepared zeolite-containing submicron-fibers by centrifugal spinning. The centrifugal spinning is a method using a centrifugal force as a driving force and was developed recently for mass production of various fibers. After careful tuning of preparation conditions and fiber compositions, obtained fiber showed high BET surface areas even after repeated water adsorption test.
To meet increasingly strict regulations on emission control, there has been a high demand on effective catalysts for ammonia selective catalytic reduction (NH3-SCR) of NOx. Although the CHA zeolite dominates the current NH3-SCR market, other small pore zeolites have been studied as equivalent or more efficient catalysts. AFX zeolite is considered to be a superior catalyst for this reaction, which has not yet been applied for industrial use. This situation is in part due to partly because of its long synthesis time (typically, one week or even longer). A more efficient synthesis can help greatly save cost and energy which will pave the way for its industrialization towards emission control. In the work, we demonstrate the ultrafast synthesis of AFX zeolite from the hydrothermal conversion of FAU zeolite, which resulted in fully crystalline product in as short as 12 min.
We carried out the synthesis in the autoclave with a typical recipe, which took 7 d to yield AFX zeolite. The ultrafast synthesis was carried out in a tubular reactor made of stainless steel tube (1/4 inch) which features fast heating and cooling. To prompt the crystallization, seeding method was applied where the autoclave product was used as a seed; the composition of the synthesis precursor was optimized where FAU zeolite was used as a raw material; and, the synthesis temperature was elevated to 210 °C. Although the crystallization time was shortened, the synthesis resulted in the co-crystallization of ANA zeolite as a byproduct. To overcome this issue, the AFX seed was found to play an important role. With a seed synthesized by an improved method, the pure AFX zeolite was obtained after a synthesis of 12 min. This ultrafast synthesis method would facilitate the mass production of AFX zeolite boost its application as a de NOx catalyst.
The synthesis of zeolites, typically carried out in batch reactors like autoclaves, takes a time so long (typically, on the order of days) that the crystallization of zeolites had been believed to be very slow in nature. Long periods of hydrothermal treatment also cause a burden on both energy efficiency and operational costs. Recently, we have reported the ultrafast syntheses of a class of industrially important zeolites within several minutes. We present herein a continuous flow method for the synthesis of ZSM-5 using pressurized hot water with extremely high temperature (370 °C) as the heating medium. With a carefully tuned gel, the crystallization could be completed in tens of or even several seconds.
Direct mixing of synthesis precursor and the pressurized hot water in a millimeter-sized continuous flow reactor could result in immediate heating up to high temperatures; consequently, the crystallization of ZSM-5, from amorphous state to full crystallinity, proceeded to completion at a remarkably fast rate in a system without the addition of any seed. The well-tuned synthesis precursor, obtained by aging the initial aluminosilicate gel at 90 °C for a certain period, triggered the nucleation and ensured the formation of ZSM-5 without any byproduct at extremely high temperatures. The crystallization rate surpassed the decomposition rate of OSDA because of the creation of a favorable environment for crystallization. When a gel aged for 16 h was employed, fully crystalline ZSM-5 was obtained after a synthesis for 6 s. SEM image shows that the ZSM-5 synthesized after 6 s exhibited well crystallized facets. These unexpectedly fast crystallization rates in a continuous flow apparatus helped us to approach the limit of the zeolite crystallization.
