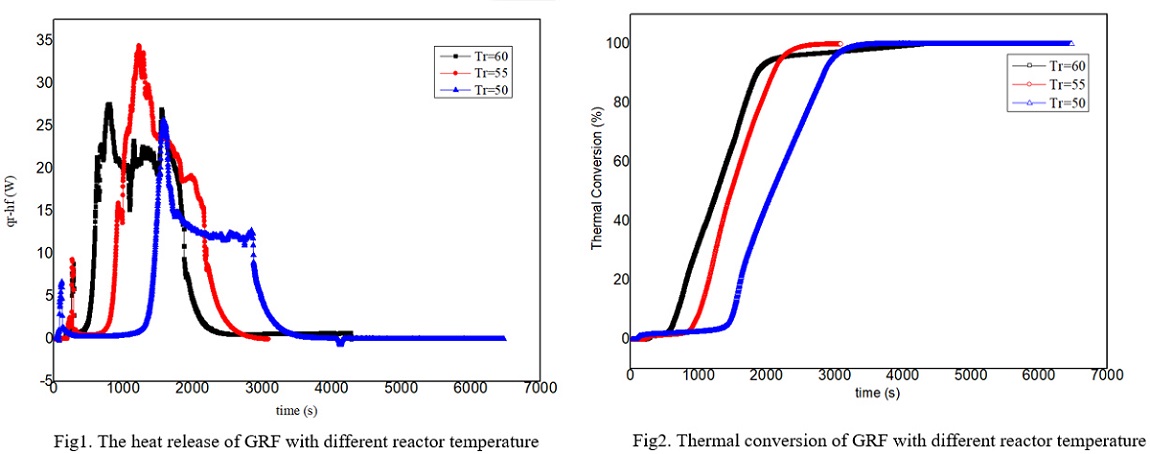
Grignard reagent is often synthesized from magnesium and halohydrocarbon in dry ether such as tetrahydrofuran(THF) or diethyl ether(DE). The Grignard reagent formation(GRF) is a strong exothermic process which has an induction period. If the material is accumulated due to improper initiation, it is easy to cause the thermal runaway after the reaction is suddenly triggered. With the development of green chemistry, more and more chemical companies have begun to develop safer Grignard reagents by changing the solvents. Therefore, it is particularly important to carry out the reaction thermal safety risk assessment for the GRF in different solvents and establish the critical criterion for the thermal runaway reaction of the GRF.
In this work, the n-butylmagnesium bromide GRF was taken as an example. The safety risk assessment of the GRF was carried out by using a cooling failure model using THF, DE, cyclopentyl methyl ether(CPME) and diethylene glycol dibutyl ether(DGDE) as solvents. RC1e and EasyMax have been manufactured by METTLER TOLEDO and used to measure the exothermic conditions of reaction. The kinetic model of GRF was obtained by the experiments. The experimental results show that the safety risk level of GRF by using THF and DE as solvent is grade 3, and it is reduced to grade 1 by using CPME and DGDE as solvent. The latter two reactions have lower exothermic and adiabatic temperature rise than the former two. The critical criterion for the thermal runaway of GRF with THF and DE was established, which has certain guiding significance for the safety of GRF. The experimental results also show that the reaction temperature and the stirring speed have influence on the induction period. In the next step, the authors intend to explore the mechanism of the factors which have the influence on the induction period of Grignard reagent.
