
Recently, lignocellulose biomass has attracted as new carbon resources. Separation of the biomass components, which are cellulose, hemicellulose, and lignin, is necessary for biorefinery, and some separation techniques have been developed for long years. Especially, organosolv method, in which a mixture of water and organic solvents is used for depolymerization of lignin and hemicellulose, has been focused on due to its potential for contribution to biorefinery.
Many researchers have investigated kinetics of delignification and pointed out the effect of mass transfer on the delignification rate. However, no one have modeled the rate of delignification including effects of chemical reaction and mass transfer.
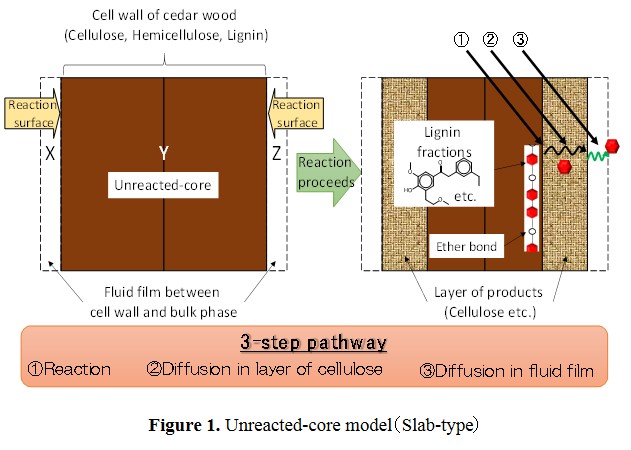
In this study, we have tried to model kinetics of delignification on organosolv method using 1-butanol by unreacted-core model, as shown in Figure 1. This model is used to express situations in which solid particles are being consumed by reactions, and the amount of the material being consumed is shrinking.
Delignification of cedar wood was carried out in a water/1-butanol mixture using batch reactor at 473 K for 0–8 h. After delignification, the products were filtered and divided into liquid and solid products. The solid products were dried, and then analyzed using elemental analyzer to measure carbon content. Chemical composition of cedar wood and the solid products were decided by NREL method.
Conversion of lignin (XL) was calculated in terms of carbon. Based on the unreacted-core model, the overall delignification rate equations were derived for the 3-step pathway shown in Fig.1. The experimental data were found to be well expressed by the rate equation derived on the basis of diffusion in layer of cellulose as the rate controlling step fot the overall delignification rate.
Acknowledgement
This work was supported by the Advanced Low Carbon Technology Research and Development Program (ALCA) as a project from Japan Science and Technology Agency.
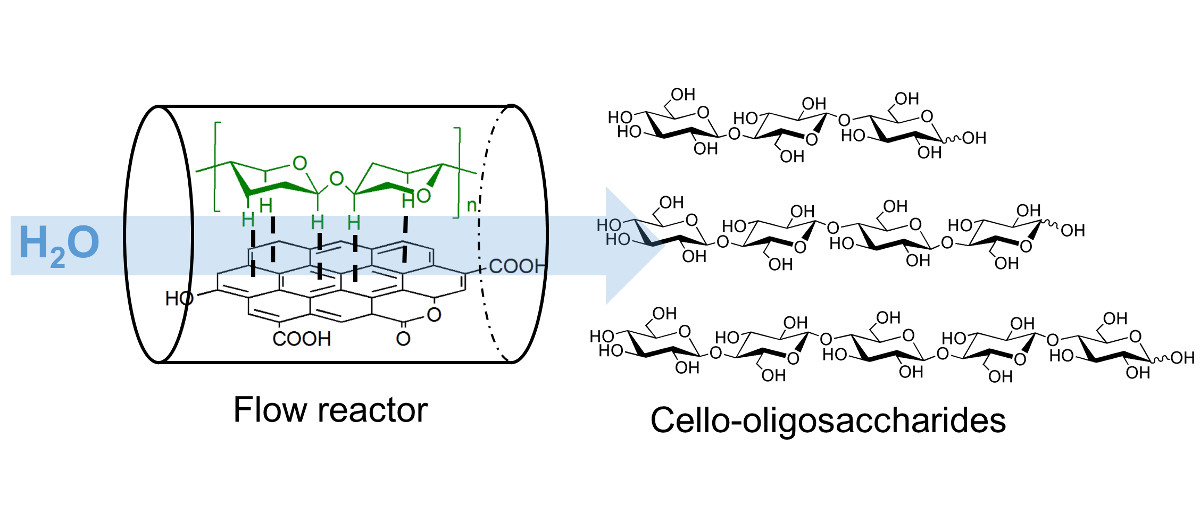
Cello-oligosaccharides are short chain bio-active molecules with the ability to improve the immune defense of plants, animals and humans. These oligosaccharides are only produced in very small quantities and their commercial use is restricted due to the absence of an efficient synthesis process.
