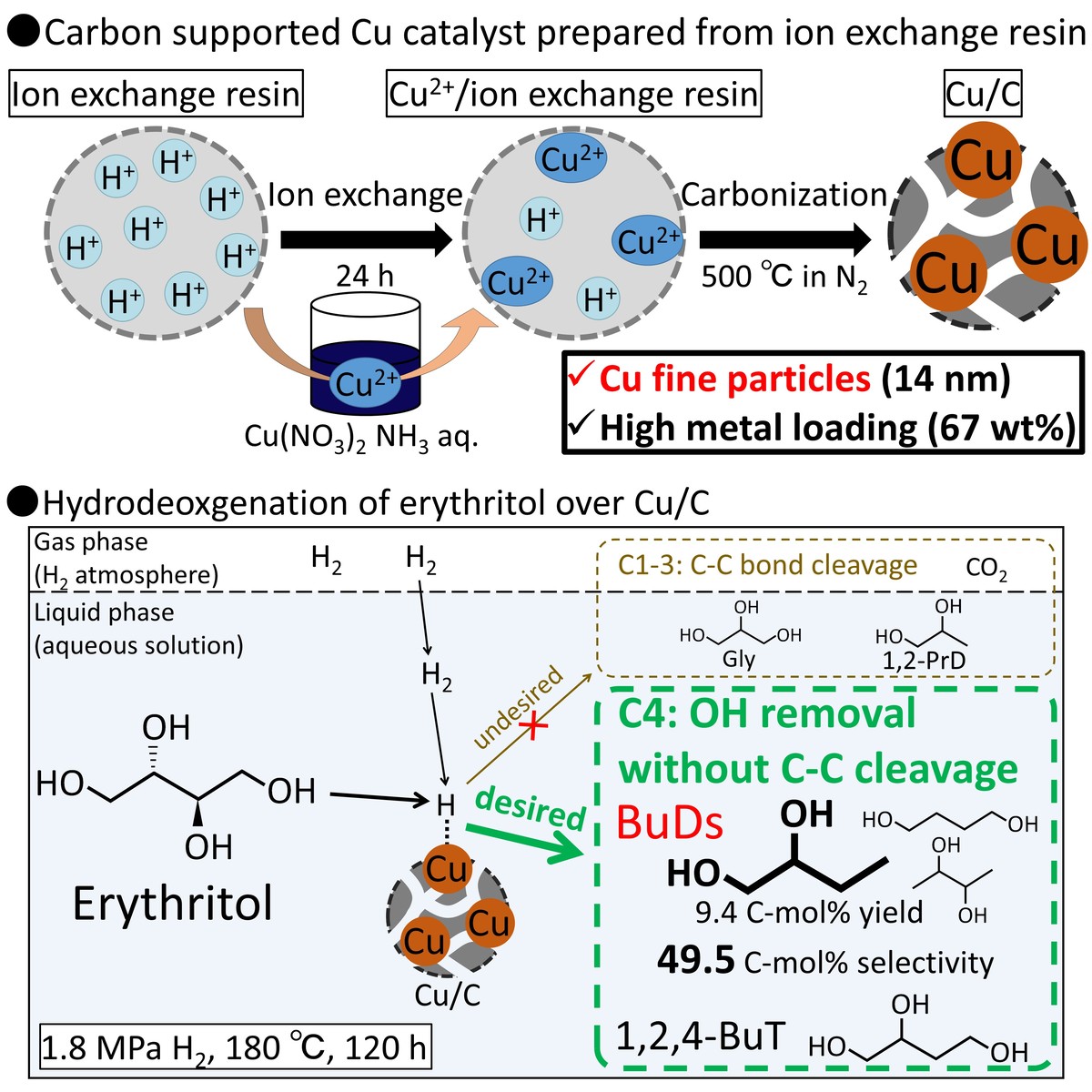
Biomass-derived polyols, such as glycerol, erythritol, and xylose, are attracting attention as renewable raw materials for basic chemical products, such as propanediols, butanediols, and 2-methyltetrahydrofuran. Because these polyols have high oxygen content (O/C = 1), deoxygenation reaction without C-C cleavage is required to convert them into the basic chemical products. In general, hydrodeoxygenation reaction is employed under pressurized hydrogen atmosphere and in the presence of metal catalyst containing Cu or Ru. To suppress oligomerization of polyols proceeding above 250 °C, catalyst with high activity at low temperature, that is, high metal dispersion, and without active site on catalysts support such as carbon support is needed. In conventional catalyst preparation methods including impregnation, it is difficult to achieve high dispersion on carbon support. To solve this problem, we focused on a new preparation method for carbon supported metal nanoparticle catalyst, which uses ion exchange resin as a precursor of carbon support. In this method, metal ions are loaded on an ion exchange resin by ion exchange and then the metal loaded resin is carbonized to convert the resin to carbon support. This study aimed at the preparation of carbon supported Cu catalyst by using the ion exchange resin as a precursor of carbon support and the investigation of hydrodeoxygenation reaction pathway of erythritol (C4 polyol) over the Cu/C catalyst. The Cu/C catalyst possessing 14 nm of Cu fine particles and 67 wt% of metal loading was successfully prepared by the ion exchange technique. We applied the obtained Cu/C catalyst to hydrodeoxygenation of erythritol to butanediols to investigate the reaction pathway by varying reaction time and hydrogen pressure. It was suggested that the main reaction pathway was 1,2-butanediol production from erythritol by sequential hydrodeoxygenation via 1,2,4-butanetriol.