Introduction
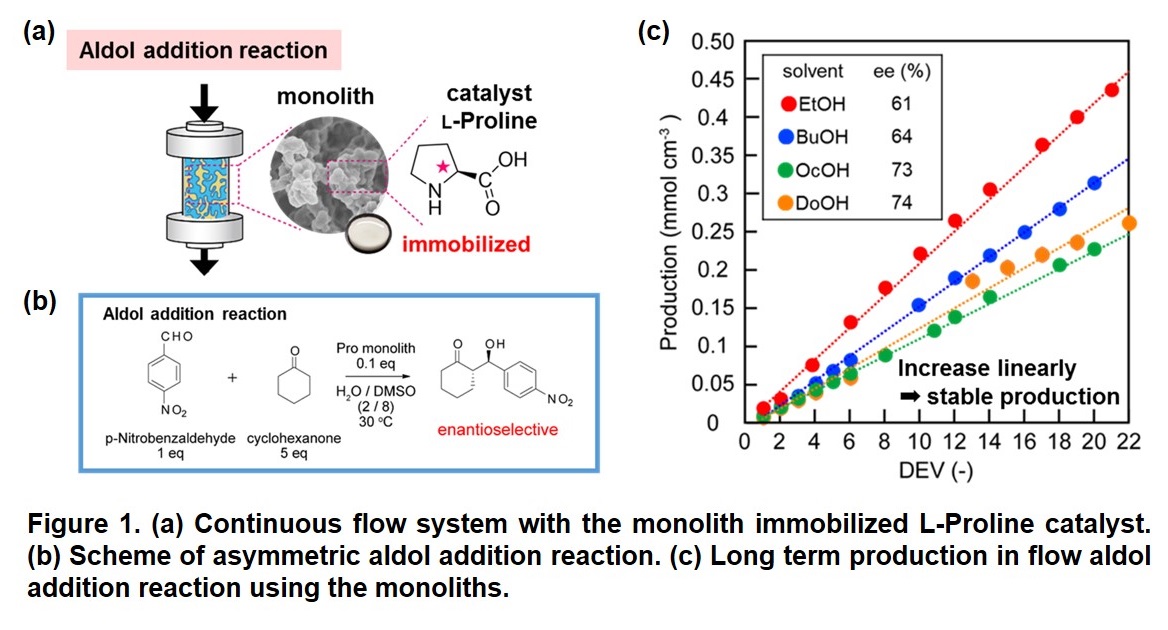
Chemical manufacturing processes in flow system have attracted attention in the field of organic synthesis. To realize energy- and cost-saving processes in the organic synthesis, using immobilized catalyst in flow reactor is a strong tool. Macroporous monolith has a three-dimensional through pore and large specific surface area contributing to its high performance in flow application. In this study, the macroporous monoliths containing L-proline-based organocatalyst were developed for asymmetric aldol addition reaction in flow system (Figure 1 (a)). The effects of porous properties of the monoliths on their catalytic activities and enantioselectivities were investigated in detail.
Results and Discussion
Macroporous monolith was synthesized by copolymerization of O-acryloyl-4-trans-L-hydroxyproline, acrylamide, and N,N'-methylenebisacrylamide in DMSO/alcohol. The porous structures of the monoliths were controlled using different porogenic alcohol (EtOH, BuOH, OcOH, and DoOH). Different length of alkyl chain of porogenic alcohol gave different porous properties, which can be explained by solubility of monomers and polymers in the solvent system.
The L-proline-containing monolith was applied to batch and flow aldol addition reaction between p-nitrobenzaldehyde and cyclohexanone (Figure 1 (b)). Interestingly, the catalytic performance of the monoliths was controlled by their porous properties in batch system. These monoliths with different porous structures were packed in stainless steel columns to construct flow reactors. With a residence time of 12 h, the flow system using the monolith was stable during over 10 days operation and achieved high enantiometric excess (ee) (Figure 1 (c)). However, the porous structure of the monoliths did not affect the catalytic activity and selectivity significantly. To reveal the relation between the monolithic structure and activity and selectivity of the catalytic reaction, the behavior of the substrate solution diffusing through the monoliths will be reported in the presentation.
Transition-metal-catalyzed cross-coupling reactions are strong tools for production of active pharmaceutical ingredients with high reaction efficiency, selectivity, and energy saving. Because flow system has advantages over batch system for cost- or energy-efficiency, safety, and automation, the development of flow cross-coupling reaction has been motivated. Particularly, transformation of inactive and inexpensive aryl chlorides under flow condition are highly desirable but have been rarely reported.
Typically, flow cross-coupling reactions require careful design of reactor because of inorganic base reagents and salt byproducts. The inorganic components insoluble in organic solvent cause serious issues of clogging in the reactor. One general solution is to use solvent system which solubilize both organic and inorganic compounds. Some reports have demonstrated that use of organic/water biphasic solvent is beneficial for complete solubility of all the components and efficient cross-coupling reaction. We postulated three-keys to achieve challenging flow cross-coupling reaction: 1) organic/water solvent, 2) immobilized catalyst, 3) efficient mixing; but never demonstrated experimentally yet.