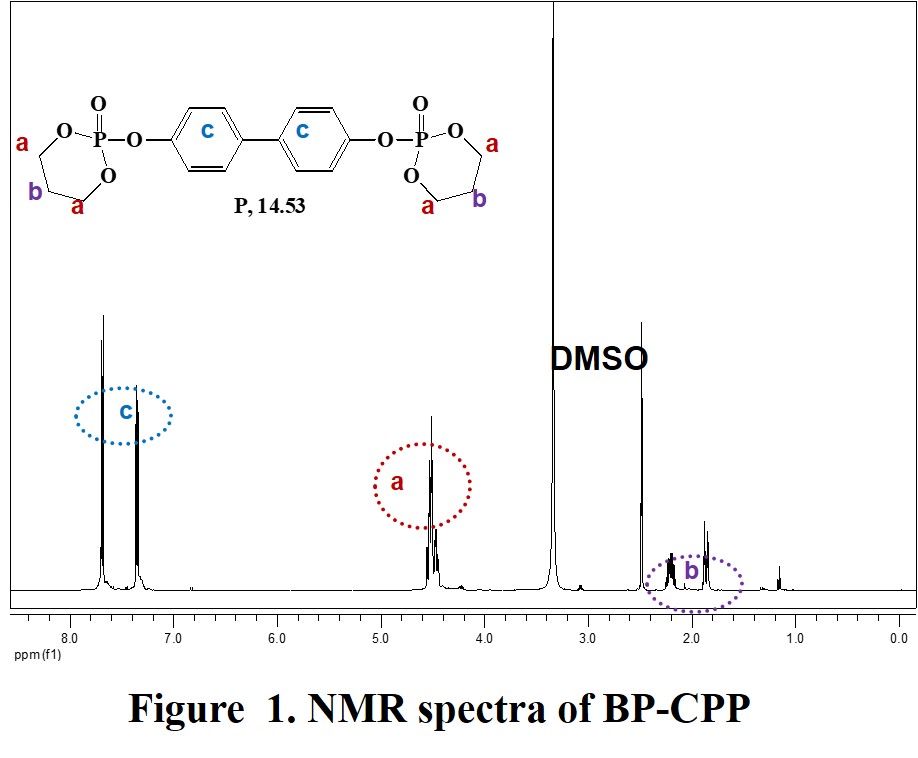
Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. Nowadays, the flame retardancy of polymers are the major concern. In this study, Polycarbonate (PC) was selected to develop an efficient flame retardant (FR) of high temperature polymer. A series of organo phosphorus flame retardants (FRs) based on aromatic phosphate and cyclic phosphate, mainly Biphenyl bis(cyclic 1,3-propanediol phosphate)(BP-CPP) and Biphenol bis(cyclic 2,2-dimethyl-1,3-propanediol phosphate)(BP-DMPP), were synthesized for polycarbonate. Their thermal stability and flame retarding efficiency as a single-component additive were investigated and compared with resorcinol bis(diphenyl phosphate) (RDP). Flame retarding efficiency was evaluated by the UL-94 test method. The V-0 rating was achieved at RDP/ BP-CPP loading of 6/2 wt% for polycarbonate, which is far better than that of resorcinol bis(diphenyl phosphate) and cyclic phosphate-based FRs. And its flame retarding performance was studied by the UL-94 and thermogravimetric analysis (TGA).
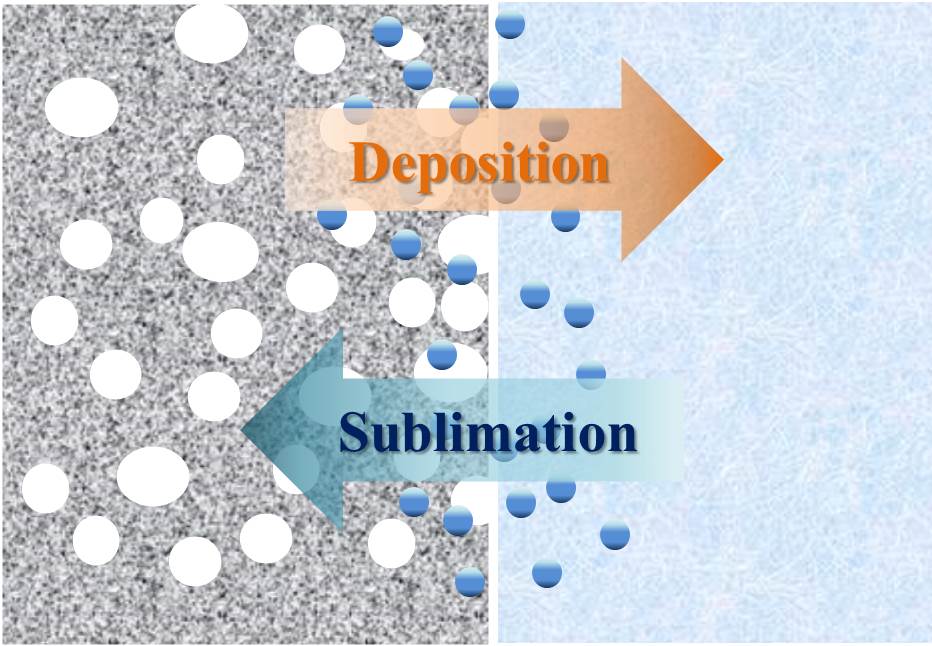
Porous materials have been widely applied to the different fields, such as adsorbents, heterogeneous catalysts, drug loading and delivery. In the current studies, the porous materials are mostly constructed by gas foaming, photolithography, templating, etc. Here, we report an innovative manufacturing process for the fabrication of three-dimension porous structures containing other ingredients including the organic and inorganic via the chemical vapor deposition (CVD). The porous materials were made by poly-para-xylenes (PPXs) replacing the origin sublimating templates via the sublimating/deposition process, and the ingredient that cannot be sublimated would be encapsulated within the PPXs porous materials. The mechanism of the porous structures is that the pores are formed by the gas vapor occupying the remaining vacancies lifted by sublimated template. By adjusting the sublimation rate of the template and the rate of PPXs deposition, the porosity can be controlled. In this report, we present three examples including the magnetic porous particles, cell-containing scaffolds and the protein-encapsulated porous particles. This novel approach overturns the general concept that vapor deposition forms uniform thin film and also provides another way to manufacture multifunctional porous materials.
