Advances in device technology have been accompanied by the development of new types of materials and device fabrication methods. Considering device design, initiated chemical vapor deposition (iCVD) inspires innovation as a platform technology that extends the application range of a material or device. iCVD serves as a versatile tool for surface modification using functional thin film. The building of polymeric thin films from vapor phase monomers is highly desirable for the surface modification of thermally sensitive substrates. The precise control of film thickness can be achieved in iCVD, creating a conformal coating on nano‐, and micro‐structured substrates such as membranes and microfluidics. iCVD allows for the deposition of polymer thin films with a variety of chemical functionality, and thus, substrate surfaces can be functionalized directly from the iCVD polymer film or can selectively gain functionality through chemical reactions between functional groups on the substrate and other reactive molecules. These beneficial aspects of iCVD can spur breakthroughs in device fabrication based on the deposition of robust and functional polymer thin films. This presentation reviews recent progress made in iCVD‐based technologies in various fields, especially for soft electronic device applications.
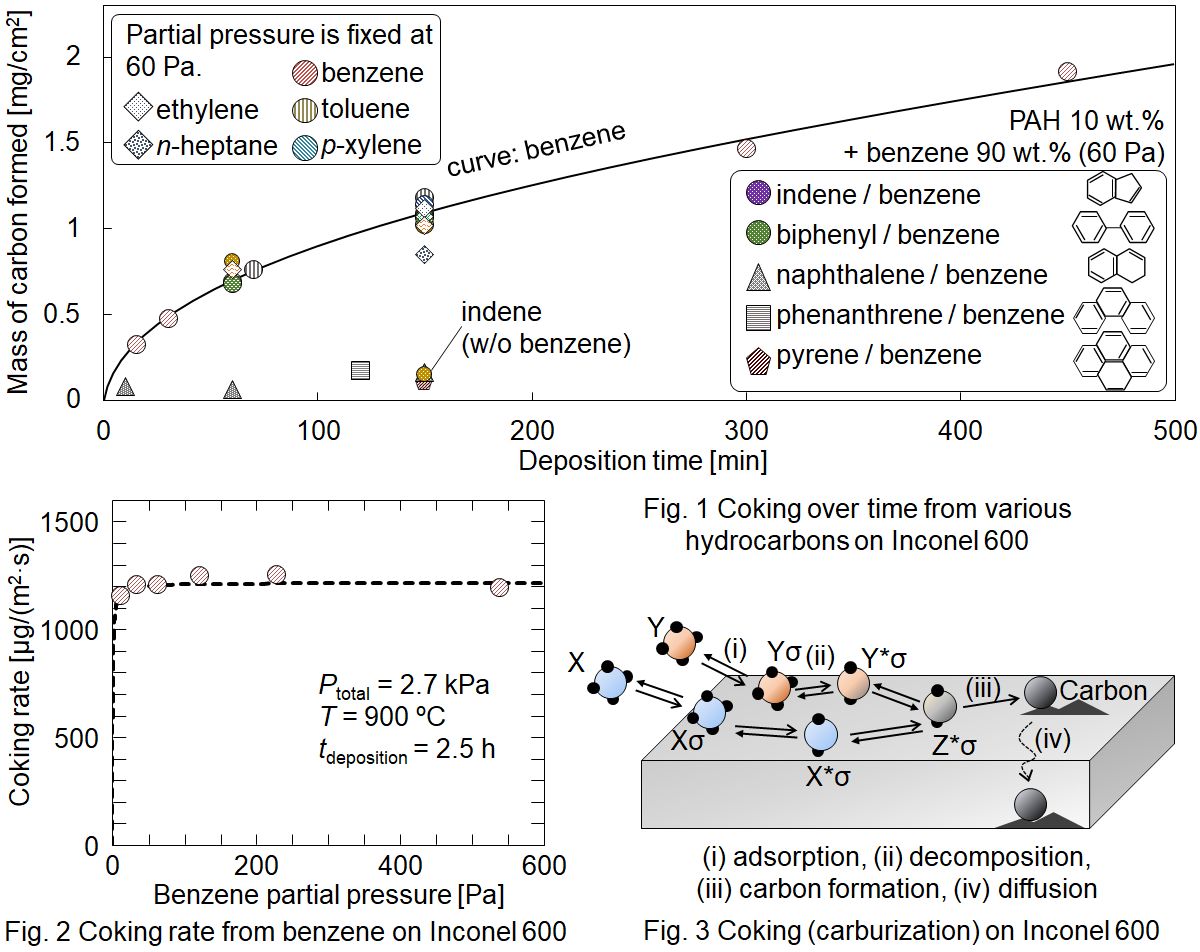
Unfavorable deposition of carbon called coke takes place in metal tube reactors used in hydrocarbon processes in the industry. The coke formation mechanism is unclear. In this study, a laboratory-scale thin tube reactor is employed for investigating mechanism of the coke formation from hydrocarbons on metal and inert substrates. Chemical vapor deposition (CVD) experiments were carried out using a quartz tubular reactor, in which 10 mm×10 mm substrates were placed. One of several hydrocarbons or a benzene solution of one of heavy hydrocarbons was supplied with diluent He to the reactor. The reaction temperature was 900 °C and the total pressure was 2.7 kPa. The space time from the inlet to the substrate was fixed at 15 ms. When benzene was supplied, coking rate was highest on the SUS 316L substrate followed by Incoloy, γ-Fe, Inconel, Nichrome, SUS 430, Ni, Cr, and α-Fe in order. This shows the catalytic activity of respective metals. On the Inconel substrate, the coking rate was approximately equal from ethylene, n-pentane, n-heptane, benzene, toluene, p-xylene, and biphenyl. The rate was almost independent of the reactant partial pressure. These resulted from solid-phase diffusion of carbon which was generated from hydrocarbons adsorbed on the metal surface near saturation. Naphthalene, phenanthrene, and pyrene exhibited ca. 10 times lower