Purpose. The aim of this paper is to enhance the dissolution rate of racemic (R,S)-(±)-sodium ibuprofen dihydrate via a bio-inspired method of growing mesocrystals.
Materials and Methods. Mesocrystals of racemic (R,S)-(±)-sodium ibuprofen dihydrate were successfully prepared from a supersaturated aqueous solution of racemic (R,S)-(±)-sodium ibuprofen dihydrate having the initial degree of supersaturation, S0, of 1.326 and the initial saturated concentration, C*, of 0.986 mol/l at 25 °C with sodium dodecyl sulfate (SDS) at a concentration of 0.10 g/l. Dynamic light scattering, scanning electron microscopy, powder X-ray diffraction, differential scanning calorimetry, and optical microscopy with cross polarizers were employed to understand the formation mechanism and to characterize the superstructures of the SDS generated mesocrystals.
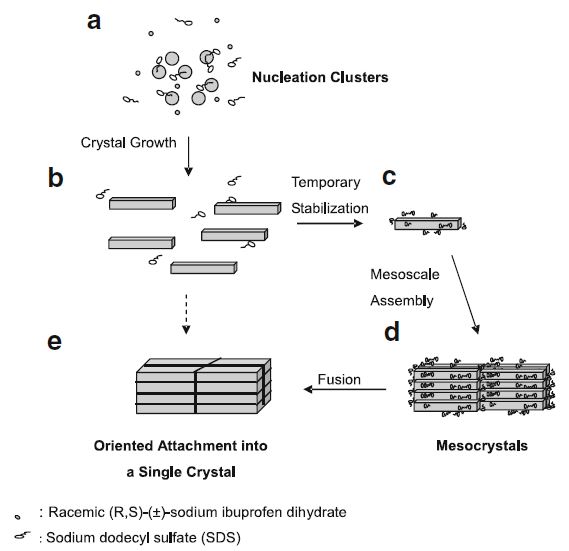
Results. The SDS generated mesocrystals were the assembly of the oriented attachment of racemic (R,S)-(±)-sodium ibuprofen dihydrate nano-sized platelets under the mediation of the side-to-side interaction between SDS and racemic (R,S)-(±)-sodium ibuprofen dihydrate. The SDS generated mesocrystals contained a mixture of the racemic compounds in α- and β-forms and the resolved racemic conglomerate in γ-form with no detectable amount of SDS. The dissolution rate of the SDS generated mesocrystals was more rapid than the one of its counterpart made by conventional crystallization pathway.
Conclusions. The crystallization of racemic (R,S)-(±)-sodium ibuprofen dihydrate in the presence of SDS yielded well-faceted, well-separated, but almost perfectly three-dimensionally aligned nano-sized platelets. This kind of bio-inspired mesocrystal superstructure has definitely opened a new doorway for crystal engineering and pre-formulation design in pharmaceutical industry. The future work is to study the mesocrystal formation of some other active pharmaceutical ingredients in organic solvent systems and
to develop an efficient method for screening the additives.
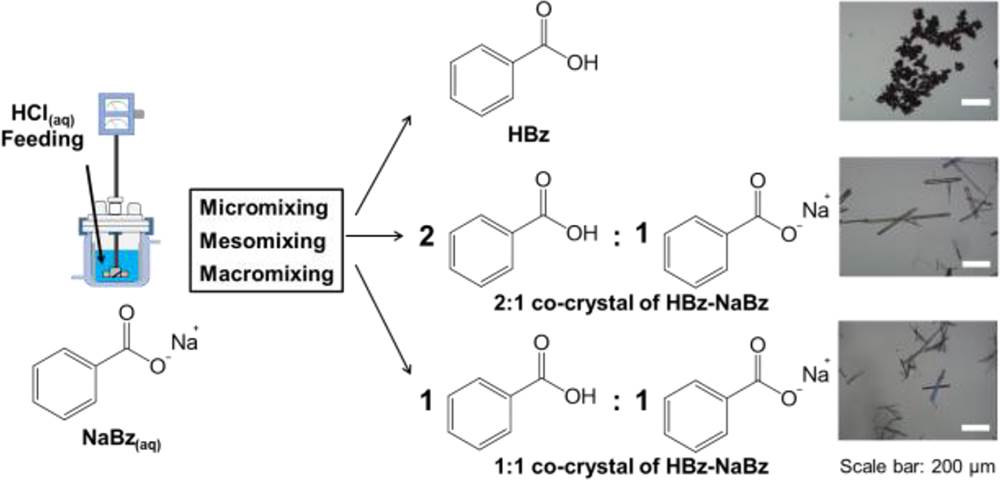
The aim of this study was to investigate the mixing effect on the stoichiometric diversity of benzoic acid–sodium benzoate (HBz–NaBz) cocrystals. The crystallization of HBz–NaBz cocrystals in a 500 mL sized glass vessel was monitored under different agitation speeds and feeding rates of HCl aqueous solution. Under good micromixing and macromixing, the HBz crystals, 2:1 and 1:1 cocrystals of HBz–NaBz were crystallized out rapidly, and all crystals were transformed to a mixture of 2:1 and 1:1 cocrystals of HBz– NaBz in a relatively short amount of time. However, the crystallization of 1:1 cocrystals of HBz–NaBz was delayed by poor micro-, meso-, and macromixing simultaneously. The compositions of the products were altered in different mixing conditions even given the identical experiment time. It was feasible to harvest pure 1:1 or pure 2:1 cocrystals of HBz–NaBz by reaction crystallization through the control of the mixing condition, concentration of reactants, and experiment time. The ternary phase diagram was also constructed for the HBz–NaBz–water system.
