CCUS (Carbon Capture, Utilization and Storage) is an important choice for a sustainable low-carbon development in China. The Chinese government attaches great importance to guiding the development of CCUS technology and has taken a series of measures to reduce CO2 emission in the past decade. This speech provides an overview of CCUS technology development which contained specific details about supported program from National Key R&D Program of China. The speech will also introduce several leading large-scale CCUS industrial demonstrations in China, by summarizing the technical characteristics, reactivity, and procedural scheme of each demonstration. An outlook on future development will be provided according to the 13th Five-Year Plan of China (2016-2020), which is focused on promoting large-scale industrial demonstration in the coal-based industry and oil and gas exploration industry.
One of the promising CO2 utilization demonstration projects is carbonation curing of concrete. CO2 curing technology has two major benefits: (i) rapid strength gain of cementitious materials, and (ii) large-scale CO2 sequestration in concrete. The gas-solid reaction kinetics between CO2 and cement-based materials were studied. According to the fitting results of different kinetic models, the carbonation curing of concrete showed a progressive product-layer-diffusion limited kinetics despite different factors. Our group also evaluated the feasibility of blending calcium silicate (CS) as intensified addition, which was found to significantly enhance the reaction rate and CO2 uptake capacity. In terms of practical feasibility, carbonation curing also improved the compressive performance of cement paste with CS. In addition, three typical industrial solid wastes - fly ash, blast furnace slag, and red mud - were studied to replace part of the cement materials to reduce CO2 emissions. The effects of carbonation curing time, pressure, and water-to-solids ratio on the CO2 uptake and compressive strength were comprehensively studied.
As one of the most important technologies for Carbon dioxide (CO2) Capture, Utilization and Storage (CCUS), the practical application of CO2 enhanced oil recovery (CO2-EOR) has been growing rapidly. Since the profitability of CO2-EOR project can be improved by the re-use of CO2 contained in the associated gas, superior CO2 separation technology is essential. JGC Corporation (JGC) and NGK Insulators, Ltd. (NGK) have developed CO2 separation system with DDR-type zeolite membrane. In many cases, the associated gas also contains hydrocarbons in which predominant constituent is methane (CH4). Since DDR-type zeolite has subnano-size pores which are desirable for CO2/CH4 separation, this membrane achieves excellent CO2 permselectivity. In addition, this system can be operated under high CO2 partial pressure conditions (~ 8 MPa) which is suitable for CO2 recovery process from associated gas with high pressure and high concentration of CO2. Recently, JGC has launched the first field test with laboratory scale DDR-type zeolite membrane at a CO2-EOR oil field. The membrane was prepared onto the inner surface of 30 channels in a cylindrical monolithic alumina substrate (diameter: 30 mm, length: 160 mm). In this field test, it has been confirmed that this membrane performed well for CO2 separation from the associated gas. It's of note that the performance observed using the associated gas was substantially equivalent to that of obtained using a simulated gas consisting of CO2 and light hydrocarbons in laboratory tests. In this presentation, the features of the DDR-type zeolite membrane as well as the summary of the first field test at a CO2-EOR oil field are addressed. In addition, the outline of the ongoing demonstration project with commercial scale DDR-type zeolite membrane (diameter: 180 mm, length: 1,000 mm) is introduced.
Along with the growth of CO2 enhanced oil recovery (CO2-EOR) industries, demand of CO2 separation technologies has been increasing to recover CO2 from associated gas which contains not only CO2 but also hydrocarbons mainly CH4. DDR-type zeolite membrane has the subnano-size pores which are preferable for CO2/CH4 separation, resulting in the excellent CO2 permselectivity. In this study, CO2 permeance and selectivity of the DDR-type zeolite membrane were examined under high pressure conditions using CO2/CH4 binary gas mixture to evaluate the potential applicability for CO2 recovery process in CO2-EOR industries. The membrane was prepared onto the inner surface of multi channels in a cylindrical monolithic alumina substrate (diameter: 30 mm, length: 160 mm). The membrane properties are investigated using one channel of the substrate. The test pressure was increased up to 8 MPaG with the temperature range of 90 - 150 deg. C. The CO2 concentration in the gas mixtures were varied from 50 to 100 %. From these experiments, CO2 permeance decreased with increasing pressure and temperature. In addition, CO2 permeance and selectivity decreased with decreasing CO2 partial pressure in the experiments with CO2/CH4 binary gas mixture. CO2 permeance obtained in experiments was correlated by the surface diffusion model coupled with adsorption isotherm equations (Langmuir equation and Dubinin-Astakhov equation). The surface diffusion model could correlate CO2 permeances well against the wide range of CO2 partial pressure. The comparison of CO2 adsorption curves estimated by the correlation was implied that the competitive adsorption between CO2 and CH4 was occurred in this system and it affected to CO2 permeability of the DDR-type zeolite membrane. As mentioned above, the amount of adsorbed CO2 decreased as CH4 concentration in binary gas increased because of competitive adsorption. In contrast, gas diffusion constant and equilibrium constant were not changed.
