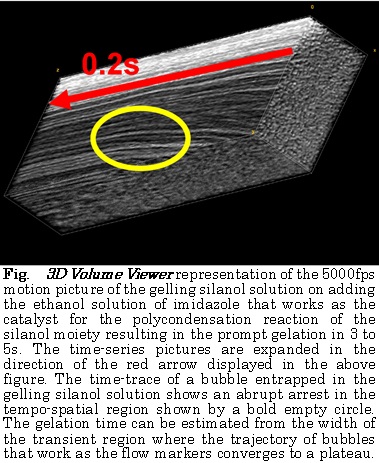
The primary distinction between gel and non-gel is ascribed to whether it macroscopically flows or not. This visible criterion for discriminating the abovementioned two states which clearly differ from each other is based on the kinematics of a gelling solution, where we need to make a decision on whether it still exhibits flowable appearance or not. In most cases, gelation is observed as irreversible arrest in the motion of the gelling solution. Therefore, the direct observation of such a transient and transitive process is regarded as the only viable approach to detecting the actually occurring gelation. Generally in chemical studies, gelation tends to be discussed in the light of the formation of a percolated network-like structure of chain-like molecules or some alternative microstructures with elongated geometry. Thus, it is almost our habitual to attempt to obtain the clues for the gelation by means of some effective physicochemical measurements which have been established in the piles of abundant theories and experiences. Nevertheless, in the present work, we attended to the direct observation of the moment at which the arrest of the flow in the gelling solution occurs. For the suitable condition for the direct observation of the very moment of the gelation, we employed high-speed motion picture which captured the gelation occurring in only a few seconds. In advance, we had found an appropriate system which undergoes such a prompt gelation, where silicate oligomers stabilized in weak acid polycondense triggered on adding ethanol solution of imidazole which worked as a highly effective basic catalyst. In the high-speed observation at approximately 104 fps, the enforced flow in the gelling solution was seen arrested at 1 to 10 ms, whereas the overall gelation of the whole solution gave the observers the visual impression that it occurred and terminated in 0.1 to 0.4 s.
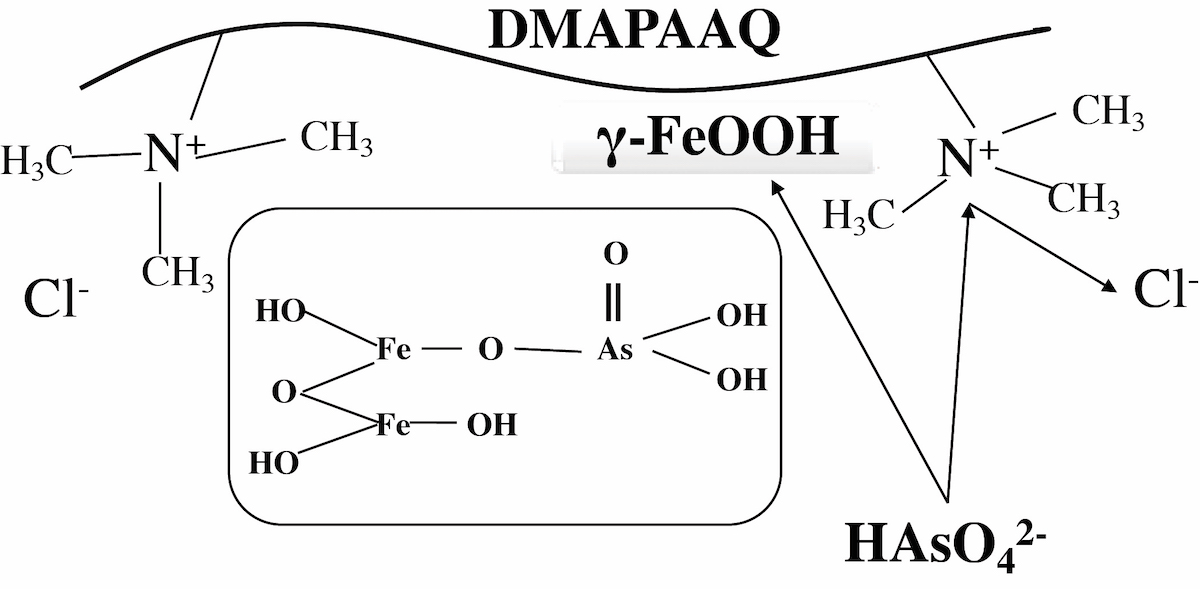
Groundwater contamination of arsenic is a global concern. The challenges in removing arsenic are low adsorption rate, lack of selectivity, regeneration and performance at natural conditions. Two forms of arsenic are predominantly found in nature, Arsenate and Arsenite. To remove Arsenate, As(V), from ground water by adsorption using a composite of cationic polymer gel impregnated with iron hydroxide. The cationic polymer gel that we used is N,N-dimethylamino propylacrylamide, methyl chloride quaternary (DMAPAAQ).
