
Hydrogen-bonding solid crystals of methane hydrate have recently gained a great deal of attention as a globally recognized new energy resource for the next generation. Despite its popularity, the phase change processes of methane hydrate are not well understood.
During the decomposition of methane hydrate, long-term stable phenomena occur under temperature and pressure conditions (around 240K) where the material is expected to be inherently unstable. Although a full degradation mechanism has not yet been elucidated, it is hypothesized that super-cooled water suppresses the decomposition reaction [1].
In this study, we observed phase changes in methane hydrate using terahertz wave spectroscopy. Terahertz radiation boasts the material permeability of radio waves and the ease of handling of light. In addition, terahertz waves are strongly absorbed by water, making them especially well-suited for detecting liquid water.
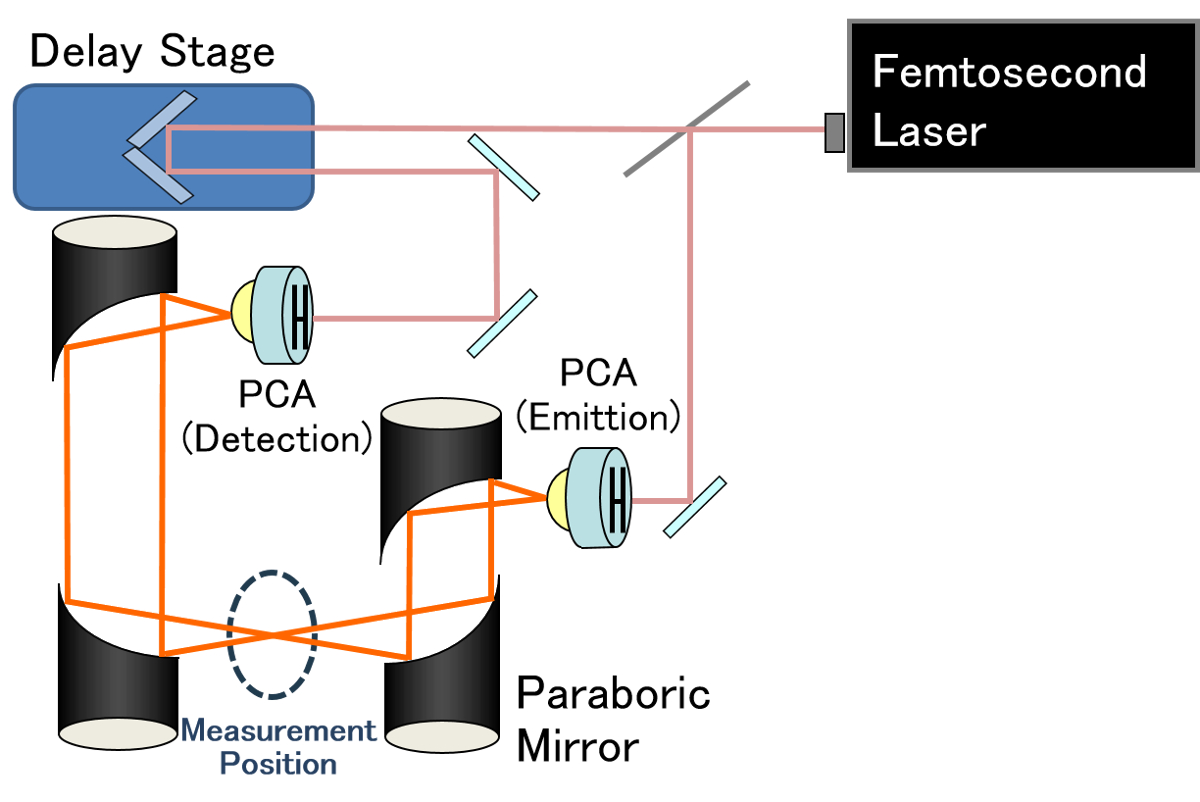
The terahertz time-domain spectroscopic (THz-TDS) system shown in Figure was used to observe phase changes in methane hydrate. This system can simultaneously observe absorption due to the presence of water and phase changes in the methane hydrate material. Experiments were conducted at 210K to 260K while maintaining approximately 3 hours at each temperature.
An increase in absorption and a leftward shift in the time waveform were observed at temperatures greater than 240K, indicating distinct changes in the sample at this temperature. In addition, this temperature coincides with the temperature at which super-cooled water exists, strongly implying an active role of super-cooled water in the phase change activity of methane hydrate.
References
[1] K. Takeya, K. Nango, T. Sugahara, K. Ohgaki, A. Tani J. Phys. Chem. B 109 (2005) 21086
Recently, the replacement of natural gas hydrates using CO2 injection has been considered as a promising natural gas production and CO2 sequestration method. This method differs from thermal stimulation and depressurization technologies in that it is non-destructive and environmentally friendly because natural gas hydrates do not need to be dissociated for production and CO2 is safely stored in the form of gas hydrates. In this study, sI and sH hydrates which were formed with methane and methane + methylcyclopentane (MCP), respectively, were used for replacement using pure CO2. To examine the effect of CO2 injecting pressure on the replacement behavior, the experiment was conducted within pressure conditions where pure CO2 hydrate is thermodynamically stable. To identify replacement efficiency, the compositions of the hydrate phase were measured by gas chromatography (GC) after dissociating the replaced hydrates. In addition, 13C NMR, powder X-ray diffraction (PXRD), and Raman spectroscopy were used to examine the structural information of gas hydrates and the changes in cage occupancy of guest molecules before and after the replacement. For sI hydrate, the replacement efficiency was found to be about 70 %, which was independent of CO2 injecting pressures. It was found from PXRD patterns that cage occupancy of guest molecules after replacement in sI hydrate were almost the same at lower and higher CO2 injecting pressures. However, for sH hydrate, the replacement efficiency was increased up to 80% with increasing the CO2 injecting pressure, which was attributed to a larger portion of a structural transition to sI hydrate at higher CO2 injecting pressure. In addition, time-dependent Raman spectra demonstrated that the structural transition of sH to sI hydrate was more significant at higher CO2 injecting pressure.
