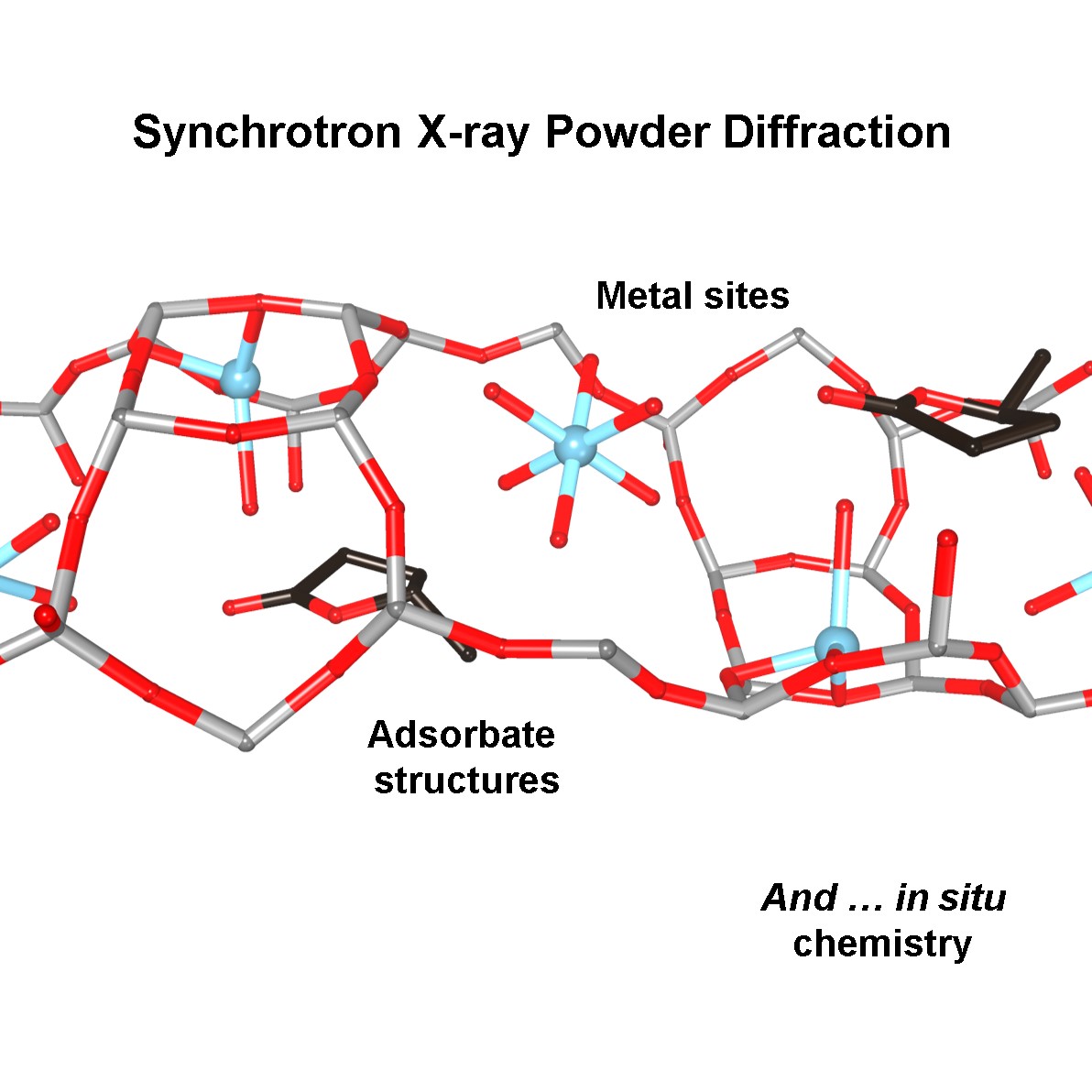
Organic-inorganic hybrid perovskites, such as methylammonium lead iodide (CH3NH3PbI3) have shown outstanding optoelectronic properties, promising extensive application in solar harvesting. Although most hybrid perovskite materials have been synthesized under solution process, recent demonstrations of the vapor phase synthesis of the hybrid perovskite nanostructures suggest there is an emerging interest in controlling their properties under highly-confined structures. In particular, single-crystalline 1D lead halide nanowires can be prepared via the vapor-liquid-solid (VLS) growth mechanism through the self-catalyzed growth mechanism, which then converted to hybrid perovskite. Since the conversion occurs on the preformed nanowire, electronic properties 1D perovskite depend on the original nanowire structure. In this study, we report the VLS growth of lead iodide (PbI2) nanowires on a c-sapphire (0001) substrate followed by conversion to CH3NH3PbI3 using methylammonium iodide, and confirm that the degree of perovskite conversion depends on the growth orientations of PbI2 nanowires. We observe two different growth directions; vertically-oriented [0001] nanowires and kinked nanowires. Photoluminescence (PL) measurements on each growth direction suggest that the oriented nanowires exhibit a higher degree of conversion compared to the [0001] oriented nanowires. In addition, [0001] oriented nanowires exhibit the position-dependent degree of conversion, depending on the presence of the catalyst tip on top of the nanowire. In particular, the conversion is observed both on the catalyst tip and the base of nanowire, suggesting methylammonium iodide incorporates into the nanowire either by vapor transport and surface diffusion. Our observation indicates that the vapor phase conversion of PbI2 to CH3NH3PbI3 is a diffusion-limited process. This finding is an important step towards a structural engineering of perovskite nanowires.
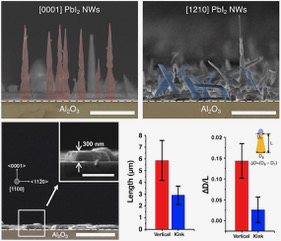
Methanol is an important industrial chemical and a raw material for the synthesis of various chemicals. It is mainly produced from synthesis gas, but the thermodynamic equilibrium in methanol synthesis results in a low conversion per pass. The use of a membrane reactor to improve the conversion per pass of the methanol synthesis has been considered. However, simultaneous membrane separation of methanol and H2O from H2 at high temperature is still difficult.
