
Metal oxides such as HfO2, ZrO2 and Y2O3 have optical, mechanical and chemical properties which raise interest for a variety of applications in fields such as sensors, optoelectronics, medical devices.... Atomic Layer Deposition (ALD) provides excellent uniformity, conformality in complex topographical structures, as well as good film purity. However, ALD is heavily dependant on the precursor choice, which may both determine film performances as well as the process Cost-of-Ownership with considerations around thermal stability, volatility, physical state. In this presentation, we will introduce recent developments on Group IV metal oxide precursors (high-temperature HfO2, ZrO2 ALD) as well as liquid lanthanide compounds (Y2O3, Sc2O3, Ln2O3) and illustrate films performances for applications such as hydrophobic coatings, anti-corrosion coatings and optical coatings.

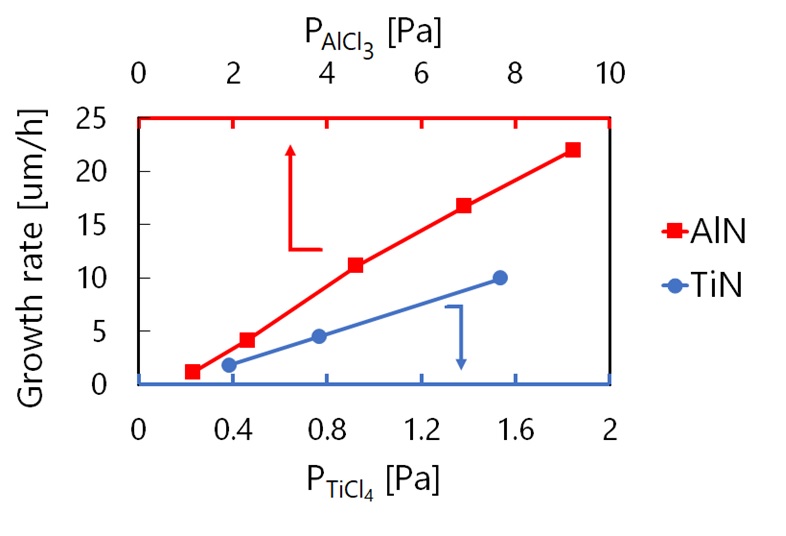
Aluminum-rich cubic titanium aluminum nitride (Ti1-xAlxN, x>0.9) is an emerging material for cutting tool coating due to high hardness, thermal resistance, and chemical stability. Cubic phase is, however, metastable at x>0.67 (hexagonal phase most stable), and physical vapor deposition (PVD) consistently yields hexagonal-TiAlN. On the other hand, synthesizing cubic-TiAlN with x>0.9 was reportedly enabled by chemical vapor deposition (CVD), whose cutting performance is satisfactory for industrial application. However, film thickness distribution was found even in the lab-scale reactor, which is the biggest hurdle for mass production. Computer-aided reactor design is the only possible solution for it; whose reliability is definitely reliant on the quality of kinetic reaction model. Kinetic analysis was therefore carried out for TiAlN-CVD at 800 °C from TiCl4-AlCl3-NH3-H2-He, by changing the partial pressure of each metal chloride and NH3 (PAlCl3, PTiCl4, and PNH3). During the TiAlN growth, growth rate of AlN ingredient increased with PTiCl4, and vice versa, suggesting that some interactive reactions should exist between them. Gas-phase reaction simulation was performed by using existing elementary reaction model, but could not explain this interaction. Thus, individual growths of TiN and AlN were examined at 800 °C and PNH3=19.2Pa. Growth rate proportionally increased with precursor partial pressure as shown in the figure but decreased with the increase of PNH3 for all growths (data is not shown). TiAlN-CVD was previously found to be diffusion limited, but the decrement cannot be rationalized by the change of mass-transport coefficient. It should rather be regarded as transient region of diffusion and reaction limited. We thus performed step coverage analysis to extract surface reaction rate constants, and found that competing Langmuir-Hinshelwood (LH) mechanism could explain the overall phenomena. Consequently, complicated interactive reaction between Ti and Al precursors are getting clarified.
