
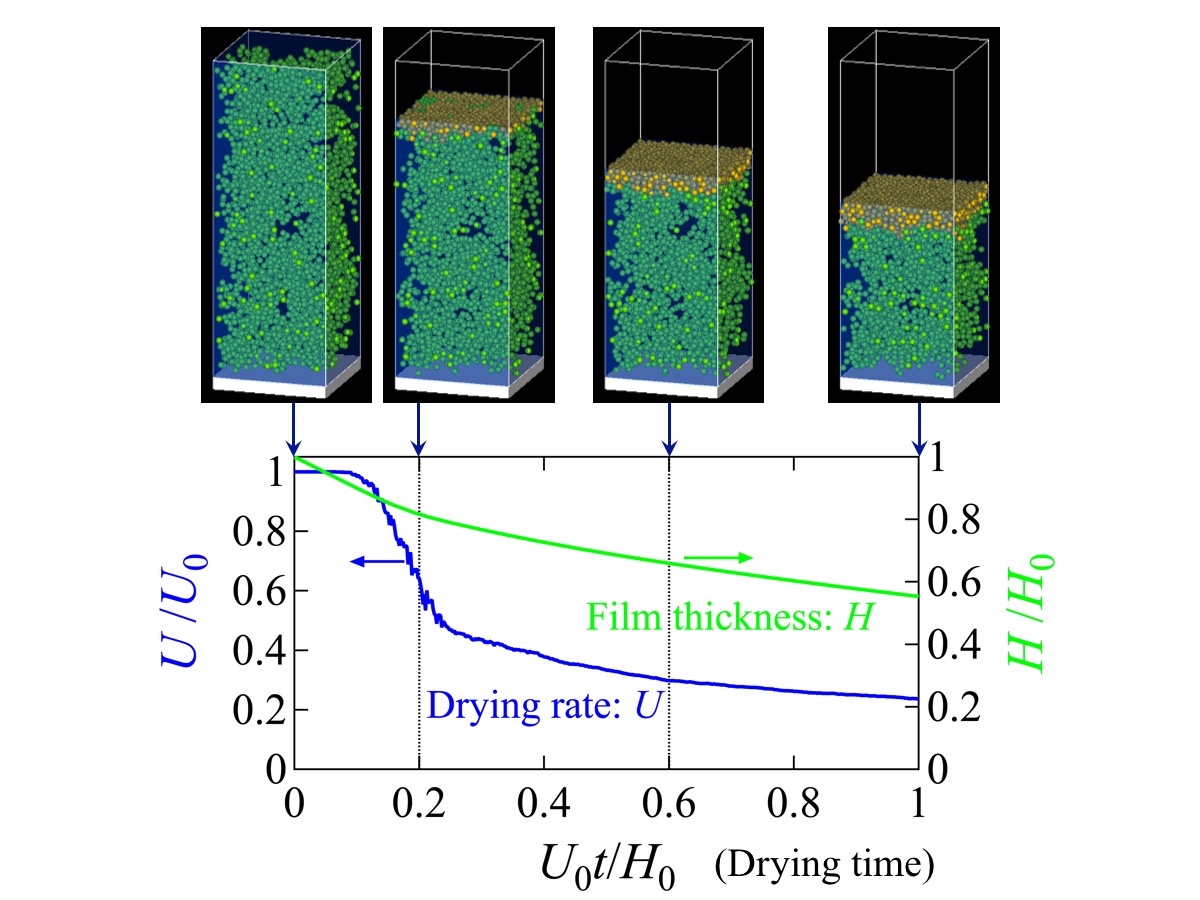
Colloidal suspensions are coated on substrates and upon drying produce various functional materials, whose quality is determined by the microstructure composed of colloidal particles. The structure formation of the particles during drying is governed by the competition between the Brownian motion of the particles and the recession of the free surface according to liquid evaporation. The Brownian motion tends to distribute the particles uniformly, while the receding free surface drives the formation of concentrated particle layers. The drying rate falls with a decrease in the liquid permeability of the growing concentrated layers. The structure formation of the particles is reflected in the drying characteristics, of which the control is required for the improvement of material quality as well as the reduction of drying time. We then construct a model to investigate how drying conditions and suspension properties affect the drying characteristics.
We have developed a simulator of colloidal suspensions: Structure of NAno Particles (SNAP). In the present study, we extend a simple mathematical model adopted in SNAP-L, where the Brownian motion of particles is described by a stochastic differential equation called the Langevin equation. We additionally construct a model to consider drying rate falling by estimating the permeability of formed concentrated layers. The drying characteristics calculated by our model is shown in Figure. Using the present model, we propose how to control the drying characteristics by initial drying rates and interactions between particles.