Cellulose has a similar structure to these oligosaccharides albeit with a high chain length. Partial hydrolysis of cellulose can produce the desired oligosaccharides but the reaction is difficult to control because the reactivity of molecules increases with the shortening of polymer chain in a conventional hydrolysis reaction.
We have invented a carbon catalyzed semi-flow process for synthesis of food grade cello-oligosaccharides by partial hydrolysis of cellulose. Carbon catalyst was decorated with oxygenated functional groups, which acted as the active sites for hydrolysis. High rate of hydrolysis was achieved by adsorbing the cellulose on carbon catalyst by a solid-state milling step. During reaction, the semi-flow process rapidly removed the dissolved cello-oligosaccharides preventing their further hydrolysis to monomers. Total cello-oligosaccharide yield of 72 % was obtained by optimizing the process at a space velocity of 70 h-1 and a temperature of 473 K.
The composition of oligosaccharides mixture was determined by developing a quantification method based on MALDI-TOF mass spectrometry. The data showed main products had chain length of less than 8 monomer units but oligosaccharide with chain length as high as 13 was also detected. Kinetic data showed the rate of hydrolysis reduced with a reduction in chain length owing to the weaker adsorption of smaller oligosaccharides on carbon surface.
Consequently, we were able to produce a drop-in cello-oligosaccharide solution from cellulose with high yield. The product solution was free from any contamination and could be readily used in healthcare and agriculture industry.
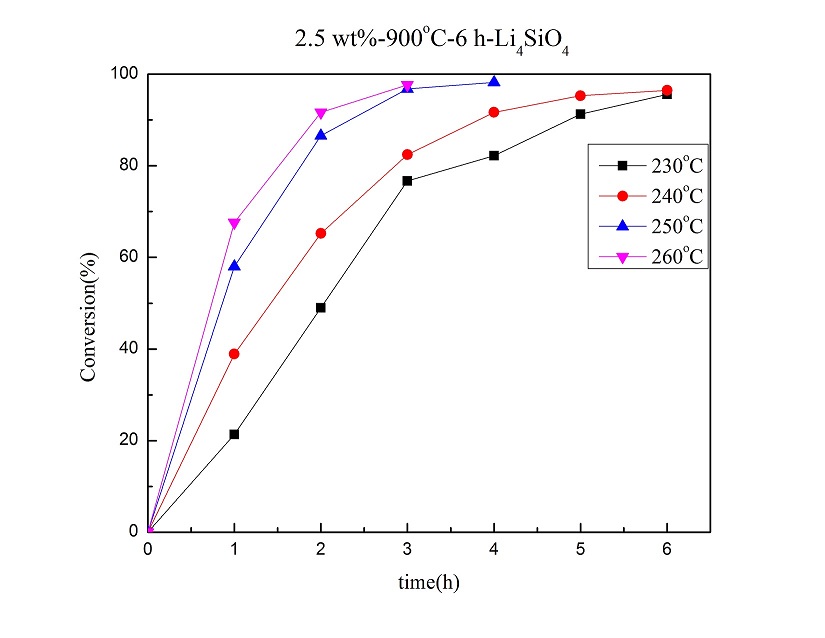
The catalytic solvent-less etherification of glycerol for glycerol oligomers using lithium orthosilicate (Li4SiO4) as a heterogeneous basic catalyst was studied. The Li4SiO4 catalyst was synthesized by solid-state reaction, and characterized mainly using various instruments, including XRD, BET, SEM, ICP-OES, ss-NMR, and the automatic titrator. The etherification reaction was performed from 230 °C to 260 °C for 6 h. GC analysis was applied to quantify the reaction products after being treated properly with silylation. With Li4SiO4 calcined at 900 °C for 6 h, the solvent-less etherification with 2.5 wt% Li4SiO4 catalyst at 240 °C for 3 h resulted in 82% for glycerol conversion and near 87% in the selectivity of di- and tri- glycerol in linear form. According to the ss-NMR and XRD analyses, Li4SiO4 was mostly transformed into lithium metasilicate (Li2SiO3) after reaction. Evidently, a significant portion of Si-O bonds of Li4SiO4 were broken during the glycerol oligomerization. Based on the Eley-Rideal mechanism, the activation energy of this catalytic reaction is 85.93 kJ/mol.
