
A preparative high-performance liquid chromatography is an extensively used technique for a purification of commercially important bio macromolecules, such as proteins and polynucleotides. Since process time is becoming more and more critical, fast and effective chromatographic methods are widely required. In this context, target molecule breakthrough point determination is of a great interest for optimization of downstream processing. Ideally, continuous analysis is preferred, such as UV absorbance or fluorescence monitoring, which in some cases however lack sufficient selectivity. An alternative is to perform fast analysis of column outlet fraction via chromatography providing equal or greater selectivity than purification step. This can be done using UPLC that however requires specialized equipment. On the other hand, fast analysis of macromolecules can also be achieved using convective chromatographic media at much lower pressure drop. This opens possibility to combine purification step and chromatographic analytics on a single chromatographic system.
In our case we implemented äKTA Explorer system to test feasibility of proposed approach. On-line analysis of preparative column outflow was performed by sequentially injecting outlet on convection based analytical column operating on the same chromatographic system where target molecules were analyzed. Cationic and/or anionic exchangers were used as chromatographic supports (along with selective protein A membrane), depending on feed sample and its characteristics. Three different case studies were tested: monoclonal antibodies purification, aggregate content and plasmid DNA (pDNA). To adjust limit of detection an algorithm varying number of injections was used. This enabled accurate monitoring of an early breakthrough for concentration below 1%. Due to its simplicity and flexibility such methodology can easily be adopted also in pharmaceutical environments.
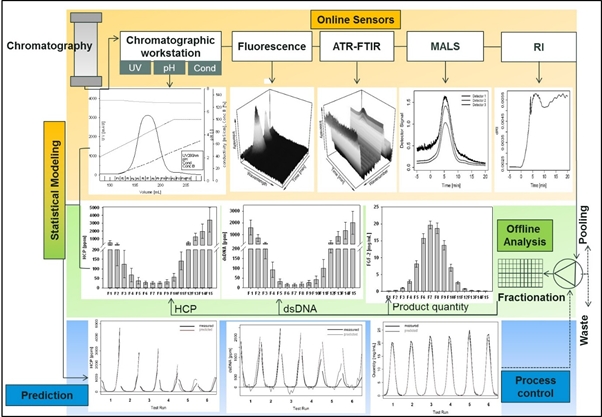
To enhance product quality and process robustness in biomanuacturing regulatory agencies have encouraged the implementation of process analytical technology (PAT). This combination of process understanding and control with real-time monitoring of quality and performance attributes also increases process efficiency and productivity. Currently, process performance is monitored by laborious and time-delayed offline analysis after each process step. This conventional approach increases hold times and overall process duration as well as the risk for batch failure. We have equipped a commercial chromatographic workstation with additional online sensors based on multi-angle light scattering, refractive index, attenuated total reflection Fourier-transform infrared, and fluorescence spectroscopy. The combination of these derived online data enables the prediction of product quantity and various purity attributes such as high molecular weight impurities, HCP and dsDNA content simultaneously during the elution phase of a chromatographic purification step. Such predictive models based solely on online data have been established for capture, intermediate and polishing steps of a monoclonal antibody produced in CHO and for fibroblast growth factor 2 overexpressed in E. coli. Online signals and corresponding offline data for product quantity and co-eluting impurities were analyzed by the statistical tools partial least square regression and boosted structured additive regression. Also chromatographic runs with varied process parameters were conducted to determine the predictive power of the models. The established methodology using complimentary online sensors enables the prediction of product quality and process performance attributes simultaneously in biopharmaceutical purification processes within a feedback time below 5 sec. The application of such models allows online pooling decisions and decreases the number of off-line analysis and hold times significantly. The costs for in-process analytics are lowered and the risk for batch failure is reduced. These findings are fundamental for the successful implementation of continuous manufacturing and real-time release in biomanufacturing.
