
A mixture of CO2 and a co-solvent is widely employed as a solvent or mobile phase for supercritical fluid extraction, chemical reaction, supercritical fluid chromatography etc. Kinematic viscosity is one of important transport properties, and is required for designing chemical process as well as estimating dimensionless numbers such as Reynolds number, Schmidt number, Prandtl number [1]. A more accurate estimation method for kinematic viscosity is increasingly demanded. The authors have proposed the estimation method using modified Eyring and activity coefficient equations [2-5].
This paper deals with the estimation of kinematic viscosities for CO2 + alkane (decane, tetradecane, hexadecane, octadecane) systems at high pressures using the modified Eyring and Wilson-VISCO model by the following equations [6].
where
The correlated results are compared with those of McAllister model [5] based on Eyring model..
where
The kinematic viscosities for ternary systems (CO2 + alkane + alkane) were then predicted using binary parameters.
References:
1) B. E. Poling, J. M. Prausnitz, J. P. O'Connell: The Properties of GASES AND LIQUIDS, fifth edition, McGraw Hill (2001)
2) K. Tochigi, K. Yoshino, V. K. Rattan: Int. J. Thermophys., 26, 413 (2005)
3) K. Tochigi, T. Okamura, V. K. Rattan: Fluid Phase Equilib. 257, 228 (2007)
4) H. Matsuda, K. Kurihara, K. Tochigi, T. Funazukuri, V. K. Rattan: ibid., 470, 188-192 (2018)
5) H. Matsuda, K. Tochigi, K. Kurihara, T. Funazukuri, V.K. Rattan: ibid., 492, 137-144 (2019)
6) K. Tochigi et al.: AIChE 2018 annual meeting, Oct.28 – Nov. 2, Pittsburgh (2018)
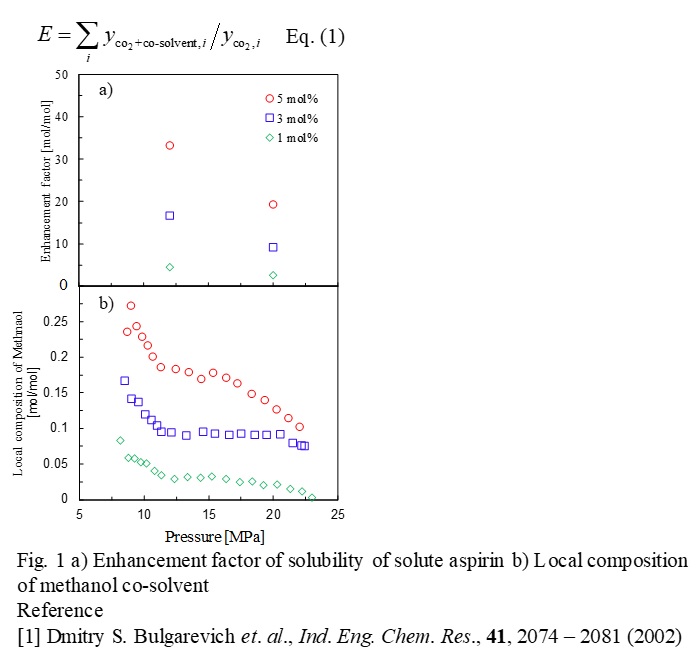
Supercritical carbon dioxide (scCO2) extractions and fractionations with co-solvents can provide many advantages for pharmaceutical and natural foods, which are no solvent residual, mild critical conditions and capability of tuning solvent property by varying pressure and temperature. Solubility data of pharmaceutical compounds are important information to design scCO2 extraction and fractionation processes. The objective of this work is to acquire the solubility data of pharmaceutical compounds in scCO2 with co-solvent and to analyze their behavior of co-solvent adding effect in term of enhancement factors (E: Eq(1)) based on local compositions. Solubility of aspirin derivatives in scCO2 with a methanol were measured at 333 K and 12.0-20.0 MPa by changing the methanol contents. The enhancement factor (E) were calculated from the measured data and correlated with thermodynamic models. Local compositions of MeOH co-solvent (xLMeOH ) were evaluated by considering the similarity of the local composition to the cybotactic region in spectroscopic measurements around an indicator, in co-solvent and CO2 system. The UV-Vis spectral data of the co-solvent with CO2 were obtained from literatures [1]. Figures 1a and 1b show the E and xLMeOH values as a function of pressure. At lower pressures (P < 10 MP), interactions of aspirin with co-solvent methanol were dominant due to low polarity of CO2. The contribution of local composition (xLMeOH ) was significant (Fig.1b). With increasing pressure, solubility of aspirin in CO2 generally increased, however, the E values (Fig. 1a) tended to decrease. The reasons for this may be attributable to a decrease in xLMeOH values (Fig. 1b). Polarity of CO2 becomes stronger at higher pressures and consequently strengthen the interact with the solute, leading to a reduction in xLMeOH values (Fig. 1b). Thus, analysis of solubility based on local composition provides better understanding in molecular interactions.
