
In a standard post-combustion carbon capture (PCC) process, the regeneration energy of the CO2 lean solvent dominates the overall energy consumption. The energy reduction in the CO2 stripper can be achieved by either formulating new solvents or optimizing the process configurations. The energy reduction achieved by stripper modifications, which include the rich-split process, the interheating process, and the integration of both configurations, have been reported in the literature. In the rich-split process, the cold rich stream is split to recover the energy contained in the overhead vapor, which was directly fed into the condenser in the traditional stripper configuration; therefore the regeneration energy can be reduced. The interheating process draws the liquid flow from the middle of the stripper and exchanges heat with the high-temperature lean solvent from the reboiler; thereby, the overall column temperature can be raised that favors CO2 desorption along the column. Therefore, the combined process, which is the rich-split process integrated with inter-heaters (IHs), takes both advantages of above modifications that can further reduce the energy requirement. However, the present work shows that energy-saving effect of the combined process is not as promising as the literature claimed. Once the design parameters of the rich-split process are selected properly, the rich-split process without IHs can achieve the same energy-saving effect as that achieved by the process of integration with IHs.
Aqueous solutions of alkanolamines are used for absorbents of the post-combustion capture of CO2. Energy requirements for the currently available systems are very high. Thus, it is important to lower the regeneration energy (ΔHreg, kJ/mol-CO2), which consists of three parts: the heat of chemical reactions for CO2 release (ΔHrxn, kJ/mol-CO2); sensible heat (ΔHsen, kJ/mol-CO2); heat of vaporization (ΔHvap, kJ/mol-CO2). In most previous works, it is assumed that the heat of CO2 release is equal to the absorption heat of CO2. However, it is a rough assumption. Thus, we present a thermodynamic model for predicting energy performance of amine absorbents (TMPEA) in order to evaluate the regeneration energy based on the enthalpies of chemical composition changes in a chemical equilibrium analysis. Firstly, we obtained the concentrations of chemical species at a low temperature Ta with a partial pressure of CO2, Pa(CO2), and at a high temperature Tr with Pr(CO2). Thus, we obtained the changes of the concentrations of chemical species from the low temperature state to the high temperature state. Secondly, the changes were expressed with reacted quantities of elementary chemical reactions. We calculated the ΔHrxn value from the enthalpies of the elementary reactions using the Hess's law. Finally, we calculated the ΔHsen and ΔHvap values according to the previous methods. We obtained the ΔHreg value as the sum of the ΔHrxn, ΔHsen, and ΔHvap values. We calculated the ΔHreg values for representative amines such as MEA, AMP, DEA, and IPAE. We found that calculated regeneration energies for MEA depends on working conditions. We demonstrated that TMPEA is useful for evaluating the regeneration energy of CO2.
Carbon dioxide capture, utilization, and storage (CCUS) is a key technology to decrease the emission of greenhouse gas, CO2. The benchmark process using a 30% monoethanolamine aqueous solvent for CO2 capture requires a large amount of energy, in particular, for the regeneration of absorbent at high temperature up to 120 °C. In a recent decade, a variety of absorbents have been investigated to reduce such high energy consumption. Phase separation solvents, which separate into immiscible CO2-rich and CO2-lean phases after CO2 absorption, have been proposed to improve the process efficiency for CO2 capture at atmospheric pressure [1]. In the present study, we attempt to extend the phase separation solvents for high-pressure CO2 capture processes. The p-V-T-x behaviors are studied for the systems of CO2 and phase separation solvents, composed of some amines and ethers at different compositions, over a wider range of temperature and pressure. The could point, inducing the phase separation, can be controlled by the basicity and concentration of amine, which are the predominant factors in the formation of ionic species. The phase separation and p-V-T-x behaviors are discussed in detail for the development of efficient CO2 capture processes at high pressure.
References.
[1] H. Machida, K. Oba, T. Tomikawa, T. Esaki, T. Yamaguchi, H. Horizoe, J. Chem. Thermodyn., 113 (2017) 64-70.
Carbone dioxide Capture, Utilization and Storage has attracted significant attention in the past two decades to reduce greenhouse gas (GHG) emissions and mitigate global warming. However, its capture cost should be further decreased to facilitate its commercial implementation. In fact, CO2 capture is considered as the most energy-intensive part.
In this research, an innovative process using chemical absorption by solid with a circulating fluidized bed has been proposed to reduce energy consumption of CO2 capture. In this process, reaction heat accompanying absorption is successfully supplied to thermal decomposition in regenerator through a heat pump, leading to circulation of whole process heat. To see the influence of the separation performance of the proposed process, some experiments have been conducted. An energy balance of the proposed process using experimental data is simulated and examined using a commercial process simulator (PRO/II, Ver. 9.1). The simulation results indicate that the proposed CO2 separation process has the large energy saving potential as compared with the conventional counterparts.
