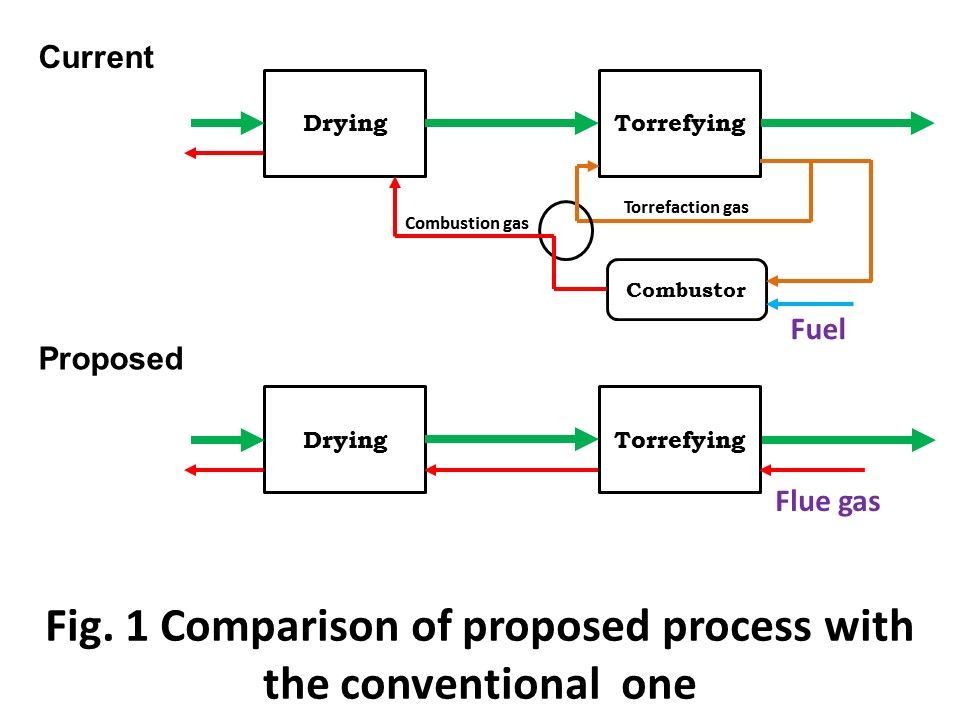
Malaysia is the second largest producer of palm oil, producing 17.3 million tons, or approximately one third of the total world supply in 2016. In parallel with the palm oil production, a huge amount of biomass residues are generated by the industry including oil palm plantations and palm oil mills: empty fruit bunch (EFB) 18.3, mesocarp fiber 11.0, palm kernel shell (PKS) 4.6, trunk 17.4 and fronds 125.3 t-wet/year. Mesocarp fiber is almost fully utilized as solid fuel for producing electricity and steam at palm oil mills. About PKS, some are utilized for electricity and steam at palm oil mills (the same use as mesocarp fiber), some are being traded as solid fuel. EFB is underutilized. There are three representative thermochemical conversion techniques developed for this kind of lignocelluosic biomass residue: torrefaction, fast pyrolysis and gasification, which produce solid fuel, bio oil and syngas, respectively. The most significant obstacle for commercialization of biomass use is not the level of conversion technology itself, but logistics. Logistics for biomass is still really immature in comparison with fossil fuels; such as coal, crude oil and natural gas. In this sense, biomass use in small scale is prioritized way to start due to its lower risk in terms of logistics. In this talk, the presenter will introduce torrefaction , of which product can be commercialized in small scale, or with less logistic risks. Combustion gas utilization for torrefaction will be introduced as a lower running cost process. The concept is illustrated in Fig. 1.
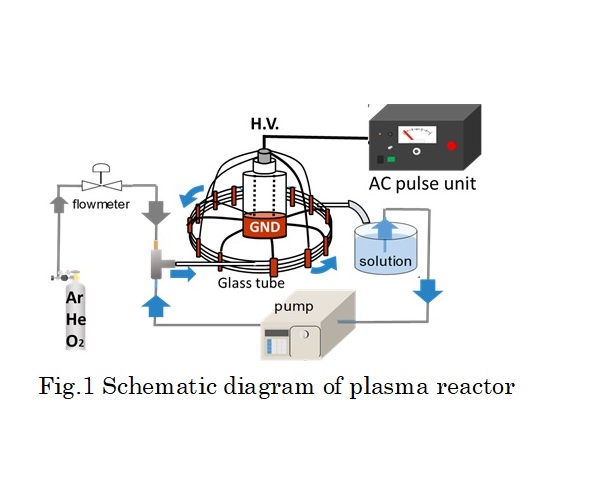
In this study, non-equilibrium atmospheric pressure pulsed discharge plasma technique using a gas-liquid slug flow in glass column is applied to water containing methylene blue (MB) degradation. The reactor was comprised of 4 rows glass column bundled by copper foils that attached on the outside of the column surface. Moreover, the foils were used as high-voltage electrodes totally in 5 parallels. As the ground electrode, copper foils were attached on column at distant of 25 mm from high-voltage electrode in the same way. The water solution and the gas were flowed simultaneously in glass column to form the slug flow system. The generated bubble motion interval was controlled by adjusting the flow rate of gas and liquid. In this time, MB solution and each gas were flowed at 1.5 and 0.15 ml/min, respectively. After the flowing bubbles in the glass column became steady, an electrical discharge was introduced into the slug flow system by using AC power supply with a bipolar pulsed output voltage to generate plasma in bubbles between electrodes. The output voltage is 9 kV at 10 kHz repetition and its pulse duration is 10 μs. The reaction was conducted totally in 120 min, and the degradation ratio was measured every 20 minute. As a result, over 90 % of MB were degraded for all of the gas species (O2, Ar, He). Especially in the case of O2, mostly all of the MB was degraded in 120 min. This implies many kinds of active species originated by oxygen were dissolved in plasma treated water. However, the amount of hydrogen peroxide was higher in Ar and He. Finally it was confirmed that the degradation rate in this method was higher than that in other plasma methods such as atmospheric pressure plasma jet.