coking rate than the other hydrocarbons, which suggests more stable adsorption and slow decomposition on the metal surface. The coking rate on inert substrates, quartz, silicon, and carbon, was ca. 40 times lower than that on Inconel. The rate per unit surface area of the inert substrates obeyed an identical Langmuir-type rate equation. As a result, coking on metal surface starts with adsorption, fast decomposition on the surface, followed by fast carbon diffusion into the substrate, and slow carbon CVD on the deactivated surface continues even after completing the carburization.
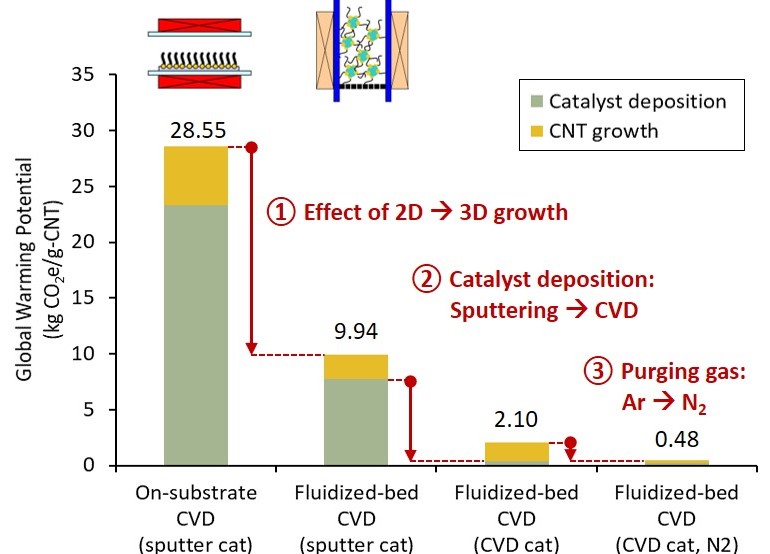
Carbon nanotube (CNT) is an emerging material with promising applications due to its superior properties. Mass production of CNT is most feasible with catalytic chemical vapor deposition (CVD). To synthesize high-quality single-wall to few-wall CNTs, catalyst nanoparticles must first be deposited on substrates. Then, using a high-temperature CVD reactor, hydrocarbons are decomposed into CNT on the substrates. The processes are intensive in material and energy. Yet, the consequential impact on the environment is rarely studied because of uncertainties in syntheses. Leveraging the experience in lab syntheses, this study aimed to evaluate the prospective environmental impact of CNT synthesized via three selected catalytic CVD methods. First, the CO2-assisted growth on flat substrates (on-substrate CVD) [Carbon, 136 (2018): p.143-149], represented the established millimeter long CNT growth. Second, the fluidized-bed growth on spherical beads (fluidized-bed CVD) with sputtered catalysts [Carbon, 50(4) (2012): p. 1538-1545], contrasted the effect of 3D CNT growth to previous 2D growth. Third, the fluidized-bed CVD with chemical vapor deposited catalysts [Carbon, 49(6) (2011): p. 1972-1979], showed a repeatable single-reactor production. We quantified the global warming potential impact of 1 g CNT production with life cycle assessment (LCA). The results for the three syntheses were 28.55, 9.94, and 2.10 kg-CO2equiv/g-CNT, accordingly (illustrated in graphical abstract). By comparing the three syntheses, we showed that the strikingly high impacts were mitigated through increasing CNT yield with fluidized bed and replacing the energy-intensive sputtering process. A scenario to substitute the remaining hotspot, argon (purging gas), with nitrogen could reduce the impact to 0.48 kg-CO2equiv/g-CNT. When considering scale-up from lab-scale (reactor diameter of 22 mm, productivity of 5 g per day) to industrial scale (diameter >30 times, productivity >3 to 104 times), the CNT production will be comparable to industrialized carbon fiber production (about 0.02 kg-CO2equiv/g-CF).
