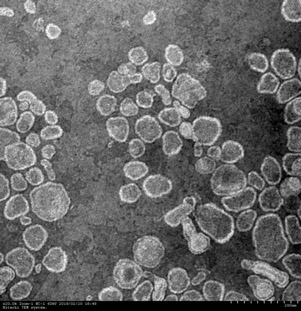
Liposome is widely recognized as an ideal drug carrier for its unique characteristics such as possession of internal space, bio-compatibility and sub-microstructure. In the conventional production methods, multi-step processing and batch production restrict production efficiency. To overcome this problem, flow production method using supercritical carbon dioxide and microfluidic system was proposed in this study.
Supercritical carbon dioxide was used as a main solvent for lipids because of its high bio-compatibility, tunable properties by temperature and pressure and low critical temperature, which is beneficial for the production of thermo-sensitive products. Moreover, supercritical carbon dioxide can be thoroughly eliminated after the process simply by depressurization and therefore, solvent separation step can be omitted from the process. Liposome was produced in the micro-channel, where slug flow of supercritical carbon dioxide phase and liquid phase is formed. Each liquid slug in the flow behaves as a tiny container separated by supercritical slugs and steadily flows along micro-channel with a constant space time. This regularity of the slug flow guarantees that the product properties have no deviation. After the production of liposome, supercritical phase and liquid phase were separated by gravitational force and continuously flows out through metering valves.
Obtained liposome was observed by transmission electron microscope (see figure). Nearly spherical structure less than 1 μm can be recognized. The size of this structure was measured by dynamic light scattering method and confirmed to be controllable in the range of 100 – 300 nm by changing flow rate of raw materials. Drug encapsulation, which defined by the ratio of total drug used in the process and entrapped drug in the structure, was measured by high performance liquid chromatograph. Furthermore, surface functionalization of liposome by chitosan is also achieved using the same method. The external surface of liposome was confirmed to be positively-charged after functionalization by dynamic light scattering method.
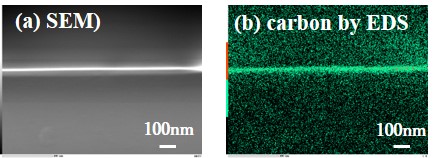
Organic photovoltaics (OPV) gains attentions because of various advantages including light-weight, flexibility, and low-cost. However, OPV suffers from low power conversion efficiency (PCE). To improve PCE, ordered bulk heterojunction (OBHJ) has been proposed as an ideal structure, in which p/n-types organic semiconductors are deeply interdigitated in nano-scale. Nevertheless, conventional technologies can fill only shallow features with the aspect ratio below 3. Meanwhile, we have developed temperature-driven supercritical fluid deposition (TSFD), which successfully filled micrometer-scale trenches (aspect ratio of 10) with anthracene. Accordingly, TSFD will be the only solution if it can fill the nano-scale high-aspect-ratio features with organic semiconductors. In TSFD, organic semiconductor is dissolved in hot supercritical CO2 and transferred to a cooled substrate under the constant pressure. The temperature drop causes supersaturation, resulting in precipitation and crystallization of the solute on the substrate. By carefully controlling the temperature drop, deposition can proceed in quasi-equilibrium, ending up with excellent crystallinity. High concentration supply of the solute leads to relatively high growth rate. As the process should start with ultra-thin and continuous film formation, followed by filling the remaining features, initial growth behavior during TSFD of tetracene was examined in this work. Dependences of supersaturation, supply concentration, and substrate temperature were studied. Small grains were found on the substrate besides the film, but completely eliminated by suppressing the flow rate fluctuation and adjusting the substrate temperature. Ultra-thin tetracene film was therefore formed with no grains. Under ultraviolet light irradiation, green emission from tetracene film was observed on the entire substrate surface. The thickness was 30 nm as shown in the figure. X-ray diffraction revealed the strong (001) orientation. The lateral size of the film was several orders of magnitude larger than those fabricated by the conventional methods, which is preferable for high PCE. Filling nano-scale high-aspect-ratio features will be realized.
High-temperature and high-pressure water has been recognized as a green chemical medium for monosaccharide reactions because dehydration and/or retro-aldol condensation can proceed without the use of a catalyst. There are some reports on the monosaccharide containing the hydroxyl groups (-OH) such as glucose and fructose. We previously reported the reaction of N-acetyl glucosamine, which contains an acetoamide group (-NHCOCH3). In this work, we investigated the reaction of sialic acid and fucose, which are monosaccharides; however, there is no report on these two monosaccharides previously. Sialic acid contains both an acetoamide group (-NHCOCH3) and a carboxyl group (-COOH). Fucose contains a methyl group (-CH3). The dehydrations of sialic acid and fucose were proceeded in high-temperature and high-pressure water. In addition, the chemical structures of the dehydration products were affected by the functional group contained in sialic acid and fucose. From the comparison of the sialic acid, fucose, and other monosaccharide, we will discuss the effect of functional groups on monosaccharide reactions in high-temperature and high-pressure water.
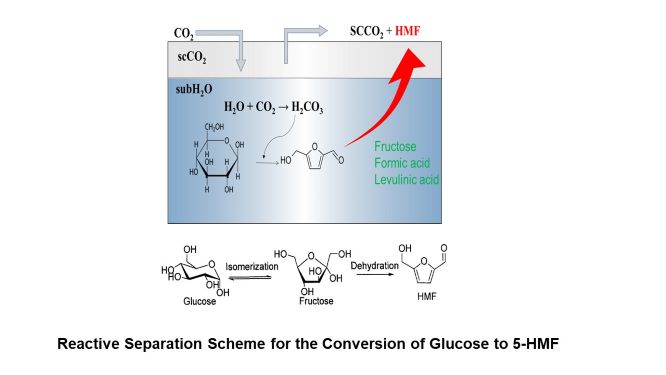
The declining amount of petroleum resources has prompted researchers worldwide to explore various alternatives including biomass as feedstocks. In this context, 5-hydroxymethylfurfural (5-HMF) has attracted growing interest because it can be produced from biomass-derived sugars, and has the potential to be further converted into fuels and fine chemicals. Acid-catalyzed conversion of fructose to 5-HMF has been widely investigated. However, glucose is more cost-effective and readily available. This research focuses on the conversion of glucose into 5-HMF employing the synergy of subcritical H2O and supercritical CO2. Carbonic acid resulting from the synergy of supercritical CO2 and subcritical H2O could acted as an acid catalyst. The operating conditions were optimized, and addition of alcohol such as isopropanol and low transition temperature mixtures was also investigated. In addition, combined reactive separation technique was also employed to further increase the yield. In this approach, since 5-HMF has low polarity, scCO2 dissolve 5-HMF. Thus, continuous CO2 flow enables simultaneous reaction and separation, and could also suppress the formation of by-products.
The highest yield (30.4%) and selectivity (62.8%) was obtained under the condition of 2 wt%, 200 o C, 20 MPa, 1.5 h. This was likely due to the higher solubility of 5-HMF in scCO2 at higher pressure. Addition of isopropanol also had positive effect on the yield of 5-HMF. These results suggest the applicability of adding isopropanol and the use of reactive separation approach employing the synergy of subcritical H2O and supercritical CO2 to the conversion of glucose to 5-HMF.