In order to obtain a phosphate rock equivalent, that is, calcium phosphates, from incineration ash of chicken manure which was obtained from power generation system using the manure, the incineration ash was treated with an aqueous solution of nitric acid to elute phosphorus. In using 0.3 M HNO3, most of the phosphorus could be eluted from 1.0 g of the ash within 0.1 h. Compared to composted chicken manure which was previously examined in our laboratory, the concentration of HNO3 should be increased for the elution. However, in case of incineration ash of chicken manure, since it was possible to remove inorganic species having a lower boiling or sublimation temperature and organic species by calcination in the power generation system, the concentration of phosphorus contained in the incineration ash together with the nitric acid extract became higher than those in the composted chicken manure. When nitric acid extract thus obtained was treated using aqueous NH3, the precipitation was formed. XRD showed that the precipitation is poor-crystallized calcium hydroxyapatite (Ca10(PO4)6(OH)2) which is one of main component in the phosphate rock. In order to confirm the certain formation of calcium phosphate species and the purity, the precipitation was calcined at 1,078 K for 5 h. XRD revealed that the calcined solid was tricalcium phosphate without evident contamination. These results revealed that phosphate rock equivalent could be easily obtained from the incineration ash of chicken manure and approximately 14% of phosphate rock imported into Japan can be replaced by the precipitation obtained from of the elution-precipitation treatment of the incineration ash of chicken manure.
Fenton reaction has been believed to be effective for degrading organic pollutants in the wastewater because Fenton reagent, ferrous ion and hydrogen peroxide, is not harmful and expensive. However, it is also well known that oxalic acid, a typical product of Fenton oxidation, forms oxalate complex of ferric ion to interfere with the regeneration of ferric ion to ferrous ion. As a result, Fenton oxidation is quenched with the generation of oxalic acid. Irradiation of UV light is often used to enhance the mineralization utilizing ligand to metal charge transfer of oxalate complex; however, energy consumption of light irradiation is costly. It is desired to accelerate Fenton oxidation to enhance mineralization.
In this study, effect of copper ion on the mineralization of phenol is examined. Mineralization of oxalic acid was enhanced in the presence of copper ion. It is well known that oxalate complex of ferric ion is hard to be decomposed by OH radicals generated by Fenton reaction. This is the reason why that mineralization of oxalic acid is inefficient on the light-assisted Fenton oxidation. In the presence of copper ions, oxalate complex of copper ion is formed. To clarify the reactivity of oxalate complex of copper ion with OH radicals, DMSO was added as a scavenger of OH radicals. Mineralization rate was significantly lowered in the presence of DMSO, indicating that reactivity of oxalate complex of copper ion is rather high. 500 mg/L phenol could be completely mineralized within 120 min in the presence of copper ion whereas more than 5 h is necessary in the absence of copper ion. Significant acceleration of regeneration rate of ferric ion was also observed in the presence of copper ion.
With the rapid and tremendous development of industries in the past few decades, several hazardous pollutants, especially arsenic, selenate, et al, were discharged into aquatic streams. Considering humans' health, the highly detrimental metal or non-metal ions beyond their permissible limits should be removed from the wastewater before discharge. The removal of metal or non-metal species from contaminated water has attracted worldwide attention of scientists. However, some species such as arsenite and selenate are extremely difficult to be removed by conventional methods.
With the aim to find an effective removal method for arsenite from wastewater, we applied supported Pt catalysts onto the removal of arsenite for the first time. The catalytic activity of the Pt catalysts on the removal of arsenite was investigated.
Arsenite was successfully oxidized to arsenate which can be easily removed by conventional precipitation method, with oxygen over supported Pt catalyst in an aqueous solution. The catalytic oxidation of arsenite with oxygen over Pt catalyst obeyed first-order kinetics, and the apparent activation energy was 50 kJ/mol. The catalytic activity of Pt catalyst was significantly influenced by the Pt particle size. The Pt catalyst possesses an excellent stability and can be used repeatedly with a high catalytic performance.
