
Formic acid (FA) is considered as a promising liquid organic hydrogen carrier for the fuel cells. FA is well-known compound to transfer into H2/CO2. With its properties, we concerned FA as the source of high-pressure CO2/H2 without any compressing procedures. These high-pressure gases can utilize for not only the high-pressure H2 for FCVs, but also various chemicals as solvents and reagents. In our presentation, we show the development of the continuous production of high-pressure H2/CO2 over 150 MPa, and easy separation from the generated gases under high-pressure conditions.
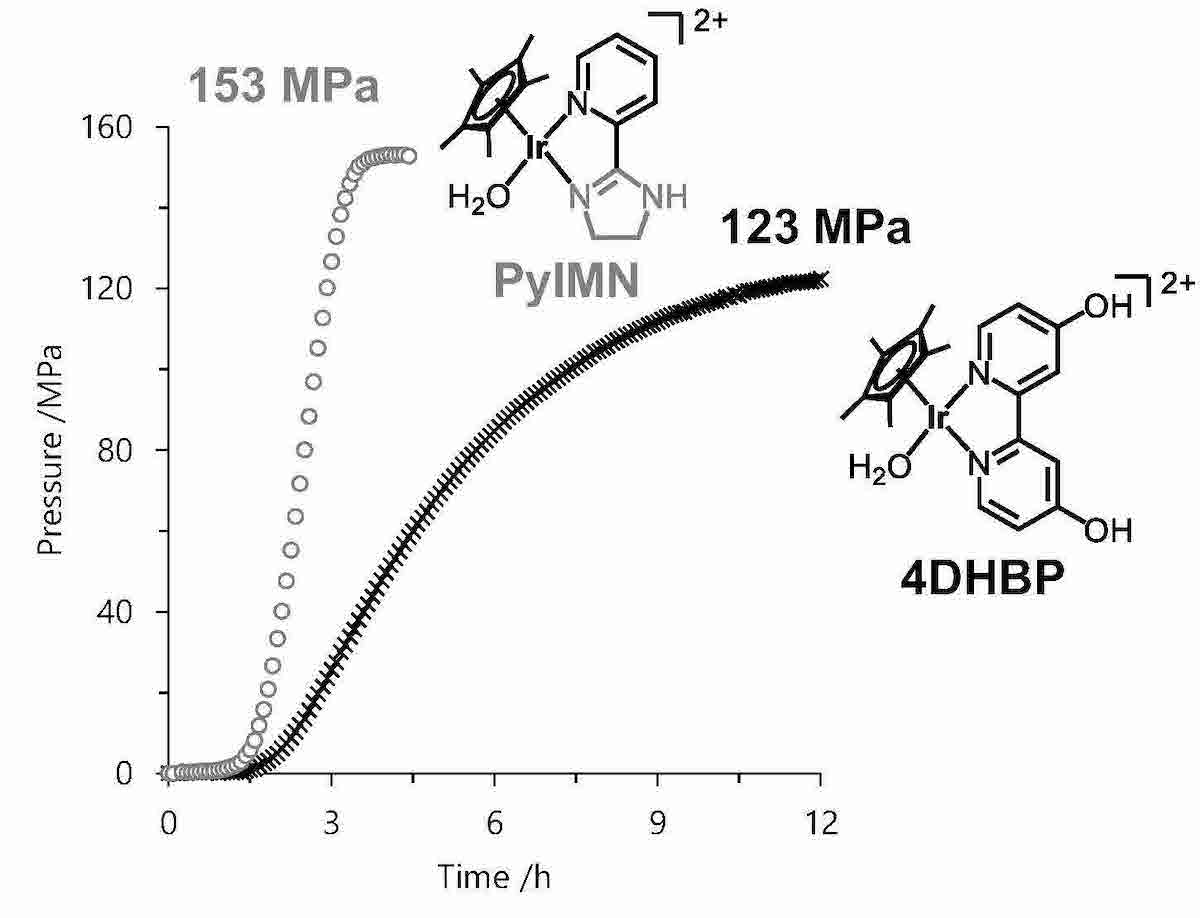
For the development of the high-pressure gas generation by the FA dehydrogenation, we used the Ir complex as a homogeneous catalyst. The catalyst can successfully produce the high-pressure gas with a high rate (TOF = 2500 h-1 at 30 MPa) at the temperature of 80oC. Whereas in the high-pressure conditions, the catalyst was deactivated gradually with decreasing the reaction rate. To overcome this, we developed higher durable catalyst by modification of the ligand, and the modified catalyst can generate high-pressure CO2/H2 for longer time. For the further development, we found that the iridium aqua complex coordinated with a pyridyl-imidazoline ligand showed the higher performances on the FA dehydrogenation at the pressure as high as 153 MPa.
