
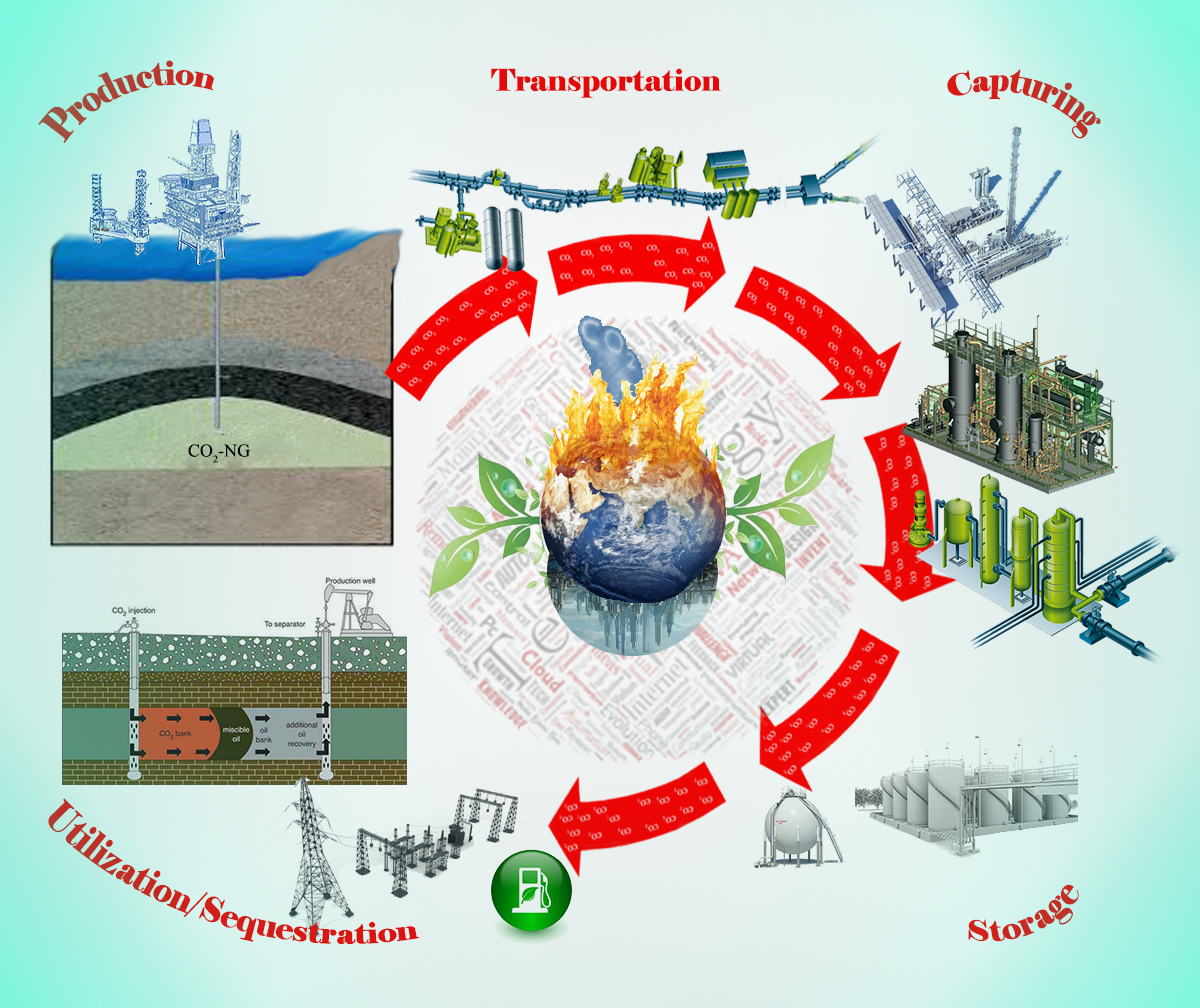
Natural gas reserves available worldwide includes varying amounts of CO2 ranging from CO2-free in Siberia to as high as 90% CO2 content in the Platong and Erawan fields in Thailand. The Natuna field in the Greater Sarawak Basin in Indonesia is the largest gas field in south east Asia, with estimated 46 trillion cubic feet of recoverable reserved. Unfortunately, it remains unexplored due to high CO2 content up to 71%. In Malaysia, CO2 content from natural gas fields varies up to 87%. Over 13 trillion cubic feet of natural gas reserves are undeveloped due to the presence of high CO2 content. The high CO2 content in natural gas is a major issue to monetizing the gas reserves. Without the removal of its CO2 content, natural gas cannot be further processed, liquefied, transported or commercially sold. However, the release of bulk CO2 into environment aggravates global warming and violates the requirement of Kyoto Protocol. Hence, there is a need for the development of technologies for the effective capture and subsequent utilization of CO2 for its conversion into value added products. In this paper, the recent advances on the potential technologies to manage the CO2 rich natural gas fields is deliberated. Challenges for gas treatment including capturing systems (from common technologies such as absorption, adsorption, cryogenic and membrane to novel technologies such as CUAS) and their applications towards a wide range of CO2 concentrations are presented. Additionally, the opportunities of separating huge amount of CO2 from such environments to produce added values products (bio fuel, synthetic-fuel, CO2-EOR, power production, etc.) are discussed.
