Among the main sustainable development goals of the European Union (EU) is a clean planet for all – the long-term vision for a prosperous, modern, competitive and climate neutral economy by 2050. The EU continues the path to a low-carbon, climate-neutral, resource-efficient and biodiverse economy in full compliance with the United Nations 2030 agenda. The adoption of process intensification methodologies that use the integration of reaction and separation in single, multifunctional units, will improve energy efficiency and competitiveness. One of these technologies is the membrane reactor (MR), where reaction and separation through membranes are integrated to achieve higher yields at lower CAPEX and OPEX.
Using selective membranes one product is remove from the reaction zone, shifting the reaction to the products, which increases the yield while also obtaining a pure product.
Among the EU projects working on MRs, FERRET, FluidCELL, BIONICO apply the MR concept for hydrogen production in a micro-CHP (combined heat and power) systems using both natural gas and biofuels (biogas and bioethanol). Hydrogen obtained from the reforming Palladium MR is used in a PEM fuel cell to produce electricity. Pd-MR were also used for the product hydrogen from syngas (DEMCAMER, CACHET II) and propene dehydrogenation (CARENA). Similarly, water gas selective membranes for CO2 hydrogenation to produce methanol using zeolite-MR (CARENA), or carbon-MR DME (C2FUEL). Oxygen membranes are used for the controlled delivered of oxygen for direct conversion of methane into ethylene (MEMERE). Supported ionic liquids membranes are used in ROMEO to improve the hydroformylation reaction. In this talk we will discuss the latest results of these EU projects on MRs.
An energy career medium is required for the world-wide utilization of renewable energy. Ammonia is one of the promising energy career medium, and carbon-free hydrogen is easily produced via ammonia decomposition. Ammonia as well as produced hydrogen will be used as energy for power generation; however, methane synthesized by reacting the produced hydrogen with CO2 will be easily used in the present infrastructure. The most serious demerit of methanation is exothermic reaction; however, combined reaction system of NH3 decomposition and CO2 methanation, heat from exothermic methanation will be used effectively for endothermic ammonia reaction,
In this study, combined reactions of NH3 decomposition and CO2 methanation were conducted at 623 K in a hydrogen-permeable palladium membrane reactor, where heat transfer as well mass transfer of hydrogen were possible. Even if commercial Ru catalyst was used for both NH3 decomposition and CO2 methanation, the levels of NH3conversion and CO2methanation were increased by membrane reactor effect, namely effective hydrogen permeation from NH3 decomposition side to CO2 methanation side.
In order to improve the performance of combined reaction system, novel catalysts were prepared for NH3 decomposition and CO2 methanation at relatively low reaction temperature of 623 K. Among the catalysts developed, Ru/BaO/Al2O3 and Ru/ZrO2 gave high catalytic activity for NH3 decomposition and CO2 methanation, respectively. As a result of the improvement of both hydrogen production rate in NH3 decomposition and hydrogen consumption rate in the CO2 methanation, the increased difference in hydrogen partial pressure led to acceleration of hydrogen permeation. Furthermore, the reaction fields of ammonia decomposition and CO2 methanation were interchanged on the inside and the outside of the hydrogen permeable membrane in consideration of their catalytic activity. It was experimentally demonstrated that membrane reactor effect was improved by development of catalysts tin the combined reaction system.
As an efficient and convenient mean of transporting and storing hydrogen, it is proposed that hydrogen is converted into chemical compounds such as ammonia, which is called hydrogen carrier. Among the carrier candidates, ammonia has some advantages like possessing a comparatively large hydrogen storage capacity (17.7wt%), being liquefied easily at 20 °C and 0.8MPa, and emitting no CO2. However, to produce hydrogen by ammonia decomposition a higher temperature, usually over 600 °C, is needed because of endothermic and slow reaction.
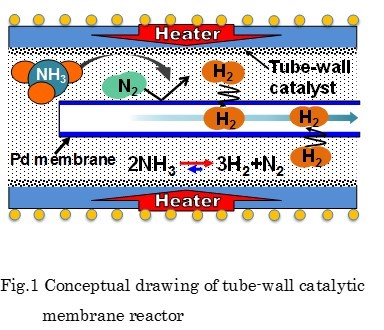
In this study, a new type of membrane reactor that consists of a tube-wall reactor and a palladium membrane tube as shown in Fig. 1 is proposed for hydrogen production via ammonia decomposition. This reactor, in which uniform and direct heat supply can be realized compared with a conventional packed bed reactor, will allow ammonia to decompose efficiently.