In this study, a oriented organometallic frame/polyvinyl alcohol (MOF/PVA) nanofiber material was prepared by electrospinning. PVA was uniformly mixed with a MOF to form a solution. In this experiment, zinc nitrate Zn(NO3)2) and 2-methylimidazole were used to synthesize ZIF-8 powder material by in-situ process, and then added to 40mg, 120mg and 200mg respectively. The 10wt% PVA polymer solution was thoroughly stirred, the precursor was disposed, and the electrospinning process was performed. The crystal structure and scanning electron microscope (SEM) were detected by X-ray Diffaction (XRD). Observing the trend of fiber arrangement and the surface morphology of ZIF-8 grown on the fiber, the weight change of the sample detected by Thermogravimetric Analysis (TGA), and the Fourier Transform Infrared Spectroscopy (FTIR) test sample The change of properties before and after mixing of different contents.
As a results, it was found by SEM observation that PVA/ZIF-8 10 wt% was formed by bead-like fibers after electrospinning. From this phenomenon, ZIF-8 was uniformly dispersed in a polymer solution to reunite ZIF-8 by electrospinning technology. ZIF-8 can be evenly distributed in the fiber. XRD analysis showed that there were indeed various characteristic peaks of ZIF-8 in the composite fiber, and it was further confirmed that ZIF-8 was contained in the fiber.
Metal-organic frameworks (MOFs) are porous material composed of metal ions and organic ligands, which have ordered crystalline structure with micro pores and high surface area. MOFs are recently focused as materials for various applications. In the development of the MOF-based mixed-matrix membranes for membrane separation processes, the size and the shape of particles are the important factor for membrane performances. Zeolitic imidazolate framework-L (ZIF-L) is one of the two-dimensional MOF which is focused as materials for mixed matrix membrane; however, the control of the shape and the particle size has not been sufficiently reported yet.
In this study, we report the effect of surfactants on the synthesis of ZIF-L. Surfactants can adsorb on the specific surface of the MOF crystal in synthesis, and can control the size and the shape of the resultant MOF particles. In the synthesis of ZIF-L, we used cetyltrimethylammonium bromide (CTAB) as cationic surfactant, sodium dodecyl sulfate (SDS) and sodium dodecylbenzene sulfonate (SDBS) as anionic surfactants, TWEEN(R) 20, 40, 80 as nonionic surfactants. We synthesized ZIF-L in the presence of various surfactants with various concentrations and characterized the crystal structures using X-ray powder diffraction, the size and the shape of the particles using scanning electron microscope, porous structure using nitrogen adsorption measurements. The characteristics of obtained ZIF-L particles are highly influenced by the species of surfactants, and the details will be reported in the presentation.