Keywords: Etherification, Glycerol, Lithium Orthosilicate, Catalyst, Diglycerol, Triglycerol
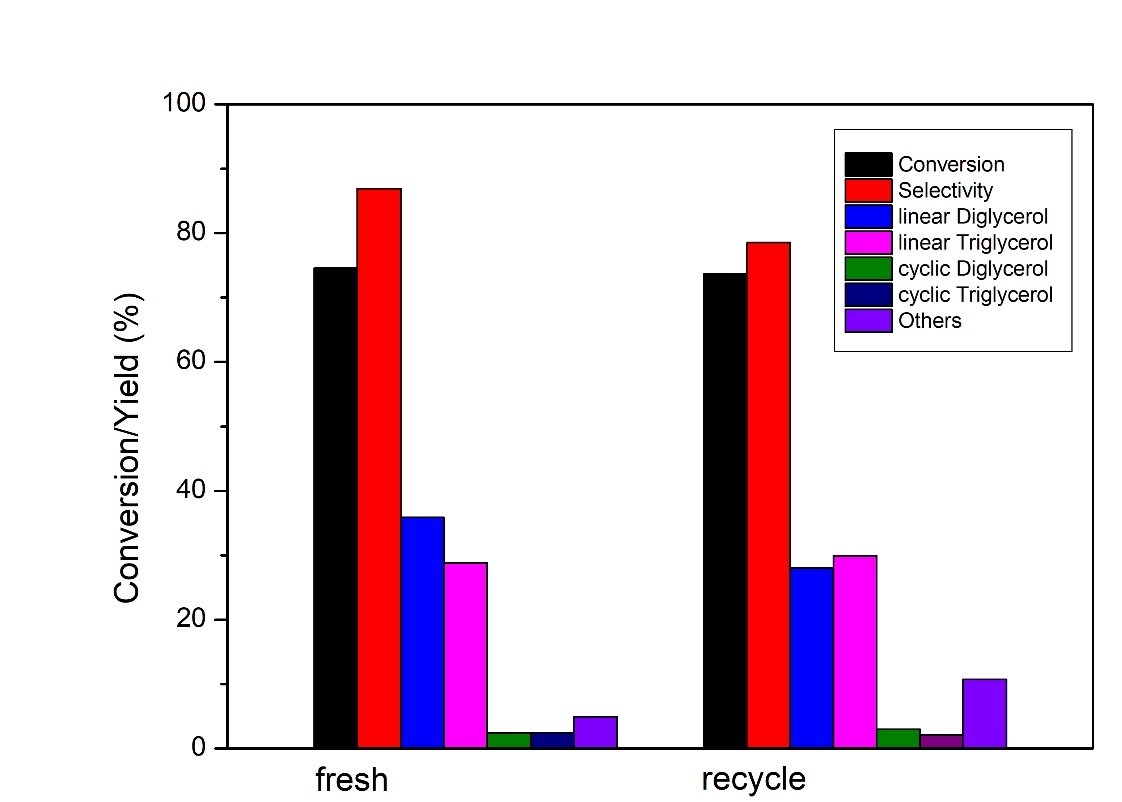
In this work, alumina supported Ca/La mixed oxides as efficient catalysts in oligomerization of glycerol were successfully prepared by the wetness impregnation method using La(NO3) and Ca(NO3) solutions as well as boehmite as support, and followed by calcination at 873K. Various instruments including BET, TPD, XRD, SEM, and ICP-OES analysis was employed for characterization of these catalysts. The catalytic etherification reaction of glycerol was typically conducted at 513 – 553K. In a typical run, the reaction was performed at 553K with a catalyst loading of 3 wt%, based on the mass of glycerol as the sole reactant and the atomic ratio of Ca/La = 2.7 on the catalyst. After 8 hours of catalytic etherification, nearly 75% of the glycerol could be converted to glycerol oligomers, while the selectivity of di-glycerol and tri-glycerol over all products was near 87%. The reusability and stability of the catalyst were tested. It was found that the recovered catalyst still showed good stability and conversion in two consecutive reactions. After 8 hours, the reaction still achieves 78% for glycerol conversion and 75% as the selectivity.
Keywords: etherification; alumina; glycerol; di-glycerol; tri-glycerol; Ca/La/alumina
In this study, Ni/Mo bimetallic catalysts supported on zeolite ZSM-5, aka Ni-Mo/ZSM5, catalysts were successfully prepared by the wetness impregnation method to heterogeneously hydrotreat palmitic acid with an aim for the production of liquid alkane fuels. A Ni-content about 10 wt% of zeolite ZSM-5 support with various Mo-contents was found on these Ni-Mo/ZSM5 catalysts. Various instruments, such as XRD, SEM, BET, XPS, NH3-TPD and H2-TPR, were employed to characterize these catalysts. Typically, the catalyzed hydrodeoxygenation (HDO) reaction of palmitic acid was carried out in a batch autoclave at 573 K with an initial pressure of hydrogen at 35 bar at this temperature. With Ni-Mo/ZSM5 catalysts, the conversion of palmitic acid could reach near 99% at 573K after HDO reaction for 4h. Furthermore, a significant portion of isomerized alkanes were obtained in the product as revealed by GC-MS. That is, both hydrodeoxygenation and hydroisoermization reactions took place conjointly with Ni-Mo/ZSM5 catalysts.
Keywords: Biofuel, Palmitic acid, Zeolite ZSM-5, Ni-Mo/ZSM5 catalyst, Hydrodeoxygenation.