We prepared the gel using a unique method where Sodium Hydroxide and Iron (III) Chloride wasSVA added to the monomer and initiator solutions, respectively, to maximize the FeOOH contents in the polymer structure of the gel.
Results suggest that the maximum As(V) adsorption capacity of our gel, DMAPAAQ + FeOOH, was 123.4 mg/g. At pH7, the maximum As(V) adsorption capacity of our gel was higher than that of other adsorbents currently being used. Selectivity analysis suggests that in the presence of Sulphate and Chlorine, the gel can selectively adsorb As(V) effectively (see Fig. 1).
Regeneration experiments were successful for eight continuous adsorption-desorption cycles with the same gel pieces with 87.6% efficiency at neutral pH levels. Conventional desorption processes use NaOH for desorption. Since NAOH is harmful to human health, we used NaCl instead.
Our newly developed gel composite, DMAPAAQ + FeOOH, adsorbed As(V) effectively and the adsorption amount was higher than the other adsorbents at neutral pH levels. The gel selectively adsorbed As(V) and was regenerated with 87.6% efficiency. The novelty in our research is in the unique preparation method of the gel and the high adsorption performances at neutral pH levels, selective adsorption of arsenic and its reusability. In future, we will examine the performance of the gel in removing As(III) and the applicability in the natural groundwater.
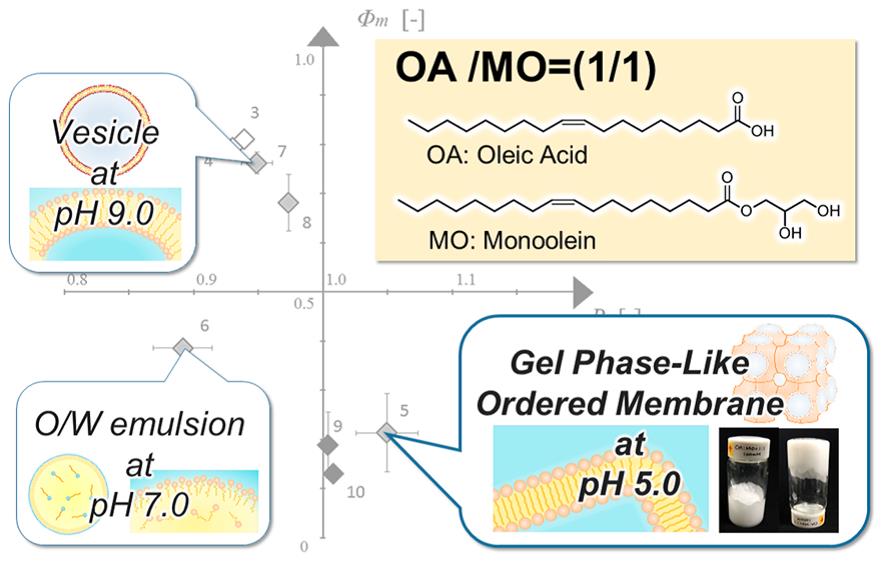
Self-assembly behaviors of fatty acid molecules are sensitive to surrounding pH conditions. Here, we developed a novel methodology to create hydrogel materials using fatty acid and its derivatives. Phase behaviors of oleic acid (OA)/monoolein (MO) mixtures were controlled by pH titration, wherein vesicle, oil-in-water emulsion, and cubic phase (cubosome) were formed at pH 9.0, 7.0, and 5.0, respectively. Rheological properties of cubosomal gel were characterized, and the mechanical properties of cubosomal gel could be improved by modifying with polymerizable fatty acid molecules. In addition, the mesoscopic membrane properties of OA/MO assemblies were systematically characterized by Raman spectroscopy and fluorescent probe Laurdan. It is concluded that the rigidity of the membrane could be key to maintain the mechanical properties of cubosomal gel materials.