CCU (Carbon dioxide Capture and Utilization) technology, in which CO2 is captured from large-scale emission sources and the CO2 is converted into high-value added chemicals and fuels, is expected to improve the economical efficiency of CO2 separation and recovery and to enable its commercialization. Among the proposed CCU technologies, organic matter synthesis from water and CO2 under hydrothermal conditions has advantages such as no use of hydrogen and no use of precious metals as catalyst and reductant. Then, in CO2 chemical absorption method using potassium carbonate, we propose that the rich absorbent solution containing CO2 be directly subjected to hydrothermal treatment so as to convert CO2 into organic compounds simultaneously with the regeneration of the absorbent solution for the following absorption process. In this study, we aim to clarify the possibility of converting CO2 into organic compounds by hydrothermal treatment of KHCO3 as a CO2 source. Furthermore, another purpose of this study is to elucidate whether it is possible to regenerate CO2 absorbent solution during the hydrothermal treatment.
A stainless batch reactor was charged with specified amounts of KHCO3 or NaHCO3 as a CO2 source, Fe powders as a reductant, Ni powders as a catalyst and distilled water. The reactor was heated in a sand bath at 300 °C for 120 minutes. After cooling the reactor, the concentration of formic acid and inorganic carbon (IC) contained in the liquid sample were measured with HPLC and a TOC meter, respectively. The results confirmed the formation of formic acid from both KHCO3 and NaHCO3. The yield of formic acid was higher when KHCO3 was used. From the results of IC in the solution after the treatment, it was indicated that most of the introduced bicarbonate was converted to carbonate after the reaction and the absorbent solution could be regenerated.
A key factor for commercialization of CCUS (carbon dioxide capture, utilization, and sequestration) is to establish an efficient and effective CO2 capture process because the capture process possesses the 50 to 80 % of total CCUS operating expense. In this study, we suggests an energy efficient CO2 absorbent comprising the aqueous blend of potassium serinate (K-Ser) and piperazine (Pz). Especially, this CO2 absorbents have high surface tension which make them proper for use in polypropylene (PP) hollow fiber membrane contactor to reduce the membrane pore wetting. By fast solvent screening test, it is revealed that the cyclic capacity of the aqueous blend of K-Ser and Pz is 25 % higher than that of 30 wt% MEA (monoethanoleamine). Vapor-liquid equilibrium was measured to estimate the heat of reaction by calculation using Gibbs-Helmholtz equation. Heat capacity was measured to calculate the sensible heat. Finally, the solvent regeneration energy of the aqueous blend of K-Ser and Pz are estimated and compared with 30 wt% MEA by summation of heat of reaction, sensible heat and heat of vaporization. The long term CO2 removal experiment using Lab-scale membrane contactor will be our future work.
For the CO2 capture from high-volume and CO2-dilute gas such as combustion gas from power plants, chemical absorption with amine is expected to be one of the most feasible technologies. However, its application to the commercial plants for the explicit purpose of CO2 emission abatement still has been limited to a small number. Although there might be several reasons, one of the most critical reasons is its low profitability. Amine solvents require a lot of energy for regeneration, which results in high running cost for CO2 separation.
Our unique phase separation type amine/ether absorbent is expected to realize the CO2 separation process with low-energy consumption (target: less than 2.0 GJ/ton-CO2). This liquid shows a single uniform phase just by mixing the raw materials; amine, ether and water. And it is splitted into two phases consisting of an amine-rich phase and an ether-rich phase by absorbing CO2. Further, it returns to its original uniform phase after CO2 desorbing. During liquid regeneration the ether phase assists the CO2 desorption from the amine phase rich in CO2 by the extraction of CO2, which results in a reduction of energy input for liquid regeneration.
In this study, 500 hours degradation test on this absorbent under cyclic thermal load was conducted in order to clarify 1) the behavior of its performance declination for CO2 capture and 2) the behavior of amine loss. Its test condition was chosen based on two assumptions that a) high-oxidation atmosphere containing O2 and SOX which is destructive condition for the absorbent, and b) adopting practical operating temperature when absorbing or desorbing.
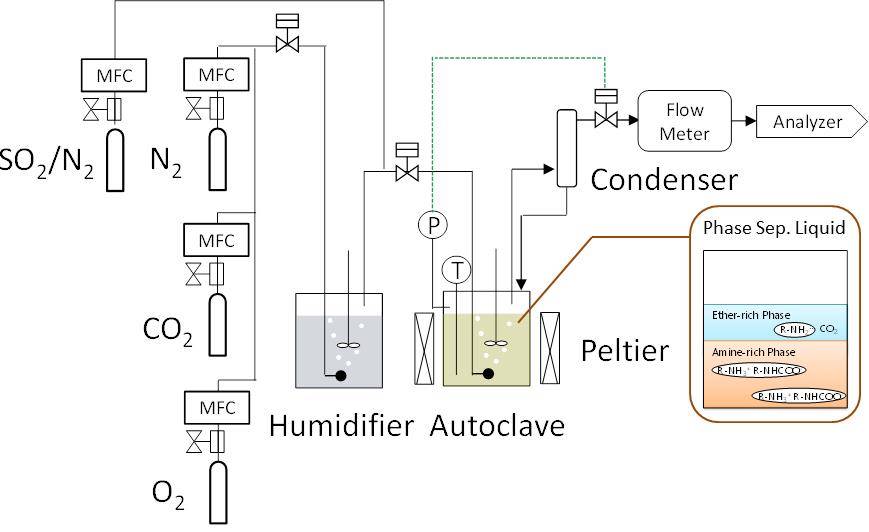
As measures against global warming, CO2 capture and storage technology (CCS) and capture and utilization technology (CCU) has drawn attention of researchers all over the world. So far, our laboratory has developed a new phase separation type CO2 absorbent to improve energy problem in CO2 separation and recovery technology. The CO2·H2 mixed gas recovered from the top of the column can be supplied as it is to the conversion process to methane, methanol and the like. In this process, H2 a component of the source gas, was applied to the bottom of the regeneration tower and reacted with CO2 freed from regeneration process to create methane, methanol and others, which makes it a CCU process. Moreover, without purifying CO2 concentration, this process reduces the energy requirement as compared to the conventional method. In this study, experimental data on stripping regeneration characteristics of phase separated absorbents were acquired. From that, CO2 separation / recovery energy was calculated.
