The excited electrons and holes generated upon by irradiating UV ray to TiO2 have powerful redox power. They produce reactive oxygen species (ROS) under aerobic conditions at the surface. These species rapidly decompose organic compounds to convert them into CO2 and H2O via carbon-carbon bond cleavage. On the other hand, they can also catalyze organic synthesis reactions such as carbon-carbon bond formation. The reactions must be carried out in dry anaerobic conditions in order not to produce ROS that can damage organic compounds. Usually, TiO2 has been used as a powder to increase the catalytic effect. However, the use of particles involves the need of incorporating extra stages in the purification process, such as separation and catalyst recycling. We focused on a composite of TiO2 nanoparticles and a polymer gel in which TiO2 nanoparticles are dispersed and immobilized in the polymer gel as a heterogeneous catalyst. The purpose of this study is to develop a novel TiO2 nanoparticle-loaded polymer gel and clarify its applicability as a catalyst for organic synthesis reaction. As polymer gels, N,N-diethylacrylamide gel, N,N-dimethylacrylamide gel, and N-isopropylacrylamide gel were used. Commercial TiO2 nanoparticle (AEROXIDE® TiO2 P-25) with 21 nm in diameter was used. The composite gels were synthesized by free radical polymerization. The 4-iodetoluene dehalogenation reaction as a model organic synthesis reaction was performed by a batch operation using 4-iodetoluene, diisopropylethylamine, dried composite gel, and acetonitrile as a solvent. The reaction was irradiated with UV light and stirred at room temperature under a nitrogen atmosphere. The composite gels catalyzed the reaction successfully.
Sodium alginate aq. is known to form hydrogel on mixing with CaCl2 aq., where Ca2+ works as the crosslinker. Megascopically, the above process is observed as instantaneous gelation. The authors have been interested in capturing the quick gelation in terms of how fast it occurs on actual mixing of these two starting aqueous solutions. First, 1wt% sodium alginate aq. was added dropwise into 10M CaCl2 aq. and the time evolution of the morphology of the droplet after the impingement onto the surface until the stabilization of its outer surface was recorded in a high-speed motion picture. The difficulty in fulfilling our observational purpose relying on the above method was revealed that the gradual morphological alteration toward the spherical geometry is temporarily too moderate to be detected by the high-speed motion picture method. In order to capture the arrest in the deformation of the gelling solution more effectively, we needed to choose a situation where the tiny surficial undulation of the gelling solution is incessantly induced by being in contact with 10M CaCl2 aq. In the intensively discharged gelling solution out of an injection syringe, it was found that the straight linearity of the gelling solution in the translational motion was observed while it kept in the state of a flowable solution. The beginning of the deviation from the as-injected linear flow implied the vanishing fluidity. Thereafter, the gelling solution was seen to behave like rod-like objects forming an entangled coil. From the discharging velocity of the gelling sodium alginate aq. at the exit of the injection syringe, the timescale for the gelation was estimated to be 10-3s in the order of magnitude.
In many countries, arsenic level in groundwater exceeds the acceptable limit. Although we removed Arsenate in our previous research, removal of Arsenite is harder. The purpose of the study is to adsorb Arsenite, As(III), from ground water by adsorption using a composite of cationic polymer gel impregnated with iron hydroxide. The cationic polymer gel that we used is N,N-dimethylamino propylacrylamide, methyl chloride quaternary (DMAPAAQ).
The gel was prepared in a unique method where Sodium Hydroxide and Iron (III) Chloride was added to the monomer and initiator solutions, respectively, in order to maximize the FeOOH contents in the polymer structure of the gel.
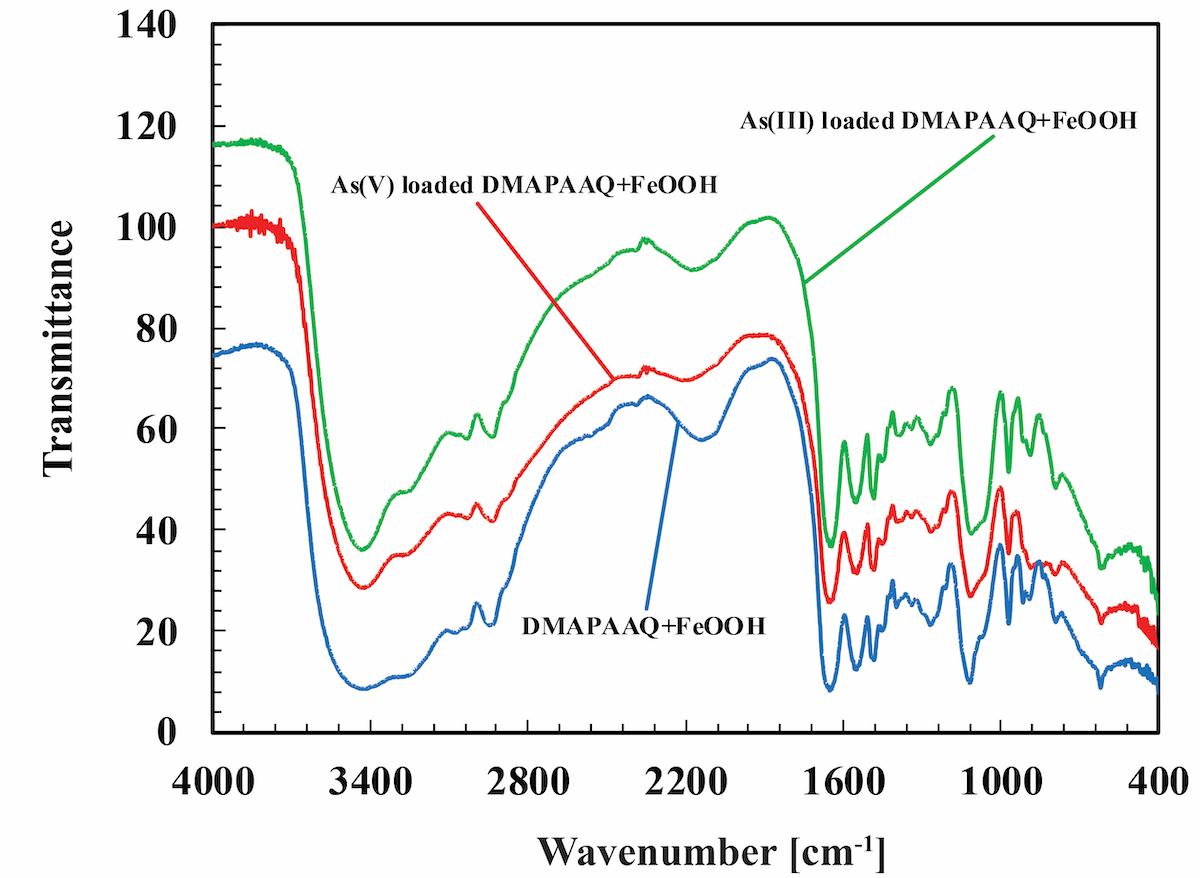
Transmission Electron Microscope (TEM) images show that our gel contains visible FeOOH particles in its structure. The Thermogravimetric Analysis (TG) indicates that the maximum weight percentage of FeOOH particles in the gel is 53.7%. Generally, there are three types of FeOOH. We performed Mössbauer spectroscopy to determine the type of FeOOH contained in the gel. The results match with that of γ-FeOOH. Previous arsenic removal experiments found that γ-FeOOH helps to remove both As(III) and As(V). Our studies suggest that in the structure of DMAPAAQ + FeOOH gel, the polymer structure of DMAPAAQ adsorbs 35.6%, whereas γ-FeOOH components adsorb 64.4% of arsenic. Hence, DMAPAAQ + FeOOH can adsorb arsenic successfully. FTIR analysis suggests that when As(III) and As(V) were adsorbed by the DMAPAAQ + FeOOH gel, changes such as shifts, appearances and disappearances in several surface functional groups occurred. These results further prove the adsorption of arsenite to the gel.
Our study successfully showed that the newly developed gel composite, DMAPAAQ and FeOOH, adsorbed As(III) effectively because of having γ-FeOOH components in the gel structure. The novelty in our research is in the unique preparation method of the gel, and It's characterization.
Catalysis by Heteropoly acids (HPAs) and polyoxometalates (POMs) having a higher demand worldwide, as it can be designed to accelerate complex reactions and be more environmentally friendly. However, recycling of water-soluble solid catalysts remains a problem. The synthesis of a recyclable composite with catalytic properties is the key to better use of HPAs and POMs. Many researches have mentioned the method of synthesis by immersing a porous carrier in a supported solution. However, the catalytic abilities of the previously studied composites after multiple uses have rarely been mentioned. In this research, a novel idea is proposed to synthesize a heteropoly acid supported composite. A complex hydrogel with catalytic properties was synthesized by mixing an anionic monomer with a heteropoly acid. The heteropoly acid particles were inserted inside the hydrogel by the interaction forces between the anions. Thus, preventing the water-soluble heteropoly acid from being lost during the catalytic reaction. The complex hydrogel is consisted of the anionic monomer 2-acrylamide-2-methylpropanesulfonic acid (AMPS) as a carrier, N,N′-Methylenebisacrylamide (MBAA) as a crosslinker and the typical keggin-type HPA: H3PW12O40, which was synthesized by one-pot method. Then the composite is divided into five groups. Following this, the five groups were washed by deionized water for 24h, 48h, 72h, 96h and 120h respectively. The existence of H3PW12O40 crystalline nanoparticles in the composite was confirmed by X窶甚ay diffraction (XRD). After comparing the samples from different groups by Scanning Electron Microscope (SEM), all the samples have similar surface structure and nanoparticle aggregation state. Nanoparticles were observed from the samples of each group by Transmission Electron Microscope (TEM). From the thermogravimetric analysis (TGA), the particle content rate of samples from each group was kept at about 17% as the washing time increased. It indicates a higher potential of this synthetic method to a wide range of applications.
