
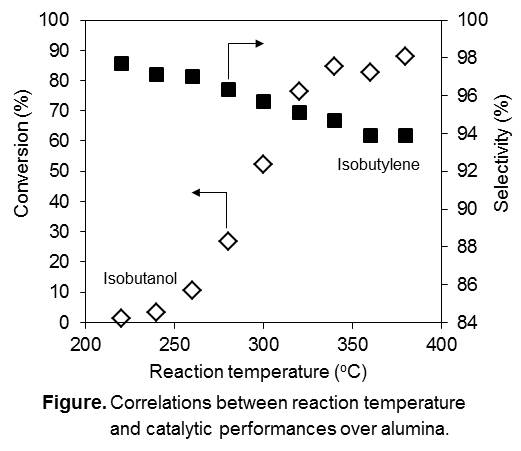
Methyl methacrylate (MMA) has been widely utilized as a raw material of poly methyl methacrylate (PMMA), a typical transparent polymer, and its global manufacturing capacity of MMA is ca. 4,500 kte/y. A few processes have been industrialized so far and ca. 30% of MMA can be produced from isobutylene. In fact, our MMA plants starting from isobutylene are well working in the world. Industrially, isobutylene is mainly derived from naphtha and it is preferable to find alternative isobutylene sources for further expansion of our activity. On the other hand, dehydration of an alcohol is a very useful reaction to obtain the corresponding olefin. As you may know, much attention has recently been paid on isobutanol dehydration to produce isobutylene, especially for the dehydration of bio-based isobutanol into isobutylene, which can be potentially utilized in MMA process. Therefore, our aim is to establish an efficient process for isobutylene production from isobutanol by dehydration. The established dehydration process could be combined with our MMA process starting from isobutylene. We have investigated the dehydration of isobutanol over alumina catalysts. The conversion of isobutanol reached 85% with 95% selectivity of isobutylene at 340oC. The main by-products were linear butenes that formed by isomerization reaction but it was reduced at high concentration of isobutanol. As a result of kinetics studies, it indicated that the reaction pathway consisting of the formation and decomposition of diisobutyl ether became preferable under high concentration of isobutanol. Characterization of alumina was executed to see correlations between crystal structure, acidic properties and catalytic performances. In addition, a long-term continuous reaction at a bench scale showed very stable performances for more than 1,500 h and a design of the potential process flow was proposed.
Industrial metallocene catalysts have to be supported on a MAO modified solid carrier. Micro-sized amorphous and porous silica is the most common used for supporting metallocence / MAO catalysts.
Commercial porous silica is made by a multi-step process with aqueous alkali metal silica as the precursor , which makes it less cost effective and not very environmentally friendly. Thus, there is a need to find a less expensive and more environmentally friendly silica precursor. Silica generated from agricultural waste is more cost effective and environmentally friendly than silica from traditional commercial processes.
In this study, spherical silica particles with a diameter of around 120 nm were fabricated from rice husk ash (RHA), and were used to support two bridged zirconcene complexes ((I) Me2Si(Ind)2ZrCl2 and (II)C2H4(Ind)2ZrCl2 ) for catalyzing propylene polymerization to produce polypropylene (PP) in a temperature range of 40-70 °C and in a solution MAO range of 0.1-0.6 wt%. Due to its small particle size, RHA-supported catalyst exhibited much higher activity than micro-sized commercial silica supported catalyst. At the optimum polymerization temperature of 55 °C and with increasing MAO concentration, polymer yield increased proportionally with the increase of number average molecular weight. Compared to (I), (II) produced more polymer molecules but with much shorter chain length, ascribed to the differences of Zr loading and bridge structure. With increasing polymerization temperature, polymer molecular weight and isotacticity decreased rapidly and resulted in a significant change of PP assembly morphology (shape and size). At 55 °C, (I) produced uniform PP assemblies which had dumbbell-like structure with smooth middle section and two fibrillar ends , while (II) produced spherical PP particles. The width of dumbbell middle part was essentially identical to the Batchelor microscale proposed in turbulent mixing theory.