In this paper, authors summarized and overviewed examples of crystallization process applied to waste and wastewater treatment, in order to remove and recover substances, and clarified strategies how to apply crystallization in the environmental fields. In case of acidic and higher concentration waste, solution crystallization was applied by combining cooling and anti-solvent crystallization. Examples of the atomic field in which Uranium recovery was available by UNH cooling and anti-solvent crystallization, followed by purification process. In application to the waste water from metal surface treatment, metal and organic acid recovery based on anti-solvent-crystallization was investigated, considering solubility in rich acid solution. In case of lower concentration wastewater, reaction crystallization of sparingly soluble crystals was applied to obtain larger crystals of better quality, by adapting an original strategy of seeding policy, and also impurity control. Phosphate crystallization makes it possible to utilize recovered phosphorus effectively as fertilizer. The MAP Process in which a magnesium compound is added recovers magnesium ammonium phosphate. The MAP Process, which requires ammonium ions in the stage of crystallization, is applied to the anaerobic digested sludge or sludge filtrate, maximum phosphate recovery rate is considered by discussing the relationship between the crystal particle size. The design guidelines on both processes have been clarified. Large scale demonstration plant is in operation at Kobe Harvest Project. On the fluoride ion removal process, Organo developed the fluidized-type and agitation type process by using crystallization process. Ni2+ recovering process from used bath of electro-less plating process by reduction crystallization, in which Ni2+ is removed as a form of Ni metal, by using reducing agents with using nickel powder as seeds Crystallization will become an effective tool to remove and recover substance from waste and wastewater having much impurity.
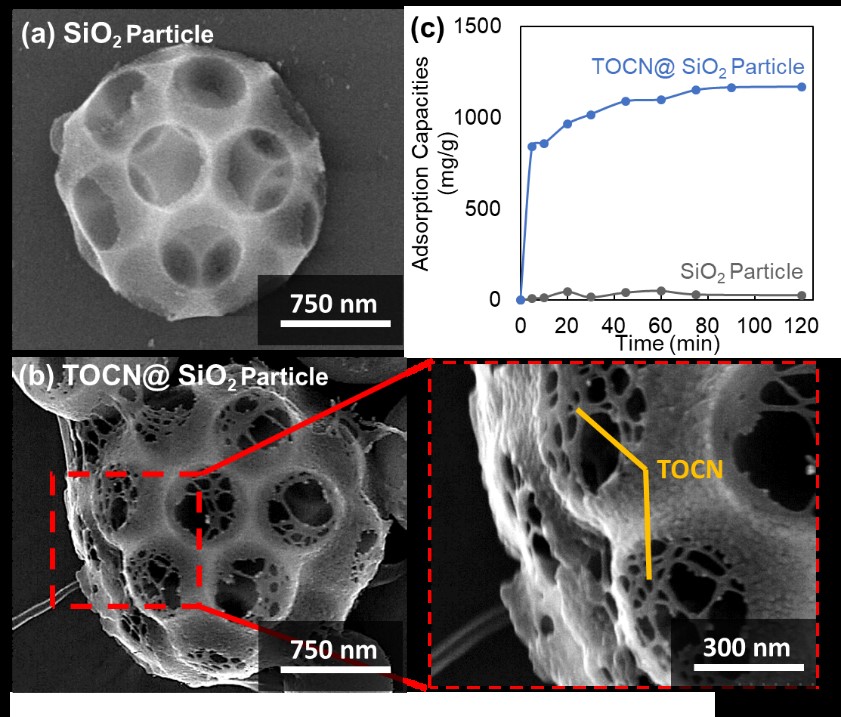
Protein adsorption is a complex process that needs to be addressed as a necessary demonstration of the successful performance of separation, drug delivery, biosensor, and other applications in biomedical. The development of adsorbent material plays a critical role to enhance protein adsorption performance. Tempo Oxidized Cellulose Nanofiber (TOCN) is a promising material owing to biocompatible, homogeneous width, highly disperse in wet-state and highly-negative charge. Even so, there is no research regarding the utilization of TOCN, especially in the particle form as a protein adsorbent material. In addition, TOCN is facing some problems such as not stable in dried-state, easily aggregated, tends to have dense morphology in dried-state that leads to the low specific area. For use as an adsorbent, stable and highly-dispersed TOCN would be desired even in the dried state. In this study, we introduced the possibility of TOCN as an effective adsorbent to specific protein considering their high content of carboxylate groups and negative charge, and a concept of TOCN decorated macroporous SiO2 (TOCN@macroporous SiO2) particle for enhanced its stability and dispersibility in dried-state. The result shows that prepared TOCN@macroporous SiO2 particle has a stable morphology with unique TOCN structure, highly negative zeta potential (-62 ± 2 mV) and surface area (30.8 m2/g) compared to pure TOCN particle. The presence of SiO2 particle allows higher stability and dispersibility which leads to a rich site of electrostatic interaction to exhibits an outstanding adsorption capacity of lysozyme which is higher than that of previous adsorbents. The material and its structure proposed in this study have a more considerable potential of application in the biomedical field.