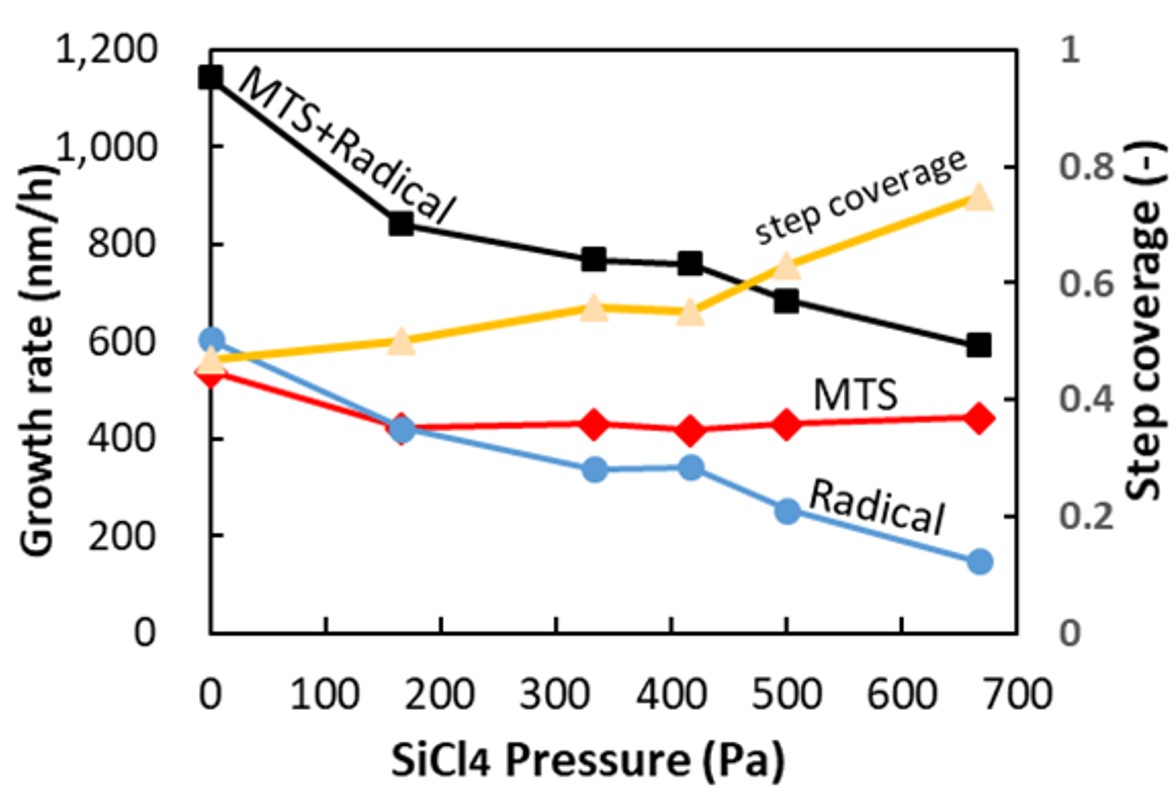
Ceramic matrix composites (CMC) consisting of SiC fibers (SiCf) and SiC matrix receive attention as a material for next-generation aerospace engines due to light weight and high heat resistance. SiCf/SiC-CMC is fabricated by weaving the SiC fibers, followed by infiltrating SiC into their interstices. Chemical vapor infiltration (CVI) from methyltrichlorosilane (MTS) and H2 is employed to infiltrate SiC fibers due to high conformality. However, perfect infiltration was unfeasible so far even by CVI because aspect ratio of the interstice is huge. We have found that SiC was formed by two growth species, one is MTS (contributes to uniformity) and the other is radical species (gas phase decomposition product of MTS, contributes to non-uniformity). Therefore, elimination of the radical species is mandatory for conformal deposition, and we proposed to use sacrificial layer for it [1]. We therefore introduced high MTS concentration (>1.2kPa), to realize higher conformality without compromising growth rate. Based on Langmuir-Hinshelwood (L-H) mechanism, we can achieve quasi-0th-order reaction at high concentration which will contribute to uniform deposition. Step coverage by MTS was thus improved from 60% to 88% using high MTS concentration. We also investigated SiCl4 gas addition into SiC-CVI for scavenging the radical growth species. Figure shows SiC growth rate and step coverage dependency on SiCl4 pressure. When SiCl4 was added, the radical was successfully scavenged, whose impact was enriched with higher SiCl4 concentration, while no impact on deposition by MTS. Consequently, combination of high MTS concentration to achieve L-H mechanism and SiCl4 addition to scavenge radical growth species is the key to achieve high growth rate and high conformality simultaneously.
