
Biomass waste is a renewable source for utilizing to generate energy products, particularly in Thailand, large amounts of biomass wastes are generated every day. Moreover, the torrefied product from biomass wastes is regarded as candidates instead of coal. However, the energy yields of the torrefied product lost so much during torrefaction. Therefore, it is essential to develop a method to increase the energy yield of the torrefied product during the torrefaction of biomass wastes. In this study, Leucaena, which is woody biomass, was pressurised at around 70 MPa at room temperature, called cold press in this work, to prepare biomass pellet. Leucaena was also pre-treated under the mechanical pressure of around 70 MPa at the temperature range of 25oC to around 250oC, called hot press in this work. Then, both cold press and hot press pellet were subjected to torrefaction process at 260, 280 and 300oC. It was found that the char yield of cold press pellet was 20.7 wt%, while the char yield of powder leucaena was only 19.2 wt%. On the other hand, the char yield of the hot press was surprisingly increased to 28.1 wt%. The energy yield of the hot press pellet torrefied at 300 oC was as high as 96.7%, while the energy yield of the cold press and powder leucaena were 89.1% and 89.5%, respectively. From the detail analyses of gas formation during the pyrolysis in TGA, it was found that the dehydration reaction was accelerated by hot press carbonization and the mechanical pressure suppresses the evolution of tar components. Therefore, the mechanical pressure is judged to be effective to increase the energy yield through the torrefaction of biomass.
Biomass waste is a renewable sources and its effective utilization is indispensable, particularly in Thailand, where massive amounts of biomass wastes are generated. Moreover, charcoal from biomass wastes is regarded as candidates for low priced raw materials for activated carbons. However, the yield of charcoal from biomass wastes is low in general. So, it is essential to develop a method to increase the yield of charcoal. In this study, Leucaena, which is a woody biomass, was pressurized at around 500 MPa at room temperature, called cold press in this work, to prepare biomass pellet. Leucaena was torrefied under the mechanical pressure of around 10 MPa at the temperature range of 25 °C to around 250 °C, called hot press in this work. Moreover, Leucaena was also torrfied under gas pressure at 250 °C in a tube-bomb reactor, and was called gas pressure sample in this study. Then, the cold press pellet, hot press pellet, and gas pressure sample were subjected to carbonization at around 900 °C by using a TGA. It was found that the char at 900 °C yield of the cold press pellet was 20.7 wt%, while the char yield of powder leucaena was only 19.2 wt%. On the other hand, the char yield of the hot press pellet was surprisingly increased to 28.1 wt%. The char yield at 900 °C of the gas pressure sample was increased to 23.1 wt%. From the detail analyses of gas formation during the carbonization, it was found that the dehydration reaction was accelerated by torrefaction under mechanical pressure and the mechanical pressure suppresses the evolution of tar components. Therefore, the mechanical pressure is judged to be effective to increase the char yield through the carbonization of biomass.
I. Introduction
Carbon neutral bio-oil is needed to replace liquid fossil fuels in order to reduce CO2 emissions and global warming. However, bio-oil produced by pyrolysis of biomass has a high oxygen content (e.g.sugars), low energy density, and is also expensive to produce. Based on previous research results it was shown that inexpensive Ni, Fe catalyst clusters can improve the quantity or quality of bio-oil produced from the biomass, but to our knowledge, very few studies have actually investigated the mixing cellulose (biomass model compound) and catalyst directly together followed by pyrolysis to produce bio-oil. In this study, Ni2Fe3 catalyst was mixed with cellulose in five different ratios in a pyrolysis batch reactor to investigate its impact on the bio-oil yield and sugar content.
Experiment
The catalyst was prepared by sol-gel method and heat-treated in flowing H2/N2 gas. Pyrolysis experiments were conducted using a fixed bed reactor, flowing 100ml/min N2 gas, at 450 °C with five different catalyst/cellulose ratios, where cellulose mass was fixed at 4g, with the addition of catalyst of 1g, 2g, 4g, 8g, and 16g.