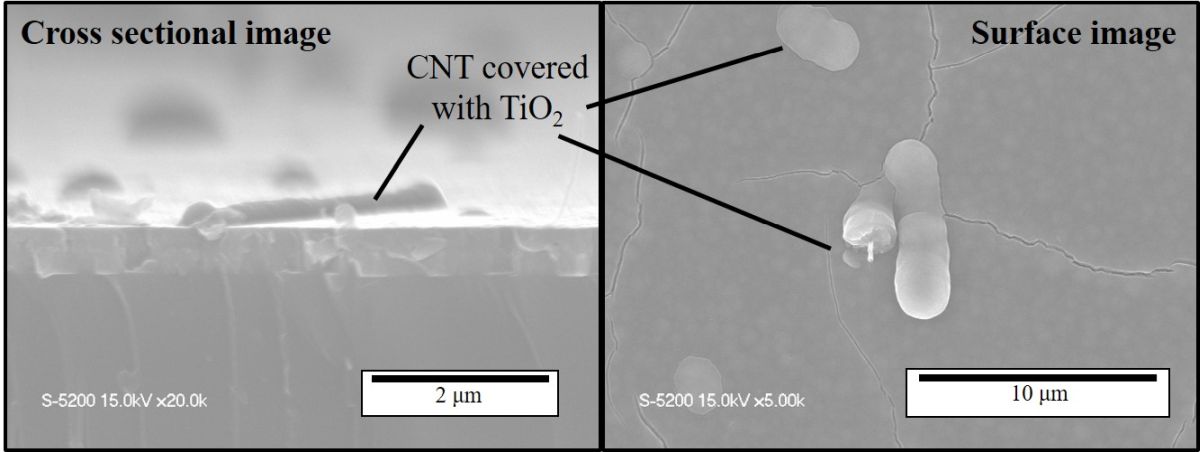
Titanium dioxide (TiO2) is applied to photocatalysts, photochromic materials, solar cells, etc. due to its characteristic electrical, chemical, and optical properties. Recent studies revealed that the photocatalytic and other performances of TiO2 thin films can be improved when silver (Ag) or carbon nanotubes (CNTs) are embedded in the films because of the changes of light absorbance and electron/hole transfer. Such films have usually been synthesized by liquid-based methods. However, the methods require many procedures, and the control of the properties and amount of the embedded nanomaterials is not easy. We recently developed a CVD technique to synthesize thin films in which well-characterized, pre-formed nanomaterials are embedded [1]. In this study, TiO2 thin films with embedded Ag nanoparticles and CNTs are synthesized by applying this technique. The properties and photocatalytic performance of the synthesized films are evaluated.
Titanium alkoxide vapor was fed into a microwave plasma field to form a TiO2 matrix. An aqueous suspension of either or both of Ag nanoparticles and CNTs was sprayed, and the resulting droplets were fed into the plasma field simultaneously. These simultaneous feedings enabled the synthesis of nanocomposite films through a one-step plasma-enhanced CVD process.
Observation of the films using a scanning electron microscope (see images) confirmed that the CNTs were successfully embedded in the film. Elemental analysis and absorption spectrum indicated the enhancement of the absorbance of visible light due to the existence of Ag and CNTs in the film. The films in which either Ag nanoparticles or CNTs were embedded showed higher photocatalytic activity than a TiO2-only film. The film with both of Ag nanoparticles and CNTs exhibited a further higher activity. The film caused 1.8 times dye-decomposing reaction rate of the TiO2-only film under visible light irradiation.
[1] Kubo et al., Thin Soild Films, 632, 55 (2017)
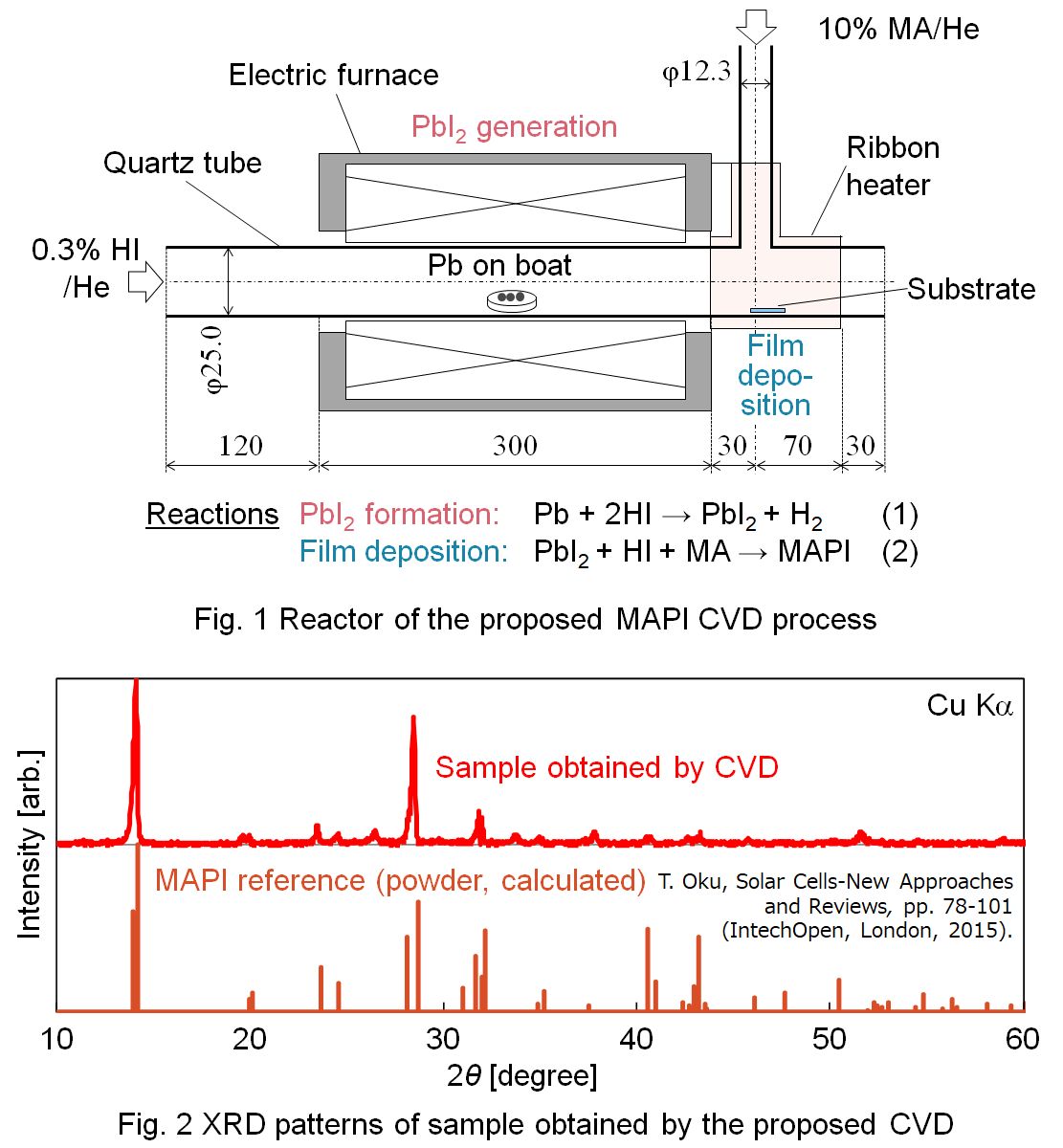
Methylammonium lead iodide (MAPI, CH3NH3PbI3) perovskite solar cells have attracted great expectations as next-generation low-cost solar cells, and development of large-scale film fabrication processes is required. Development of a chemical vapor deposition (CVD) process is required for its capability for large-area and high-purity fabrication of thin films. Although a CVD process for fabricating MAPI was proposed,1) it includes solid reactants that should be sublimated and therefore the flow rate control is difficult. In this study, a novel CVD process to synthesize a MAPI thin film from gaseous reactants, hydrogen iodide (HI), monomethylamine (MA, CH3NH2), and lead (Pb) vapor is proposed. As shown in Fig. 1, the reactor consists of two tubes. Molten Pb was placed in a tube heated at 873 K, and 0.3 % HI in He was supplied for generating PbI2 vapor in Reaction (1). PbI2 and HI were mixed with 10 % MA in He supplied from the other tube, to form a product film on a 10 mm×10 mm quartz substrate heated at 423 K in Reaction (2). The total pressure was approximately 2 kPa. Flat red-brown films were obtained at 423 K for 30, 60, 180 min. The film thickness was 0.4, 1.0, 2.8 μm respectively. The mass of the samples are on the liner plot. XRD patterns and UV-vis spectra of the obtained samples are in good agreement with the reference pattern of MAPI. MAPI was successfully synthesized by a proposed CVD method. XRD patterns of obtained samples show that the PbI2 peak decreased and the MAPI peak increased with time. The result indicates that the MAPI crystal grew with time, which can also be indicated by the result of observation with SEM.
1) M. M. Tavakoli, L. Gu, Y. Gao et al., Sci. Rep., 5, 14083 (2015).
Utilization of CO2 by converting it into useful chemicals is sought. Catalytic hydrogeneration of CO2 to methanol is an attractive pathway as it is an important feedstock. So far, for this reaction, Cu/ZrO2 has been proposed as a promising catalyst and the interface of Cu-ZrO2 is considered as the active site. Therefore, high Cu loading and small Cu particles are essential for maximizing the number of the active site. Here, we successfully produce highly-loaded CuO (60 wt% as Cu) on ZrO2 nanoparticles by flame spray pyrolysis (see the left side of the figure) that can be scaled up to kg/h of the production rate. By decreasing the precursor feed rate, the particle size decreases because of the shorter particle residence time in the flame, which hinders the particle growth (as shown in the figure). Notably, when the catalyst was prepared at the feed rate = 2 mL/min, any Cu species were not detected by XRD indicating that Cu species were present as an amorphous and/or very small particles that are below the detectable limit. This catalyst showed about 5 times higher selectivity to methanol than a commercial Cu/ZnO/Al2O3 catalyst. In contrast, by preparing the catalyst at the higher feed rate (5 and 10 mL/min), CuO peaks appeared in the XRD pattern and their selectivity to methanol is about 50% less than that prepared at 2 mL/min of the feed rate. Therefore, the catalyst prepared at the feed rate = 2 mL/min that consists of smaller CuO and ZrO2 particles than that prepared at the feed rate = 5 and 10 mL/min would be beneficial as they maximize the interface of Cu-ZrO2 sites.