Thus Pt catalyst has a splendid prospective application on the removal of arsenite from contaminated water due to its outstanding catalytic performance, high chemical stability and recyclability.
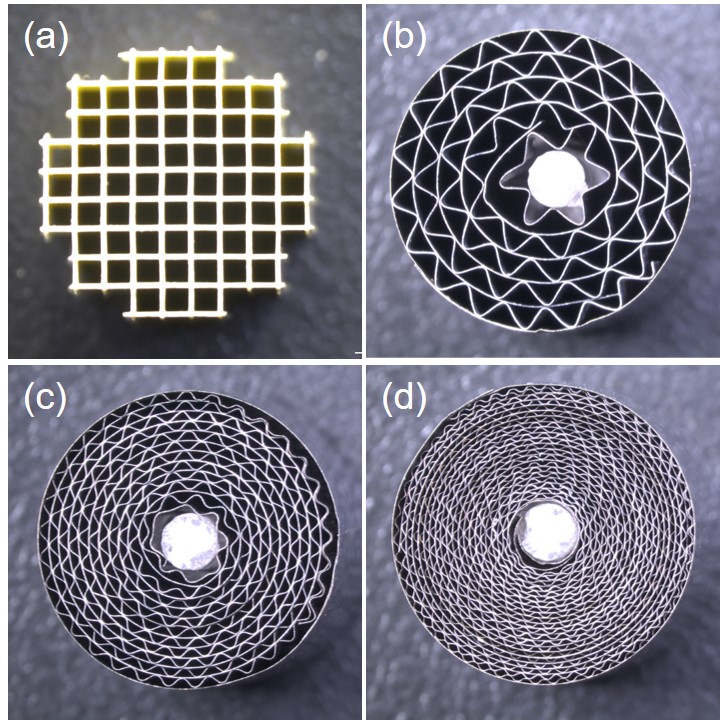
The automotive three-way catalyst (TWC) is composed of platinum group metals (PGMs), which are mainly deposited on washcoated cordierite ceramic substrates via wet impregnation processes. Another substrate that consists of thermally durable Fe–Cr–Al metal foil is also used due to their attractive features such as higher thermal conductivity and lower pressure drop compared with conventional ceramic substrates, whereas the honeycomb catalysts are prepared via the same wet impregnation and/or slurry coating regardless of the different substrate materials. We recently succeeded to prepare an active Rh nanolayer catalysts on the Fe–Cr–Al metal foils using a pulsed arc-plasma (AP) deposition. Regardless of the very small geometric surface area of metal foils with nonporous and smooth surface, this new catalyst delivered a high catalytic performance for simulated TWC reaction with high turnover frequency compared with conventional Rh powder catalyst (Rh/ZrO2). A miniature honeycomb (11 mm×10 mm, 900 cpsi) was easily fabricated using flat and corrugated metal foils after coating of Rh nanolayer (Fig. b), and it showed steep light-off for NO, CO and C3H6 and complete conversion in the simulated TWC test at GHSV = 120000 h–1. Due to unnecessary of conventional wet processes such as impregnation and washcoating, we successfully fabricated the metal honeycomb converter with much higher cell density of 4,000 cpsi (Figure c) and 9,000 cpsi (Figure d), the activity of which was enhanced. The AP-processed honeycomb catalysts with surface-modified foils are promising not only for automotive TWC applications, but also for other industrial catalysts. It is a simple dry process compared to conventional wet catalyst preparation and thus the substantial decrease of PGM use may also be expected.