We also demonstrated the high-pressure H2/CO2 gas separation by cooling the gas to change the supercritical to gas-liquid phase. Using our system, more than 99 mol% of H2 (96 mol% of purity) and 94 mol% of CO2 (99 mol% of purity) were separately obtained as a gas and liquid, respectively, under the high-pressure conditions.
Ours system has a potential to be used as a simple and easy to handle system for the generation of high-pressure CO2/H2 and these separated high-pressure gases can be used for the variety of application such as fuel cell vehicles.
Carotenoids exist in plants usually as the (all-E)-configuration which have higher crystallinity and lower solubility in organic solvents compared to the Z-isomers. When they are converted to Z-isomers (cis forms), bioavailability increases due to the lower crystallinity and higher solubility [1, 2]. We have studied Z-isomerization of carotenoids such as lycopene and beta-carotene and behavior of the isomers in extraction process and fine particle formation process. Since Z-isomers have higher solubility in supercritical CO2, extraction of carotenoids from plant materials is enhanced. Fine particle formation process is also influenced due to the difference in the crystallinity as well as the solubility.
Z-isomerization behavior of lycopene in the oleoresin was studied by adding various vegetable oils and the key component to enhance the isomerization was revealed. When the plant materials were pretreated to convert Z-isomers, the extraction rate of carotenoids by supercritical CO2 or subcritical dimethyl ether increased considerably [3].
For particle formation by SAS process, smaller particle was obtained by Z-isomerization pretreatment, whereas (all-E)-form of carotenoid produced larger crystal form of particles [4].
Reference
[1] K. Murakami, M. Honda, R. Takemura, T. Fukaya, M.Kubota, Wahyudiono, H. Kanda, M. Goto, Biochem. Biophys. Res. Commun., 491, 317, 2017
[2] M. Honda, Yo Watanabe, Kazuya Murakami, Nguyen Ngoc Hoang, Wahyudiono, H. Kanda, M. Goto, Eur. J. Lipid Sci. Technol., 2017, 1700293 (1-8), 2017
[3] K. Murakami, M. Honda, Wahyudiono, H. Kanda, M. Goto, Sep. Sci. Technol., 52, 2573-2582, 2017
[4] T. Kodama, M. Honda, R. Takemura, T. Fukaya, C. Uemori, Wahyudiono, H. Kanda, M. Goto, J. Supercritical Fluids, 133, 291-296, 2018
The history, principles and potential of Process Intensification (PI) will be introduced. A range of “tools” that are particularly suited for the development of PI-based processes will then be described, with particular reference to the re-engineering of pharmaceuticals to improve bioavailability, and to provide targeted response and controlled release modalities. Examples of PI success stories and “not-so-successful” case studies will be presented, as well as some lessons learned from the coal-face during my travels along the Journey to Technology Transfer & Commercialisation.
Curcuma xanthorrizha Roxb, known as Temulawak or Javanese ginger, is a plant species. Its rhizomes are used as a medicinal herb. It contains curcumin as an active compound and ethereal oils mainly consisted of sesquiterpenes. In this work, Curcuma xanthorrizha Roxb ethanolic extract was micronized with an addition of PVP using supercritical antisolvent (SAS) method. The ethanolic extract was obtained from dried Curcuma xanthorrizha Roxb using soxhletation. For the micronization, the extracted compound solvent was a mixture of acetone and ethanol (90:10 (v/v)), while the supercritical CO2 was used as an antisolvent. The SAS process was carried out at temperatures of 40, 60, and 80oC, pressures of 8, 10, and 12 MPa, and PVP concentrations of 0, 2, and 4%. The produced particles were analysed by SEM (Scanning Electron Microscopy), FTIR (Fourier Transform Infrared Spectroscopy) and HPLC (High Performance Liquid Chromatography). The effect of operating conditions on the particles size and morphology was evaluated. Based on the HPLC analysis, the extract mainly contained curcumin. By using this process, the extract microparticle had smaller size than standard curcumin (6.42 ± 3.45 μm) and PVP (33.25 ± 18.83 μm). Microparticle extract with size ranging from 0.6 – 5.95 μm were successfully formed. The particle size decreased as the temperature increased, whereas pressure did not significantly affect the particle size or morphology. The best result was predicted at a pressure of 12 MPa, temperature of 40oC, and 2% PVP concentration in solution. Extract microparticles have amorphous form and it does not form crystal. From the experimental result, it can be concluded that SAS method with an addition of PVP is very effective to reduce the size of particles and increase the bioavailability of extract.
Keywords: Micronization; Curcuma xanthorrizha Roxb; Supercritical antisolvent; PVP; Curcumin;