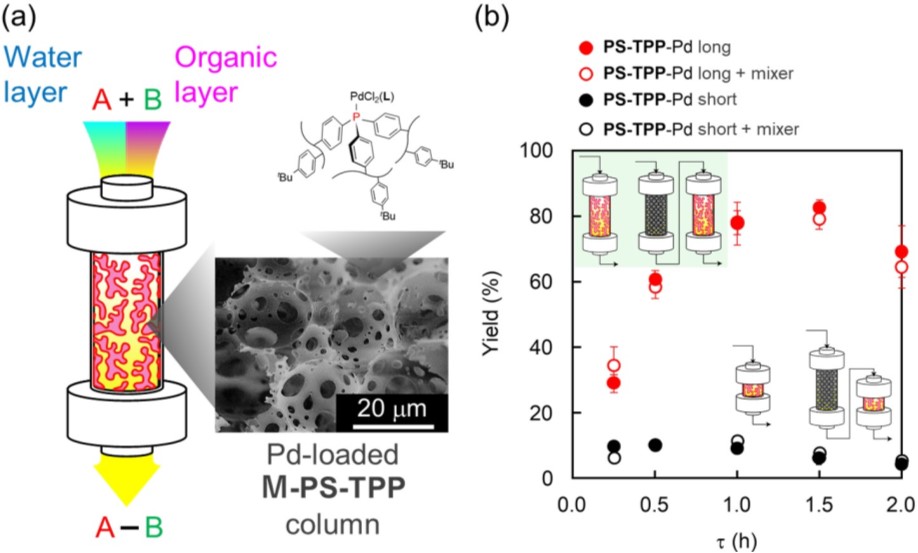
Herein, we first report an application of macroporous polystyrene-triphenylphosphine monolith (M-PS-TPP) to palladium (Pd)-catalyzed Suzuki-Miyaura cross-coupling reaction in flow system (Figure 1a). The M-PS-TPP was synthesized by copolymerization of three-fold cross-linking triphenylphosphine, divinylbenzene, and p-tert-butylstyrene. The macroporous structure of M-PS-TPP was fabricated via polymerization of water-in-oil high internal phase emulsion (HIPE) template. The M-PS-TPP had characteristic window and void structure with the pore sizes of 2–5 and 10–20 micrometer (Figure 1a, inset). Pd-loaded M-PS-TPP column was applied to flow Suzuki-Miyaura cross-coupling reaction between 4-chlorotoluene and phenylboronic acid in THF/water biphasic media containing K3PO4. Tuning the column length and residence time (tau) achieved efficient mixing and high production efficiency in the reaction (Figure 1b).
Silver nanoparticles have caught a wide attention due to its unique physical and chemical properties. Silver nanoparticles embedded in polyvinyl alcohol-polyvinylpyrrolidone (PVA-PVP/Ag) free-standing films are prepared by microwave irradiation within few minutes with varying ratios of the polymers. PVA and PVP perform as reducing agents and stabilizing agents as well as support for silver nanoparticles. The catalyst is characterized by UV-Vis spectrometry, SEM and TEM where the analytical results affirm the reduction of silver ion to silver nanoparticles in the polymer matrix. Good dispersion of silver nanoparticles in the film is confirmed by TEM analysis and the size of silver nanoparticles is found to be in the range of 2-10 nm. This film is used as catalyst for the synthesis of a β-enaminone, 4-phenyl-amino-pent-3-in-one from acetyl acetone and aniline, where silver nanoparticles act well as the catalytic agent of the composite catalyst. A typical reaction shows 89% conversion of aniline to β-enaminone at 600C with this catalyst. Parametric effects on the reaction are studied and heterogeneous kinetic model is proposed for the reaction, where Eley-Rideal model shows good fitting of experimental data. The model parameters are also evaluated and found satisfactory with a high value of correlation factor.
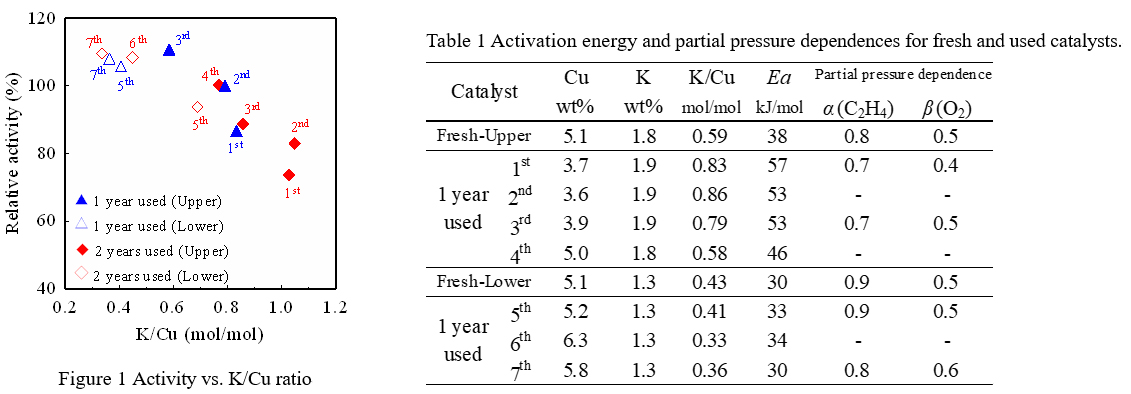
In industrial Vinyl chloride monomer (VCM) manufacturing, the oxychlorination process is most important in terms of elemental efficiency. In this study, we report the reaction kinetics analysis of deactivated catalysts in Oxygen-based oxychlorination plant.
