
There is still a large demand for advanced combustion technologies for the stable production of power and electricity in our societies. Recently, numerical simulations have made outstanding achievements, and the number of research projects with Computational Fluid Dynamics remarkably increases with developing a computer performance. Under such situations, flamelet based tabulated chemistry has received much attention due to its high accuracy and relatively a low computational cost. In this work, using this approach based on a non-premixed flame, piloted methane/air jet flame was simulated by large eddy simulation. The numerical solutions showed good agreements to experimental data of temperature and major chemical species. Although the numerical solutions of temperature slightly overestimated the experimental data in the downstream, this difference was also reasonable because the effect of radiation was not included in the present simulation. Flame index, which is the indicator of local combustion mode, suggested the coexistence of premixed and non-premixed regions in the computational domain. Specifically, a premixed region was located in the inner side of the piloted jet while the combustion mode in the outer side of the piloted jet was non-premixed mode. To evaluate the influence of the flame type which is calculated prior to the main simulation, the look-up table based on premixed flame was constructed and used in an additional simulation. The detailed data obtained from the above simulations provided reliable insights for understanding the transport phenomena and chemical reactions of the chemical species in turbulent jet flames.
The combustion and heat release characteristics of hydrogen-methane/air (H2-CH4) diffusion flames on micro-jet array burner are focused in this research. The effects of H2-CH4 co-combustion and equivalence ratio on the flame structures, temperature, free radicals, heat release rate, and NO emission distributions of the H2-CH4/air diffusion flames are numerically investigated. The results show that the simulation results of OH distribution and flame length based on GRI 3.0 and Bilger mechanism are agreed well with the experimental results, showing an acceptable and repeatable results of H2-CH4 combustion based on those mechanisms. Furthermore, uniform and small flames can be formed at various H2-CH4 ratios. The intensity and distributions of heat release rate can be adjusted by H2-CH4 ratios and equivalence ratios. Finally, the dramatically enhancement of NO emissions at CH4 addition conditions are considered from the promotion of prompt NOx when CH4 added to the H2 flame.
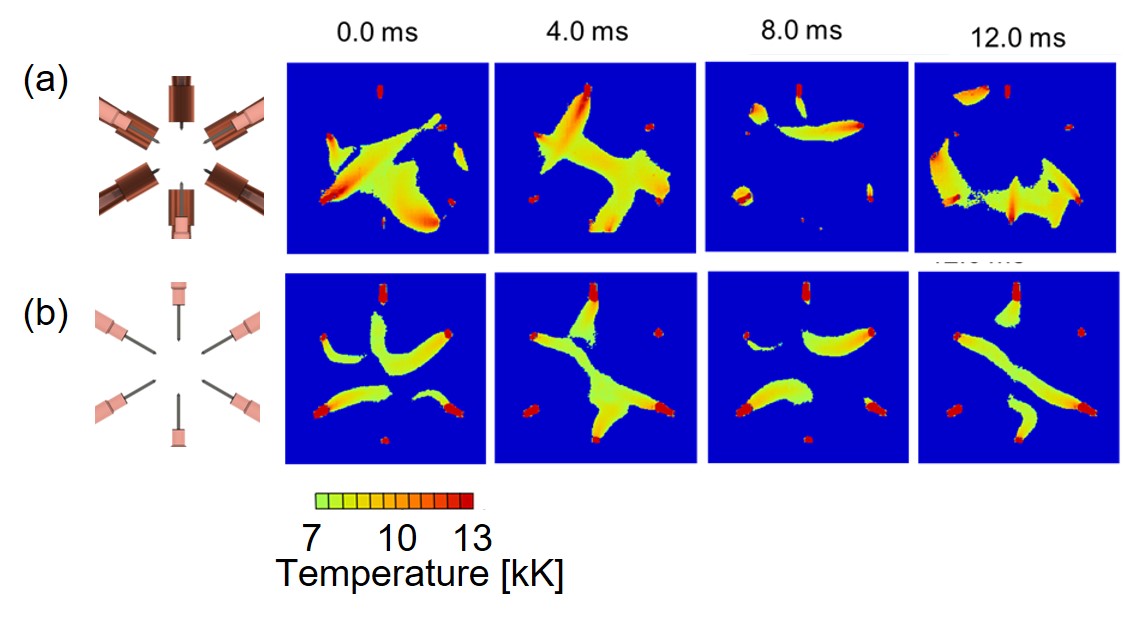
An innovative multiphase AC arc (MPA) was drastically improved by diode rectification technique with bipolar electrodes. Temperature fields of the diode-rectified MPA (DRMPA) were successfully visualized on the basis of a high-speed camera with appropriate band-pass filters. The DRMPA had been developed to solve the electrode erosion issue, which was critical issue for MPA to be utilized in industrial fields. Diode-separation of AC electrode into a pair of cathode and anode led to drastic improvement of the electrode erosion characteristics. However, fundamental phenomena in the DRMPA are still poorly understood because of its novelty, although spatiotemporal characteristics of the arc and/or electrode temperature are necessary to develop the DRMPA for industrial applications. The purpose of the present study is to investigate temperature fluctuation characteristics of the DRMPA. Six phase AC arcs with and without diode-rectification were generated and their temperatures were measured by the high-speed camera system with appropriate band-pass filters on the basis of Boltzmann plot method. Figure shows electrode configurations and the two-dimensional temperature field of the DRMPA (a) and the conventional MPA (b)during one AC period of 60Hz. Results indicated that the arc temperatures of both DRMPA and MPA were fluctuated in the range from 7,000 to 13,000 K. The arc temperature near the electrode was higher than 10,000 K, while the temperature in the center region in the furnace was about 7,000 K. These obtained results suggest that the DRMPA is a promising heat source to fabricate attractive nanomaterials at a high productivity.