Activated carbon has been widely used to prevent emissions of volatile organic compounds (VOCs) in industrial processes because of its high adsorption ability. Regeneration of used activated carbon is required for its reuse in the industrial processes with the aim of SDGs. Supercritical carbon dioxide (scCO2) is a promising solvent for the regeneration of activated carbon because of its high diffusivity into the microstructure and ability to operate at a moderate temperature (critical point : 304 K) resulting in less damage to the microstructure of the adsorbent.
We have studied the scCO2 regeneration of the activated carbon used in exhaust facilities of semiconductor production factories, suggesting long used activated carbon could not be regenerated because of high boiling point adsorbates [1]. The experimental results show these high boiling point adsorbates were not removed from activated carbon by the scCO2 regeneration, and these adsorbates were caused by propylene glycol monomethyl ether (PGME) and propylene glycol monomethyl ether acetate (PGMEA) as main VOC components at practical semiconductor manufacturing processes [1]. The activated carbon with PGME and PGMEA was influenced by heat treatments (200-500 °C) at the exhaust facility, and high boiling point adsorbates were observed in the case of heating temperature of higher than 250 °C, which suggested that heating temperature of 200 °C prevented high boiling point adsorbates.
In this study, practical applications of the scCO2 regeneration process of activated carbon used at the exhaust facility of a semiconductor production factory were studied while considering the performance of the exhaust processor with the scCO2 regenerated activated carbon and reduction effects of industrial wastes and CO2 emissions.
Reference
[1] Ito, Y., I. Ushiki, Y. Sato and H. Inomata, Kagaku Kogaku Ronbunshu 45, 29-34(2019)
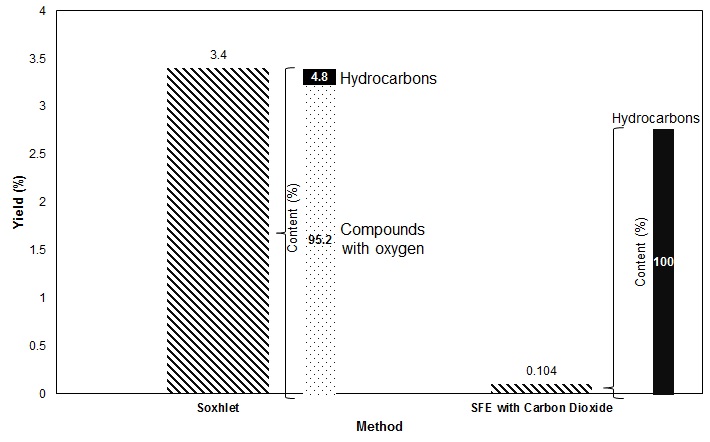
High Density Polyethylene (HDPE) is a type of polyethylene used in a lot of products, from containers for high purity chemicals, package for food to bottles for water. It is impact resistant, long lasting, and easily molded. This type of polyethylene is manufactured at low temperatures and pressures, using Ziegler-Natta and metallocene catalysts or activated chromium oxide (known as a Philip catalyst). In this process, impurities like oligomers, additives, and products of side reactions are generated. These impurities increase the toxicity of the polyethylene, and may migrate to the packed product if found in high concentrations. For the purpose of extracting them, many extraction methods like soxhlet, accelerated solvent extraction, and microwave extraction have been known in the literature. However, these methods have many disadvantages like long extraction times, use a lot of solvent, and require further clean-up of the solvent. To overcome these problems, supercritical fluids have become an alternative because of their tunable solvating properties by changing pressure and temperature. In this research, we use supercritical carbon dioxide because it does not require further clean up, has an easy achievable critical point and functions as a good swelling agent for polymers, which facilitates extraction. We succeeded on extracting hydrocarbons from C16 to C22 at 22 MPa and 60 °C using 1 L/min flow rate and studied the effect of time on the extracted yield. These results were also compared to soxhlet which selectivity for hydrocarbons was found to be less than the shown by supercritical carbon dioxide extraction (see Fig. 1)
Polyvinyl chloride (PVC) consumes less petroleum, which is an exhaustible resource, and is cheaper than other plastics. Foaming of the PVC is also a subject of interests in engineering because the foam is superior in thermal insulation, cushioning and light weight property. In the forming, use of carbon dioxide (CO2) as plasticizer/blowing reagent has been attracting attentions due to its characteristics which form microcellular plastics. Unfortunately, in the case of the PVC, the PVC does not dissolve CO2, and use of CO2 as plasticizer/blowing reagent is impossible.