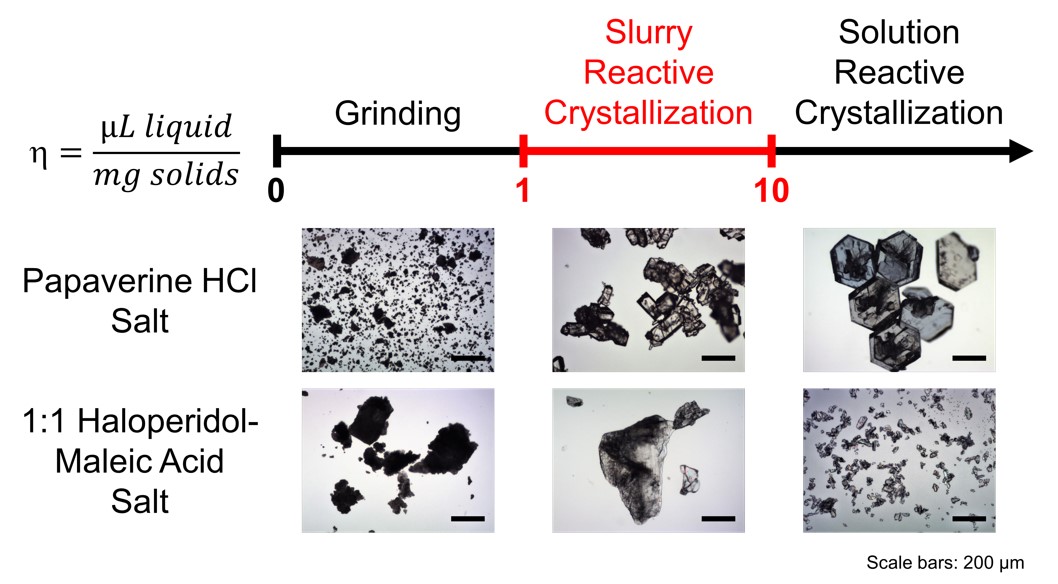
Papaverine HCl was successfully suspended by slurry reactive crystallization with the use of isopropyl alcohol (IPA) at 25oC, a solid-to-liquid ratio of 0.19 g/mL, an aging time of 8 h, a yield of 82.0 w/w %, crystal sizes of 200-400 μm, and the value for enthalpy of fusion of 154.5 J/g. The poor solubility of papaverine in IPA and better solubility of papaverine HCl in water-containing IPA had made the homogeneous nucleation of papaverine HCl dominate. Crystal size and crystallinity of papaverine HCl were time and temperature dependent. On the other hand, the 1:1 haloperidol-maleic acid salt was also successfully suspended and generated by slurry reactive crystallization with the use of water at 25oC, a solid-to-liquid ratio of 0.18 g/mL, an aging time of 8 h, a yield of 82.0 w/w %, crystal sizes of 500-1000 μm, and the value for enthalpy of fusion of 84.9 J/g. The poor solubility of haloperidol and 1:1 haloperidol-maleic acid salt in water had made the heterogeneous nucleation of 1:1 haloperidol-maleic acid salt dominate. Crystal size and crystallinity of 1:1 haloperidol-maleic acid salt became less sensitive to time and temperature. Comparing with grinding, solution reactive crystallization by cooling and solution re-crystallization by cooling, slurry reactive crystallization was a simple, robust, straightforward, low-constant-temperature, low-solvent-volume and environmentally benign process giving comparable yield, particle size distribution, and crystallinity. Moreover, the use of a poor solvent in the slurry reactive crystallization enabled the recycling of the mother liquor without any significant loss in yield and crystallinity up to three cycles.