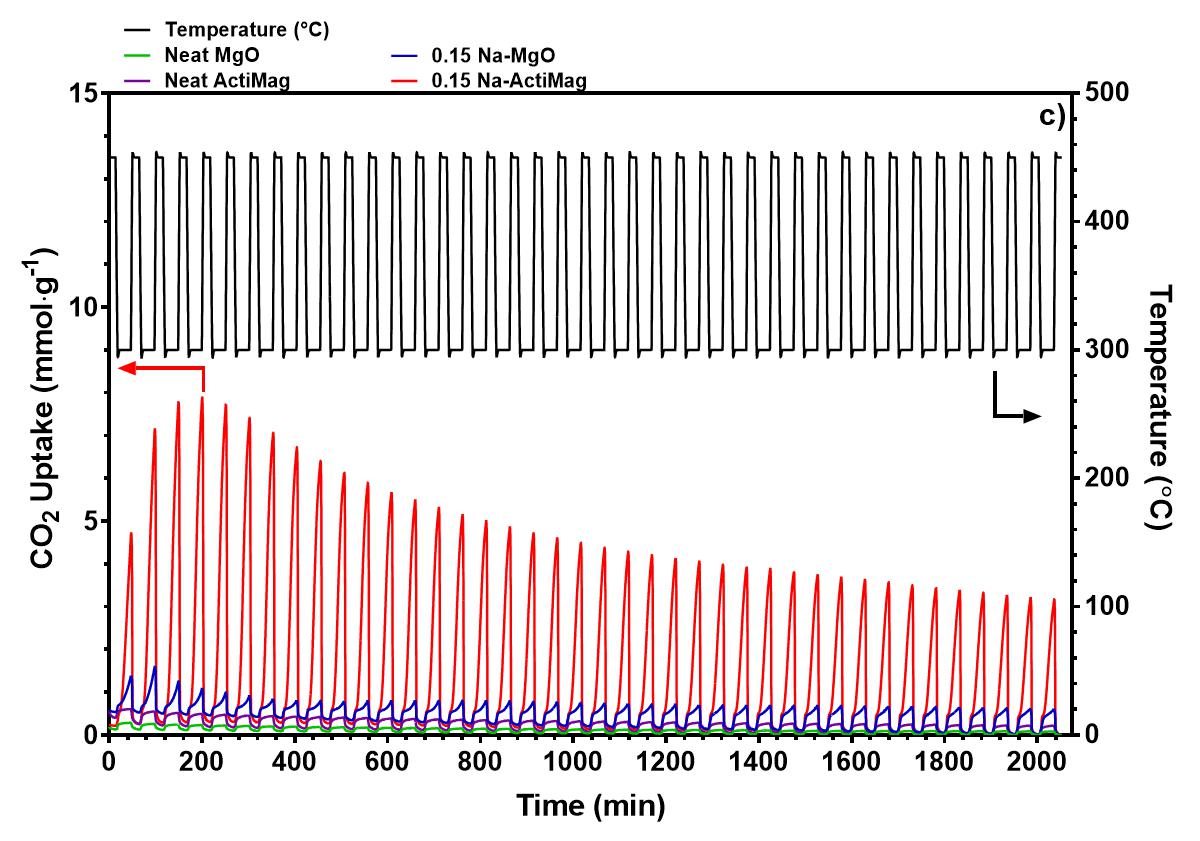
High surface area magnesium oxide (MgO) has garnered interest as an in-situ CO2 sorbent for the water-gas shift reaction (WGSR) for its low regeneration temperature and uptake of CO2 gas at intermediate temperatures of 300 °C upon modification with alkali nitrate salts. Semi-industrial scale production of these high surface area MgO sorbents (>200 m2·g-1) can be achieved via Calix's flash calcination process. Upon modification with NaNO3, the CO2 uptake of MgO in a pure CO2 environment after 1 h improved by a factor of 27 to 11.1 mmol CO2·g-1. The sorbent retained 60% of its CO2 capacity after 40 adsorption-desorption cycles, outperforming a commercial MgO control. The higher uptake of the NaNO3 modified high surface area MgO is attributed to a higher ratio of NaNO3 to MgO, resulting in larger and more similarly sized crystallites of MgO and NaNO3. This improves the extent of interphase boundaries between the molten salt and gaseous CO2, improving the rate of adsorption of CO2 into the sorbent. The molten salt also influences the sintering of MgO and NaNO3, which further increased the crystallite size and surface area in the first few cycles. When applied as a sorbent in the Cu catalysed WGSR, the NaNO3 modified MgO sorbent exhibited a CO conversion of 27% at 300 °C, while CO2 output remained relatively high. The high CO2 output is attributed to the loss of basic sites when the NaNO3-MgO sorbents are mixed with the Cu catalyst, which lowers the retention of CO2 at 300 °C, and impedes the effect of NaNO3 in capturing CO2 in the WGSR.