Removal of heavy metals from ground water and soil has been a common problem both in industry and agriculture, since high concentration of heavy metals are harmful to environment and human health through water and food. In this study, a simple and effective removal method of various harmful anionic ions such as arsenic acid, selenium acid, and chromium acid from soil and water was proposed. Generally, these anions were removed by cationic adsorbent or cationic ion exchange resign. However, various adsorbents have low ability to remove heavy metals from low concentration solutions, and it sometimes desorb those ions when interfering ion exist. We present new ion removal method by using hydrogel. Anionic hydrogel of 2-Acrylamido-2-methylpropanesulfonic Acid Calcium salt (AMPS-Ca) and cationic hydrogel of N,N-Dimethylamino Propyl Acrylamide (DMAPAA gels) were used to remove anionic heavy metal ions. Each heavy metal ion could be partially removed by both hydrogels. It was found that removal of arsenic and selenium by AMPS-Ca hydrogel as attained by the formation of heavy metal calcium salts in the hydrogel. On the other hand, the crystallinity of the heavy metal salt was not found in the DMAPAA gel. It is found that removal of heavy metals by each hydrogel was made by adsorption or formation of heavy metal salt in the hydrogel. In addition, the various hydrogels used in this study could absorb water as well as sodium acrylate. Therefore, it is suggested that they also provide a method of solidify the heavy metal contaminated sludge discharged in tunnel excavation work. The contaminated sludge will be recycled after the treatment of the hydrogel.
Introduction
The demand for lithium-ion batteries has rapidly increased in recent years, and the supply will be insufficient in near future. Therefore, the establishment of lithium recycling process is required to solve the problem of the increase of the waste lithium batteries. However, conventional lithium recovery processes are complicated and costly. We have presented new simple lithium recovery process1) using hydrogel with a quaternary amine ((3-Acrylamidopropyl) trimethyl ammonium chloride)(APTAC). In this research, the reaction mechanism of lithium and carbonate ion are investigated.
Result and discussion
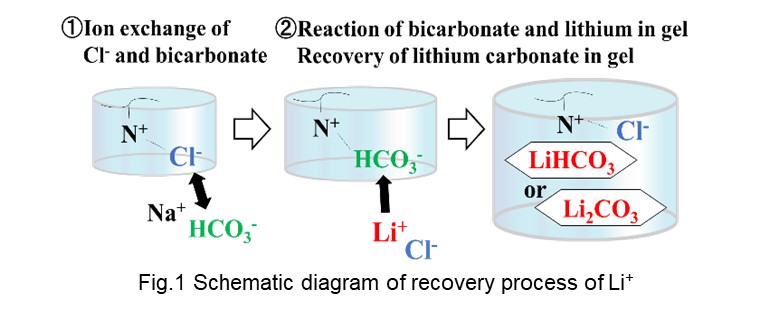
A schematic diagram of the process is shown in Figure 1. At first, APTAC gel was immersed in the solution containing anion which exchanged Cl-. The gel was then immersed in LiCl aqueous solution. The Li+ reacted with the anion in the gel to produce lithium salt. The Li+ was recovered as the lithium salt within the gel.
We investigated which anion was suitable for Li+ recovery and found that the HCO3- recovered the lithium ion best among PO43-, HCO3- and CO32-. HCO3- reacted with Li+ more than their stoichiometric ratio, 1.0, when the initial concentration of Li+ was more than 1000 ppm. It is considered the Li+ initially reacted with HCO3- to form metastable LiHCO3 when its initial concentration was below 1000 ppm. Then H+ in a part of the LiHCO3 exchanged with Li+ in the solution to form stable Li2CO3 when it was higher than 1000 ppm. Therefore, Li+ was recovered more than double of the stoichiometric ratio.
The elemental composition of the gel after the Li+ recovery was analyzed by XPS. The gel had the C-O single bond that was not exist in the original APTAC gel. We found that Li+ reacted with HCO3- and was recovered as LiHCO3 or Li2CO3 in the gel.
Gel bead can be used as a carrier for drugs and fertilizers and its diffusion characteristics has been studied for controlled release of chemical substances. In our study, the effect of the addition of the dispersed phase on the diffusion rate of substances in hydrogel was investigated. The oxygen diffusion rate in hydrogel containing microbeads was measured to study the effect of the size and amount of microbeads on the diffusion inhibition.
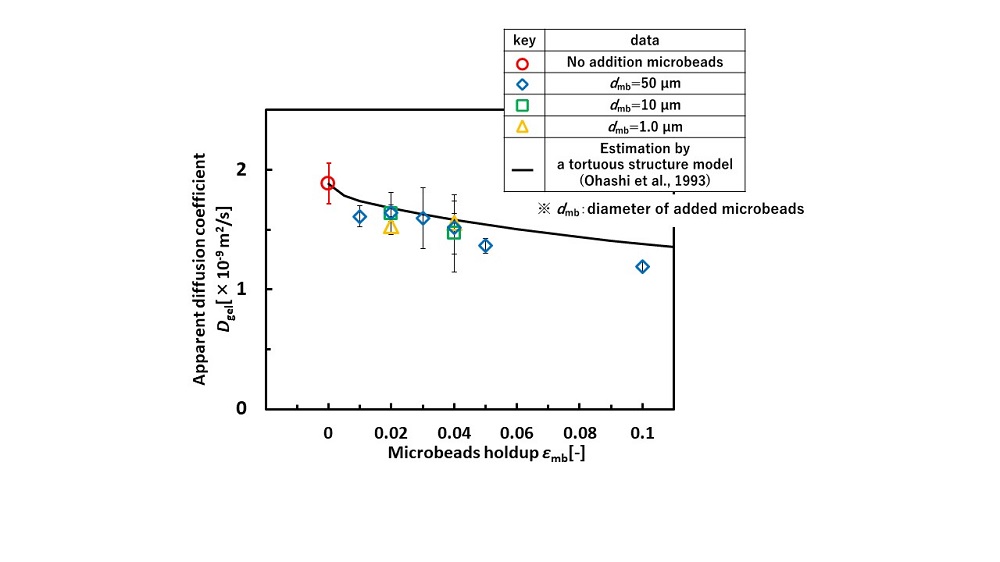
Figure 1 shows the effect of microbead addition on apparent diffusion coefficient of oxygen in hydrogel. The apparent oxygen diffusion coefficient was calculated by solving one-dimensional unsteady diffusion equation with measured data of dissolved oxygen concentration profile in the gel. First, the apparent oxygen diffusion coefficient in the gel containing the microbeads was smaller than that without the microbeads. It was confirmed that the addition of the microbeads inhibited oxygen diffusion.
Next, the apparent diffusion coefficient decreased with an increase in the microbeads holdup. On the other hand, the diffusion rate did not depend on the microbead size. To develop the estimation method of apparent diffusion coefficient, diffusion in hydrogel containing microbeads was considered by analogy with the diffusion theory in porous medias (Ohashi et al., 1993) where diffusion path is lengthened by solid obstacles. The experimental values agreed well with the model. This suggests that the apparent diffusion coefficient can be estimated from the volume fraction of the added dispersed phase.
Heat dissipation is one of the most important issue for designing high performance devices. Thermal interface materials (TIMs) are used to promote heat transfer at the solid-solid interface between IC chip and heat radiator in electronic devices. Presently, composite materials of thermally conductive fillers and polymers are widely used, however polymers have problems of low thermal stability and low thermal conductivity. Here, we propose and report Ag aerogel films composed of Ag foils sandwiched with Ag porous layers (Fig. 1a,b). The film was fabricated by simultaneously forming Ag particles by gas-evaporation and depositing Ag particles on an Ag foil. The boat-foil distance was carefully optimized to make the Ag porous layer soft and uniform. The steady-state measurement showed a thermal resistance as small as <20 mm2K/W (Fig. 1c), which is similarly small as the high performance TIM of an indium sheet. Our aerogel TIM, made of Ag having a melting point (962 °C) much higher than indium (157 °C), can expectedly be used for thermal management under high-temperature conditions.

Hydrogel have good flexibility and biocompatibility, so they cause little damage by implantation in the body. Furthermore, since hydrogels can release drugs, they are expected to be applied to a local drug delivery system. The amount of drug used can be reduced and side effects can be alleviated by the local drug delivery system. However, the hydrophilicity of hydrogel interfere result in suppression the controlled release of hydrophobic drugs such as anticancer agents or antibiotics. We have developed hydrophobically-modified gelatin (HMG) and could prepare a hydrogel by only hydrophobic interaction. HMG hydrogel do not require the use of chemical crosslinkers, which are often toxic to the body. In addition, HMG hydrogel have hydrophobic segments in its structure, which can absorb hydrophobic drugs and expect to control their release. As a factor to control the amount of adsorbed hydrophobic drug in the hydrogel, we focused on the strength of hydrophobicity of hydrophobic group. HMG with different alkyl chain length (C4~C12) of modified hydrophobic group were synthesis. The swelling ratio of these hydrogels were decreased in increasing the chain length of alkyl group modified to HMG. This result means that the increase of alkyl chain length intensifies the hydrophobic interaction. Then, HMG hydrogel was adsorbed with uranine used as a model for hydrophobic drugs. The adsorption test exhibited that the amount of hydrophobic drug adsorbed in HMG hydrogels could be controlled by varying hydrophobic alkyl chain length. The controlled release of the drug was confirmed by drug release experiments in vitro. In conclusions, HMG hydrogel could control drug adsorption and delivery.
Novel organic-inorganic hybrid material was developed as a LED sealant.
Polysilsesquioxane with -SH group (PSQ-SH) and acrylic monomer were selected as inorganic and organic components, respectively. Trimethylolpropane Trimethacrylate (TRIM) which is a trifunctional monomer, has been selected as an acrylic monomer, and curing of the material was performed by the Thiol-ene reaction.The LED light emitting substrate was sealed using the prepared PSQ-SH/TRIM hybrid material, and a light emission test was performed. However, there was a problem that cracks occurred in the hybrid material during lighting. Therefore, Ditrimethylol propane tetraacrylate (DTMPA), which is a tetrafunctional monomer, was newly selected to prepare a PSQ-SH/DTMPA hybrid material in order to enhance the strength and heat resistance of the material. The organic component can be reduced by the increasing the number of the functional group in the monomer, and a more inorganic rich material can be prepared. The heat resistance can be improved by increasing the inorganic components in the hybrid material. The mechanical strength of the hybrid material can also be improved by increasing the polymerization density. Therefore, the characteristics of novel PSQ-SH/DTMPA hybrid material were evaluated and compared with PSQ-SH/TRIM hybrid material. As the contents of the evaluation, the heat resistance was evaluated by confirming the weight reduction start temperature using the Thermalgravimetry-differential thermal analyzer(TG-DTA) analysis, the mechanical strength was also evaluated by bonding the glass substrates with a hybrid material and measuring the sealing strength. The obtained results did not improve with respect to the heat resistance even if the monomer was changed.However, the mechanical strength was greatly increased by changing the monomer.