In the PVC molding process, the mixture of PVC, organic plasticizer, and stabilizer (antioxidant reagent) was heated up because PVC is a thermosetting plastic. In this study, we are focused on the plasticizer instead of PVC as a CO2 reservoir for the foaming of the PVC during the heating stage. In theory, solubility of the CO2 into the plasticizer is high at low temperature. The CO2 would separate from the PVC mixture during the heating stage which result in the formation of bubbles in the PVC mixture matrix. As the separation of the CO2 and the thermal curing take place at the same time, the control of the foaming process would be difficult.
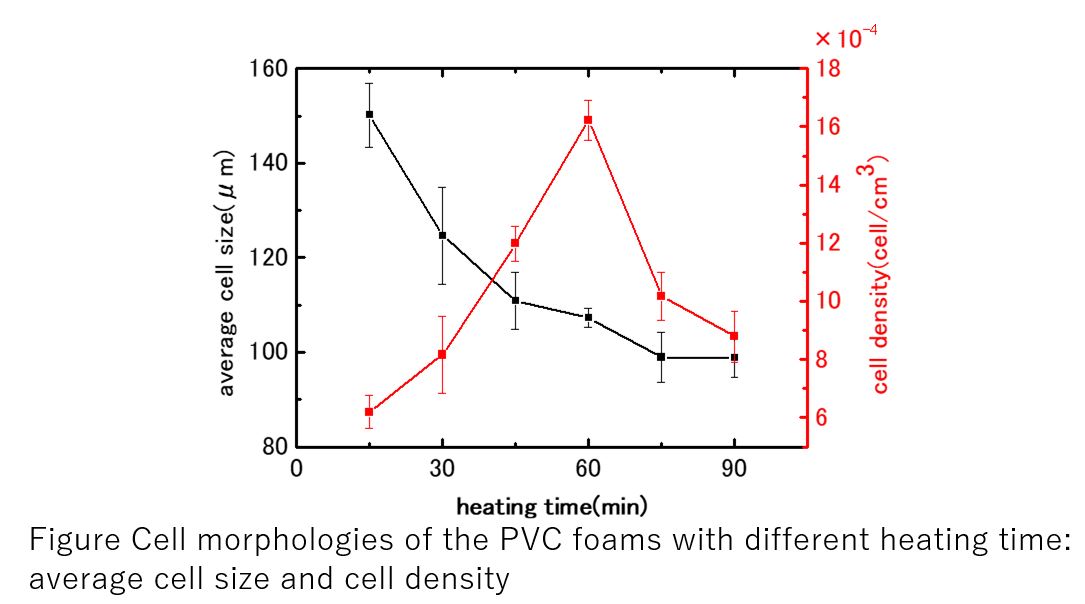
In this study, we examined the preparation of PVC foam using carbon dioxide as a blowing agent, Diisononyl phthalate (DINP) as plasticizer, and LOX-13 as a stabilizer by a batch process. As described above, foaming and thermal curing occurs at the same time, the structure of the PVC foam strongly depends on the heating time and temperature. So, the relationships between foam characteristics and the heating time and temperature were examined.
Fast flow liquid-liquid extraction process using CO2 microemulsion has been developed to extract and separate hydrophobic compounds from liquid. The process can be adapted to aqueous ethanol solution of a biaryl compound, which is a model of solution obtained after Suzuki-Miyaura cross coupling reaction. Ethanol aqueous solution of the biaryl with salts and CO2 are mixed in a micromixer at 40 °C and 20 MPa, which forms microemulsion of CO2-rich phase/water-rich phase. Because of large interface area of phases fast extraction can be achieved. After extraction, CO2-rich phase and water-rich phase are separated in a separating cell. The level of water-rich phase is maintained by controlling the aperture of a control valve in the bottom outlet line to keep the differential pressure between lines from bottom and top of the cell at constant. Pressure is controlled by a backpressure regulator equipped in the top outlet line. The above process can extract more than 80% biaryl continuously to CO2-rich phase.
This study investigated effects of water/ethanol ratio of the model liquid and extraction pressure on biaryl extraction ratio at 40 °C. Biaryl extraction ratio increased with increasing water/ethanol ratio at 20 MPa. It is probably because the solubility of biaryl in liquid decreased with increasing water/ethanol ratio since the biaryl is a hydrophobic substance. Biaryl extraction ratio increased with increasing extraction pressure. Carbon dioxide density increases with increasing pressure under a constant temperature, so the solubility of the biaryl should increase at higher pressure conditions. Thus, it is considered that the higher solubility in CO2-rich phase resulted in higher biaryl extraction ratio under higher pressure conditions. In the presentation, relationship between biaryl solubilities and water/ethanol ratio or extraction pressure will be discussed based on the biaryl solubility data.