Reference: K. Suga et al., Langmuir, 34 (5), 2081-2088 (2018). K. Suga et al., J. Chem. Eng. Japan, 52 (3), 311-316 (2019). K. Suga et al., Langmuir, 32 (30), 7606-7612 (2016). K. Suga et al., Langmuir, 30 (43), 12721-12728 (2014).
In recent rising environmental problem, competition and interest for CO2 gas capture materials have been increased. Solid CO2 adsorbents such as activated carbon and zeolite have been used for CO2 capture, however, those CO2 capacity or adsorbed kinetics were dramatically decreased in high humid condition. We have been investigated hydrogel film for CO2 capture to show relatively high CO2 capacity in R.H > 95%, however, CO2 absorption kinetic of this hydrogel film was too slow to spend over 1 hour. In this study, we developed an aeroporous hydrogel film (as shown in Fig.1) which consist of phase separation structure for hydrophilic hydrogel phase and hydrophobic porous gas diffusion phase. In this concept, CO2 gas can smoothly penetrate through gas diffusion phase inside film and finally absorb to hydrogel film phase for short time. We prepared aeroporous hydrogel film by mixing between amine containing hydrogel particle (GPs) and acetylene black (AB) using planetary beads milling in dry state, forming film by uniaxial pressing, and humidifying under R.H > 95%. After mixing GPs and AB, obtained powder showed core-shell structure, especially primary AB particle with 30 nm coated on the surface of GPs with approximately 5 μm from its characteristics using SEM, EDS, and particle size distribution laser diffraction type of particle size distribution measurements. After preparation the film, prepared self-standing film shows submicron ordered phase separation of GPs and AB, and a lot of voids was further observed in AB phase from TEM observation. CO2 absorption and desorption kinetics of this film showed higher than that of only GPs film evaluated by pressure swing adsorption method using humid CO2 and N2 gases. We supposed that this result revealed that gas transfer micro-channels in the aeroporous hydrogel film could be maintained under high humid condition and attributed to accelerating CO2 absorption and desorption rate.

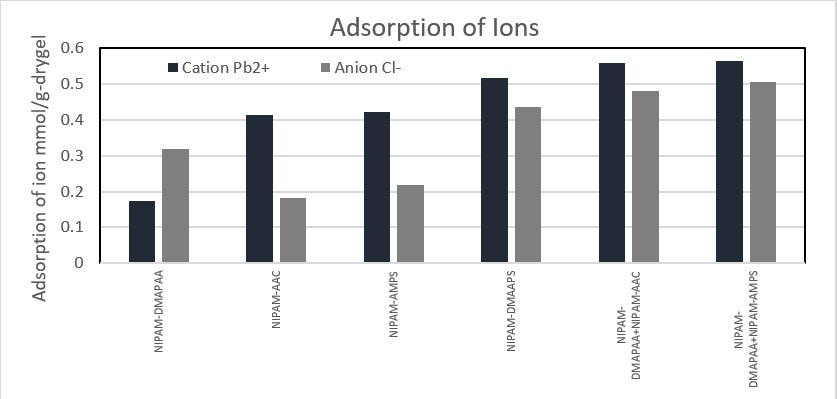
Clean water is a rare commodity to get in several areas, take Batam in Indonesia for example. In 2018 approximately 70 percent of the waterways in the area is polluted by heavy metal contaminant. In this research an efficient and economical metal separation technique is proposed for the adsorption of heavy metal ions in solutions containing heavy metal ions. This technique adsorbs undesired cations and anions at the same time by deploying both cationic and anionic gel to adsorb heavy metals in target solution. The model system consisted of metal ion (PbCl2 or ZnCl2), cationic copolymer gel, and anionic copolymer gel, metal adsorption was successfully conducted. Copolymer gel consisted of N-isopropylacrylamide (NIPAM) as a thermosensitive agent, Acrylic Acid (AAc) / Acrylamidomethylpropane sulfonic acid (AMPS) as cation adsorbent and N,N-Dimethylamino propylacrylamide (DMAPAA) as anion adsorbent. With the existence of NIPAM as one of the major constituent of the gel, gel also exhibits thermosensitive properties, hence gel can desorb and adsorb ions at different temperatures. Figure 1 shows us that adsorption of both cation and anion does occurred in every type of gel. However gels that are able to adsorb cation and anion simultaneously (Dimethyl(acrylamidopropyl)ammonium propane sulfonate (DMAAPS), NIPAM-DMAPAA+NIPAM-AAc, NIPAM-DMAPAA+NIPAM-AMPS) recover more ions compared to other gels. Simultaneous usage of cation and anion gel also increases the reusability of gels. Unlike systems that uses sulfobetaine gel such as DMAAPS gel, this system enables gels to adsorb both cation and anion without any inhibition from inter-chain, intra-chain, and intra group bonding, thus reducing the probability of the system reaching its equilibrium due to the different charges in the target solution. The tested system, where anionic and cationic gels deployed at the same instant, has the potential to efficiently recover heavy metal ion from target solution.