II. Results & Discussion
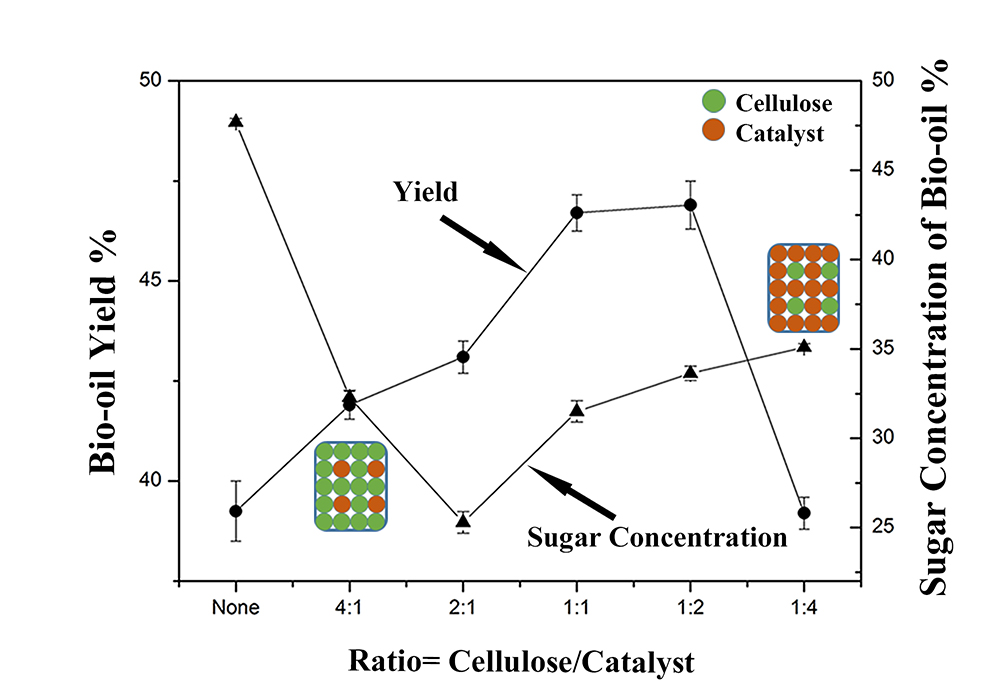
In Figure 1, bio-oil yield and sugar concentration measured by GC-MC is plotted versus cellulose/catalyst ratios, the yield increase as the catalyst is added initially and then decrease. The sugar content decreased initially as the catalyst is added and then increased slightly. The reason is that when the cellulose ratio is low, the metal catalyst can help transfer heat uniformly and provide sufficient reactive surface (figure inset). However, when the catalyst ratio is high, the catalyst has little physical contact with the cellulose, which auto-aggregates at high temperature. A high catalyst ratio not only decreases the yield of bio-oil but influences the overall catalyst acitivity. Research work is continuing on evaluating the catalyst reusability.
Recently, many biomass gasification plants are operated as local power plants. However, the management relies on FIT because the energy is the cheapest product. To establish the economically reasonable technology for biomass conversion, it is required to develop a new scheme for producing valuable chemicals selectively with simple operation. To realize it, we propose a multi-stage pyrolysis with instant selective separation scheme. Utilizing the difference in the decomposition temperatures of main biomass three components, hemi-cellulose, cellulose, and lignin, biomass was pyrolyzed sequentially at three step optimal temperatures for recovering volatiles selectively in vapour phase. Thus produced volatiles, which contained saccharides derivatives, were further converted into valuable chemicals by installed second higher-temperature zone in each stage pyrolyzer. In this study, the effective pyrolysis conditions for the selective valuable chemicals production were examined using pulverised cypress to clarify the validity of proposed method.
As the first step, pyrolysis was conducted at 300 °C for the selective decomposition of hemicellulose into saccharides derivative volatile. Thus produced volatile was immediately heated up to 650 °C, and furan was selectively obtained at the yield of 0.623 g/g-(hemicellulose in biomass). As the second step, the obtained char was subsequently pyrolyzed at 370 °C. Through the second-step pyrolysis, cellulose was selectively decomposed and the anhydrosugar of levoglucosan was obtained as the product. The second-step pyrolysis avoided lignin decomposition, which resulted in avoiding undesirable heavy tar production. The remaining solid product, char, in the second step pyrolysis can be used high calorific carbon materials for power plant. Thus, the validity of the proposed scheme for selective co-production of valuable chemicals and energy was clarified.
<Gasification is known as one of the most promising processes for utilization of coal and biomass, e.g. for production of fuels and chemicals. During the gasification, besides gases, a number of volatile hydrocarbons are discharged together with tar. Tar is a complex mixture of components, typically poly-aromatic. How to deal with the viscous tar is one of the major issues in the biomass/coal gasification process since it can cause quite a few problems in the different applications.
A large number of catalysts have been researched to eliminate tar in gas products. The two most catalysts are Ni-based catalysts and dolomites. When Ni-based catalysts are used, tar concentration in the product gas can be reduced significantly by means of reforming but quite rapid catalyst deactivation (both by coke fouling on Ni catalyst surface and by sintering). Dolomite, a kind of inexpensive and abundant natural mineral, is very attractive as a tar cracking catalyst and it was found to be effective on coking resistance during tar steam reforming.
In this study, tar was treated in a two-step reactor, where catalyst sample was filled in the secondary step. Tar gasification using calcined dolomite was discussed at different temperatures and various introduction gases. The combined use of dolomite and nickel-based catalyst was also compared. Gas yield and carbon deposit formations were determined. Additionally, changes of the catalyst pore feature, surface area were analyzed.>