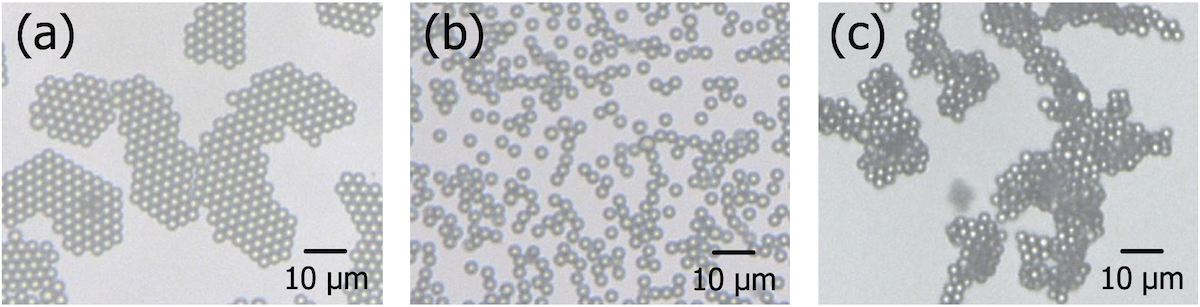
Self-assembly is an attractive technique to fabricate colloidal crystals, which are promising for various applications. However, few works focused on the formation process of colloidal crystals based on interaction forces acting on particles, which play a critical role in self-assembly. Here we produced colloidal crystals and controlled their size by adjusting the balance between the attractive force induced by the depletion effect and the repulsive force due to overlapping of the electric double layers of particles.
We used polyethylene oxide (PEO) as a depletant and sodium chloride (NaCl) as a salt and systematically investigated the effect of their concentrations. Figure 1a shows a typical image of depletion-induced colloidal crystals composed of 3 μm polystyrene particles under the condition of 0.4 g/L PEO and 10 mM NaCl. The decrease in the depletants resulted in a dispersion state (Fig. 1b), while the increase produced aggregates (Fig. 1c). Under a lower salt concentration condition, a larger amount of the depletants (i.e., the stronger attractive force) was necessary for the crystallization because of a longer Debye length.
Further, the depletants of 0.7 g/L without salts resulted in a formation of large crystals with an average size of 33 μm while the addition of 1 mM NaCl decreased the size to 15 μm. In addition, the crystal formation without salts was much slower than that with 1mM NaCl because of a higher energy barrier caused by a longer Debye length. These results suggest that a higher energy barrier produces a fewer number of "nuclei" of colloidal crystals, resulting in larger domains during assembly. Hence, we demonstrated that the formation process of crystals and their size can be controlled by adjusting the balance between repulsive and attractive force.
Figure 1. Images of (a) depletion-induced colloidal crystals, (b) dispersion state, and (c) aggregates
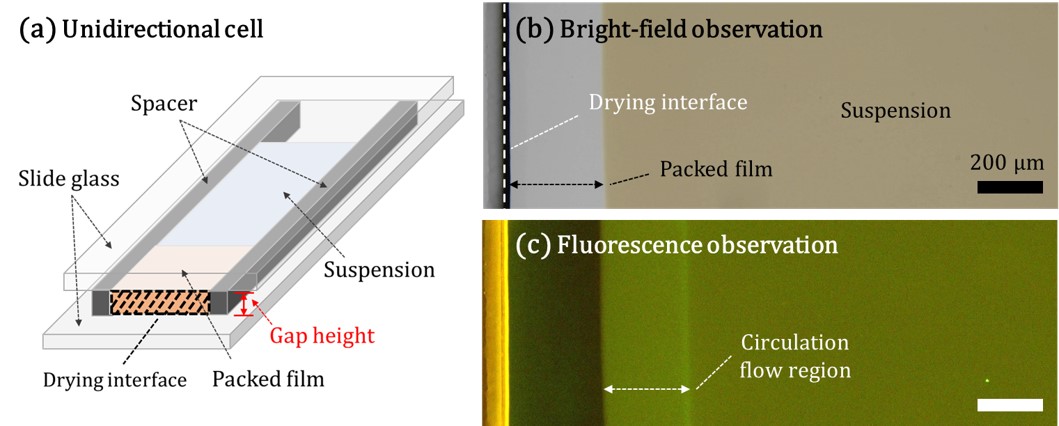
Drying of colloidal suspension is a common industrial process and widely used for manufacturing products, such as paints, ceramics and electrodes. During drying of a colloidal suspension, particles move towards the drying interface, and they form particulate films. Many papers have reported drying kinetics of colloidal suspensions and directional drying is frequently used in those studies. Suspension is introduced into a narrow space between two glass plates. Evaporation occurs from one open side, which induces flow to the drying interface. Because flow is confined in a narrow space, it has been widely accepted that only horizontal flow is induced by drying. However we have found that there is a complex circulation flow. We used a unidirectional drying cell (Hele-Shaw cell) to dry silica colloidal suspensions. Fluorescent particles were added to trace flow inside of the drying cells. A circulation flow appeared around the interface between the packed layer of particles and the suspension although the suspension was confined in a narrow space with a height of 100 μm. The width of circulation flow region was strongly dependent on the growth rate of packed film and the gap height of cells. The origin for the circulation flow and possible impacts of the flow on film formation processes are discussed.