Alumina (γ-Al2O3) and silica(SiO2) have been widely used as adsorbents or catalysts/catalyst supports for hydrodeoxygenation in bio-oil, because of their high surface area, high thermal and chemical stability. The HDO is one of the promising methods for upgrading bio-oil by removal of oxygen-containing compounds. In this study, SiO2/γ-Al2O3 composite particles with different Al to Si ratios were synthesized by spray pyrolysis combined with sol-gel process. The spray pyrolysis method has advantages of synthesizing spherical micron-sized particles in one step with controlled size and morphology of the product particles. Also, mass production is possible with this method as it is a continuous reaction process. In order to control specific surface area and pore structures, the particles were prepared by adding CTAB as a template. The product particles were analyzed by BET, XRD, FE-SEM and TPD. Also, the produced gas and liquid products from HDO were analyzed by GC with TCD/FID.
This study reviewed the technologies that have been applied to hydrogen liquefaction processes so far. First of all, various types of hydrogen liquefaction processes were closely reviewed, and their advantages and disadvantages were compared. In particular, the effect of refrigerants and types of refrigeration cycles on energy efficiency of the process were analyzed. Then, recently developed innovative conceptual approaches to the hydrogen liquefaction process were reviewed. Lastly, a new approach was suggested to increase an energy efficiency of the hydrogen liquefaction process in South Korea.
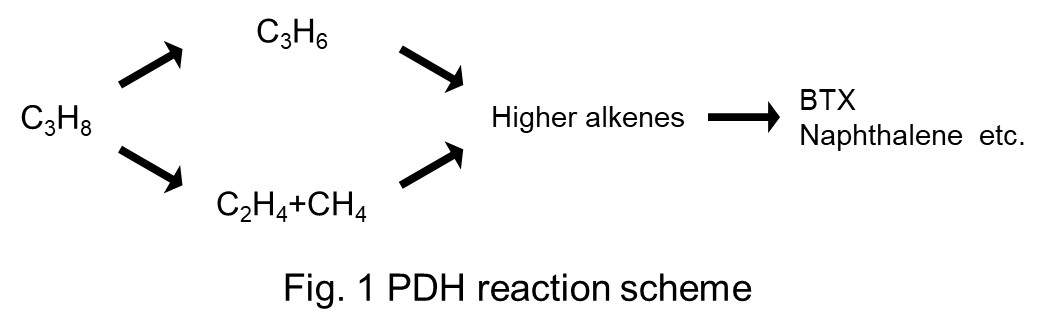
Propane dehydrogenation (PDH) is one of the most promising candidates of propylene production process to meet the growing demand of propylene in the future. However, precious metals such as Pt have been required for high catalytic performance. Therefore, there is an urgent need to develop new catalysts based on non-precious metals for PDH reaction.
The scheme of PDH reaction is shown in Fig. 1. The initial reaction step from propane is dehydrogenation to propylene or cracking to ethylene and methane and then aromatic compounds can be produced through the excessive reaction. Therefore, it is required to increase the selectivity of dehydrogenation pathway and depress the excessive reaction. To meet these requirements, we focused on Brønsted acidity derived from Ga-species and diffusivity of zeolites which have large micropores.