Carbon capture and storage plays an important role in greenhouse gas reduction. Monoethanolamine(MEA) is now widely applied in industry. To overcome the energy penalty of this methods, phase separation solvent was suggested by Machida et al. Since the phase-separation solvent under developing composes of multiple components and separates into two liquid phases after CO2 absorption, it takes time to determine the composition.
Fourier transform infrared spectrometer (FTIR) can simultaneously collect spectra of chemicals within a short time. In order to establish an in-situ method to analyze the components of solution, process FTIR (reactIR) was applied during CO2 absorption. Calibration samples of amines, ethers and CO2 absorbed samples were used to build the model. TOC was used to verify the accuracy of method and model. From FTIR spectra, not only concentrations of components can be determined, phase separation was also observed. Therefore, this method has a prospective application in liquid composition monitoring in plant step.
As a countermeasure against the global warming, early introduction of CCS to large scale CO2 emission facilities, such as thermal power stations, is expected. In general, since CO2 capture process using amine-based solution requires a large quantity of heat for stripping CO2 and regeneration of rich loading solution, process improvement as well as exploring novel solutions is a key to reduction of energy penalty. High concentration 2-Amino-2-methyl-1-propanol (AMP) solution precipitates carbonate with absorption of CO2 and can be expected to reduce sensible heat of regeneration energy by sending the separated carbonate to the stripper. The solution of AMP50wt% promoted by adding Piperazine (PZ), has been tested and successfully operated with solid-liquid separation by a centrifuge. However, the CO2 recovery rate was up to 65% since PZ regeneration reaction did not occurred and PZ was circulated without regeneration. In this study, as a result of searching for new promoter, N-Methyl-1,3-diaminopropane (MAPA) was selected. MAPA primary-carbamate can form an eight-member ring which is easily regenerated, so it is expected to improve CO2 absorption rate in the low CO2 loading range without decrease in the CO2 loading capacity. The operating condition of the small-scale CO2 recovery test was updated by testing AMP/MAPA solution at various temperatures and CO2 loadings. No compounds derived from MAPA were detected in the precipitation solid by results of Raman spectroscopy and GC-MS. Precipitation characteristics of AMP carbonate was empirically obtained by batch tests to determine a stable condition of precipitation process, and cooling temperature was determined 53.4 °C. The injection of semi-lean liquid, which is separated from AMP carbonate by the centrifuge, to the middle inlet seems to be effective to keep the absorption rate even at the top of absorber where the CO2 concentration decreases. The rate-based 10 stages model of absorber using AMP/MAPA solution was built on Aspen Plus and CO2 recovery rate associated with precipitation rate was simulated to determine the position of middle inlet. Injecting the semi-lean liquid to the fifth stage showed the highest CO2 recovery rate. Aqueous solution of AMP 40 wt% + MAPA 5 wt% was evaluated by small-scale apparatus of CO2 capture and recovery with the middle injection process. As a result, the regeneration energy decreased, and CO2 recovery rate reached 90 %.
Global warming and its harmful implications are a major concern for the government and scientific communities worldwide. CO2 being a greenhouse gas is considered to be a major contributor of the global warming. To reduce the anthropogenic emissions of CO2, capturing CO2 from the industrial processes is a must. The solvent based CO2 capture processes is considered to be the most mature and promising technology to capture CO2 from the exhaust streams. Alkanol amines are widely used as a solvent in these processes. However, they are energy intensive and there are still need for development of novel solvents that can make the CO2 capture technology more energy efficient. Potassium salts of amino acids have recently emerged as potential candidate to be used both as a single solvent or as a promoter in the CO2 capture technology. In this work, the potentials of Potassium Salts of Glycine (KGly) as promoter is explored via a kinetics study of reactions of CO2 with different blends of N-Methyldiethanolamine (MDEA) and KGly. Stopped-flow technique was used to carry out the investigations. The experiments were performed over a temperature range of 298 to 313 K and solution concentration of 0.2 and 0.8 mole/l in different MDEA/KGly proportions. Obtained kinetics data were interpreted using zwitterion and termolecular mechanisms for the potassium salts of glycine. The individual rate constants of the participating reactions were regressed and their corresponding activation energies were estimated