Metal-organic frameworks (MOFs) are novel porous materials composed of metal ions and organic ligands. MOFs have ordered porous structures and show adsorption and molecular sieve properties. As one of the applications, MOF-based mixed-matrix membranes (MMMs) have been reported for gas separation. The morphology of MOF particles is the important factor for membrane performances. It is reported previously that MMMs prepared by stacking two-dimensional (2D) MOFs horizontally in the membrane plane can exhibit high gas selectivity by reducing the permeation of non-permeable component. Zeolitic imidazolate framework-7-III (ZIF-7-III) is one of the 2D MOFs which is composed of zinc ion and benzimidazole and forms layered crystal structure with micropores which diameter is approximately 0.29 nm. In the previous report, composite membrane was prepared using ZIF-7-III nanosheets which was made by exfoliation of layered ZIF-7-III particles. The initial ZIF-7-III particle size should be influential on membrane performances; however, there has been no report on the control of ZIF-7-III particle size.
In this study, we report the control of ZIF-7-III particle size using water-in-oil microemulsion. Microemulsion was prepared using heptane as continuous phase, water as dispersed phase, cetyltrimethylammonium bromide (CTAB) as surfactant and 1-hexanol as co-surfactant. Synthesis of ZIF-7-III was conducted in microemulsion, and obtained white powder was characterized using powder X-ray diffraction and scanning electron microscope. Reaction conditions such as reaction temperature, concentration of CTAB were highly influential on the morphology of resultant ZIF-7-III particles. The details will be reported in the presentation.
Metal-organic frameworks (MOFs) are an emerging class of nanoporous materials. Owing to their highly ordered nanopores, MOF membranes are promising for higher selectivity and permeability than polymer membranes which dominate industrial membrane separation. However, high cost of support materials and lack of robust membrane fabrication methods are problematic. Here, to establish a versatile fabrication method, we investigated the formation process of MOF membranes on hollow fibers, originally used for water purifier filter, which are cheap and easily available.
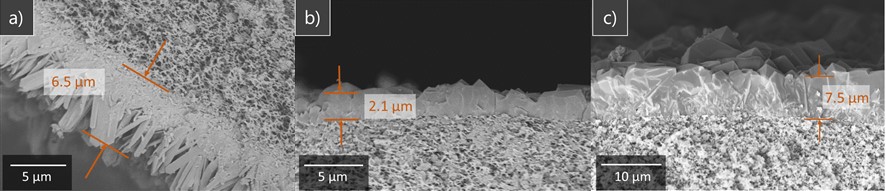
Here we focused on zeolitic imidazolate framework-67 (ZIF-67) composed of Co2+ ions and 2-methylimidazole (MIM) linker, which is a family of MOFs, and applied a secondary growth method. In this method, seed crystals were deposited on the fiber by immersing the fiber in MIM and Co(NO3)2 aqueous solutions alternately, followed by the seed crystals growth by immersing seeded fibers in a mixed aqueous solution of MIM and Co(NO3)2 (growth solution). We first investigated effects of concentration ratio of growth solution (R = [MIM]growth / [Co2+]growth). A small R resulted in the deposition composed of needle-like crystals with many gaps (Fig. 1a), while a large R produced a thin film densely packed by rhombic dodecahedral crystals (Fig. 1b). However, resultant films showed no separation selectivity.
To improve the membrane performance, we modified the growth process. Immersing seeded fibers in a growth solution with a small R was followed by subsequent growth under a large R so that ZIF crystals first grow vertically and then merged with each other by filling gaps in the second growth. This two-step process successfully produced a thick and dense membrane (Fig.1c) with separation factor of 1.15 for air and propane, which suggests that the two-step growth reduces cracks. Hence, we demonstrated that the stepwise control in the crystal growth direction enables the membrane densification.