In conclusions, it was possible to suppress the generation of cracks at the time of lighting by using the PSQ-SH/DTMPA hybrid material compared to PSQ-SH/TRIM hybrid materials in the light emission test.
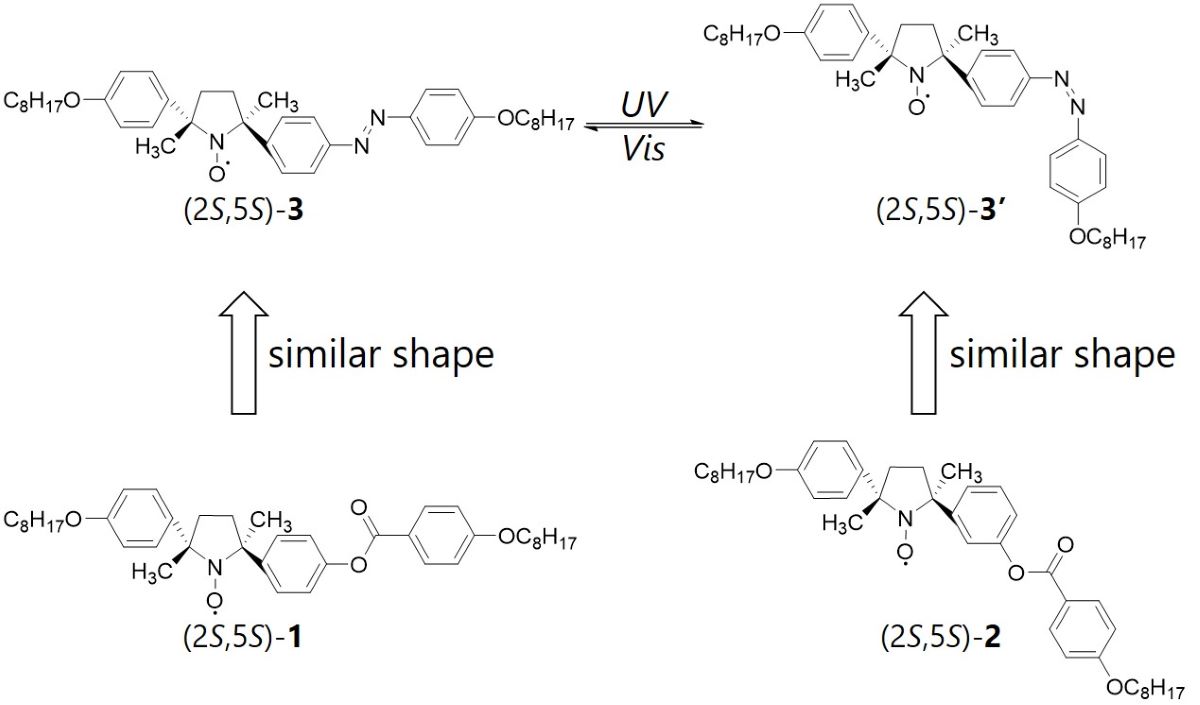
Rod-shaped nitroxide radical (NR) compounds with a five-membered ring NR moiety in the mesogen core (LC-NRs) show unique intermolecular magnetic interactions in their liquid crystalline (LC) and isotropic liquid (Iso) phases above room temperature; their magnetic susceptibilities increase abruptly at the phase transitions from the crystalline (Cr) to LC phases. This phenomenon is called magneto-LC effects. This effect is considered to originate from inhomogeneous intermolecular magnetic interactions. In fact, we confirmed that the effect was enhanced by adding a small amount of (2S,5S)-2 to (2S,5S)-1 (Figure). We also synthesized a new LC-NR with an azobenzene moiety (2S,5S)-3 whose trans and cis isomers have similar shapes to (2S,5S)-1 and (2S,5S)-2, respectively. Since an azobenzene moiety shows the photoisomerization, we expected to change reversibly in inhomogeneity of intermolecular magnetic interactions of (2S,5S)-3 due to the change of the molecular shapes during the photoisomerization. Here, we report the synthesis of a new LC-NR with an azobenzene moiety in the mesogen core and the effect of the photoinduced phase transitions on magnetic properties.
We observed the phase transition behaviors under ultraviolet (UV) or visible (Vis) light irradiation by polarized optical microscopy and measured UV-Vis light absorption spectra. We also measured electron paramagnetic resonance (EPR) spectra under continuous irradiation of UV or Vis light.
Photoinduced phase transitions occur only between LC and Iso phases under UV or Vis light irradiation for (2S,5S)-3. This is likely to arise from the increase of the ratio of cis-isomer by the UV light irradiations, which could reduce the orientational order parameter. EPR spectra indicate that magnetic susceptibility increases under UV light irradiation and decreases under Vis light irradiation. We confirmed that bidirectional switching of the magnetic properties of (2S,5S)-3 occurs under alternating irradiations of UV and Vis light.
With the increasing demands on wearable devices, the stretchablity of various electrical devices have drawn much attention recently. The most commonly used method to enhance mechanical performance of stretchable polymers is introducing nanofillers to form a composite system. Polydimethylsiloxane (PDMS) is the most widely used silicon-based elastomer, and has been widely used in many medical, biological, and microfluidic applications. In the literature, various nanofillers, such as carbon nanotubes, graphene sheets, and silver nanowires, have been well dispersed in PDMS to demonstrate the flexibility and versatility of PDMS composites.
On the other hand, because of the chemical inertness, it is difficult to include ionic fillers in PDMS. PDMS composites with ionic filler usually have problems like filler aggregation, delamination, phase separation, or fractures, under stretching conditions. To address this problem, in this work, a water-in-oil type emulsion is used as a carrier for water-based ionic materials. The relationship between applied sheer rates and droplet size will be examined to elucidate the emulsion process. Moreover, emulsion stability will also be carefully characterized to investigate the effectiveness of various emulsifying agents. In summary, this research provides a new method for PDMS composite synthesis and opens a new avenue to stretchable polymer applications.
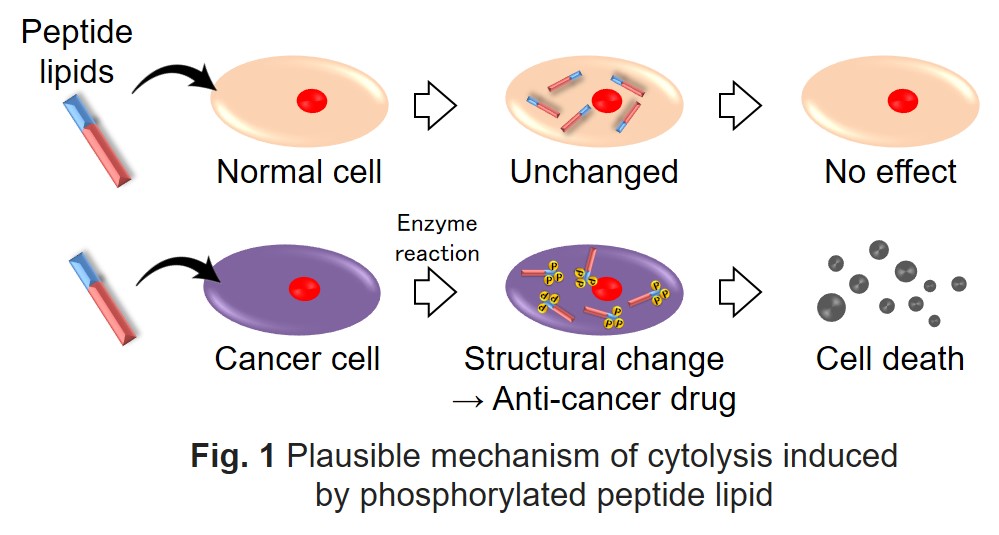
In the last two decades, many reports revealed the novel functional properties of natural and synthetic peptide lipids. Previously, we also reported the high self-assembling ability of various peptide lipids and demonstrated high cytotoxicity of some peptide lipids to animal cells. Peptides can be substrates for many enzymes in a living system. For example, tyrosine kinase adds a phosphate group to a tyrosine residue in a target protein, which plays a key role in signal transduction on cell growth and differentiation. Gene mutation in tyrosine kinase leads to uncontrollable signal transduction and causes many cancers. In other words, tyrosine kinase activity of some cancer cells is different from other cells.
Here, we prepared novel peptide lipids that underwent phosphorylation of its peptides by tyrosine kinase in animal cells and evaluated their cytotoxicity. First, we synthesized different types of peptide lipids by solid-phase peptide synthesis, which were composed of fatty acids (C8 – C16) and peptides containing tyrosine (Fig. 1). The peptide sequences were designed according to tyrosine kinase substrates. The synthesized peptide lipids were purified by HPLC and confirmed by MALDI-TOF MS. When a phosphate group is added to tyrosine in a peptide lipid by tyrosine kinase, the hydrophilicity of the peptide lipid increases. We conjectured that a peptide lipid is transformed to anti-cancer drugs in cancer cells. We expected that phosphorylated peptide lipids should have cytotoxicity different from the original peptide lipids (Fig. 1). The present study proposes the novel effective treatment of drug-resistant cancer cells based on kinase activity.
Lipid vesicles are closed bilayer membrane(s) consisting of amphiphilic lipid molecules such as phospholipids formed in aqueous media. Lipid vesicles can encapsulate various water-soluble biochemical molecules into their internal water phase, therefore lipid vesicles have been studied as biochemical microreactors surrounded by lipid bilayer membranes, those are structurally similar to biological cells. However, the utilization of lipid vesicles as microreactors is limited by their preparation method because it is generally difficult to obtain vesicles containing biochemical molecules such as enzymes with high encapsulation efficiency and controlled diameter.