A tube-wall reactor was made from aluminum tube with 8mm in inner diameter, 1mm thick and 100mm long. The inside wall was anodized to 0.1mm-thick alumina layer for the support of Ru catalyst, where the evaporation-to-dryness method using RuCl3 aqueous solution was applied for the Ru loading. Ether a rolled Pd77Ag33 tube (3.2mm in outer diameter, 0.2mm thick and 100mm long) or a composite Pd/Al2O3 tube (0.002mm thick and 90mm long), prepared by chemical vapor deposition was inserted at center of the tube-wall reactor. Ammonia decomposition was carried out in the range of 325 - 425 °C at 0.1 MPa to compare the differences between 1) the packed-type and the tube wall-type, and 2) the tube-wall reactors with and without hydrogen separation by Pd membrane. When performances between the tube-wall reactors with and without hydrogen separation by the 0.2mm-thick rolled Pd alloy membrane were compared, an increment of conversion was clearly observed.
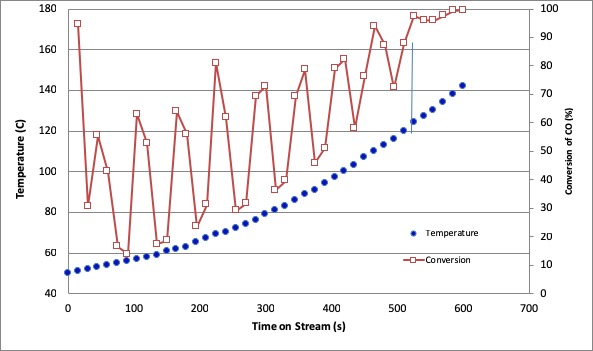
CO oxidation process in the catalytic converter hasn't showed best performance particularly when it is cold start-up, since the catalyst inside the converter is not active during this period. The purpose of this experiment was to develop the forced unsteady state operation procedure of CO oxidation using Pt/γ-Al2O3 with 0.05%-w and space velocity of 0.406 mmol s-1gram-1catalyst. The catalytic converter was gradually ramped-up, while introducing the feed gas containing CO in the air. The feed gas was modulated following a square wave model with switching time variation at 3, 6, 15, and 30 s and various operation modes. To gain the intrinsic reaction rate, the external mass transfer criterion was determined. Ramping-up the temperature from 50 until 150 °C increased the CO conversion with different profiles between steady state and dynamic flow rate. The dynamic system with modulated CO feed flow gave lower light-off temperature and higher average CO conversion than the steady state system which gave light off temperature 115 °C and average CO conversion of 48.86 %. The switching time of 3 s gave highest average CO conversion during ramping-up, which was 79.35 %. Meanwhile the dynamic operation system with modulated compressed feed flow gave higher light off temperature and lower average CO conversion than steady state system.
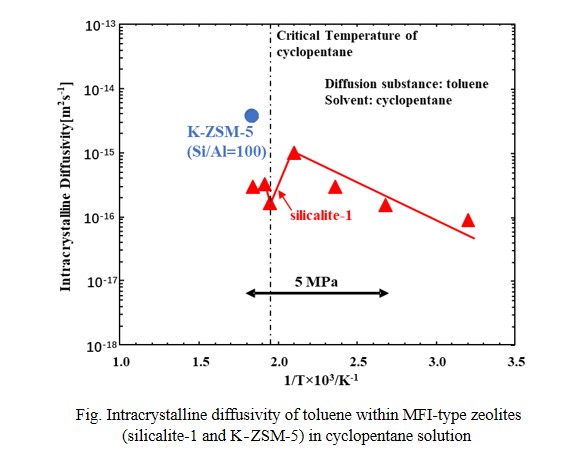
In this study, intracrystalline diffusivity of toluene within MFI-type zeolites (silicalite-1 and K-ZSM-5 (Si/Al=100)), possessing micropore which size is close to molecular size of toluene, in sub-, and super-critical fluid of cyclopentane was investigated using the constant volumetric method. This study clarified that concentration of toluene in sub- and super-critical fluid of cyclopentane (critical point: 511K, 4.5MPa) can be measured using Raman spectroscopy. Using change in toluene concentration with time due to adsorption of toluene onto the zeolite and the intracrystalline diffusivity was calculated using theoretical equation. Logarithm of intracrystalline diffusivities of toluene within silicalite-1 (MFI-type zeolite without Al in its framework) measured under 5 MPa was correlated with a single line against inverse number of temperatures. In contrast, above 473 K, the intracrystalline diffusivity of toluene within silicalite-1 was decreased and took minimum value around critical temperature. Formation of clusters between solvent molecules and diffusion substance near the critical point of solvent was considered to lead to the increase of diffusion resistance at pore mouth of silicalite-1. In addition, the intracrystalline diffusivity of toluene within K-ZSM-5 in supercritical fluid of cyclopentane at 543K was also measured. The intracrystalline diffusivity showed much higher value than that within silicalite-1. By increasing toluene concentration at outer surface of the zeolite, formation of the cluster may be suppressed and decreased diffusion resistance at pore mouth, which leading to increase the diffusivity.