There is growing interest in mAb processing to improve individual unit operation performance and overall process train efficiency through linked processing. In the downstream process, several types of chromatography are typically performed to achieve the target of purity of therapeutic mAb. AEX chromatography is commonly operated under product flow-through conditions to bind negatively charged impurities such as HCP and virus. CEX chromatography focuses to separate the mAb related impurity. Ichihara et al., (mAbs J. 10-2, 325-334, 2018) reported the operating CEX chromatography in the flow-through mode for mAb aggregate removal. To intensified flow-through chromatography, controlling the feed concentration through single pass TFF (SPTFF) is one of the ideas to get the higher loadings from the isotherm binding condition, especially for AEX column. The use of SPTFF technology can help facilitate these process intensification efforts by modifying process intermediate volumes and concentrations without the need for recirculation. However, the impact of concentration change on other chromatography is not evaluated well on the fully combined in-series operation.
In this work, SPTFF by Pellicon® 3 30kD is used prior to a flow-through chromatography polishing step to boost chromatography resin loading. Then, Eshmuno® CP-FT resin was challenged with various concentrations of mAb feed and evaluated the difference of impurity removal. And results compared the performance of previously reported SPTFF-AEX results (Merck Application Note, AN5364EN00 2017), and evaluate the impact of connected flow-through polishing on feed concentration.
The biopharmaceutical downstream process is designed to achieve high product yield with efficient impurity removal. “Process derived impurities” such as host cell proteins (HCPs), lipid, DNA and fermentation ingredients and “Product related impurities” like aggregates and fragments should be reduced to meet each specification of drugs for quality and safely.
Economical pressure on biopharmaceuticals, especially monoclonal antibodies (mAb), has motivated to develop high capacity chromatography resins and new purification technologies including periodic counter-current chromatography and multi cycle batch chromatography using smaller particle size resins with high flow rate. Thus, load amount onto chromatography resins are tend to increase.
Higher amount loading of not only target proteins but also impurities on chromatography resins affect selectivity and life time of chromatography resins. Increased risk of fouling on chromatography resins by accumulation of impurities should be mitigated as much as possible to minimize burdens on each step to develop stable process with lower COGs.
We developed new impurity reduction method using co-precipitation of impurities without loss of target proteins. The method reduced HCPs and DNA in harvested cell culture fluid expressing mAb one third and one thousandth respectively. We also evaluated the effect on the quality of eluate from protein A chromatography and other benefits on the process.
1. Introduction
There is increased interest in continuous processing for monoclonal antibody (mAb) purification in bind and elute mode. This is because such continuous chromatography offers higher productivity and cost effectiveness than conventional batch mode. JSR developed and conducted a DoE for continuous chromatography process of mAb in bind and elute mode using a high-capacity Protein A resin, Amsphere™ A3.
2. Experimental
The mAb breakthrough was monitored by HPLC. The concentration of Protein A purified mAb was determined from the measured absorbance at 280 nm. Host cell protein (HCP) was quantified by CHO HCP ELISA kit, 3G (Cygnus Technologies). A harvested cell culture fluid (HCCF) contains IgG1 subclass mAb.
A three factor and three level DoE was designed using Minitab (Minitab, Inc.) to determine the impact of purification conditions on productivity (g/L/hr) and HCP concentration. The tested factors were IgG-loading, residence time for washing step and washing volume.
3. Results and discussion
Productivity of the continuous process with Amsphere A3 was calculated on various purification conditions. The results showed that the process time for sample loading and washing steps have significant effect on the productivity. The total amount of HCP in the eluates showed a positive correlation with the sample loading amount, especially when the flow rate for the loading step was relatively faster. That is, washing conditions have smaller effect on the HCP clearance performance with shorter residence time of sample loading step.
4. Conclusions
Amsphere A3 showed high productivity with continuous process because of its high DBC even for high linear flow velocity. Also, we found that the HCP clearance performance is independent from the residence time for washing step. This indicates that higher productivity and good HCP clearance can be achieved together by increasing flow rate and decreasing process time of washing step.