As an ideal catalyst for PDH reaction, we have synthesized high silica Ga-Beta from dealuminated zeolite using dry gel conversion method. Ga species was well incorporated into *BEA structured zeolite frameworks and worked as Brønsted acid sites. The high silica Ga-Beta with Si/Ga ratio up to 177 can be synthesized. On the PDH reaction, synthesized Ga-Beta showed the highest propane conversion, the highest propylene yield and the highest selectivity of dehydrogenation on the initial reaction step in Ga-based zeolites. The combination of large microporosity and acid sites derived from Ga incorporated into the zeolite frameworks is likely to lead to the excellent catalytic performance of the Ga-Beta. Moreover, the Ga-Beta with higher Si/Ga ratio shows longer catalyst lifetime on PDH reaction since coke deposition rate was depressed by the decrease of the acid amount. This work provides new insights for PDH reaction over Ga based zeolite catalysts, which would contribute to the progress in activation and transformation of light alkenes to value-added chemicals.
Formic acid is regarded as a promising energy carrier because hydrogen can be produced at mild conditions. Formic acid can be decomposed according to the following two pathways.
Dehydrogenation : HCOOH → H2 + CO2 (1)
Dehydration : HCOOH → H2O + CO (2)
Selectivity of the reaction pathway (1) is very important issue not only because low selectivity will lead to wasting much energy carrier, but also because CO is a poison for fuel cell catalysts in the subsequent conversion of hydrogen into electrical energy. Hence, an efficient catalyst for dehydrogenation of formic acid should be developed.
Considering the separation and reusability of catalysts, heterogeneous catalysts are more suitable for practical applications than homogeneous catalysts. Catalyst support is also crucial for H2 selectivity. Al2O3 or TiO2 support may have the activity to decompose formic acid without generating H2. In this work, carbon supported metal catalysts were used for formic acid decomposition.
Catalysts were prepared from a weak-acid-cation-exchange resin (WK11, Mitsubishi Chemical Corporation) and metal cation solutions. The resin and the aqueous solutions of [Pd(NH3)4]Cl2 and Ni(NO3)2 were stirred at room temperature for 24 h. Treated resins were filtered and dried followed by the heat treatment in N2 steam at 500 °C to prepare Pd/C, Ni/C and Pd-Ni/C catalysts.
Thermogravimetric analysis and powder X-ray diffraction patterns provided that the Pd and Ni particles were highly loaded and highly dispersed. A fixed bed reactor was used for the decomposition experiments in gas phase. Formic acid was able to be completely decomposed below 200 °C for all catalysts. H2 selectivity was above 85 % for Pd/C and Pd-Ni/C catalysts, indicating that they are suitable for H2 production from formic acid. Liquid phase decomposition was also performed.
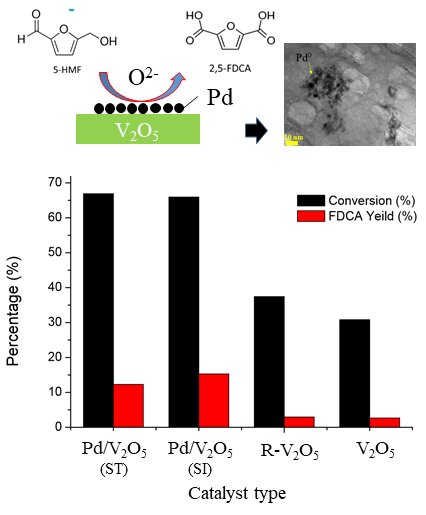
2,5-furan dicarboxylic acid (FDCA) derived from the oxidation process of 5-hydroxymethylfurfural (5-HMF) is a valuable platform chemical for a wide range of industrial applications - particularly the bioplastic industries. This research aims to incorporate 1 wt.% palladium nanoparticles (Pd NPs) into vanadium(V) oxide (V2O5) which was used as an alternative heterogeneous catalyst for HMF oxidation. The incorporated Pd/V2O5 catalyst was prepared by colloid-chemical reduction step and immobilization step, where the combination of these two steps proceed differently - stepwise (ST) and simultaneous (SI) process. For the colloid-chemical reduction step, an aqueous solution of PdCl2.H2O (0.086 mmol Pd, 250 ml DI) was mixed with 2 wt.% of polyvinyl alcohol (PVA) solution - uses as a nanoparticle stabilizer blocking part of the active sites by covering the majority of the metal surface. The mixed solution, then, was reduced by adding slowly of 0.1 M NaBH4 solution. In case of ST process, adding 3 g V2O5 powder for Pd NPs immobilization was done after the reduction step while the simultaneous (SI) one was done at the same time. Morphology, particle size and distribution, as well as, the chemical composition of synthesized samples are characterized by various techniques i.e. TEM, BET, X-ray diffraction, UV-vis spectrophotometer. The modified Pd/V2O5 catalysts obtained from the two different processes (ST and SI) affected the surface characteristics – different Pd NPs size and distribution as well as specific surface area. The potential oxidation of 5-HMF over incorporated Pd/V2O5 catalyst was higher than the one without Pd NPs – raising 5-HMF conversion percentage about two times.