Recently, we developed a novel vesicle preparation method by the combination of multiple emulsification and solvent evaporation techniques (Kuroiwa et al.: J. Am. Oil Chem. Soc., 93, 421-430, 2016). In this method, at first, water-in-oil (W/O) emulsion containing water-soluble enzymes was prepared by membrane emulsification. Next, water-in-oil-in-water (W/O/W) multiple emulsion containing above W/O emulsion as a dispersion phase was formulated by microchannel emulsification with water-soluble polymeric emulsifiers. After removal of organic solvent in the oil phase of W/O/W emulsion by solvent evaporation under the ambient condition, finally we obtained enzyme-containing lipid vesicles. The diameter of lipid vesicles reflected that of the starting W/O emulsion droplets. For porcine pancreas lipase, the activity-based encapsulation yield was determined to be ca. 30%. These value was quite high compared with values for conventional vesicle preparation methods. Lipase-catalyzed reaction inside lipid vesicles was carried out by supplying a substrate (fluorescein diacetate) from the external water phase to internal water phase of lipid vesicles. The hydrolysis of the substrate was proceeded as comfirmed by fluorescence of hydrolytic product, fluorescein, from internal water phase of vesicles. This result suggests that the substrate permeated through lipid bilayers of vesicles and then it was hydrolyzed by lipase inside vesicles. We also evaluate the encapsulation characteristics of a proteolytic enzyme.
Structural color is color produced from materials having microscopic structures that can interact to visible lights via interference and/or scattering phenomena. Artificial structured materials have been widely developed by fabricating various nano-sized structures using synthetic organic or inorganic materials. In contrast, development of structural colored materials based on natural biomolecules have not studied enough although very vivid structural colors are widely observed in nature, for example, in butterfly wings, beetle shells, and bird feathers.
In this study, we developed a novel structural colored material using millimeter-sized molecular aggregates consisting of natural amphiphilic lipids including phospholipids. A lipid mixture consisting of phosphatidylcholine, cholesterol, and additives (various monoalkyl compounds) was dissolved in n-hexane and placed onto aqueous phases with various pH values. After evaporative removal of n-hexane, molecular aggregates with visible size (larger than 1 mm) were formed in the aqueous phase. The shape of molecular aggregates was film-like or spherical, depending on lipid composition and pH value in the aqueous phase. Small/wide angle X-ray scattering measurements revealed that the molecular aggregates exhibited the liquid crystalline polymorphism and their phase structure was related to their macroscopic shape. Among them, spherical aggregates with cubic liquid crystalline phase could be converted to aggregates exhibiting colorization by controlling the salt concentration of their external aqueous phase. The reflection spectra of visible lights from the colored molecular aggregates suggested that their colorization was attributed to the internal structure of the molecular aggregates. Electron microscopic analysis demonstrated the existence of periodic structures with the characteristic distance corresponding to wavelength of visible lights. We believe that our finding would contribute to development of novel lipid-based colorization materials that are potentially applicable for foods, cosmetics, and display materials.
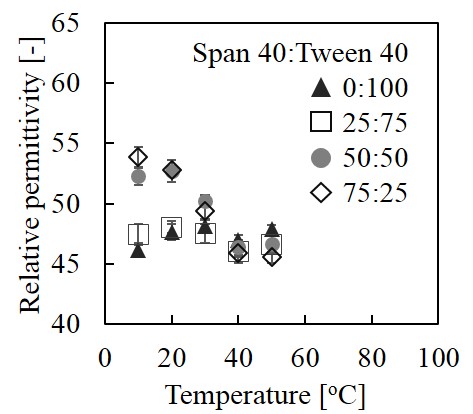
Amphiphilic molecules form self-assembled aggregates, such as micelles and vesicles. These aggregates are expected to be applied as a drug carrier and a microreactor. Polarity of the hydrophobic region is an important parameter to enbed hydrophobic drugs and substances. In this study, the relative permittivity was evaluated by using fluorescent probes. These probes were synthesized by conjugation of 1-pyrenemethanol and dicarboxylic acids, succinic acid (P-C3-COOH), suberic acid (P-C7-COOH) and dodecanedioic acid (P-C11-COOH). Aggregates were prepared by non-ionic sorbitan surfactants, Span 40 and Tween 40. The mixture of these surfactants forms various kinds of aggregates, Tween 40-rich and Span 40-rich mixtures tend to form micelles and vesicles, respectively. Figure 1 shows the relative permittivity of aggregates using P-C3-COOH. Relative permittivity of vesicles at low temperature was apparently higher than that of vesicles at high temperature, it is due to the localization of P-C3-COOH. Rigid structure of vesicles prevented P-C3-COOH to insert into the deep hydrophobic region. Increase of temperature change vesicles more fluid, resulting in decrease of relative permittivity. The measurement using P-C7-COOH shows a similar result as that using P-C3-COOH, while it seems that behavior of P-C11-COOH was different from that of P-C3-COOH and P-C7-COOH. The relative permittivity of micelles did not change regardless temperature. On the other hand, relative permittivity of vesicles was increased from 10 to 30 °C. It was related to phase transition temperature of vesicles. These results help to assume localization of drugs or substances in the aggregates, such as the considering about to apply as a drug carrier or a microreactor.
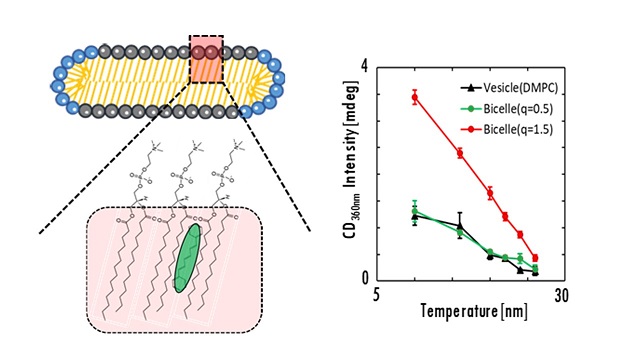
Bicelle is a disk-shaped self-assembly with bilayer membranes like vesicle, and its size is small as well as micelle. In addition, bicelle with a large disc diameter aligns to the magnetic field. Because of these unique properties, bicelle has been attracting tremendous attention as NMR measurement medium. In the formation and application of bicelle, the ordered structure of bicelle bilayer membranes is particularly important. In this study, bicelles were prepared by DMPC and DHPC, then the ordered properties of the bicelle bilayer membranes were characterized by combining the fluorescent probe method and induced circular dichroism spectroscopy (ICD). ICD is a phenomenon that CD activity occurs when achiral molecule DPH is ordered highly within a chirality lipid membrane. The ICD intensities at 360 nm decreased with increasing temperature, suggesting that the motion of the DMPC molecule was limited at low temperature, and the orderly orientation of DPH in the bilayer membrane was lost. The ICD intensity of bicelle was stronger than that of conventional vesicle. Furthermore, the aggregated bicelles, such as stacked bicelle, can be designed by modifying the membrane surface properties of bilayer region.
References: P. Walde et al., Langmuir, 13, 1668 (1997). S. Taguchi et al., Colloids and Interfaces, 2 (4), 73 (15 pages) (2018).
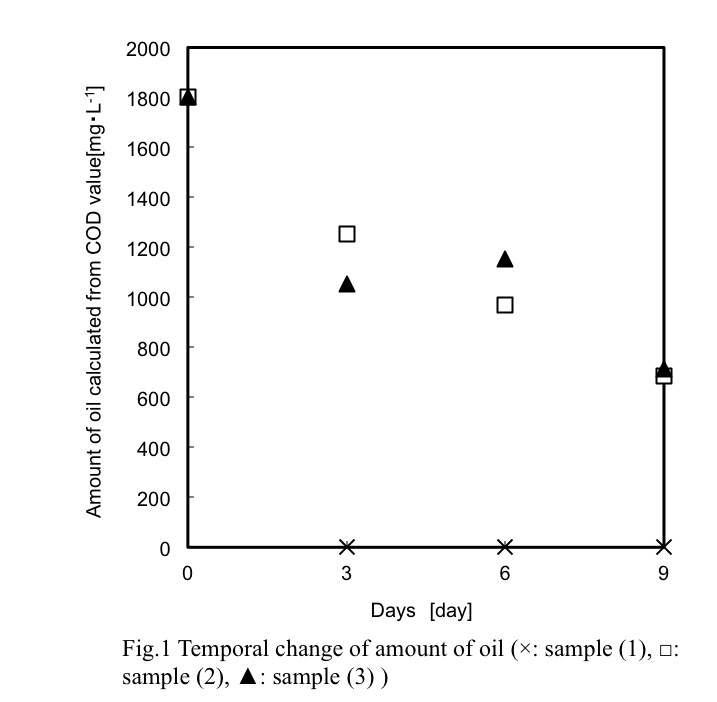
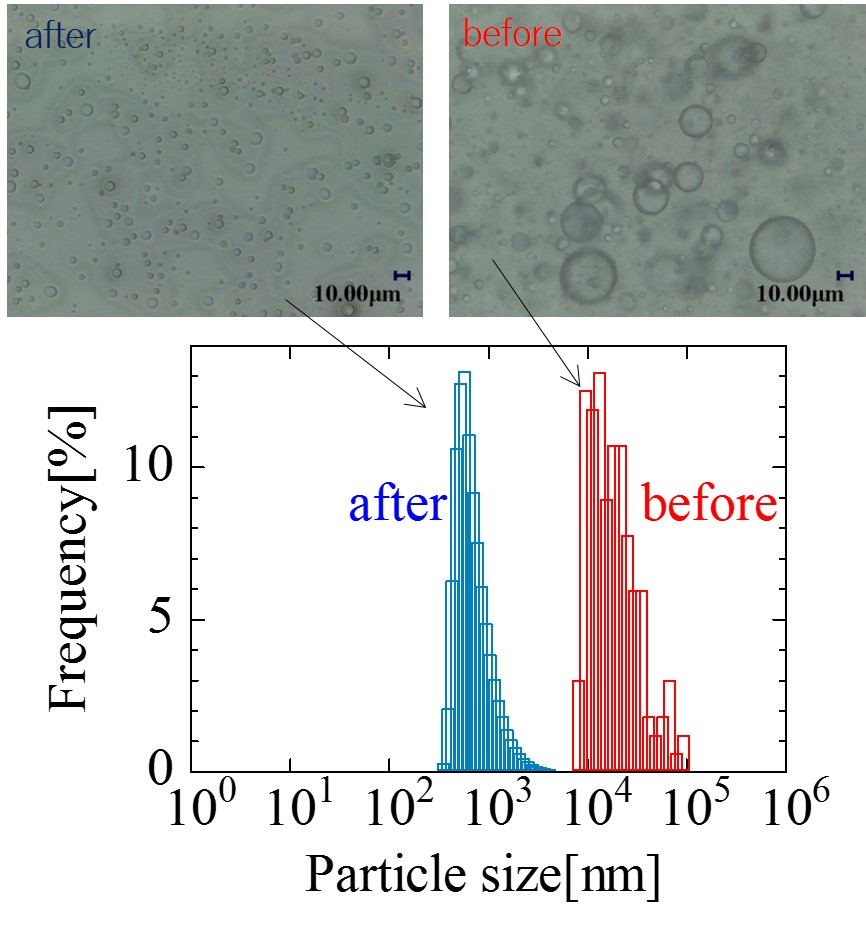
The "gas-liquid two-phase mixed flow" mechanism that fine bubble generation uses rapidly swirling water which shear gas and making small bubbles in water. Based on this mechanism, sending liquid such as oil into the swirling water instead of gas and it able to be emulsified. At present, evaluation methods for emulsions can be considered as particle size distribution and dispersion stability. However, in systems where the separation behavior is not good, it is a problem to catch only some dispersed oil particles and use them as a whole evaluation. In order to express numerical evaluation including the oil particles which can not be dispersed, we use chemical oxygen demand (COD) as numerical evaluation. The figure shows the temporal change of the amount of volatilizing oil calculated from the COD of emulsions sample (1)-(3). The dispersed phase used was of three types: sample (1) only one oil, sample (2) an anionic and a nonionic emulsifier were mixed and added to the oil, sample (3) an anionic and an another kind of the nonionic emulsifier were mixed and added to the oil. It was confirmed that COD decreased and sample (1) has a large amount of volatilized oil because there were many oil particles that can not be dispersed. The performance of the emulsion could be expressed numerically, including the oil particles which could not be dispersed. We also directly analyzed volatile components and compared them with COD results.
