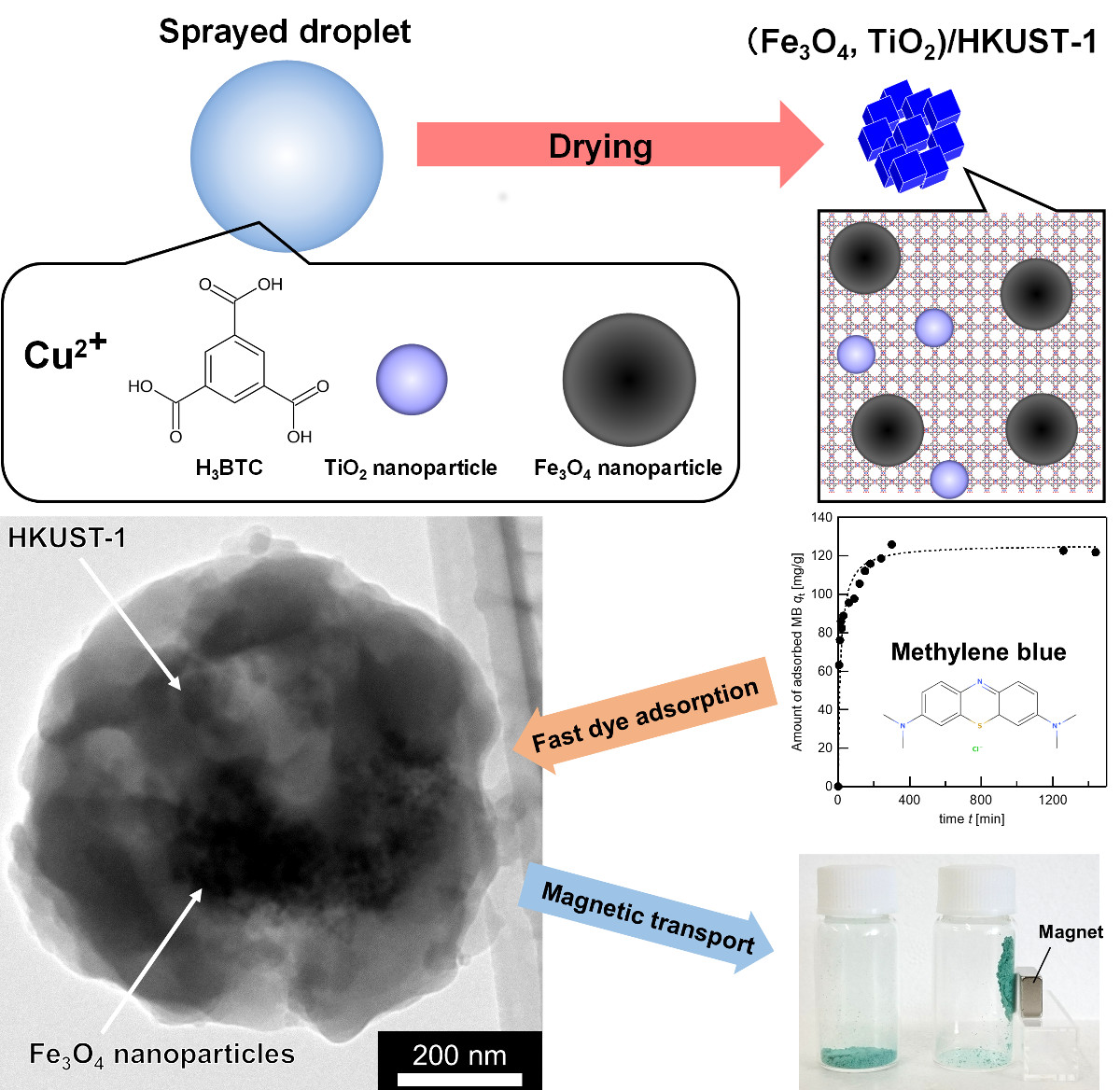
Metal–organic frameworks (MOFs) incorporating other functional nanoparticles have attracted research interest due to the synergistic functionality when combining these components. In this study, we demonstrated the fabrication of MOF composites incorporating one or two types of nanoparticles by a spray-assisted synthetic process. This process enables to synthesize MOF nanoparticle agglomerates in a short time (less than 10 ms) by self-assembly of metal ions and organic ligands during solvent evaporation from sprayed droplets of the MOF mother solution. We fabricated HKUST-1 (Cu3(BTC)2; BTC3- = 1,3,5-benzenetricarboxylate) and incorporated either Fe3O4 or TiO2 nanoparticles or both.
Nanocomposites of HKUST-1 with incorporated nanoparticles can be obtained after spray-drying of the mother solution. The amount of nanoparticles incorporated can be controlled by simply changing the concentration in the spraying solution. Further, by adding both Fe3O4 and TiO2 nanoparticles to the solution, they can both be incorporated into the same HKUST-1 crystals. HKUST-1 incorporating Fe3O4 nanoparticles have superparamagnetic properties due to the incorporated magnetic Fe3O4 nanoparticles. The nanocomposites have a high surface area (>1,200 m2/g), giving very high adsorption capacities for methylene blue (>700 mg/g). Furthermore, all HKUST-1 nanocomposites exhibit good stability for reuse as an MB adsorbent.