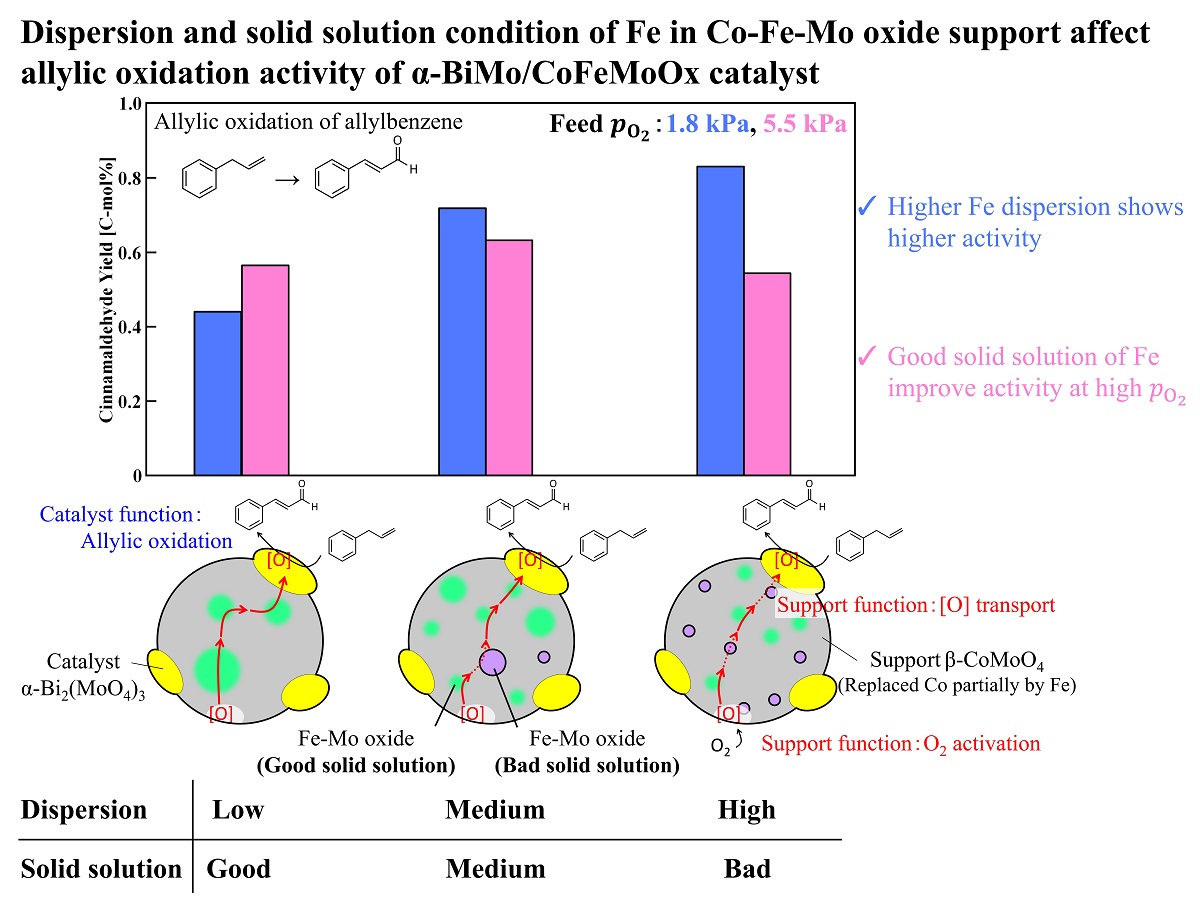
The allylic oxidation is one of important reaction in chemical industry to convert olefin into unsaturated aldehyde for valuable intermediates. It is generally reported that Bi-Mo oxide catalyst loaded on Co-Fe-Mo complex oxide support is effective for allylic oxidation. In this reaction, the oxygen atom in Bi-Mo oxide is consumed in allylic oxidation, gaseous oxygen is uptaken in Co-Fe-Mo complex oxide, and the lattice oxide of the support is transferred to Bi-Mo oxide to regenerate the active center. It is commonly reported that Fe atoms, which is dissolved in CoMoO4 matrix in the support, are responsible for the lattice oxygen transfer and this step is considered to be the rate-limiting in allylic oxidation. Therefore, the allylic oxidation activity can be enhanced by improving the dispersion of Fe atoms in the support. We herein investigated the effect of preparation condition on the dispersion of Fe atoms in the support and the catalytic activity for the allylic oxidation. Co-Fe-Mo complex oxide was prepared by co-precipitation method and the initial pH value of the metal precursor solution was varied to control the precipitation rate of each metal which can be related to the dispersion. When the starting pH value was high, the Fe atoms in the support was highly dispersed without forming greatly agglomerated Fe2(MoO4)3. Furthermore, the allylic oxidation of allylbenzene to cinnamaldehyde was performed by using α-Bi2(MoO4)3 loaded Co-Fe-Mo complex oxide prepared. The catalyst with higher dispersion of Fe showed the higher cinnamaldehyde yield, which could be due to the improvement of the oxygen transport capacity and the promotion of reoxidation of the active center.