Metal-organic frameworks (MOFs) are new porous materials discovered in the 20th century. MOFs are synthesized through self-assembly of metal ions and organic ligands, and they have regular arrays of micropores and a large surface area. In addition, by selecting components, MOFs exhibit various crystal structures with tunable pore size. From these characteristics, MOFs are expected to be applied to the medical field, especially drug delivery systems (DDSs). In DDSs, the drug with necessary amount are transported to the right place at the right time by spatial and temporal control. Generally, a carrier for containing or binding the drug is required for DDSs. Although it is common to use a liposome and a polymeric micelle as the carrier, the high inclusion method of drugs has not been established. In recent studies, therefore , MOFs have attracted much attention as a new DDS carrier with high drug inclusion.
In this study, the drug-release behavior from MOFs by a pH change was investigated. As model MOFs, we selected zeolite imidazolate framework-8 (ZIF-8) and Universitetet i Osolo-66-NH2 (UiO-66-NH2). It is reported that the crystal structures of ZIF-8 and UiO-66-NH2 collapse under acid and base conditions, respectively. As model drugs, indomethacin and ibuprofen were selected. We found that ZIF-8 included indomethacin and its dissolution rate from ZIF-8 was the fastest at pH 5.0. On the other hand, UiO-66-NH2 included ibuprofen and its dissolution rate from UiO-66-NH2 was the best at pH 8.5. These results will lead to the development of the new pH responsive DDS with high drug capacity.
Hydrogels are used in applications such as separation, reaction, and drug delivery systems. The diffusivity of solute in hydrogels is an important parameter. For example, the concentration and the diffusivity of solutes, such as oxygen and NH4+, in the hydrogel can affect the activity of ammonia-oxidizing bacteria (AOB) immobilized within the hydrogel for an ammonia wastewater treatment system. The goal of this study is to develop a novel method to measure the diffusivity of solute in spherical hydrogel using a microsensor. A microsensor measures the chemical environments at the scale of the micrometer-sized sensor tip, as well as the concentration of solute in hydrogel. Millimeter-sized spherical hydrogel is suitable for the experiment using the microsensor and the analysis of diffusion kinetics. The spherical polymeric hydrogel was prepared using a production method combining sedimentation polymerization and two-fluid atomization, developed in our previous study. Oxygen, NH4+, and N2O were used as model solute for AOB-catalyzed reactions. The experiment on oxygen diffusion was carried out as follows. Firstly, a microsensor was inserted in the center of the spherical hydrogel and the hydrogel was placed in anaerobic water purged with N2 gas. Then, water was purged with air and the dissolved oxygen (DO) concentration in the hydrogel was measured continuously. The DO concentration was approximately 0 during initial period, and increased with time up to approximately 230 mmol/m3 in air-saturated water. Diffusivity of solute in hydrogels was successfully analyzed using the solution of the Fickian diffusion equation within a sphere.
Pd catalyzes various kinds of reactions such as automobile exhaust emission treatment and organic synthesis. In this study, we focus on polymer gel as a heterogeneous catalyst carrier. The gel is a polymer of a three-dimensional network structure swollen with a solvent and can be handled like a solid, but the inside of the gel is also like a liquid. The aim of this study is to develop a polymer gel supported Pd catalyst using various polymer gels, to clarify the influence of the absorption and adsorption ability of the gel and the holding state of Pd(II) ion on the catalytic activity to construct a design guideline for a high performance gel catalyst. The model polymer gel was copolymer gel of allyl mercaptan (AM) having a thiol group interacting with Pd(II) ion and multicomponent, and gel having amino group and ammonium group in side chain. The various kinds of gels were synthesized by free radical polymerization. Pd(II) ion-adsorbed gels were prepared through the Pd(II) ion adsorption experiment, and subsequently Pd nanoparticle-loaded gels were prepared by the reduction of Pd(II) ions using NaBH4. The model organic synthesis was Suzuki-Miyaura coupling (synthesis of biphenyl with phenylboronic acid and iodobenzene as reactants) using both catalysts Pd(II) ion-adsorbed gels and Pd nanoparticle-loaded gels. The adsorption properties of Pd(II) ions of various gels, characteristic properties of gel supported Pd, and Suzuki-Miyaura coupling reaction characteristics using gel supported Pd were investigated.