
In recent years, many studies have been made in finding alternative additive to enhance the mechanical and thermal properties of polyvinyl alcohol (PVA). In this study, PVA-clay and PVA-grog composites were fabricated by solution casting method with varying additives composition. Clay and grog particles have been pre-treated by milled and sieved into micron size. The properties of composites were investigated with tensile test, scanning electron microscope, X-ray diffraction, fourier transformed infrared spectroscopy and thermogravimetric analysis. The results showed that PVA molecules could not intercalate into clay and grog particles during solution casting. The structure of composites were suspected to be phase separated instead of exfoliation or intercalation structure. The addition of clay or grog had slightly improved the mechanical strength of composites but FTIR result suggested that the improvement was not caused by hydrogen bonding between filler and PVA. The surface morphology from SEM showed that high loading of clay or grog in PVA has resulted in rougher surface and agglomeration of fillers. Based on XRD result, the average crystallite size of PVA-clay composites decreases with increasing clay loading while the overall crystallinity of PVA-grog composites increases with increasing grog loading. For thermal stability, increasing clay or grog loading have caused the thermal stability of the composites decreased slightly. Overall characterization results of the composites suggested that micron sized clay or grog particles are not suitable to enhance PVA mechanical and thermal properties.
Poly(N-isopropylacrylamide) (PNIPAM) nanogels were synthesized by emulsion polymerization using sodium dodecyl sulfate (SDS). After removal of SDS by dialysis, the surface tensions of the PNIPAM nanogel aqueous dispersions were measured by the pendant-drop method, and it was found that the surface tensions of the nanogel dispersion below the lower critical solution temperature (LCST) of PNIPAM were much smaller than those of water and comparable to those of the SDS aqueous solution. The stability of the aqueous foams generated by nitrogen bubbling thorough the PNIPAM nanogel dispersion was investigated below and above the LCST of PNIPAM. The foam prepared below the LCST was stable in some degree, whereas almost no foam was formed above the LCST. Moreover, the foam prepared below the LCST was quickly collapsed by changing the temperature above the LCST. This rapid defoaming represents that the surface activity of the PNIPAM nanogel can be switched off by the temperature increase across the LCST. In addition, we succeeded in controling the temperature dependence of the Pickering foam stability by copolymerizing the hydrophilic monomer, N,N'-dimethylacrylamide (DMAM) with NIPAM.
We report a microfluidic preparation of poly(ethylene glocol)-based hydrogel microparticles with tunable structures using phase separation in aqueous droplets, followed by cross-linking reaction. The process consists of water-in-oil (W/O) emulsion droplet formation through microfluidic emulsification, subsequent transition from the W/O emulsion droplets to water-in-water-in-oil (W/W/O) double emulsion droplets by internal phase separation, and gelation of the reactive monomer phase in the W/W/O emulsion droplets. We discuss the effect of the rate of gelation on the resulting structures of the hydrogel microparticles.
Advanced materials with low density and high strength have transformative impacts in construction, aerospace and automobile industries. These materials are realized with integrating well-designed modular building units into interconnected structures. Here, we present a hierarchical design strategy to demonstrate a new class of carbon-based closed-cellular structures (CCS). The building units are prepared by a multi-scale approach starting from functionalized graphene oxide nanosheets, leading to the microfluidic synthesis of solid-shelled bubbles with shape diversity at the micro-scale, followed by the assembly into meso-scale 3D structures. Subsequently, these are transformed into self-interconnected and structurally-reinforced CCS, resulting in graphene lattices with rhombic dodecahedral honeycomb structures at the centimeter-scale. The 3D suprastructure simultaneously exhibits the Young's modulus above 300 kPa while retaining a light density of 7.7 mg/cm3 and the elasticity against up to 80% of the compressive strain. The fabricated 3D CCS opens a new pathway for designing lightweight, strong, and superelastic materials.
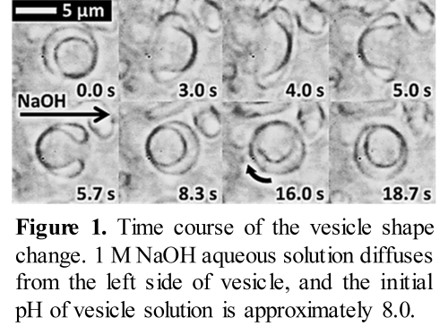
Biological functions are maintained by various molecular motors, where the driving force is obtained from a chemical potential difference within the microscale. Here, we show in detail artificial vesicles that generate mechanical work from a local pH gradient. The vesicles are composed of oleate and oleic acid and exhibit rhythmic shape change[1] shown in Figure 1. This cyclic motion involves both rotation of the entire vesicle and reversal motion that reverses the vesicle structure of inside and outside, which constitute relaxation and excitation processes against a pH gradient, respectively. A cycle is constituted of these two elementary processes. In our previous work, vesicle motion was observed under an unsteady pH gradient formed by a diffusion of a base solution. As a result, the pH and pH gradient around the vesicle varied throughout the observation period. Therefore, quantitative aspects such as the driving force strength were unclear.
In this study, we observed periodic shape change under a quasi-steady state pH gradient, meaning that the pH gradient can be regarded as constant throughout the observation period. For this purpose, a device maintaining a quasi-steady state pH gradient over a sufficient period of time was made. With this setup, the pH and pH gradient around a vesicle exhibiting a shape change could be estimated. From the motion analysis of the vesicle, the driving force for vesicle motion was evaluated as a function of the pH and pH gradient. As a result, a quantitative discussion of the self-excitation and relaxation processes is provided. The present results demonstrate that this vesicle can be regarded as a molecular assembly machine working under a pH gradient.
Acknowledgements
E.N. gratefully acknowledge the financial support from JSPS KAKENHI Grant Number 19K15425.
Reference
[1] E. Nawa, Y. Nishigaki, D. Yamamoto, A. Shioi, Soft Matter, 2013, 9, 7832.
In recent years, poorly water-soluble drugs are increasing, and improvement of water solubility is considered as an important issue. Several methods have been proposed for improving water solubility, and one of them is a method of dispersing a drug by utilizing hydrophobic interaction between a water-soluble polymer having a hydrophobic moiety and a hydrophobic drug. In this method, there is a problem that only a polymer having a hydrophobic part can be used. However, it has been found that the water-soluble polymer itself inhibits the crystallization of the drug even in the absence of the hydrophobic moiety, and that it is possible to disperse the poorly water-soluble drug. This has resulted in more selectable polymers and the ability to use biocompatible polymers. In this study, we tried to use ionic polymers to disperse poorly water soluble drugs via ion-ion interact of water soluble polymers and hydrophobic drugs.
It was confirmed that with Glycol Chitosan as a cationic polymer, Indomethacin, an anionic poorly water-soluble drug, could be dispersed in water. In addition, with anionic polymer Chondroitin sulfate C sodium salt, dispersion of Lidocaine, a cationic poorly water-soluble drug, was also confirmed. Confirmation of the dispersion was conducted by UV measurement and the presence of the ion-ion interaction was conducted by IR measurement. Compared with pure water, the dispersibility of Indomethacin and Lidocaine were improved about 1,600 times and about 50 times, respectively.