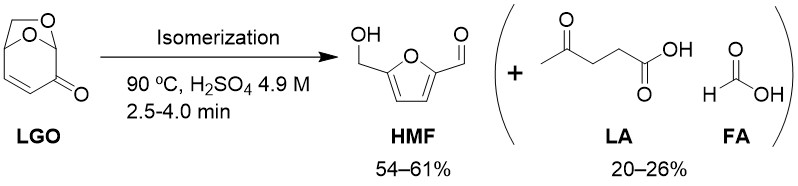
Hydroxymethyl furfural (HMF) is a biorenewable important intermediate molecule for synthesis of a variety of furan derivatives that have a potential in fuel, solvent and polymer applications. Levoglucosenone (LGO), anhydrosugar available from cellulose pyrolysis, has recently been identified as a HMF feedstock. According to the recent studies, LGO can be converted to HMF under milder conditions only with acid and water as catalyst and solvent, respectively. To further explore the potential of this reaction, in this study, we demonstrate the HMF synthesis below 100 °C within a short time of a few minutes at high yields. HMF forms from LGO by the isomerization under acid catalysis, but LGO and HMF suffer from side reactions, excepting the hydration of HMF to levulinic acid (LA) and formic acid (FA), to form humins as unwanted polymeric side products. In the initiation step of this research, we employed high concentration of sulfuric acid (4.9 M) to promote the reaction at low temperatures and carried out the reaction in a batch reactor. However, the yield of HMF was limited to below 30%, and the formation of humins was visually apparent. This was likely caused by the presence of HMF and LGO with high concentration sulfuric acid for a long time to achieve the desired reaction temperature. To solve this problem, the reactor was changed to a microtube reactor, which enabled quick heating of the solution and precise control of the reaction time. As a result, the HMF yield was improved to above 60% in the reaction at 90 °C for 2.5 min. The highest total yield of HMF, LA and FA, indicating the reaction selectivity, was 87% at 4.0 min. When using conventional feedstock such as glucose or fructose, the product yield was much lower even with longer reaction time (<6 min).
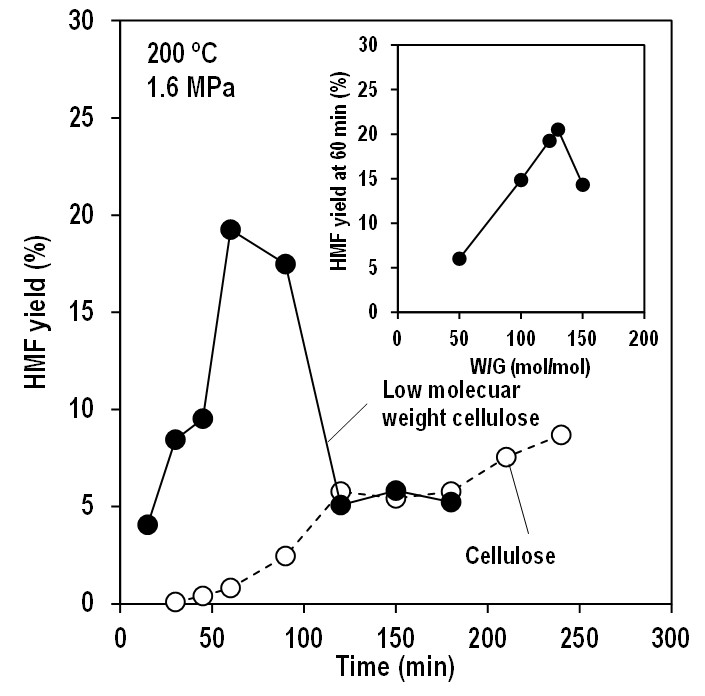
Cellulose is one of the most important biomass resources which can be converted to value-added chemicals, e.g. mono- and oligo-saccharides, furans and organic acids. Among them, hydroxymethylfurfural (HMF), which is precursor of biofuels, polymers, medicine and the other chemicals, is one of the most important target compound. Normally, direct conversion of cellulose to chemicals is difficult because it is stable and insoluble any solvents. Because of these cellulose properties, special solvents and catalysts are developed, however, they are normally expensive and sometime toxic. For these reasons, direct conversion of cellulose to HMF by using eco-friendly way is still challenging research. Here we report that direct conversion of cellulose to HMF using saturated steam, i.e. only water. It is well known that high-pressure, high temperature water, such as subcritical or supercritical water, can promote chemical reactions including hydrolysis and dehydration of saccharides. To directly convert cellulose to HMF, firstly, glyosidic bonds in cellulose must be hydrolyzed, and then, obtained glucose must be dehydrated. Therefore, hydrolysis followed by dehydration must be proceeded in one pot. Because of high hydrolyzability of high-pressure, high-temperature water, cellulose and obtained HMF are easily converted to undesired compounds. To avoid this undesired reactions, we used saturated steam, whose hydrolyzability might be milder than subcritical water. Because of mild hydrolyzability, cellulose was effectively converted to HMF up to 21 %. In addition, we found that pretreatment of cellulose and amount of added water (W/G, molar ratio of water and glucose unit in cellulose) strongly affect to HMF yield. The obtained results indicate that possibility to produce HMF from cellulose using only water, without using special solvents and catalysts.