With record heat spreads all over the world, global warming has become an issue that cannot be ignored for human beings, and greenhouse gas emissions must be curbed and reduced. In particular, carbon dioxide (CO2), which accounts for the majority of greenhouse gas emissions, is the most important target of emission control and reduction. Although CO2 is a very stable compound with high standard redox potential, it can be converted to industrially useful substances such as carbon monoxide, formic acid and hydrocarbons reduction. It is possible to reduce not only greenhouse gases but also produce a resource. Various studies have been conducted to reduce CO2 using renewable energy, excess thermal energy and electrical energy. We have tried to reduce CO2 electrochemically using metal catalyst electrode and convert it to useful substance. Many studies have revealed that the main products of CO2 electrochemical reduction differ depending on the metal species. In the metal species, this study focused on zinc(Zn). Zn is known to produce carbon monoxide (CO) as a main product of CO2 electrochemical reduction as well as gold and silver, while having abundant reserves. In addition, it is expected to selectively generate CO because the overpotential of hydrogen generation is also large and hydrogen generation is suppressed. In this report, surface modification of Zn electrode has been performed by anodization. By changing the conditions of anodic oxidation, different surface modifications were successfully performed and it was successful that prepared Zn electrodes showed quite different product selectivity such as CO and formic acid in CO2 electrochemical reduction. In order to clarify the factor of this product selectivity, it carried out by surface analysis and electrochemical characteristics of the electrode. It was revealed that the product selectivity can be controlled by changing the anodization condition.
Recent manufacturing processes for high-performance crystal particles require the advanced control technologies of crystal structure and morphology. In order to develop the crystallization process for the desired crystal particles, it is important to understand the crystallization behavior from a microscopic viewpoint and actively control the nucleation and crystal growth in solution. We have studied the crystallization behavior of aqueous microdroplet of salt solution using electrodynamic balance (EDB), where a charged droplet could be electrically levitated in space over the periods of hours. It enabled us in situ observation of crystallization behavior in a levitated microdroplet with enough spatial and time resolutions. In this presentation, we directly observed the nucleation and crystal growth in the aqueous NaCl microdroplet . It was found that the finally produced crystal particles had different several morphologies which were mainly influenced by the diameter and the charge of a levitated droplet.
In essence, ionic liquids (ILs) are salts with melting points at or below 100 °C, which are composed of organic cations and organic or inorganic anions. Owing to their distinctive properties, such as low melting point, wide liquid range, extremely low vapor pressure, sound thermal stability and wide electrochemical windows etc., ILs are used in a number of application areas. However, some recent studies are noteworthy, showing that ILs are flammable during thermal upsets. Other studies have also indicated that the gas phase of decomposition products from ILs is spontaneous combustion and easy ignition. To accomplish the objective, thermogravimetric is used to examine the thermal stability of 1-ethyl-2,3-dimethylimidazolium nitrate ([C2mmim][NO3]; C7H13N3O3). Under the dynamic heating conditions, [C2mmim][NO3] is considered to be decomposed until approximately 280.0 °C. Meanwhile, lower hydrophilicity leads to better thermal stability of [C2mmim][NO3] than [C2mmim][X] (X represents halogen). However, due to the limitations of the dynamic heating experiment, [C2mmim][NO3] is found to be decomposed before the lowest measured decomposition temperature under dynamic conditions. Moreover, the combination of the homemade combustion test device and high speed camera are used to investigate the combustion process of [C2mmim][NO3]. The results indicate that the flammability of [C2mmim][NO3] is caused by the combustible decomposition products (see Figure). Therefore, the entire decomposition and combustion process of [C2mmim][NO3] is fully characterized based on the results of thermogravimetric with fourier transform infrared spectrometer.