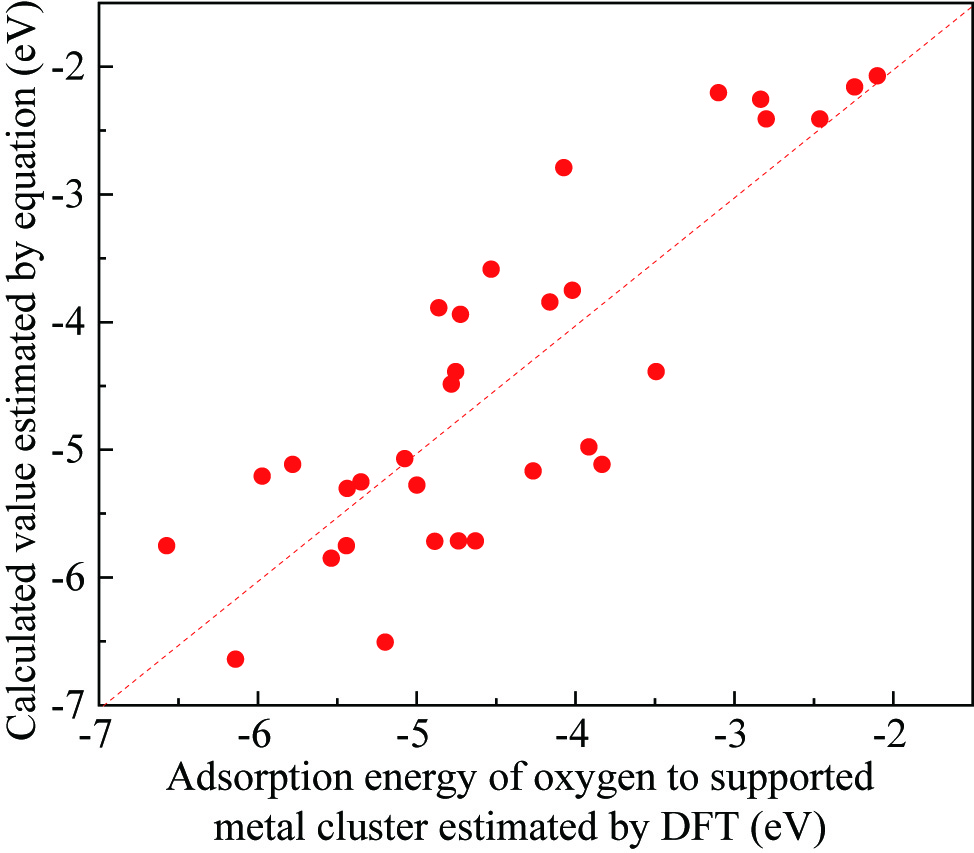
This study carried out the Density Functional Theory (DFT) calculation, to understand the catalytic activities of supported noble metal catalysts. The adsorption energies of the oxygen on the supported noble metal clusters (EDFT) were estimated for several structure models (e.g. Pt3/TiO2, Pt3/Al2O3, Pd3/TiO2, Pd3/Al2O3, Rh3/TiO2, Rh3/Al2O3 etc.). As a result, it was found that the EDFT varies depending on the chemical potential of oxide support (mMOx), d-band center of noble metal (ed) and the number of atoms (n) contained in the structural model. We proposed the empiric formula, Ecal=C1(mMOx-mO)2n/(n+1)+C2ed+C3, to estimate the EDFT values. The unknown parameters, C1, C2 and C3, can be determined so that the Ecal values corresponds the EDFT values. The figure shows the relationship between the Ecal and EDFT values. This correspondence suggests that the EDFT values can be estimated by obtaining the mMOx and ed values by the DFT calculation. The Brønsted–Evans–Polanyi (BEP) principle implies that there is a linear relationship between the activation energies (Ea) of the supported noble metal catalysts and EDFT values. Namely, there is a possibility that the catalytic activity (Ea) can also be estimated by the mMOx and ed values via Ecal values. To verify this possibility, this study carried out the experiments. The supported precious metal catalysts, with different noble metal (Pt, Pd, Rh) and different support oxides (Al2O3, SiO2, TiO2 etc.), were prepared by the impregnation method. Then, the propylene (C3H6) oxidation activities for prepared catalysts were analyzed by a fixed bed flow reactor. Moreover, the Ea values were obtained by the Arrhenius plot. As a result, a well matched correspondence was found between the Ecal and Ea values.