The development of efficient downstream processing plays an importance in therapeutic antibodies (mAb) purification. As an example, a continuous multicolumn chromatography technology in ProteinA capturing step has been attracted attention from biopharmaceutical companies. Recently an integrated flow-through (FT) process is considered to be one of effective approaches for mAb purification. FT mode has an advantage to increase loading amount of mAb per column volume. Further an integrated FT-FT process technology is expected to achieve much less installed chromatography resins, buffer and tanks to use in comparison with a conventional polishing step consisting of bind/elute (B/E) mode and FT mode. This technology will promise pool-less manufacturing for economical mAb process in near future.
Cellulose based chromatography resins are well-known to be stable to caustic condition for cleaning and less nonspecific adsorption properties. These unique properties are suitable to apply an integrated FT-FT process technology for mAb purification. In the past few years we succeeded in developing a unique cellulose based mixed mode resin, Cellufine MAX IB, which can remove impurities effectively in FT mode for mAb purification under high salt condition. The resin is further tested to use integrated FT-FT mode for mAb purification by examining mAb recovery and impurities removal. In this poster, we report development of efficient mAb polishing process by integrated FT-FT mode which consists of a combination of Cellufine MAX IB resin with other cellulose based cation exchange or hydrophobic chromatography resins.
To accelerate process development and optimization of chromatography, it is essential to use mechanistic models to simulate and predict the implementation of such operation for certain products. The universal prerequisites for chromatography modeling involve adsorption isotherm and mass transfer mechanism, where users can choose the models based on the balance of predictability and calculation ability. Unfortunately, most software packages are still not user-friendly because of complexity of models and input parameters.
In the study, different mechanistic models for various simulation platforms that depend on the same isotherm and mass transfer coefficients were tested for linear gradient elution (LGE) of proteins. The model system was separation of basic proteins such as lysozyme and IgG by ion exchange chromatography (IEC). Our model (Yamamoto model) was used for determining the two parameters (A, B) needed for steric mass action (SMA) isotherm model from LGE experiments. As for mass transfer, lumped kinetic model and linear driving force model (LDF) were used as only one lumped mass transfer coefficient (km) is included, which reduces the computational burden in determining individual effect from axial dispersion and pore diffusion, and such. The km value was determined also from LGE experiments based on Yamamoto LGE-HETP model, which includes the compression factor for the peak sharpening effect in LGE.
By a set of LGE experiment, the major parameters (A, B, HETP) can be obtained, and thus the adsorption and mass transfer models for mechanistic modeling can be constructed. Different simulation platforms have given similar outputs in comparison, which demonstrates that modeling for protein chromatography can be achieved from limited LGE data in different computational tools, albeit the coefficients definition varies in the software.
Reference Yoshimoto, Yamamoto, Simplified methods based on mechanistic models for understanding and designing chromatography processes-Yamamoto Models and Approach-, in Preparative chromatography for separation of proteins, 2017
The changes from stainless steel equipment to single-use solutions as well as switching from batch to continuous operations are currently strong discussed topics in the field of downstream processing. Besides the product yields and the purity grades, the footprint reduction and higher productivities result in decreased cost of goods (CoGs). With these developments, not only economic but also ecological goals will be achieved through buffer and materials savings. In the course of the present evaluation, we compared continuous and single use solutions to conventional platform processes and quantified their economic and ecological impact. In detail, we have compared a cell culture antibody process consisting of conventional broth clarification by centrifugation and batch capture by protein A affinity chromatography with a continuous process consisting of continuous clarification by depth flocculation and continuous precipitation and/or periodic counter current chromatography (PCC). Our results show potential cost saving strategies and how ecological goals and improved costs of goods can be combined. Possible savings of 30 % can be achieved by combining novel technologies and buffer savings of 60 % can be realized by the use of precipitation or PCC. The scenarios were evaluated for an existing facility with a bioreactor scale of 1,000 L as well as for two theoretical facilities scaled for either an orphan drug production of 50 kg/year or a blockbuster production of 1,000 kg/year.