CO2 activation is an attractive pathway to utilize CO2 obtained either from large industrial point sources such as heavy industry and power plants, or captured directly from air. CO2 can be activated by reacting it with methane to obtain CO or syngas as a product. An often suggested process to do so is the dry reforming process.
CH4 + CO2 → 2 CO + 2 H2
However, the dry reforming process produces a mixture of CO and H2 and can thus not be operated flexibly to adjust the H2/CO ratio. Furthermore, only one mole of CO2 can be activated per mole of CH4 used. These issues can be addressed by aiming at the production of only CO and water from the same reactants instead.
CH4 + 3 CO2 → 4 CO+2 H2O
A promising implementation of this net reaction is to combine methane cracking and metal oxide redox reactions in a two-reactor moving bed process. In the ideal embodiment of this process, methane is first cracked to solid carbon and hydrogen on an iron substrate in the first reactor. The produced hydrogen is used to reduce an iron oxide material to the aforementioned iron substrate, producing H2O. The iron and the carbon deposited on its surface are then exposed in a second reactor to CO2, yielding a CO/CO2 product gas mixture and regenerating the iron oxide. Here, we report on the gas production and operating parameters of this process in a one-reactor laboratory-scale moving bed, in which this two-reactor concept is emulated.
In this study, we efforted to CCU that converse from CO2 to CO using the reverse cycle chemical looping process. Reaction rate by TGA was calculated, and product gas was analyzed by gas chromatography in FBFR.In addition, oxygen carrier before and after use were analyzed by XRD and SEM, and changes in composition and surface observed. At a reaction temperature of 1123 K or more, it was observed by XRD that the calcium ferrite structure was changed.Forthermore, even if redox reaction were performed multiple cycles, there was no difference in reaction rate in any cycles. At reaction temperature of 1023 K or less, calcium ferrite changes mixure of CaCO3 and Fe3O4. At any tempture, used calcium ferrite surface has been changed to be rough when observed by SEM, so that, surface area is expected to increase.
Two major problems that arise along with economic and population growth in the World are the increase of energy consumption and amount of plastic waste. Recently, crude oil is used as one of energy sources. However, it is not renewable energy. Therefore, alternative renewable energy sources such as plastic waste are needed. Plastic waste is composed of long-chain hydrocarbon compound, so it could to be converted into liquid fuels via catalytic pyrolysis method. Catalytic pyrolysis is the process decomposition of organic materials using catalyst in the absence of oxygen. The plastic waste used in this research is Polypropylene (PP). Fuel generated from PP plastic pyrolysis process, is expected to increase energy supply and reduce the amount of plastic waste in the World, especially in Indonesia. The purpose of this research is to study the catalytic pyrolysis of PP plastic and to refine products of catalytic pyrolysis of PP plastic with distillation methode. And also to study the effect of temperature on the liquid fuels, the effect of adding the catalyst material zeolite on the products of PP plastics pyrolysis and determine physical and chemical properties liquid fuels of pyrolysis products before and after distillation. Pyrolysis is operated at a temperature of 350OC and 450OC during 60 minutes and using natural zeolite catalysts 60 and 100 mesh.
The GC-MS analysis showed that the pyrolysis liquid oils from all samples mainly consisted of hydrocarbon compounds such as C5H12 and C8H16. The produced liquid oils have density, viscosity and high heating values (HHV) of 0.78 - 0.79 g/ml, 2.15 – 2.52 and 10540 – 10650 cal/g respectively, which are similar to conventional gasoline. The liquid oil has potential to be used as an alternative source of energy or fuel production
It is well known that ammonia is produced through a catalytic reaction at high temperature and pressure from pure nitrogen and hydrogen. This catalytic chemical process is a massive and high energy-consuming process, but a very important one for nitrogen fixation.
Here, we show a non-catalyzed one-step synthesis of ammonia from atmospheric air (nitrogen source) and water (hydrogen source), based on an interfacial reaction between the air plasma gas phase and the water phase, at 25 °C and atmospheric pressure. In the plasma/liquid interfacial reaction (P/L reaction), atomic nitrogen, which is produced through electro-discharge, abstracts hydrogen from the H2O molecules on water phase surface at the P/L interface, and then NH is produced without any catalyst. Formed NH is reduced further in the water, affording NH3, which then dissolves in the water phase.
In the presentation, the mechanism of this reaction was considered from the relationship between the quantitative result of atomic nitrogen and the amount of ammonia production by the P/L reaction. In addition, we have obtained results suggesting that the yield of P/L reaction is greatly improved by devising the discharger to produce excited nitrogen more efficiently.
The PL reaction doesn't require hydrogen gas, and the only sources are nitrogen and water. It is a reaction system at normal temperature and normal pressure, and all that is required is electricity. Therefore, it is considered to be suitable for small-scale ammonia water production regardless of the production site.