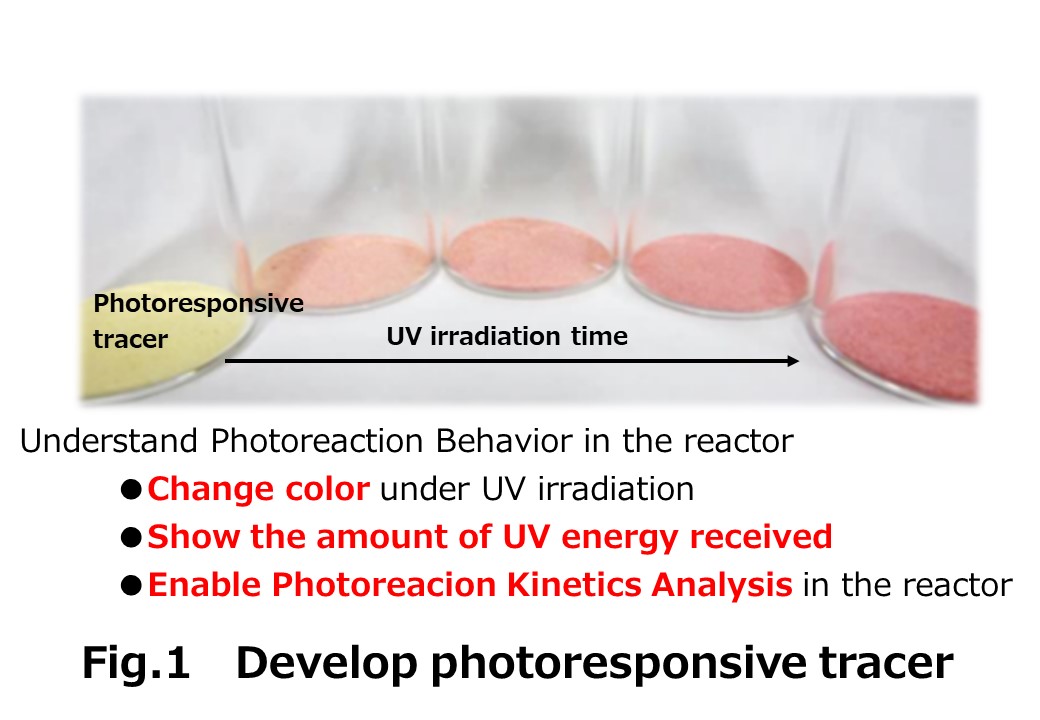
A photoresponsive tracer that changed color under ultraviolet (UV) irradiation was synthesized by immobilizing a photoacid generator and methyl orange on a poly(vinyl chloride) (PVC) carrier resin to understand photoreaction behavior in a dry powder photoreactor. The tracer changed color as a function of the received cumulative radiant energy.Tracer particles were fluidized in a fluidized bed photoreactor under various conditions and exposed to UV irradiation from the side of the reactor. The color change of the tracer during irradiation was recorded using a video camera. By analyzing the recorded images, the color transition of the tracer could be continuously detected as a change in the green component of RGB color value. The apparent photoreaction rate constant of the tracer in the fluidized bed photoreactor was successfully estimated by analyzing the rate of change in the green RGB value during irradiation. The transmission of UV into the powder layer were found to obey the Lambert-Beer law. The photoreaction behavior in the reactor was successfully reproduced by a simple two-phase model consisting of a reaction zone and a non-reaction zone. The present method for evaluating the performance of dry powder photoreactors by use of a photoresponsive tracer is very simple. Since the reaction behavior can be visualized, it is intuitively easy to understand and useful for designing dry powder photoreactors.