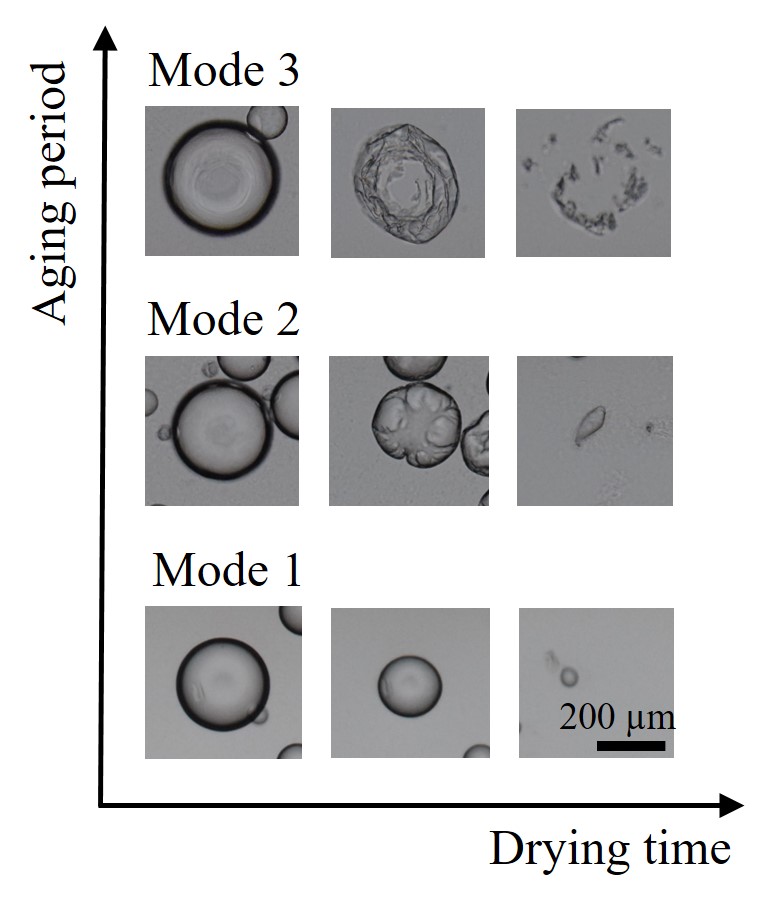
Some solid particles stabilize water-oil interface and particle-stabilized emulsion forms. We prepared Water-in-Oil (W/O) emulsions using hydrophobic silica particles as a stabilizer. Drying of droplets was observed. Due to evaporation of water, droplets shrunk. We found that the ways to droplets shrunk varied depending on the storage time (aging time) of droplets as shown in the Figure. Immediately after preparation, droplets shrunk isotropically during drying (Mode 1). After several-days aging, droplets shrunk but wrinkles formed on surface of the droplets (Mode 2). Further aging induced buckling of drying droplets (Mode 3). We revealed that those changes closely related to particles affinity to water. Particles were hydrophobic and they did not have good wettability to water in praparation process. However wettability of water increased during aging. Formation of wrinkles and buckling of droplets suggested that the droplet interface became solid owing to packing of particles. Because particles at the interface were compressed due to drying-induced shrinkage of droplets, particles packing occurred at some point. We consider that wettability of particles is a key issue in packing. Simple drying experiment of particle-stabilized water droplets would provide us with important information about how packing of particles occurs on two-dimensional interface.
Performance of emulsion significantly depends on interface control. This study focused on interfacial layer formation of particles for oil-water emulsion system and its emulsion stabilization effect. In order to investigate interfacial localization, adsorption, and desorption of clay particles in the presence of surfactant, interfacial modulus of oil-water interface was measured under the control of surfactant loading. Depending on the balance of wetting property of particles between oil and water, localized particles responded to surfactant differently, especially depending on ionic characteristics of surfactant and the oil phase. Natural particles responded rapidly to surfactants and enhanced to desorb to the other phase as the concentration of surfactant increases. Organically surface-modified clays localized at the interface with the presence of surfactant and slowly formed viscoelastic complex interface of particles. (this work was supported by the National Research Foundation of Korea(No. NRF-2018K2A9A1A06086766))