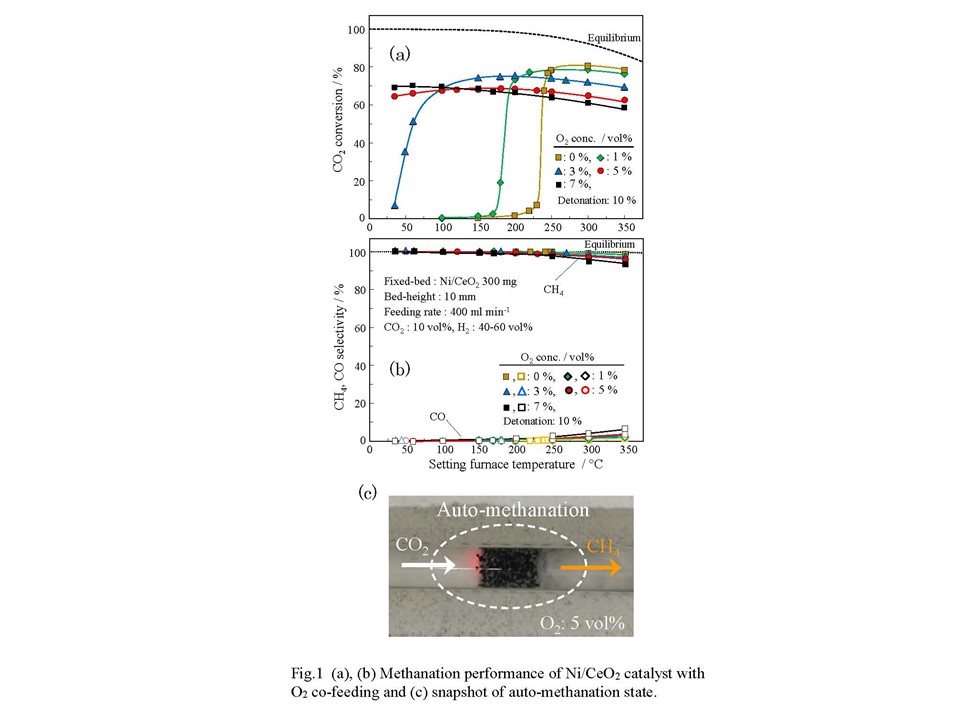
The CO2 methanation has received attention as a reaction converting CO2 into a useful resource such as methane (PtG technology). When assuming that methanation is used as a reaction to process CO2 emitted from an industrial process, the presence of oxygen may have a negative influence, such as an oxidation of the active site. In this study, to examine the influence of O2 on the catalytic function, the methanation performance of a Ni/CeO2 catalyst was evaluated using a raw material gas containing oxygen of 1–10 vol%. As a result, the methanation activity at a lower temperature was greatly improved, and the ability to produce a high methanation performance was realized even in a region at room temperature. We found a novel route of CO2 methanation that can be driven with no heating, even in ambient, which we named as auto-methanation. No one in the World has reported such transformation route yet.
A granular Ni/CeO2 catalyst was prepared using evaporation to dryness of Ni(NO3)2·6H2O on CeO2 support. The CO2 methanation was investigated using conventional flow-type reactor by introducing feed-gas at setting temperature of 350 °C down to room-temperature. The raw gas contained CO2, H2, O2, N2. The CO2 concentration was fixed at 10 vol% with 400 ml/min.
Figures 1(a) and 1(b) show CO2 conversion and product-selectivity. When supplying oxygen of 1 vol% and 3 vol%, the drastic change in conversion shifted to the lower side, which was near room temperature. At oxygen of 5 vol% and 7 vol%, about 65 and 70% conversions were obtained even at room temperature without external heating. Methane selectivity was almost 100% at lower temperature. Figure 1(c) is snapshot of the auto-methanation state. A combination of methanation and combustion might offer a novel CO2 transformation technique, which will open a new process.