In a well-known efficient CO2 absorption process, Rectisol, chilled and pressured methanol is used as the absorbent to capture of CO2 physically. Unfortunately, because of higher solubility of CO2 in methanol under lower temperature and higher pressure, this process requires strict conditions (-40 ~ -60 oC and 3~8 MPa) and this leads in high demand of both operating and equipment costs. Therefore, a rotating packed bed (RPB) was evaluated to capture CO2 with NaOH in aqueous methanol as well as pure methanol solution under ambient conditions to relax low-temperature and high-pressure constraints. The experimental results suggest that with the NaOH-methanol solution, the absorption could be almost completed (~100%) within one gas/liquid pass in an RPB at room temperature and atmospheric pressure. Furthermore, the apparent overall mass transfer coefficient could be elevated to almost 5 s-1, which is one of the highest values among the RPB-related chemical absorption processes. Under this intensified centrifugal field, it is clear that CO2 capture can be highly enhanced with this good combination of physical-chemical absorbent, NaOH-methanol solution.
High purity CO2 recovery with lower energy consumption is required for effective CO2 Capture and Utilization/Storage (CCUS), and membrane separation would be suitable for the CO2 separation. Our research group has investigated and developed amine-containing polymeric membranes for effective CO2 capture. For example, amine-immobilized poly(vinyl alcohol) (PVA) membranes show excellent CO2 separation properties even over smaller H2. It was found recently that alkanolamines, such as 2-(2-aminoethylamino)ethanol (AEAE), elevated the CO2 separation properties, and mechanism of the preferential CO2 transportation was elucidated. Under humidified conditions, carbamate formed by an interaction between CO2 and the secondary amino group of AEAE was hydrolyzed to give bicarbonate ion, which was the major migrating species through the membrane. The hydroxyl group of alkanolamines facilitate the interaction to enhance bicarbonate production upon hydrolysis of the carbamate.
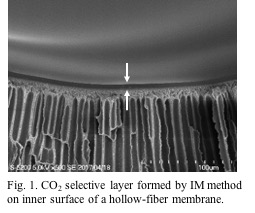
For pilot-scale demonstration, a hollow membrane module was prepared by in-situ modification (IM) method (Duan S., J. Membr. Sci., 2006). An aqueous solution of amines and PVA was circulated in a commercial hollow-fiber membrane module, and a CO2-selective layer was formed on the inner surface of hollow fiber membrane (Fig. 1). The thickness of the active layer was determined by circulation period, concentration of the solutes, and flow rate of the solution. The optimization of the IM method explained that 5 min circulation was enough to obtain higher CO2 permeability and CO2 selectivity. While the amines were physically immobilized in a polymer matrix, decrease in the CO2 separation performance was not found at all under 90 % relative humidity at 50 °C for about 300 h, which was triggered by the leakage of the amines. The IM method is preferable for mass production, and the scale-up is also feasible. The membrane modules developed would be suitable for biogas upgrading and air capture to obtain high purity CO2 in the permeate.
CO2 capture from dilute sources (e.g., flue gases, air) and subsequent storage or conversion will play a key role in the carbon-constrained future. The low driving force for CO2 capture from these dilute streams has positioned adsorbent materials and adsorption technology as a leading approach for addressing this global challenge. The creation of robust materials-enabled advanced adsorption separators and their manufacturing into low-cost, energy-efficient devices will be the focus of the talk. Engineering novel materials—such as solid-supported amines, metal-organic frameworks, and polymers of intrinsic microporosity—into structured contactor adsorption devices shows promise for these CO2 capture applications. Structured sorbent contactors can help manage kinetic and engineering factors associated with the separation, including pressure drop, sorption enthalpy effects, and external heat integration (for temperature swing adsorption). Monoliths, fiber sorbents, and 3D printed materials will be discussed as the three main classes of existing or emerging structured sorbent contactors. Recent developments in their manufacture; advantages and disadvantages of each structure relative to each other and to pellet packed beds; recent developments in system modeling; and finally, critical needs in this area of research will all be discussed.