Keyword(s): Thermosensitive, Simultaneous, Adsorption, Heavy Metal.
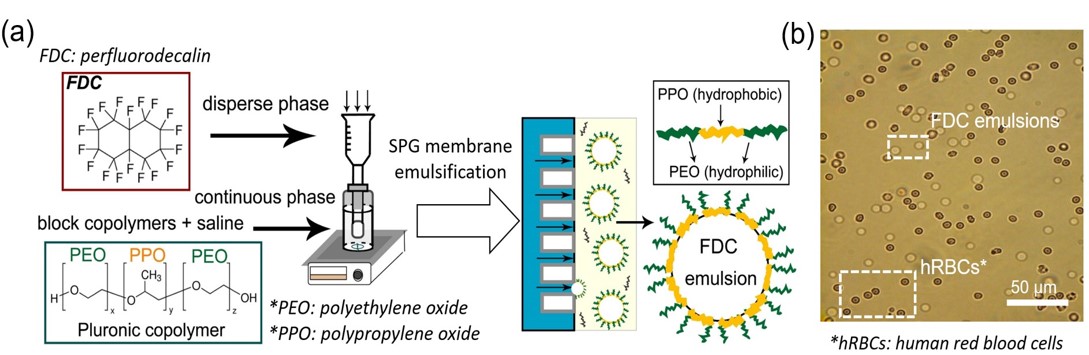
Perfluorocarbons (PFCs) have long been investigated for use in many fields including contrast agent, cancer therapy, and oxygen carriers (OCs) for a long time. In the case of OCs, PFC-based materials aim to provide adequate amount of oxygen to treat ischemic diseases or to facilitate tissue growth in 3D cell culture system for tissue engineering. Nano-sized PFC-based OCs have been studied for several decades due to the high oxygen carrying capacity and low side effects of PFC. In addition, micro-sized OCs are also attractive since they can avoid cellular uptake via endocytosis. Size control has been proposed as a requirement for developing OCs and uniform size distribution is crucial for their function of oxygen supply, durability, and biological interactions. However, it is hard to be accessed by traditional methods, such as mechanical agitations, that produce wide size distribution.
Shirasu porous glass (SPG) membrane emulsification technique is capable to produce monodisperse emulsions with precisely-controlled size. We have, for the first time, prepared PFC-based OCs using SPG membrane emulsification (Fig. 1a).1 By changing the membrane pore size, we were able to precisely control the size of emulsions and fabricate emulsions similar in size to human red blood cells (Fig. 1b). We also fabricated PFC emulsions using different types and concentrations of Pluronics (F127, F68, P85 and P103) as surfactants. The F127- and F68-stabilized micro-sized PFC emulsions were highly stable even during autoclave sterilization. The obtained emulsions were further loaded with oxygen-sensitive probe on their surfaces to indicate oxygen concentration. Their oxygen delivery to cultured cells was demonstrated using genetically-engineered HeLa cells that express EGFP in response to hypoxia.
Reference
1) Fu, X., Ohta, S., Kamihira, M., Sakai, Y., Ito, T. Langmuir 2019, 35, 4094-4100