Lactic acid (LA) is the carboxylic acid that can be applied for using in food, pharmaceutical and chemical industries. An application of using lactic acid as the initial raw material for biodegradable polymer such as polylactic acid (PLA), has been an attractive issue for scientists and engineers. Although lactic acid is commercially produced by fermentation of aqueous glucose using microorganisms, biological processes have various drawbacks for example low reaction rates, and this will lead to the long reaction times and the huge reactors required. The low concentrations of products usually in water will require high energy consumption for separation and purification of lactic acid from water. Therefore chemical processes to synthesis lactic acid from petrochemical resources and other biomasses are still challenged. In this study cellulose, one kind of biomass, is used as the raw material to convert to lactic acid via hydrothermal process in a presence of catalyst. The reaction is conducted in either high pressure reactor or microwave reactor at 200oC, three types of catalysts are used, ZrO2, Al2O3 and mixture of ZrO2 and Al2O3. It is found that the catalytic conversion of cellulose to lactic acid in the high pressure reactor is quite low, the yield obtained from using ZrO2 and Al2O3 are 8.02% and 6.63%, respectively. In the case of hydrothermal process in microwave reactor using ZrO2 as catalyst, it is found that the production yield of lactic acid is higher about 35%. Therefore the microwave reactor can be used as an alternative method to increase the production rate of lactic acid.
In this study, the non-isothermal reaction characteristics and kinetics of biomass devolatilization and char gasification were investigated by TGA. Four kinds of devolatilization atmosphere (N2, N2+CO2 (10%), N2+CO2 (50%), N2+steam (10%)), three kinds of gasification atmosphere (N2+CO2 (10%), N2+CO2 (50%), N2+steam (10%)) and different heating rates (5, 10, 20, 30, 40 K per min) were adopted. Meanwhile, the gasification behaviors of in-situ char and ex-situ char were also compared. The results show that the volume reaction model and shrinking core reaction model can describe well the reaction behavior of biomass devolatilization and char gasification, respectively. With the increase of heating rate for a certain atmosphere, the typical reaction temperatures for both biomass devolatilization and char gasification increased obviously. At a given heating rate, the adopted inert and reactive atmospheres displayed a very weak influence on biomass devolatilization, regardless of reaction behavior and kinetics. However, the devolatilization atmosphere indeed had a remarkable effect on the following char gasification even for the same gasification agent. The in-situ char produced from the atmosphere of N2+steam (10%) had a higher reaction rate and a lower E than the ex-situ char generated from the N2 atmosphere, indicating the necessity of adopting in-situ char for gasification reaction analysis. For the adopted atmospheres, the calculated values of E for char gasification were ranked from large to small as N2+CO2 (50 %), N2+CO2 (10 %),N2+steam-2 (10 %),N2+steam-1 (10 %).