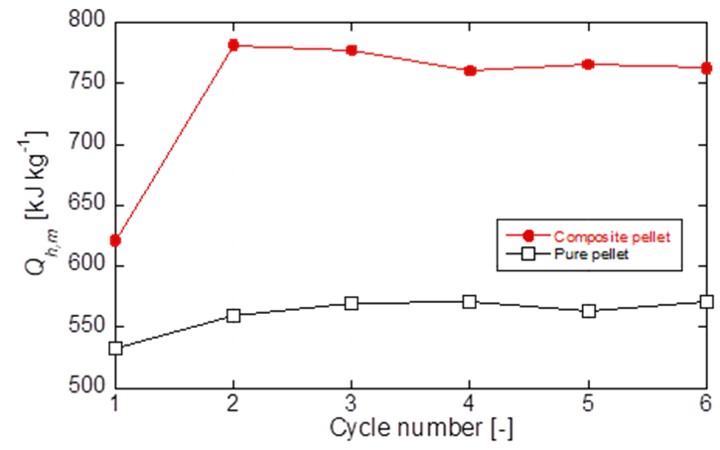
Thermochemical energy storage (TCES) has grown more attention in energy storage and conversion field, for its benefits in better reversibility, lower cost and safer chemical compounds, and higher energy releasing and storage density than conventional sensible and latent heat storage system among thermal energy storage systems. Calcium oxide/water/calcium hydroxide (CaO/H2O/Ca(OH)2) TCES system adapts for heat storage and releasing potential at 400 °C to 600 °C. Calcium carbonate (CaCO3) is used as a precursor for the TCES. However, the raw CaCO3 material from nature shows insufficient performance during heat storage capability and durability experiments. A composite material impregnated with silicon-related material of 0.6 wt% was developed and demonstrated superior reactivity and durability on repetitive operations than a conventional material studied previously. Instead of powder figure TCES materials, real reactors favor materials in pellet figure, then, the composite material in the pellet figure was developed and discussed kinetically TCES material performance in this study. The developed composite pellet material was evaluated by thermogravimetric analysis. The composite pellet was compared with a pure CaCO3 reference pellet on both hydration and dehydration processes. The heat output and storage density and their reaction rates of both materials were compared and discussed under the same operating condition. According to 6 cycles experiment, the composite pellet maintained stable reaction conversion and showed an increase of the hydration reaction rate. It was demonstrated that the additives for the composite had the positive effect and heat output density, Qh,m [kJ kg-material-1], of the composite pellet increased 32.7% in comparison with the pure pellet as shown in Fig. 1. Morphology change of samples before and after experiments indicated that the vapor diffusion and heat transfer were guaranteed by the additives which performed well as effective agglomeration prevention.
About 60% of the energy input in the chemical industry is discarded from the plant. Energy saving can be achieved in the entire plant by recovering these waste heats and reusing them as power and heat sources in the power plant. An adsorption heat pump has been developed for the purpose of regeneration of such unused energy.
In this study, saturated humid air was supplied to a device packed with 13X zeolite particles of 4 mm in diameter. The time variation of temperature in the apparatus was measured experimentally. Then, the maximum temperature was estimated from the relationship between heat balance and adsorption equilibrium. The trend of the maximum temperature calculated from the heat balance is consistent with experiment. Further, it was found from the result of the heat balance equation that the sensible heat of the moist air supplied and the heat of adsorption of the zeolite are mainly distributed to the sensible heat of the zeolite. In the future, it is important to make effective use of the sensible heat of this zeolite. In order to extract more thermal energy from the device, it is necessary to improve the heat transfer between the packed bed and medium.
A double pipe heat exchanger having a zeolite packed bed on the annular side was proposed as an apparatus. Flow direction of the humid air supplied to device was changed in two different ways. The one of them is supplying humid air vertically to the device and another is supplying the air in parallel. The influence of flow direction on heat transfer between packed bed and medium is studied with numerical simulation.