
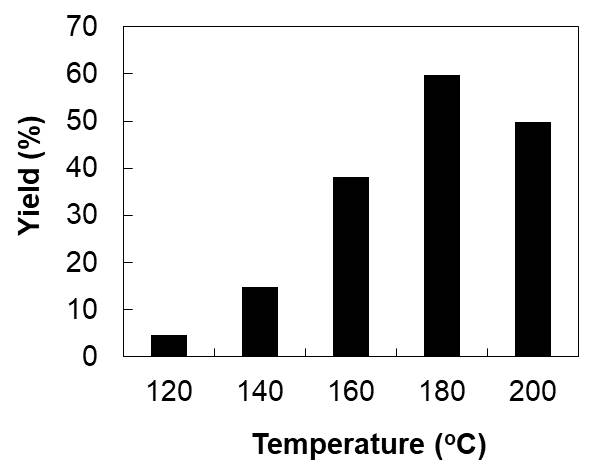
Rutin (quercetin-3-O-rutinoside) is a flavonoid glycoside which is widely distributed in many plants such as buckwheat and onion. It has several functional properties such as antioxidant, anti-inflammatory, anticarcinogenic and other effects in human body. However, it has low absorption efficiency in the body and lower antioxidant activity and bioavailability compare with its aglycon, quercetin. Quercetin can be obtained via hydrolysis of the glycosidic bond between quercetin regidue and rutinose one. Conventionally, a liquid acid catalyst such as hydrochloric acid is used for the hydrolysis. Although the high yield of quercetin can be obtained by using the conventional method, there are some problems such as high impact to the environment and high cost due to considerable after treatments such as neutralization. Therefore, in this study, solid acid catalysts like graphene oxides (GOs) were employed as alternative catalysts and microwave (MW) irradiation was used as a tool of rapid heating of the starting material. The objectives of this study are to explore suitable operating conditions for high efficient hydrolysis of rutin and to develop a new isolation process of quercetin after the hydrolysis of rutin under hydrothermal conditions. As a result, we found that relatively high yield of quercetin was achieved at hydrothermal conditions (160-180oC) for 15-30 min in the presence of graphene oxide with microwave irradiation as shown in Fig. 1. In the conference, a suitable reaction and separation conditions for higher yield of quercetin by using this technique.
Development of drug delivery systems (DDS) and regenerative medicines (RM) are important in improving the quality of life (QOL) of the patients in medical care. Hyalurinic acid (HA) is a biocompatible polymer with high molecular weight (~600 KDa) alternating co-polymer consisted of two saccharide repeating units. HA have properties such as strong water retaining ability, high viscosity, gelation, biocompatibility and biodegradability which makes it a promising material for drug delivery systems and regenerative medicines. It is known that desirable forms and functions can be designed by controlling the molecular weight of the HA. Current methods for degrading the HA are by enzymes or acid or base degradation which require multiple steps, toxic chemicals and long reaction times (hours). In this work, we studied the decomposition of HA in high temperature water without any catalysts using batch type and flow type reactors. Results revealed that the hydrothermal treatment of HA with long reaction times lead to low molecular weight HA with different chemical structure from the initial HA and undesirable decomposion products. On the other hand, the hydrothermal treatment of HA conducted at short reaction times (below 10 s) using the flow reactor lead to low molecular weight HA having the initial chemical structure and no decomposition products. The estimation of molecular weight of the hydrothermal treated HA was possible by a kinetic model assuming random depolymerization. Hydrothermal treatment using a flow reactor system is an efficient method to obtain low molecular weight HA without chemical changes in the product HA and undesirable degradation products.
The catalytic activities of metal/metal oxide depend strongly on their exposed crystal facet. However, the active facet is thermodynamically unstable and the catalyst deteriorates with morphological change during the usage. Therefore, the regeneration process of facet-controlled catalyst has significant importance in the chemical engineering.
In previous study, it is reported that supercritical hydrothermal treatment with carboxylic acid enables the morphological control of AlOOH during the dissolution-recrystallization process [T. Fujii et al. Crystal Growth and Design, 16, 1996–2001 (2016)]. In this study, to develop the method for reactivation of degenerated catalyst, the change of exposed facet for ceria nanoparticles under supercritical hydrothermal condition with carboxylic acid is investigated.