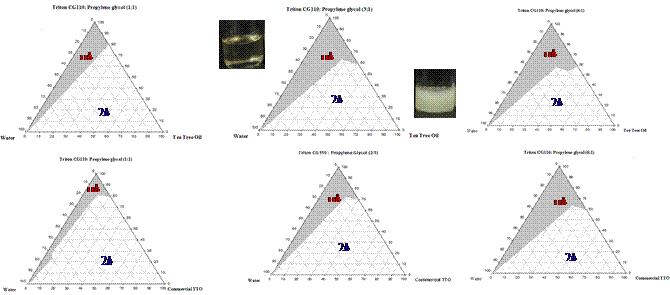
In this study, a plant-derived alkyl polyglucoside (APG) surfactant with alkyl chain of C8- C10 was formulated with the essential oil of Melaleuca alternifolia (tea tree) in the presence of propylene glycol as co-surfactant. This nonionic surfactant with a tradename as Triton CG 110 is reported to possess a CMC at 1748 ppm at 25oC. Typically, tea tree oil (TTO), commonly used in variety of cosmetic and health care products, was extracted from tea tree leaves by Triton CG110-assisted hydrodistillation. The extraction yield was 6.68% under the conditions with (1) 645.4 ppm Triton CG110, (2) at a liquid/solid ratio of 24.5, and (3) for 128 mins as the extraction time. The pseudo-ternary phase diagrams were investigated at the different weight ratios of surfactant mixtures (Smix = CG/PPG) as 1:1, 3:1, 6:1 with hydrodistilled TTO and commercial TTO by water titration method at room temperature. One-phase region consisting of micelles and microemulsion (μ_phi) was painted in gray in Figure 1. The greater the amount of Triton CG110, the wider phase compositions of microemulsion formed. For example, the largest microemulsion region was occurred at a surfactant mixture of 6:1. No liquid crystalline phase was observed at all ratios of Smix under the cross-polarized microscope. Particularly, the effect of storage temperature on selected microemulsion made of 1% TTO with various concentrations of Triton CG100/PPG (3:1 w/w) were recorded. Shelf stability was examined on microemulsions right after preparation and in one month with dynamic light scattering. These results also showed that microemulsion made of 1%TTO, 9% Triton CG100/PPG (3:1 w/w) was insensitive with time and temperature during long time. Antimicrobial activity and antioxidant activity were evaluated with microemulsion made of 1% TTO.
Recently, Pluronic surfactants (PEO–PPO–PEO triblock copolymers, where PEO: poly(ethylene oxide)x and PPO: poly(propylene oxide)y) were found to show CO2-responsive aggregation behavior in water. In earlier paper, mesoporous silica prepared in a hexagonal liquid crystal/water system was swollen by CO2 and the mesopore size was tuned by pressure of CO2 atmosphere. It suggests various potential applications of CO2-responsive surfactant. To clarify mechanism of CO2-responsive behavior of Pluronic surfactant assemblies in water, this study examined effects of temperature, pressure, PEO and PPO lengths on growth of Pluronic surfactant micelles in water under high-pressure CO2 atmosphere.
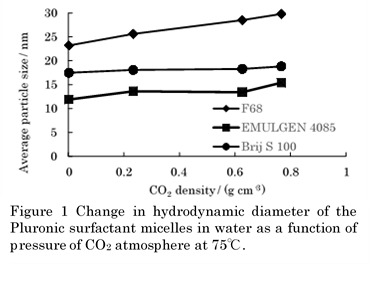
Aqueous micelles of Pluronic surfactants F108 (x = 150, y = 55) and F127 (x = 100, y = 65) showed no significant change in hydrodynamic diameter of micelles even at high CO2 pressures of 100–300 bar. In contrast with those Pluronic surfactants, F68 (x = 80, y = 30) and F88 (x = 100, y = 20) displayed clear micellar growth with increasing CO2 pressure. Figure 1 shows relationship between the diameter of aqueous micelles and density of CO2 atmosphere at 75 °C and the surfactant concentration 1 wt% for F68, a nonionic EMULGEN 4085 having the C14H29-tail and the PEO (x = 85) close to that of F68, and a nonionic BrijS100 (x = 100) having the C18H37-tail and the PEO chain (x = ~100) similar to that of F88. The tendency that the higher the CO2 pressure (or CO2 density) the larger the micellar diameter was considerably small for nonionic surfactants without a hydrophobic PPO chain, compared with Pluronic surfactants having corresponding PEO units. Especially the Pluronic surfactants with PEO lengths x = 80–85 were found to exhibit significant micellar growth with increasing CO2 density.
Real-time monitoring of small molecules, such as reactants, dissolved gas molecules or metabolic products, during biological conversion is of paramount importance for understanding the metabolic pathway, optimizing conversion efficiency and enhancing the reproducibility of bioprocesses. Generally, detection of such small molecules during biological conversion are largely dependent on chromatography-based techniques, such as high performance liquid chromatography and gas chromatography. However, these techniques are time-consuming, requiring more than 10 minutes for the separation of sample in the column. Moreover, off-line sampling process is inevitable for the analysis which has a risk of causing contamination. For these reasons, development of a novel method that is suitable for real-time monitoring of biological conversion is required. Here, we propose the plasmonic detection of small molecules in biological conversion by using plasmonic nanostructures produced using capillarity-mediated inverse transfer. Nanoparticles assembled at air/water interface are transferred onto solid substrates through the reconstruction of the assembly interface along the direction of gravity by a capillary tube, enabling the transfer of nanoparticles on solid substrate via direct contact. Small molecules are preconcentrated on plasmonic nanostructure through electrostatic attraction and chemisorption. Preconcentration of small molecules increases the number of molecules affected by strong near-field enhancement at the proximity of the metallic surfaces; thereby, allowing the detection of trace amount of small molecules in the solution.
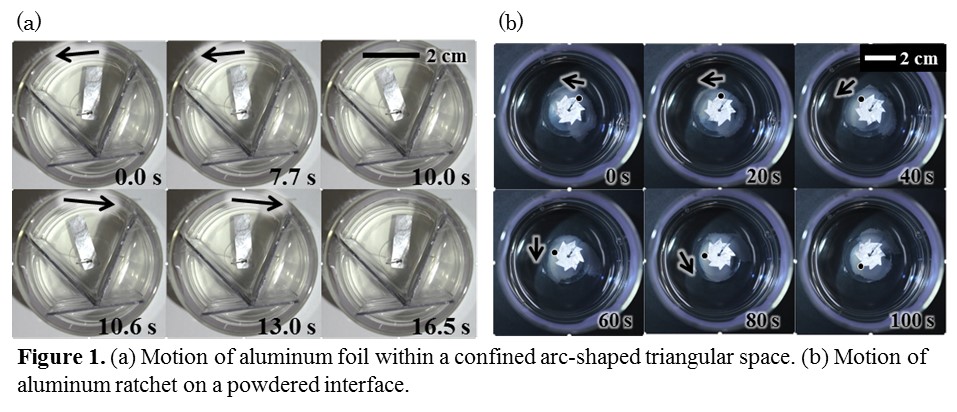
A living matter moves as an efficient energy conversion system under a non-equilibrium state. The study of this motion may be associated with creation of innovative energy conversion system. Fundamental study with a model system involving similar characteristics to living matter is one of the powerful approaches for understanding and application of such types of motion. In this study, we have studied the motion of aluminum float driven by the spontaneous flow at oil/water interface: The oil and water phases contain di(2-ethylhexyl) phosphate (DEHPA) and Fe2+, respectively, whereby spontaneous instability develops at the interface. Spontaneous flow is generated by a local compression of the adsorbed DEHPA layer by an interfacial flow, and the generated flow compresses the surrounding DEHPA monolayer to cause the subsequent flow. This suggests that spatiotemporal control of compression points rectifies the flow pattern, resulting in a regulated motion of a float.
