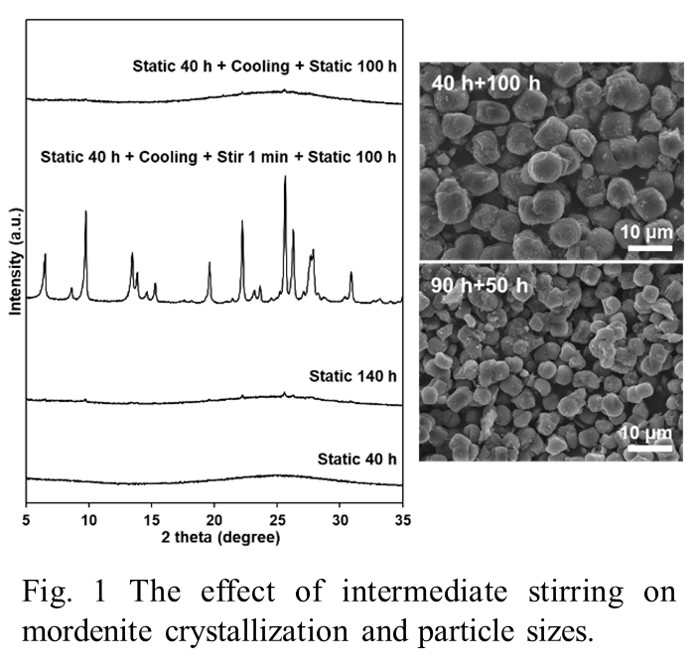
Crystallization of zeolites in hydrogel system usually requires long synthesis time, leading to products in the form of polycrystalline aggregates with broad particle size distribution. In the present study, we demonstrated that the crystallization of mordenite in an organic-free dense hydrogel system (0.275 Na2O: 0.025 Al2O3: 1 SiO2: 25 H2O) can be induced by an intermediate stirring method—quenching and opening the reactor in the middle of synthesis to stir the substances for 1 minute. Moreover, the particle size and size distribution of the crystalline products was found to vary with the intermediate stirring timing. These results suggest the crystallization in this system may follow the autocatalytic nucleation theory, and the number of nuclei changing along with synthesis time can also be estimated. Our observations on the gel particles show that worm-like particles (WLPs) formed large spherical condensed aggregates (CAs) with ca. 10 μm diameter through aggregation and coalescence. The formation of spherical CAs can take place at the very beginning of the synthesis (5 h) under static condition. Based on the results, we speculated that mordenite nuclei can form inside WLPs, and the formation of large CAs as well as the increasing packing density of the dense hydrogel phase further reduce the chance for these nuclei to contact with liquid phase and grow.
Modification of zeolites by the incorporation of divalent Zn in the silicate framework is attracting extensive attention because it results in higher anionic charge density and creates new active acid sites. However, it is a great challenge to incorporate Zn into the framework of highly siliceous MFI-type zeolites without Al. In addition, Zn tends to precipitate under basic synthetic conditions. In this study, ball milling, a mechanochemical method, was employed to prepare Si–Zn oxide composites from mixtures of fumed silica and ZnO as the starting materials for the zeolite synthesis. The dispersion of Zn on an atomic level inside the silica matrix was realized by mechanical forces. Then, the Si–Zn oxide composites were subjected to hydrothermal treatment in the presence of an additional Si source and a structure-directing agent to synthesize MFI-type zincosilicate zeolites with high Zn contents (Si/Zn=36.9–13.4) but negligible Al impurity (Si/Al>900). The successful incorporation of Zn into the zeolite framework was confirmed by several characterization techniques, including DR UV-Vis, FT-IR, XPS and solid-state 29Si MAS NMR. Co2+ ion exchange experiments showed the superior selectivity and capacity of MFI-type zincosilicate zeolites for divalent cations as compared with those of aluminosilicate analogs. The mechanochemically treated Si–Zn oxide composite plays a crucial role in the synthesis of MFI-type zincosilicate zeolites with high Zn contents and minor extra-framework Zn species.
Zeolites are crystalline microporous aluminosilicates that are widely used as adsorbents, ion-exchangers, and catalysts owing to their large micropore volumes with well-defined pore structures, ion-exchange abilities, and solid acidities. It is well known that the zeolites degrade through several mechanisms, such as interactions with metal cations and steam. High-temperature (> 800 °C) steaming conditions, to which the zeolites are exposed in regenerators for removing cokes in fluidic catalytic cracking (FCC) processes, have been a hindrance for the degradation of FCC catalysts, and over 400,000 t/yr of catalysts has been discarded. More recently, zeolites also have been utilized in ammonia selective catalytic reduction (NH3-SCR) of NOx under high temperature steaming conditions. Therefore, hydrothermal stability is one of the most important factors for the application of zeolites. Here, we provide a simple method to improve hydrothermal stability of zeolites. Several types of commercially available zeolites were treated and showed excellent stability under very high temperature (up to 1150 °C) steaming conditions. The target temperature was achieved after 75 min of ramping and was kept for 3 h for testing using a house-made durability test apparatus, followed by cooling to room temperature for over 3 h. Water was introduced through a syringe pump and mixed with dry air from a gas cylinder to achieve 10 vol.% steam at atmospheric pressure. Samples before and after hydrothermal treatment were characterized by X-ray diffraction, scanning electron microscopy, nitrogen adsorption/desorption, and so on.
