
The challenge for applications of viruses, virus-like particles, or other extra cellular bionanoparticles in gene-therapy or in cancer therapy is the high dose with up to 1014 particles per dose. Downstream processing is one bottleneck in the production of these bionanoparticles. It has been often overlooked that a heterogenous population of bionanoparticles is present and they must be separated from the product. The heterogeneous populations of bionanoparticles are released from the cells, carrying different host cell proteins, DNA and RNA fragments. Additionally, cells produce extracellular vesicles (microvesicles and exosomes) with similar size and surface properties. The biological activity of these different particle populations is not fully understood. Particle characterization and biological activity assays require the separation of these populations with high purity. Here we compare the results obtained for the purification, as well as particle populations' separation, of HIV-1 gag VLPs produced in CHO cell culture using different downstream processing approaches. These approaches include polymer grafted anion exchangers, monoliths (anion exchange and hydrophobic interaction), a combination of flow-through and heparin affinity chromatography, and membrane adsorbers. Several analytical tools including Nanoparticle Tracking Analysis and Mass Spectrometry were used for VLP characterization. An outlook will be given how continuous ultracentrifuges will complement these purification approaches.
PEGylation is a well-known technique to develop biopharmaceuticals, such as proteins and DNAs. It can increase the hydrodynamic radius to reduce their kidney excretion and prolong their in vivo half-life. On the other hand, the control of PEGylation reaction is difficult because of the formation of heterogeneous mixtures of target PEGylated product, its isomers, unreacted proteins and PEG reagents. As the differences in the sizes and the surface properties such as charge and hydrophobicity of the isomers are sometimes small, several purification steps are always combined to obtain the target product from the reaction mixture. Among the purification method, ion exchange chromatography has an advantage for the separation of isomers on the basis of their difference in size and charge with a single operation. However the separation mechanism is not fully understood. In this study, synthetic PEGylated DNAs were used as a model of well-defined structure isomers to investigate the charge shielding effect of PEG on the charged biomolecules. Poly T possessing 9 to 95 thymine bases, an amine group for conjugation and middle base was reacted with NHS-activated PEG reagents. The retention factors on anionic exchange chromatography, Q sepharose HP and QA monolith, were determined by liner salt gradient experiment. Elution salt concentration of PEGylated DNA was decreased depending on the molecular weight of PEG. However, the decrease was not pronounced when the number of bases increased. On the other hand, the number of binding site was practically unaffected by the PEGylation which means that the conjugated PEG chain did not disturb the interaction of DNA with cationic ligand while the interaction was weakened by the conjugated chain.
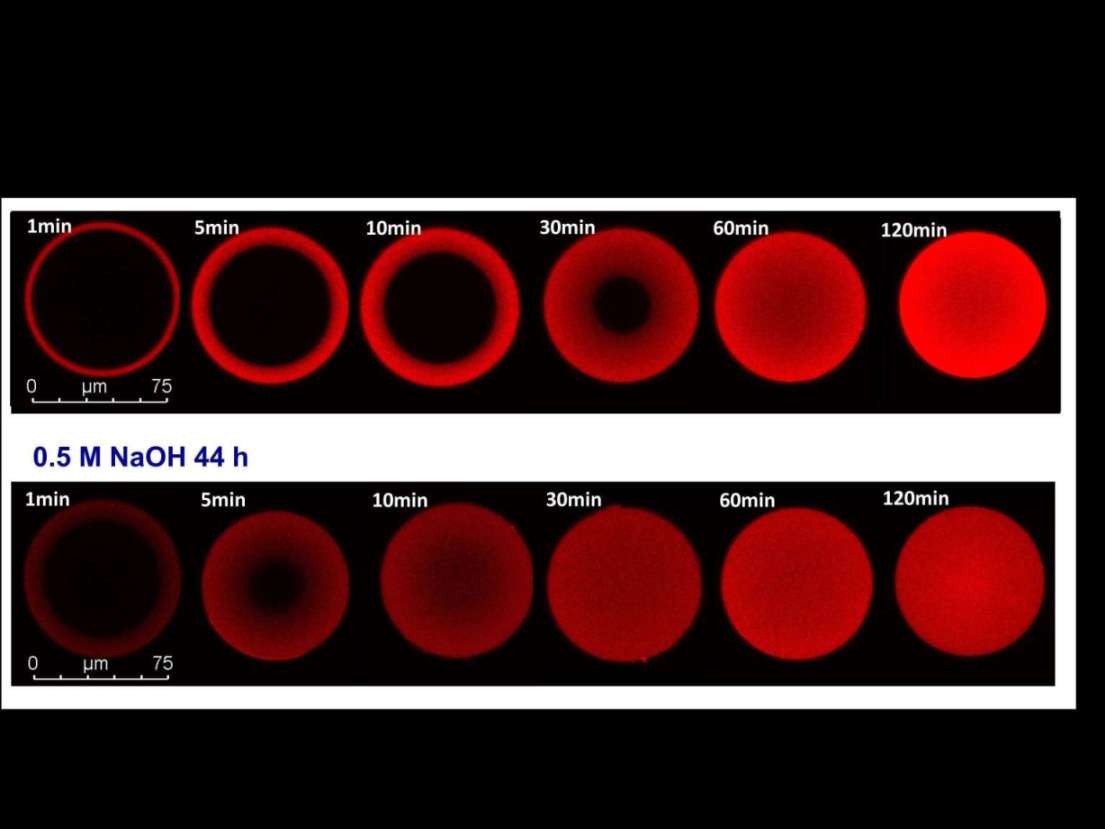
Protein A affinity chromatography is still the preferred option for antibody capture. In the past years, significant improvements have been made with respect to alkaline stability of the recombinant Protein A ligands, with resistance to 1 M NaOH over extended time periods as the latest milestone. We have investigated how exposure to alkaline conditions can alter antibody binding kinetics of Protein A resins. Break-through curves of fresh resins and resins after different time periods of alkaline exposure were compared using mAb and polyclonal IgG. As expected, equilibrium binding capacity dropped to some degree at long incubation times. In contrast, mass transfer of IgG was enhanced which was reflected by steeper break-through curves. Fitting of the break-through profiles with a pore diffusion model revealed effective pore diffusion coefficients which were increased by a factor of 2-3. Adsorption isotherm measurements showed that even after alkaline exposure, typical highly favorable isotherms were obtained, albeit with association constants that