As an example, a rectangular aluminum foil is placed in an arc-shaped triangular space as shown in Fig.1(a). The aluminum foil exhibits a reciprocal motion. This motion repeats itself many times. Such a motion was not obtained without the confined space. The motion of the aluminum foil results from the compression of DEHPA layer between the aluminum foil the wall, because the compression induces the next flow. As the other example, a ratchet gear made of aluminum foil is placed at the interface where powder are dispersed in the oil/water interface(Fig.1(b)). The ratchet rotated in one direction, that lasted for more than 10 minutes. This rotation may be considered to be caused by the compression of DEHPA layer around the aluminum foil assisted by the powder acting as a wall. These motion may lead to the creation of an innovative energy conversion system.
Graphite consists of many carbon sheets. A single layer of the carbon sheets is called graphene. Graphene is a material having good electrical conductivity and high mechanical strength. Industrial applications of graphene are expected in the wide range of fields. However, graphene is usually aggregated in solvents because of strong hydrophobic interaction. This causes the difficulty of the manipulation and the loss of electrical conductivity of graphene.
We previously succeeded in dispersing graphene using poly(3-hexylthiophene-2,5-diyl) (P3HT), which is the conductive organic polymer, in an organic solvent. However, P3HT dispersed graphene only in an organic solvent, which leads to large environmental concerns. Here, we aimed at the preparation of graphene aqueous dispersion using poly(3-(4-sulfophenyl)thiophene-2,5-diyl) (P3ST), which is a water-soluble polythiophene derivative.
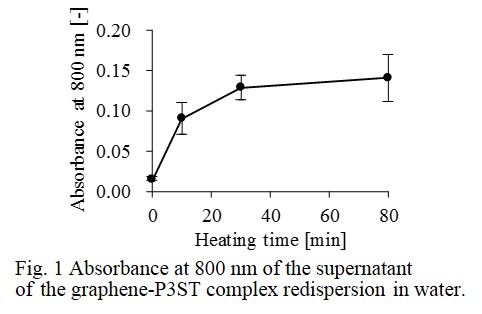
P3ST is obtained by deprotecting neopentyl groups of poly(3-(4-neopentylsulfophen-1-yl) thiophen-2,5-diyl) (P3NST). We mixed graphite flakes with P3NST in chloroform and conducted ultrasonication for exfoliation and dispersion of graphene. After the removal of chloroform, the graphene-P3NST complex was heated at 220~230 °C for deprotecting neopentyl groups of P3NST. Then, we added water and redispersed the graphene-P3ST complex in water by ultrasonication. After the redispersion was left for 20 h, the supernatant was collected. The supernatant was diluted 2 times with water and its absorbance at 800 nm was measured to evaluate the dispersion of graphene.
Fig. 1 shows the absorbance at 800 nm of the dilute solution of the supernatant. The x axis shows heating time for deprotecting neopentyl groups of P3NST. The results showed that dispersion of graphene in water was improved by heating the graphene-P3NST complex. The deprotection of neopentyl groups produced sulfo groups in the polythiophene derivative and the sulfo groups would generate electrostatic repulsion between the graphene nanosheets in water to stabilize the dispersion.
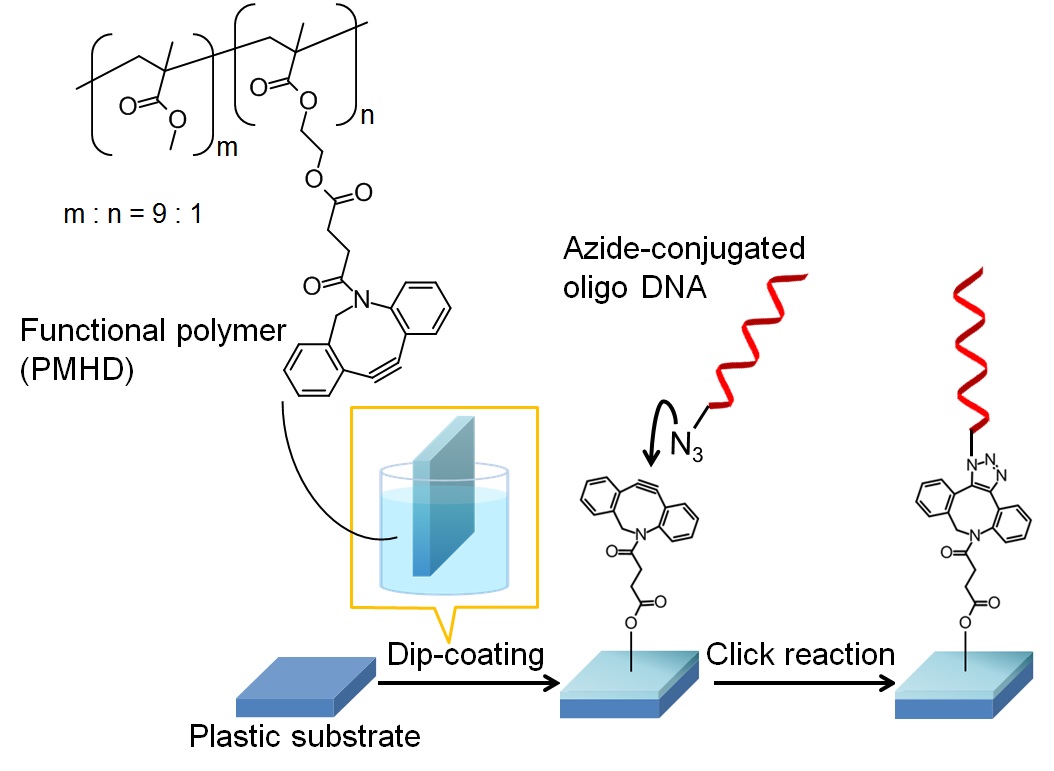
Cu-free click reaction is one of the most famous reactions for conjugating DNA, proteins and cells. Recently, a Cu-free clickable surface is attracting as a platform of immobilizing biosubstances for preparing biosensors. In previous studies, inorganic substrates (e.g., silicone and glass) were used, and they needed surface activation by plasma and chemicals. In this study, we synthesized a Cu-free clickable functional polymer, and a Cu-free clickable surface was prepared on a plastic substrate by polymer coating without pre-treatment.
We synthesized a methyl methacrylate (MMA) and hydroxyethyl methacrylate (HEMA)-based backbone copolymer. Then, dibenzocyclooctyne (DBCO), a Cu-free clickable functional group, was conjugated to the hydroxy group of PMH to get a Cu-free clickable polymer, poly(MMA-r-HEMA-DBCO) (termed PMHD). PMHD was dissolved in ethyl acetate, and a plastic substrate was coated with the polymer solution and dried to prepare a Cu-free clickable surface. AFM and SEM observations revealed that dip-coated surfaces were flat compared with bare substrates.
We also synthesized an azide-conjugated fluorescent substance and estimated the amount of clickable moieties on the polymer-coated surface using Cu-free click reaction between the surface and the fluorophore. The surface density of Cu-free clickable moieties was proved to be 63 pmol/cm2 on PMHD-coated PMMA substrates. This meant that a Cu-free clickable surface was easily prepared on a plastic substrate by polymer coating. To investigate the potential of the functional surface as a platform of a biosensor, azide-conjugated oligo DNA was immobilized on the clickable surface. In consequence, 5.4 pmol/cm2 azide-conjugate oligo DNA was immobilized and hybridized with the complement DNA on the PMHD-coated functional surfaces.
In summary, we synthesized a functional polymer (PMHD), and succeeded in the preparation of a Cu-free clickable surface by simple polymer coating. The investigation of DNA immobilization revealed that the functional surface had high potential as a scaffold of biomatters.
In recent years, separation membranes have attracted a lot of attention: that is widely applied to seawater desalination treatment, separation/recovery of exhaust gas, etc. The materials of the separation membrane are classified into inorganic or organic one, and the latter has generally advantages of low cost, low density and easy handling. In the widespread use, it is indispensable in our lives.
At present, a new preparation process using phase separation in liquid phase has been developed. The outline of this process is 1) selection of a polymer, its solvent and anti-solvent, 2) mixing of them, 3) emergence of phase-separation at specific compositions and 4) drying and formation of porous separation membranes. However, the formation mechanism of the membrane is not clear in the drying process and should be clarified for design of the membrane structure. In this study, poly(vinyl alcohol) (PVA), water and γ-buthyrolactone(GBL) were selected as ingredients for the membranes. Then, the concentration changes of mixed samples have been investigated in the formation of the porous membranes of PVA by the drying process.
As a first approach, preparation of the phase diagram for the three components system was carried out.When the concentration of the liquid sample changed by adding a component slightly, the turbidity was measured. The binodal curve was found by connecting of the turbidity points in PVA-GBL-water systems. The trace of concentration change in drying processes were drawn in the obtained phase diagram. The structures of PVA on the trace line was measured by SEM and DSC measurements were carried out. The relationship of drying rate and PVA structure was elucidated with the structural and thermal data.
Plastics have some practical characteristics; in recent years, the modification of plastic surfaces has received considerable attention for new application. The display of reactive functional groups on plastic surfaces is one approach of the modification of plastic surfaces and it is very important for adhesion and immobilization of biomolecules and polymers. In this study, we displayed amino groups and immobilized gold nanoparticles (AuNPs) on plastic surfaces. Preparation of AuNPs-modified surfaces will realize new applications such as fabrication of conductive plastic surfaces, immobilization of thiol compounds on a surface of a plastic substrate.
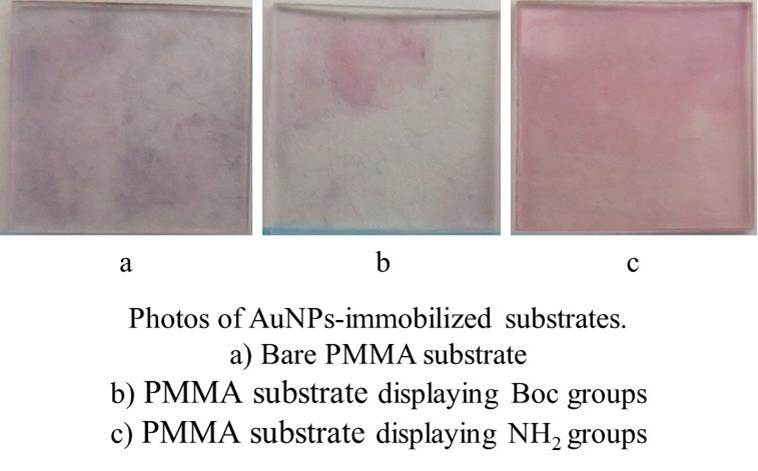
We synthesized a methacrylate-based copolymer containing amino groups in the side chains, in which the amino groups were protected with tert-butoxycarbonyl (Boc) groups. The protection groups of amino groups in this copolymer were hydrophobic, leading to the display of the amino groups on the outermost surface of a PMMA substrate after dip-coating. We deprotected the Boc groups and displayed amino groups on the surface of the substrate. Quantitation of the amino groups was carried out using a cleavable fluorescent compound. The amount of amino groups displayed on the surfaces was 10.3 pmol/cm2.