The 11wt%CuCl2-3.4wt%KCl/Al2O3 (Upper) and 11wt%CuCl2-2.7wt%KCl/Al2O3 (Lower) catalysts were prepared according to the conventional impregnation method. These fresh catalysts were subjected to an endurance test in a commercial plant. After two years operation, the catalyst layer was divided into seven positions from the inlet and sampled (1st to 4th were upper catalyst, 5th to 7th were lower catalyst). The chemical properties of fresh and used catalysts were evaluated using a fixed-bed reactor and some instruments.
The figure 1 shows a relationship between oxychlorination activity of the catalyst sampled from each position of the reactor and the ratio of potassium and copper elements (K/Cu). The activity shown here is the relative activity when the initial catalyst performance is 100%. In the used catalysts, the K/Cu ratio increased with operation time due to a decrease of copper accompanying sublimation. As a result, a tendency of activity decreasing due to an increase in the K/Cu ratio was confirmed.
The table 1 shows the activation energy and the partial pressure dependences of raw material gases of the fresh and used catalysts, which was estimated by a kinetic study. A correlation was found between partial pressure dependences and the K/Cu ratio caused by sublimation of copper, but it was slight. On the other hand, the activation energy of the deactivated catalyst tended to increase significantly with the increment of the K/Cu ratio.
Furthermore such an increase tendency of activation energy was utilized for predicting catalyst life. As a result, the simulation applying the increasing tendency of activation energy showed a good agreement with the measured value after 2 years.
Oxygen-based oxychlorination is one of the efficient processes for producing 1,2-dichloroethane of a key material in vinyl chloride production. Despite abundant researches about the commercialized CuCl2-KCl/Al2O3 catalyst, its deactivation characteristics has not been fully understood. Besides, a carbon deposition property has not been examined in detail. Therefore, this study focuses on the clarification of the deactivation factor by XANES analysis of the commercialized catalyst as well as a measurement of the deposited carbon by temperature programmed oxidation (TPO).
The CuCl2-KCl/γ-Al2O3 catalyst was extracted from the industrial reactor after one- or two-years operation. The performance of the used catalyst was then evaluated by a fixed-bed reactor at laboratory scale. The deposited carbon was measured by TPO and the Cu oxidation state was investigated by Cu K-edge XANES analysis.
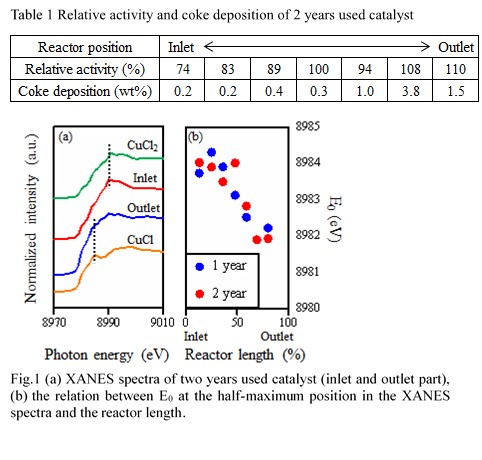
Table 1 shows a relative activity and the amount of coke on the two-years used catalsyt. The relative activity was defined as the conversion of the used catalyst devided by the conversion of the fresh catalyst. Althogh the catalyst at the inlet part was deactivated to 74-89%, the activity at the outlet part was still high. As for the amount of the coke, the more coke was observed at the outlet part, comparing to that amount at the inlet part. Fig. 1(a) shows XANES spectra of two years used catalyst. While the spectrum of the catalyst at the inlet part was similar to that of CuCl2, the catalyst at the outlet part was similar to that of CuCl. The Cu species of the outlet part catalyst were reduced state. Fig. 1(b) exhibits the relation between Edge jump energy (E0) and the reactor length. The E0 value was decreased from the inlet side to outlet side. The difference of the oxidation state might cause a deactivation of the catalyst and coke deposition property.