were reduced to some extent. Based on these altered binding properties we could identify a narrow window of specific operating conditions, where the alkaline treatment eventually led to an increase of the dynamic binding capacity. These conditions varied for different resins and strongly depended on the NaOH concentration and incubation time. Confocal laser scanning microscopy measurement showed that the accelerated mass transfer involved a transition from a shrinking core behavior with sharp fronts to a situation with more diffuse profiles typical for solid diffusion or pore diffusion with lower binding strength. Linear pH gradient elution studies showed that the desorption behavior was not changed significantly, as pH values at the peak maximum were almost the same as for fresh resin. The results of this study will contribute to a deeper understanding of phenomena associated with performance change of affinity media upon alkaline regeneration.
Polyacrylonitrile (PAN) nanofibrous membrane was prepared by electro-spinning technique. After heat treatment, alkaline hydrolysis and neutralization reaction, the ion exchange membrane (namely P-COOH) was further chemically grafted with chitosan molecule. The obtained P-Chitosan membrane (namely P-CS) was then covalently immobilized with Procion orange MX-2R dye from simulated textile wastewater to be used as a dye-ligand membrane. Fiber diameter, porosity, pore size, immobilized dye-ligand concentration, and protein binding capacity were characterized. Furthermore, the membrane was applied to evaluate the binding capacity of lysozyme under various operating parameters (e.g., pH, chitosan mass per volume ratio, immobilized dye concentration, ionic strength, and temperature) in batch mode. The experimental results were directly applied to purify lysozyme from chicken egg white by newly designed membrane chromatography module. The results showed that the recovery yield and purification factor were 98.9 % and 56.9-fold, respectively, in a single step. The binding capacity remained consistent after five repeated cycles of adsorption-desorption operations. This work demonstrates that the dye-ligand nanofibrous membrane holds great potential for purification of lysozyme from real feedstock.
Protein precipitation by polyethylenglycol is a simple, robust and cost effective first capture step for purification. The resulting structure of the precipitate particles has a significant impact on process design and process performance like recovery and dissolution kinetics. Current technologies for visualizing and reconstructing 3D precipitate structures are either very time consuming and expensive (Cryo-TEM) or generate a single average value (a fractal dimension) for a population of precipitate particles (light scattering). We developed a new method using light microscopy to reconstruct the complete three dimensional structure of individual precipitate particles with a resolution of 0.1-0.2 μm. This methods was then used to reconstruct and characterize the three-dimensional structure of particles generated by batch precipitation by PEG as well as continuous precipitation in different shear stress environments. Both methods, batch and continuous precipitation, generate particle structures of very diverse nature, while the average fractal dimension is significantly different between the two (2.40 for batch in low shear environment and 2.52 for continuous in high shear environment). Besides the overall average, when investigating both populations in detail, the distributions of fractal dimensions of individual particles overlap significantly. We could also confirm that the generation of PEG-precipitated particles is diffusion limited, as simulations predict a fractal dimension of 2.4 for diffusion limited precipitation and 1.7 for reaction limited precipitation. A close inspection of the 3D structure of the precipitate also shows monofractality of the particles from micro to nano scale visualized by light microscopy. We showed that the fractal dimension and 3D reconstruction is a valuable tool for characterization of different shear stress conditions and mode of operation. The current switch from batch manufacturing to continuous manufacturing has to take the 3D structure and population of different protein precipitates into account in their design, engineering and scale up.