Metal-organic frameworks (MOFs), which consist of metal ions and organic ligands, possess high porosity and large surface area. An interesting feature of MOF is that particle downsizing from micron to nanoscale changes its adsorption characteristics. For application purposes, those nanosized particles have to be shaped into millimeter-sized beads or pellets. However, mass transfer is a typical problem especially when nanoparticles form aggregates with randomly-packed structures. It is thus necessary to establish a process to assemble MOF particles into ordered structures, or “supraparticles”, in which gaps between constituent particles serve as mesopores to improve mass transfer.
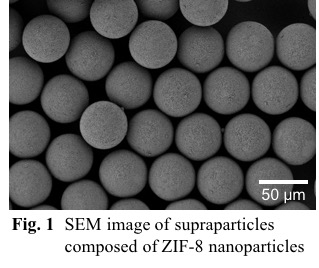
Here we focus on zeolitic imidazolate framework-8 (ZIF-8), which is a subclass of MOFs, as a building unit. We synthesized ZIF-8 particles with the size of ca. 200 nm using a central collision type microreactor and prepared ZIF-8 suspension droplets by mixing a suspension with an oil phase in a microfluidic device, followed by the drying of droplets to form ZIF-8 supraparticles. As shown in Fig. 1, monodisperse supraparticles successfully formed and the supraparticle size can be controlled by the ZIF-8 particle concentration and the microchannel width. Adsorption isotherms of N2 in the supraparticles showed a hysteresis between adsorption and desorption branches possibly derived from mesopores. Importantly, no hysteresis loop was observed in ZIF-8 powders, which indicates that the supraparticles have hierarchical structures with the intercrystalline mesopores. Furthermore, BET surface area of obtained supraparticles is independent of supraparticle size, which indicates the successful fabrication of ordered structures with interconnected pores via a bottom-up self-assembly. The pore size distribution and pore volume are possibly determined by the arrangement and monodispersity of ZIF-8 particles. Detailed investigation on the effects of the supraparticle morphology on mass transfer is now under progress.
Assembling of small particles onto larger particles is a useful technique toward various microstructural material design. Although many colloidal hetero-aggregation systems have been reported, most systems suffer from serious aggregation and solidification at high solid concentrations (c.a. > 15 vol%) which was induced by very strong attractive interaction between small and large particles. Herein, we successfully prepared a flowable 50 vol% silica/silica toluene suspensions comprising from micron-sized silica particles covered with submicron-sized silica particles based on surface design. The micron-sized particles stabilized with polyacrylic acid partially complexed with oleylamine (PAA-OAm) and submicron-sized particles stabilized with polyethyleneimiene partially complexed with oleic acid (PEI-OA) were mixed in toluene. The cross-sectional FE-SEM image of the in-situ solidified suspensions showed that sub-micron particles were attached on micron particles. It is expected that the hetero-assembly was driven by the acid-base interaction between free carboxyl/amino groups of PAA/PEI on two kinds of particles. It was also found that decreasing the OA content of PEI-OA fixed on submicron-sized particles resulted in gelation of concentrated suspensions under low coverage of submicron-sized particles on micron-sized particles. Contrary, when the OA content increased, the suspension showed flowable state with small apparent viscosity and little hysteresis in the flow curves. These results support that increasing oleyl chains on the hetero-assembled particles suppress aggregation in concentrated suspensions owing to steric and osmotic repulsion between the hetero-assembled particles in toluene. In summary, colloidal hetero-assembly of binary system in flowable 50 vol% suspensions were achieved by controlling the acid-base interaction between small/large particles and stabilization of resulting hetero-assembled particles by repulsive interaction.
The aims of this study were to impregnate polyethylene glycol (PEG) 4000 in low-cost silica fume (SF) to form phase change material (PCM) composites with cementitious value, and to provide a quick-fix design for PCM (1) with tailor-made thermal properties and behaviors by particle blending of two types of polyethylene (PEG)/silica fume (SF) composites having different PEG wt% loading, and (2) with enhanced physical properties by turning the powdery PEG/SF composites into round granules through spherical agglomeration. The simple composite blending method was used to broaden and tune the application temperatures in response to variable conditions and environments without the need of searching for new materials to mitigate global warming. Spherical agglomerates of PEG/SF composite exhibited a good homogeneity in thermal properties and low Carr's indices indicating of excellent flowability, packability and compatibility, and offering an enhanced contact area for heat transfer and uniform mixing with other building materials. Noticeably, the agglomerates displayed higher heat capacity values of solid phase, Cps, and liquid phase, Cpl, than those of the composite determined by temperature-history method. The thermal stability of PEG75/SF composites was also attested by the small enthalpy loss, and the highly reproducible melting and solidification behaviors after more than 100 temperature cycles