In this study, we developed a new concept on chemically stabilized ionic liquid (IL) membranes which are organosilica membranes with IL like properties prepared from silylated ILs. ILs have been used in membrane separation for more than a decade due to their special features such as non-volatility, thermal stability and ability to dissolve a large range of organic molecules. Gas/vapor permeation tests revealed that permeation separation performance of the membrane is strongly controlled by the affinity of the permeate molecules toward the IL, and its permeation and separation mechanisms are explained by a combination of the selective adsorption/pore blocking effect and the solution-diffusion mechanism.
The separation factors for methanol/H2 in the binary system were 12–48 in the temperature range from 100 to 200 °C. The membrane also showed high H2O/H2 separation performances in methanol/H2O/H2/CO2 quaternary system, and the presence of H2O and CO2 in the feed stream did not affect the methanol permeation performance. Methanol and H2O permeances of the developed membrane were higher than those of the Li-Nafion membrane, and comparable to values of the zeolite membrane. The silylated IL-derived organosilica membrane showed an excellent potential for the simultaneous separation of methanol and H2O from methanol synthesis gas.
Synchrotron X-ray powder diffraction has begun to stretch its strategic presence from the sheer determination of crystal structures into the mechanistic and kinetic information of microporous zeolite materials. Not only can this technique be used to reveal the internal framework structure of zeolites and their active sites, but it can also be combined with other analytical tools to suit the particular needs for in situ or operando gas storage and separation and catalytic studies.
With a keen interest in materials and catalysis, our research group has focussed on the fundamental aspects of zeolite materials. We have developed a new structural methodology by combining state-of-the-art synchrotron X-ray facilities with other characterisation techniques, that can:
(i) Directly visualise how organic molecules behave and interact with the BAS of zeolites.
(ii) Provide mechanistic information for important industrial processes.
(iii) Engineer new catalytic system for industrial processes.
(iv) Explain the reaction mechanisms and kinetics of new biomass conversion reactions.
(v) Design a novel enzyme-mimic inorganic system.