Ion exchange chromatography is a widely used method for purification in all types of biomolecules in current biotechnological downstream processes. Knowledge on the binding behavior of proteins provides valuable insight for understanding the molecular mechanisms of protein interactions in a biological context. However, thermodynamic parameters such as enthalpy and entropy changes accompanied by protein adsorption and desorption are still unknown in ion exchange chromatography. This work relates the change in molar adsorption enthalpy, Δh0, with the salt concentration needed to elute a model protein with a gradient elution, IR. Bovine serum albumin (BSA) was adsorbed on four anion exchanger solid phases possessing grafted ligands (Toyopearl GigaCap Q-650M, Toyopearl SuperQ-650M, Toyopearl Q-600C AR, Q Sepharose XL). A linear relation was observed between the Δh0 and IR. From the highest Δh0 to the most negative: Toyopearl SuperQ > Q Sepharose XL > Toyopearl Gigacap Q-650M > Toyopearl Q-600C AR. The same experiments were also done with a non-grafted conventional solid phase, Q Sepharose FF. However, it did not fit in the same trend due to the ligand disposition. Δh0 was found to be endothermic for Q Sepharose FF and Toyopearl SuperQ-650M and exothermic for the other solid phases. Endothermic Δh0 indicate that entropic effects play a major role on the adsorption of BSA on these two solid phases.
Flexible adaptation to constantly changing market demand is getting increasingly important in the manufacturing workflow of biopharmaceuticals. Moreover, fast development times and reliable scale-up are required and are key to success.
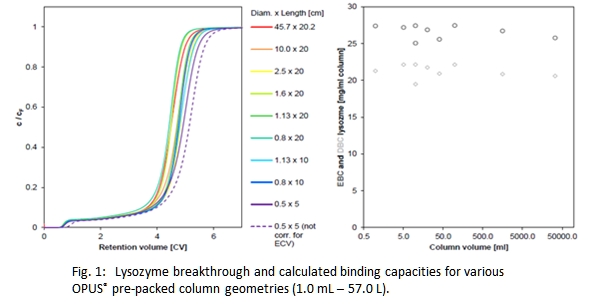
Pre-packed chromatography columns have been commonly adapted in industry during the recent years and allow to reach these requirements, however data showing scalability throughout various column sizes applied in early stage process development up to commercial manufacturing has been not available so far. This presentation confirms the scalability of column performance throughout Repligen's OPUS® pre-packed column line including MiniChrom, ValiChrom and OPUS® Process Scale columns covering a range of 0.5 to 60 cm inner diameter.
Scalability has been demonstrated for various resin types and functionalities and has been proven for both column packing performance and protein separation under isocratic conditions.
Experimental data on OPUS® packing performance has been compared to traditional self-packed columns and found at least equal if not better.
Furthermore a statistical data analysis using advanced software tools has been performed for more than 30.000 pre-packed columns supplied into global industry over a time span of 10 years.
Extra-column effects can have significant influence on separation performance. These effects are caused by dead-volumes, valves, column hardware and tubings. For small scale chromatography systems, and especially with very small columns, the extra-column band broadening can be dominant over the actual broadening of the column that is used for separation. This issue has long been recognized and has been investigated extensively by many researchers. However, though it is often overlooked, also for preparative and pilot scale chromatography extra-column effects can be an important factor. This is particularly valid, if the column size is relatively small. We have investigated the Aekta Pilot system volume contribution to band-broadening by non-binding tracer experiments on columns of various sizes using columns packed by axial compression to ensure consistent packing performance. Additionally, the influence of non-standard mixing behavior occurring in the bubble trap during step elution was investigated using a simplified model of interconnected reactors. Other flow parts of the chromatography system were modelled by dispersed plug flow reactors and CSTRs for dead zones, e.g. column in- and outlet and mixers. Essentially, the effective mixing volume of the bubble trap is affected by the flow rate and the density difference of the two buffers. As a consequence, the influence on the separation in the column varies with these parameters. Experimentally, the band broadening effect of the bubble trap on the chromatographic separation was investigated by performing linear gradient elution and step elution experiments using bovine serum albumin at analytical loads. There was no observable band broadening effect in linear gradient elution. However, during step elution the salt transition profile was affected leading to significant peak broadening of the BSA elution profile.