Developing efficient and low-cost routes for the synthesis of zeolites is of high significance, considering the ever-emerging applications and increasing consumption of these materials. Our group has established the ultrafast synthesis of zeolites and demonstrated that the continuous flow synthesis could offers a number of advantages for zeolite production. Sharp viscosity increase taking place in the zeolite formation, however, renders a continuous flow synthesis very challenging. We herein show that the emulsion system creates a unique opportunity to address the viscosity issue, allowing the zeolite products to smoothly flow out of the reactor in forms of a suspension. The emulsion-based, continuous flow syntheses of three important zeolites – ERI, *BEA and CHA were achieved, proving this method to be a general approach.
Recently, co-authors have made it possible to densify B4C (Boron carbide) even at low pressure firing. It is expected that basic research and practical application research on B4C based ceramics will be promoted.
Evaluation on sliding characteristics was carried out mainly using B4C - SiC (Silicon carbide) ceramics prepared by low pressure sintering as test specimens. As a result, we've got some interesting findings. First, According to the observation using TEM(Transmission Electron Microscope), it was confirmed that at the interface of B4C and SiC phases voids were not observed or if any few , indicating that both were firmly bonded. Secondly, in polishing and subsequent sliding tests, it was found that a step having a height of several tens of nanometers was formed on the surface due to slight difference in wear rate between B4C and SiC. Since the step spreads over the entire sliding surface, it has a structure like a relief, we called this a relief structure.
Generally, it is said that the coefficient of friction is governed by the true contact area and the shear force at the interface. Specifically, the friction coefficient is influenced not only by control factors such as load and speed, but also by complicated phenomena such as the reaction occurring at the interface with the mating material and the lubricant, as well as the chemical and physical state of the solid surface. However, there are very few research reports that systematically examined them.
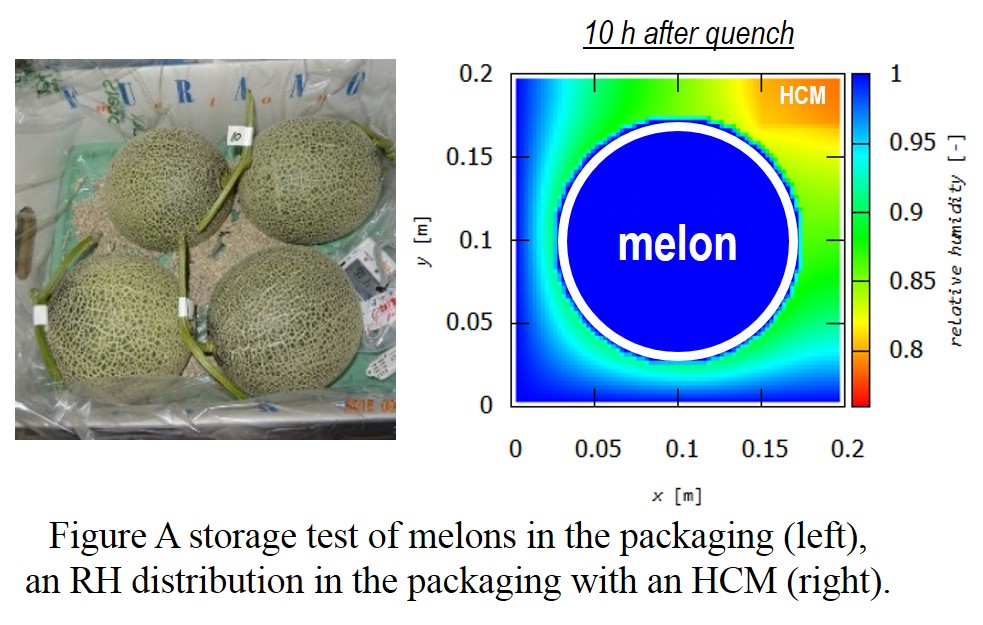
Modified-atmosphere packaging is a readily available technique to extend storage life of fruits. This is achieved just by keeping fruits in packaging bags made from high gas barrier films. Meanwhile, this is often not effective for fruits with high rates of transpiration (evaporation of water) such as melons. The relative humidity in the packaging (RH) reaches 100% due to the transpiration, resulting in condensation followed by mold growth on their surfaces. Recently we succeeded in decreasing the RH to 50% using humidity control materials (HCMs). Although condensation can be prevented, the RH is so low that melons lost too much water and were spoiled. Since the RH depends on multiple factors of HCMs (property, utilization), the RH ideal for melons (90%) is expected to be achieved through their optimization. However, this has been time-consuming because it requires multiple storage tests for data acquisition.
Simulation-assisted approaches to design HCMs have been studied in architectural engineering. Humidity distribution in rooms can be predicted through solving the modified equations for heat and mass transfer. The aforementioned factors can readily be optimized to achieve the target RH with a small number of experiments. In this work, we established a time-effective method to design HCMs for the packaging through this approach.
Three porous carbons with various water adsorption isotherms were investigated as HCMs. RH distribution of the packaging containing one of the HCMs was calculated when this was quenched (25 °C to 3 °C). The ideal environment, where melon was surrounded by atmosphere with 90RH%, was observed when the HCM with type IV isotherm (5 g) was located at the center of the packaging. This result corresponds with that of the actual experiment, where the RH was 92%, indicating reliability of our approach.