[1]K. Shima et al., Society for Chemical Engineers Japan 47th Autumn meeting, Hokkaido University, O302 (2015).
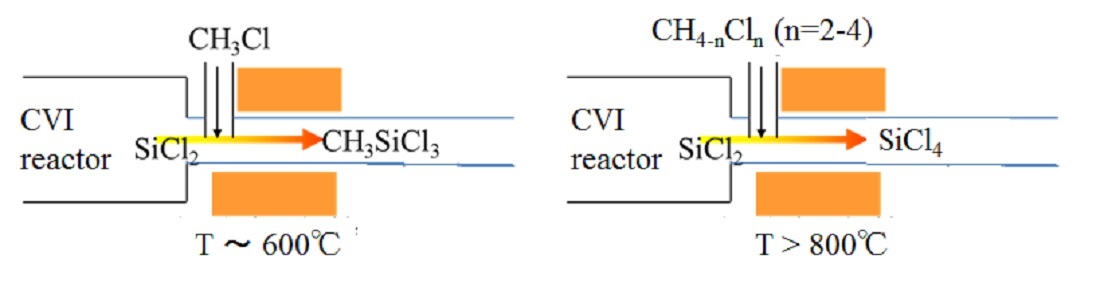
Silicon carbide (SiC) is widely used for structural materials due to high mechanical strength and corrosion resistance. Chemical vapor infiltration (CVI) from methyltrichlorosilane (MTS) is commonly adopted for its conformal deposition capability and high productivity. However, hazardous by-products, polymerized SiCl2 such as (SiCl2)n and X-(SiCl2)n-Y, are known to be piled up in a exhaust system of reactor. It compels a periodic cleaning work that takes several days for mass-productive reactor. To make matters worse, these are stable in vacuum i.e. reactor, while explode via hydrolysis when being exposed to air. Accordingly, we have to develop the way to inhibit (SiCl2)n production without resorting to unsafe experiments. First, the reaction mechanism to form (SiCl2)n was studied theoretically. The rate constants of elementary reactions starting from SiCl2 to (SiCl2)n and Cl-(SiCl2)n-Cl (n=1-3) were calculated with quantum chemical calculations, followed by coupling with existing model for H/C/Si/Cl system. Reaction kinetic simulation using the updated model revealed that SiCl2 is stable at a process temperature, typically above 900 °C; meanwhile, during temperature drop in the exhaust system, SiCl2 reacts into SiHCl3 and SiCl4 at 500-800 °C and (SiCl2)n below 400 °C. As the reaction mechanism was clarified, practical way to inhibit (SiCl2)n generation was sought. Here, we propose to inject additive gas into the exhaust system, which reacts with SiCl2 to form stable species. When CH3Cl is added at 600 °C, residual ratio of SiCl2 decreased to 0.1% in 2 sec (product: MTS). When CHnCl4-n (n=2-4) is added above 800 °C, the residual ratio decreased below 1% in 1 sec (product: SiCl4). It is recommended to add CH3Cl around 600 °C or CHnCl4-n (n=2-4) above 800 °C into exhaust gas to inhibit (SiCl2)n production. This conclusion has already been verified experimentally. Although this work was conducted for SiC-CVI, the same solution is applicable to Si-CVD from SiHCl3 or SiH2Cl2.