In the experiment, spherical ceria nanoparticles was treated under supercritical hydrothermal condition at 400 °C with decanoic acid in batch-type reactor. As the modifier concentration increased, the morphology of nanoparticles changed from spherical to cubic shape exposing (100) facet. Moreover, the size distribution became narrower and the nanoparticles below 4 nm disappeared after supercritical hydrothermal treatment at higher modifier concentration. This narrowing of the size distribution accompanying with the morphological change from spherical to cubic implies that the morphological change was induced by the dissolution-recrystallization process affected by the organic modifier. Our previous study revealed that the in-situ surface modification by organic molecules during nanoparticles formation in supercritical hydrothermal condition enables the formation of ceria nanocubes exposing (100) facet, having high catalytic activity [J. Zhang et al. Advanced Materials, 19, 203–206 (2007).]. These results indicate that morphology and exposed facet can be controlled not only by in-situ surface modification, but by the post-treatment with organic modifier.
In APPChe 2019, we will discuss on the detailed mechanism of morphological change during the supercritical hydrothermal treatment and the feasibility of this method for the reactivation of facet-controlled catalysts.
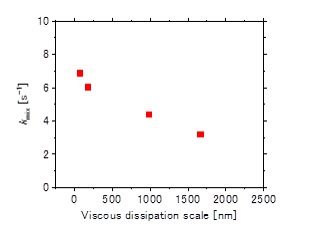
Reaction kinetics of hydrothermal synthesis was evaluated through the formation of nickel oxide nanoparticle as a model in the wide range of high-temperature compressed water. Mixing state in supercritical hydrothermal synthesis was considered to find reaction control conditions. Rapid mixing can be achieved by low kinetic viscosity with small eddy size. Figure 1 shows the relationship between the mixing rate and Kolmogorov scale at mixing point; rapid mixing achieved with short mixing length in supercritical water. Based on the mixing study, the reaction rate of nickel nitrate was conducted changing pressure and temperature. Acceleration of reaction rate in the supercritical region was observed and investigated in terms of the solvent effects with the changes in dielectric constant using Kirkwood model. The role of water in terms of reactant was also investigated, and it was found that the reaction is retarded under dilute conditions where the water dissociation suppressed such as high temperature or low pressure. The fundamental kinetics study would pave the way for the practical use of supercritical water as a medium of nanomaterials synthesis.
With the advent of electric cars that do not consume any petroleum fuel and the commercialisation of shale oil, the petrochemical industry is facing a big turning point. There is a need for an economically feasible process for petroleum fuels, which is less burdensome to the environment, and at the same time, makes it possible for a direct chemical conversion to the olefins. Upgrading bitumen through subcritical hydrothermal processing is a promising process. By using physicochemical properties of water at near critical conditions, it is possible to crack the asphaltene to light oils. Besides, since the operating temperature is lower than other processes at a high temperature, there is an advantage of suppression for the coking formation. However, since the operating temperature is relatively low, it is necessary to advance the reaction sufficiently. Therefore, a highly active catalyst capable of activating the reaction at a relatively low temperature is required.
In this study, highly concentrated Cr-substituted CeO2 nanoparticles were synthesised by the nonequilibrium reaction using a flow-type reactor under sub/supercritical hydrothermal condition. The oxygen storage capacity (OSC) of Cr-substituted CeO2 nanoparticles was compared according to the loading amount of Cr and the presence of the surface modifier. In a batch reaction, Cr substitution hardly proceeded, however in the flow-type reaction, more than 30 mol% of Cr was substituted. Moreover, the activity of Cr substituted CeO2 nanoparticles was evaluated using the bitumen upgrading under subcritical water conditions. The weight fraction of asphaltene decreased, and the gases and light oils increased with increasing the concentration of substituted Cr. Finally, by analysing the correlation between the OSC and the product of the nanocatalyst, the possibility of upgrading the low-temperature bitumen is suggested.