AuNPs, which were 13 nm in diameter, were immobilized on the plastic surfaces by simply immersing PMMA substrates in Au-colloidal solution for 30 minutes. The acrylic substrate displaying amino groups turned red after immersion (Fig. 1), comparing to the bare substrate and the substrate displaying Boc groups. We measured absorbance of these substrates after immersion at 520 nm, which was maximal absorption wavelength of Au-colloidal solution. Absorbance of the substrate displaying amino groups was the highest among the three different kinds of substrates. We conjectured that electrostatic interaction between amino groups and negatively-charged AuNPs led to the immobilization of AuNPs on the surface.
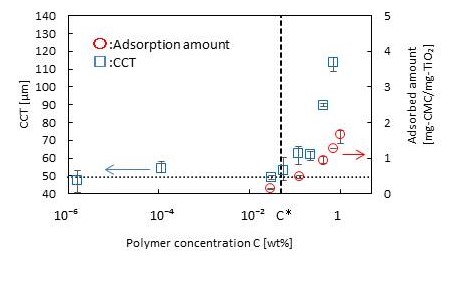
In drying of particle dispersion films, drying defects such as cracking or the like are caused by capillary forces acting between the particles. Previous studies have shown that cracks develop when the dry film thickness exceeds a critical thickness, referred to as critical cracking thickness (CCT)[1]. Polymeric additives can increase CCTs as they (i) adsorb on particle surfaces to induce steric repulsive forces between the neighboring particles, (ii) adsorb at air-liquid interface to reduce the surface tension, and thus the capillary forces, or (iii) show physical crosslinking between particles. However, few quantitative data are currently available to clarify how polymers suppress the propagation of cracks. In this study, we aim at investigating the effects of polymer adsorption on critical cracking thicknesses of drying particle dispersions.
We used Carboxymethyl cellulose (molecular weight: 1850000 g/mol, Dai-ichi Kogyo Seiyaku) as polymer. The sample dispersion containing TiO2 (diameter: 150 nm, concentration:15 wt%) and polymer was dried on a tilted hot stage. The dried film surface was observed by an optical microscope to find a position where cracks developed with a length of 30 μm or longer. The film thickness at that position was measured using a laser focus displacement meter to determine CCTs.
The results showed that CCT was nearly constant at polymer concentrations lower than the critical concentration of c* ~ 0.05 wt%, and agreed with that in polymer-free particle dispersions. At polymer concentrations above the critical value, on the other hand, the CCT monotonically increased with increasing the polymer concentrations. The adsorption measurements indicated that the onset of polymer adsorption on the particle surface emerged at the same polymer concentration, c*, indicating that crack propagation was suppressed by polymer adsorption on the particle surface.
References
[1] K.B.Singh and M.S.Tirumkudulu,Phys.Rev.Lett.98(21)(2007)218302
Shape of particles in dense suspension influences on the rheological properties. We focused on wrinkles generated by buckling as the periodic structure on the surface of elastomer particles, and evaluated the suitable method to control the obtained particle size and the shape of wrinkles.
Polydimethylsiloxane (PDMS) was used as the base material for wrinkled particles. The monomer and curing agent were mixed (10:1 (w/w)) to obtain the uncuring PDMS sol, and then the sol was mixed in a 15wt% polyvinylalcohol/H2O solution by the following methods, magnetic stirrer (ST), homodisper (HD), and homogenizer (HG). The PDMS droplets were filtrated with the porous membrane to fractionate the droplet size. In order to cure PDMS, the droplet suspension was heated at 80 °C for 3 hours. Finally, the surface of the PDMS particles were oxidized with a mixed acid solution to form wrinkle structure. The size of PDMS were measured from the image of optical microscope and/or dynamic light scattering.
The order of PDMS droplet size was roughly ST > HD > HG, and the particle size distribution was very broad from several 10 nm to above 100 μm. To separate out the particles with desired size, the HD pre-mixed PDMS suspension (1000 rpm, 5 min) was filtrated by using a shirasu porous glass membrane (pore size: 1 μm). The filtrated PDMS suspension shown a more narrow size distribution (d50 ~2.5 μm (Figure)). However, after surface oxidation of mixed acid, surface wrinkling on the filtrated PDMS particles was not observed by a SEM. Only PDMS particles of 15 μm or more in the diameter obtained from HD pre-mixed suspension were buckled into wrinkling. The wavelength of wrinkle was about 450 nm (d~15 μm), which tends to increase slightly with the particle diameter.
The organic luminescent material easily loses its luminescence once it is exposed to moisture, and the highly reactive cathode with low work function will be easily corroded by moisture and oxygen. To solve this problem, We demonstrate flexible moisture barrier film encapsulation for organic electronics using low temperatures process method. To realize the flexible barrier, the best practice is to make the barrier as multilayer structure. Low temperatures process applied the atomic layer deposition(ALD) method and sputtering method to deposit barrier films. Inorganic layers work as moisture barrier and very thin thickness provide the flexibility and planarization. The moisture permeation characteristics of the optimized Al2O3/SiNX multilayer films were finally measured using MOCON's WVTR measuring instrument and WVTR values lower than the detection limit of the measuring device (less than 5.0 x 10-5 g/m .day) were obtained.
This study is to investigate the optimal time of applying current assisting during employing hot-pressing Bi-Sb-Te films to improve the thermoelectric properties. First, an average 5 um of grinded P-type of Bi-Sb-Te powers was used to formulate a slurry paste to coat on a polyimide substrate for the current assisted hot pressing. It shows that the resistivity decreases with increasing the current density, and then increases with further raising the current density. The main reason is speculated that increasing the electric driving force at low current density can eliminate defects by reducing the obstacles of carrier transmission and formatting Sb-rich phase to form a bridge between grains. However, the excessive current density will generate stronger electric driving force causing a large amount of joule heat to increase the resistivity. Also, the case of “hot pressing and current assisting simultaneously” or the case of "current assisted hot pressing after completion of hot pressing" cannot achieve the best improvement of the thermoelectric properties. Analyzed by surface reaction method, the best thermoelectric efficiency can be obtained at 1000 A/cm2 with 3 min hot pressing followed by 7 min current-assisted hot pressing. Applying hot pressing ahead can increase contact area between particles to make current density distribution more uniform, which can generate less extra joule heat and larger electric driving force, leading to further reducing resistivity to 1.04 mホゥ·m and increasing power factor to 2880 μW/K2m. Compared with the case of hot pressing and current assisting simultaneously for 10 min and the case of current-assisted hot pressing for 7 min after hot-pressing for 10 min, the power factor increases 34% and 20%, respectively.
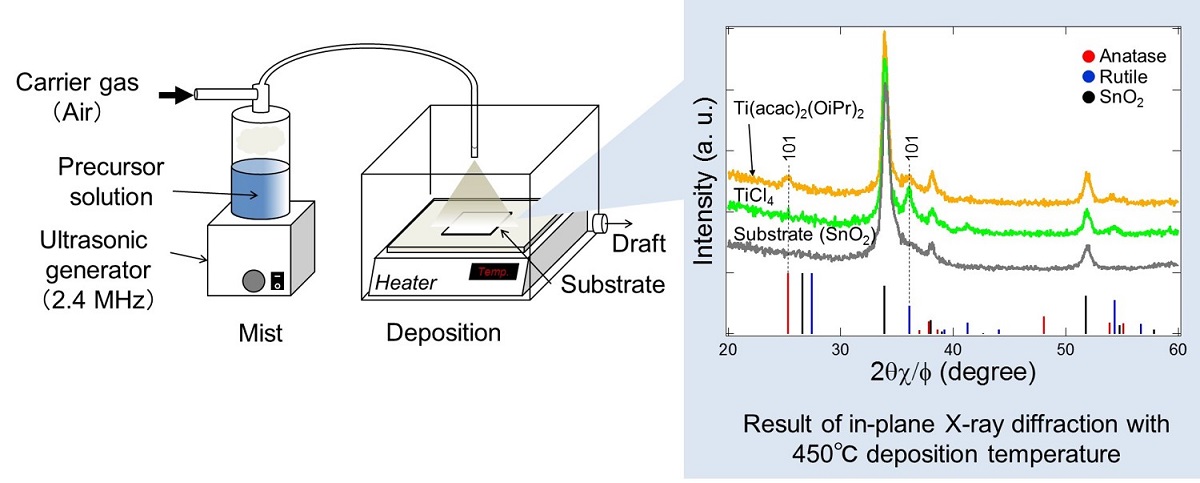
Perovskite solar cells have been intensively studied because they have high efficiency over 20%1 and are fabricated by a low-cost wet process. The ideal cell structure is a simple planar type with a multi-layer of substrate/electrode/electron transport layer/perovskite power generation layer/hole transport layer/electrode. Titanium dioxide (TiO2) is used as a compact blocking layer as well as an electron transport layer. The underlying TiO2 layer would also play a role in determining the structure of a perovskite layer. To improve the efficiency of the solar cell, it is important to control the structure and physical properties of the TiO2 layer. The ultrasonic spray method is one of the wet coating processes. It enables to control film structure by nuclear growth on the substrate and to deposit large-area and continuous film, having advantages of applicability of the Roll to Roll process upon scale-up to mass-production. The aim of this study is to analyze the relationship between coating process, film structure and physical properties of TiO2.
Titanium bis acetylacetonate diisopropoxide (Ti(acac)2(OiPr)2) and titanium tetrachloride (TiCl4) were used as soluble precursors in ethanol. The ultrasonic spray method was used to deposit TiO2 films on two different types of SnO2 films. The structure of the TiO2 films was investigated by X-ray diffraction and X-ray absorption spectroscopy. In the case of Ti(acac)2(OiPr)2 solutions, TiO2 films had anatase and rutile structures with (101) orientation, while the films deposited with TiCl4 solutions showed only a rutile structure with (101) orientation. The local epitaxial growth of rutile TiO2 on rutile SnO2 structures would be the origin of the observed rutile (101) orientation. We will discuss the effect of the structure of the TiO2 films on the physical properties of perovskite solar cells.
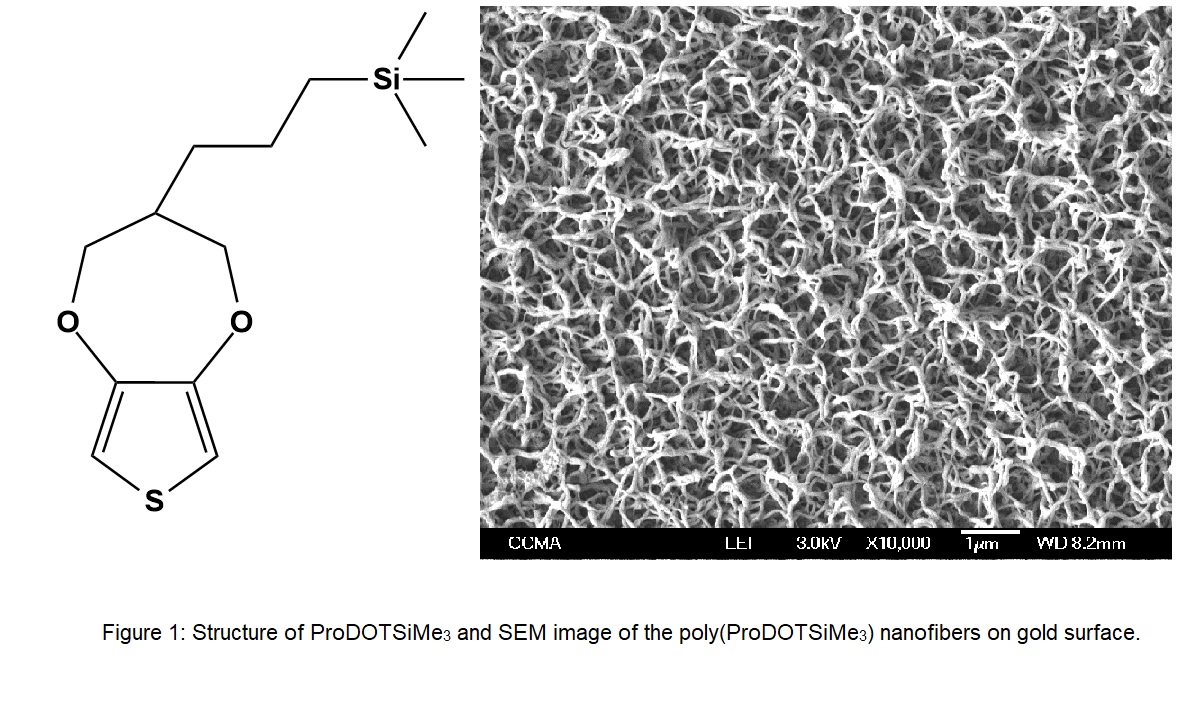
Fluorinated compounds often exhibit a very low surface energy, and these can be used as antifouling and antifogging agents. However, high F-content compounds are generally expensive and bioaccumulative. Our previous research found that non-fluorinated surfactants having trimethylsilyl groups decreased aqueous surface tensions as low as a short or middle fluorocarbon-tail surfactant can (i.e. ~22 mN/m). To develop fluorine-free water-repellent materials comparable to fluorinated one, this study synthesized 3,4-propylenedioxythiophene monomer having 3-trimethylsilylpropyl group (ProDOTSiMe3, Fig. 1) and obtained poly(ProDOTSiMe3) films on gold surfaces by electrodeposition of the monomer.
Electrodeposition was carried out by placing a gold plate (working electrode), a glassy carbon electrode (counter electrode), and SCE in a glass cell containing 10 mL dehydrated acetonitrile solution with 0.1 M tetrabutylammonium perchlorate and 0.01 M ProDOTSiMe3. Water repellency and morphology of the surfaces were also studied. SEM observation clarified that the gold surfaces after electrodeposition were covered with dense poly(ProDOTSiMe3) nanofibers with a large roughness. Water contact angle on the gold surface was 141.6 °ツア 4.7 °, similar to the values 136.8 °and ≥ 150 °for 3,4-propylenedioxythiophene monomers having two isopropyl-terminated alkyl chains[1]. Interestingly, the poly(ProDOTSiMe3) surfaces exhibited parahydrophobicity due to the wenzel-state.
References
[1] Thierry Darmanin, Claudio Mortier, Julian Eastoe, Masanobu Sagisaka, Frederic Guittard., RSC Adv., 2014,4, 35708–35716
A probe needle for a probe card which is employed in a current energization inspection of an integrated circuit chip in a semiconductor wafer manufacturing process has been made of tungsten, rhenium tungsten, beryllium copper, etc. Particularly, tungsten is a material which is excellent in wear resistance and the excellent toughness is popularly used. However, the contact resistance is raised and the liability of probe card deteriorates on the inspection because tungsten is easily oxidized due to the frictional heat which is generated by the frictional wear or the generation of heat. In order to solve this problem, it is desirable to use gold or platinum material, which is strong in corrosion resistance, as a probe needle. However, in case of gold, it cannot be used in contact with the pad due to its ductility. In addition, there is no economical efficiency because the process cost is increased.
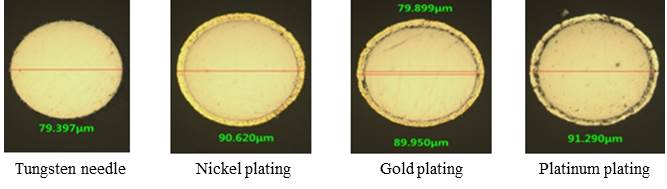
In this study, we platted on the surface of tungsten needle with 1 ~ 5 um of gold, platinum, and nickel to prevent the oxidation of tungsten needle by frictional heat which can be used sufficiently as a probe from being corroded by heat. The undercoat was conducted with nickel, and then, gold, platinum, or nickel was plated. The change of electric resistance after plating was examined. Because the needle thickness is very thin, the over-plating occurred at the tip of tungsten needle. To reduce over-plating, new zig for needle was invented and plating conditions were optimized.
Figure1. The microscope cross-sectional images of bare tungsten needle, the plated tungsten needles with nickel, gold, platinum.
High-chromium cast iron, which is a wear resistant material, is a ternary white cast iron generally containing 7% or more of Cr. It forms many carbides of high hardness, for example M23C6 and M3C, M7C3 (M = Fe, Cr)(1).Therefore, it is used as a material for rolling rolls used in rolling processing and slurry pumps for slurry liquid transfer. However, the rolling conditions are becoming more severe in terms of energy saving and quality improvement in rolling processing, and the slurry pump has problems of wear and corrosion due to high concentration slurry liquid. In order to cope with the current situation, it is important to change the solidified structure by adjusting the Cr and C content, in order to improve the low toughness, corrosion resistance, and wear resistance. Changes in the composition and cooling rate that affect the solidification structure are factors related to the mechanical properties of cast iron. Direct observation of the solidification process in which carbides and base structures appear is thought to be important because it leads to the elucidation of the growth mechanism and can be expected to control the size, amount and formation position of the crystal. Direct observation of metals under high temperature is carried out to clarify the growth mechanism of the metal structure. For this purpose, it is necessary to use a laser microscope which is not affected by the radiation emitted from the molten metal. In this research, our purpose to establish the observation condition of the growth process of base structure and carbide using laser microscope and to elucidation the formation of structure
(1)Tomoo Sato, Taiji Nishizawa, Joo Ishihara, hardness of carbides in iron and steel, Journal of the Japan Institute of Metals, Vol. 23, No. 7, pp. 404-405, 1958
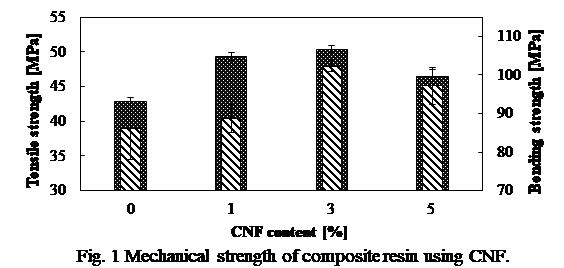
Cellulose nanofiber (CNF) is a micro-refined nano-level fibrous material that can be produced from chemical and mechanical treatment of variety of woody biomass materials. CNF has attractive characteristics, it's density is 5 times lesser than that of steel while being 5 times stronger. Moreover, it is produced from natural biomass resources therefore it is entirely environmentally friendly material1). Recently, its compounds with resins have received increased attention as alternative to pure metals owing to their light-weight and noncorrosive characteristics. In this use, defibrating and surface hydrophobization have main roles in obtaining high quality CNF. CNF is hydrophilic, while phenolic resins are hydrophobic; therefore, mixing them could result agglomeration and consequently lower the strength of the composite. In addition, the form of CNF is likely to influence the properties of the composites. Application of thermoplastic resins, such as polyethylene and polypropylene, in the formation of CNF composite materials have been widely reported. However, to the best of our knowledge, few studies have been conducted using thermosetting resins in the synthesis of CNF-reinforced composite materials. In this study, reinforcing of phenolic resin with CNF as a model for thermosetting resins was investigated. Various tests were conducted to evaluate the performance of the composites produced with CNF (produced at different physico-chemical conditions) in different proportions with the resin. Various techniques, such as Fourier-transform infrared spectroscopy, mechanical testing, and scanning electron microscopy were utilized to understand their properties. Fig. 1 shows mechanical strength of typical CNF composite. As shown, bending strength as well as tensile strength increased approximately 20% when 3 wt% CNF was used. This study suggested that reinforcement of the resin using CNF resulted in stronger and more promising composites.
References: 1) Akihiro Sato et al., Composites Part A: Applied Science and Manufacturing, 83, 72-79 (2016)