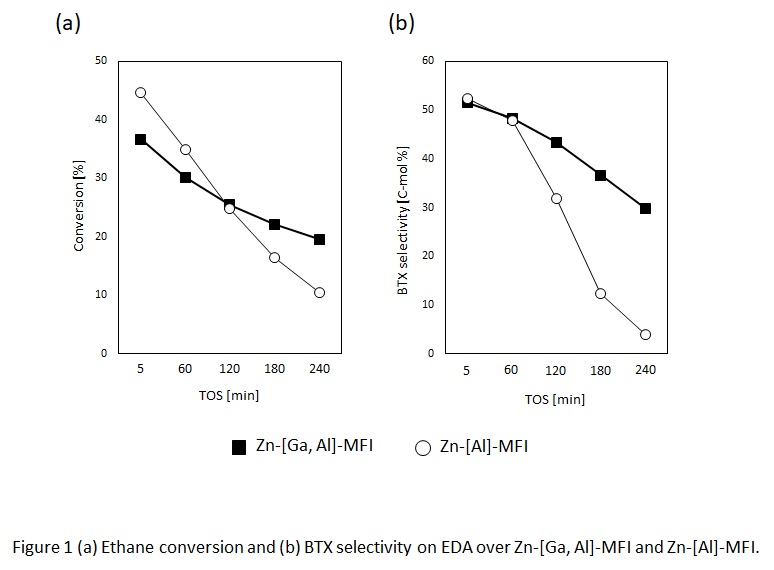
Benzene, toluene and xylene (BTX) are value-added chemicals as basic petrochemical products. These chemicals are mainly obtained from naphtha by the catalytic reforming process. Meanwhile, direct conversion of light alkanes to BTX is a promising process to utilize natural gas as a feedstock for their production. Zn2+-exchanged ZSM-5 (Zn-[Al]-MFI) zeolite was known to be an effective catalyst for converting ethane to BTX. However, the catalyst was rapidly deactivated by a coke deposition. Here, we examined incorporation of Ga into the zeolite framework (gallosilicate). Ga species in the zeolite framework is known to promote aromatization of light olefins. Additionally, gallosilicate zeolite has lower acidity compared to alminosilicate zeolite. In this study, we prepared Zn2+ ion exchanged MFI type galloaluminosilicate (Zn-[Ga, Al]-MFI) for ethane dehydroaromatization (EDA) reaction in order to improve catalyst life time as well as BTX yields.
Both Zn-[Ga, Al]-MFI and Zn-[Al]-MFI samples showed typical XRD patterns of MFI zeolite. From NH3-TPD spectra of the samples before Zn2+ exchange, the acidity is higher in the order of [Al]-MFI > [Ga, Al]-MFI > [Ga]-MFI, indicating that the substitution of Ga for Al weakened the acidity. Then, we concluded that Ga is successfully incorporated into the zeolite framework. The amount of Brønsted acid of [Al]-MFI decreased after Zn2+ ion exchange. Meanwhile, the amount of Brønsted acid of [Ga]-MFI did not change after Zn2+ ion exchange, indicating that proton near Ga sites is hardly exchanged. This result suggested that proton near Al sites of [Ga, Al]-MFI was selectively exchanged with Zn2+ ion. The conversion of ethane and the BTX selectivity on EDA reaction were shown in Figure 1a and 1b, respectively. The catalyst lifetime has been successfully improved because the deactivation by coke deposition on the strong acid sites was suppressed by substitution of Ga for Al.
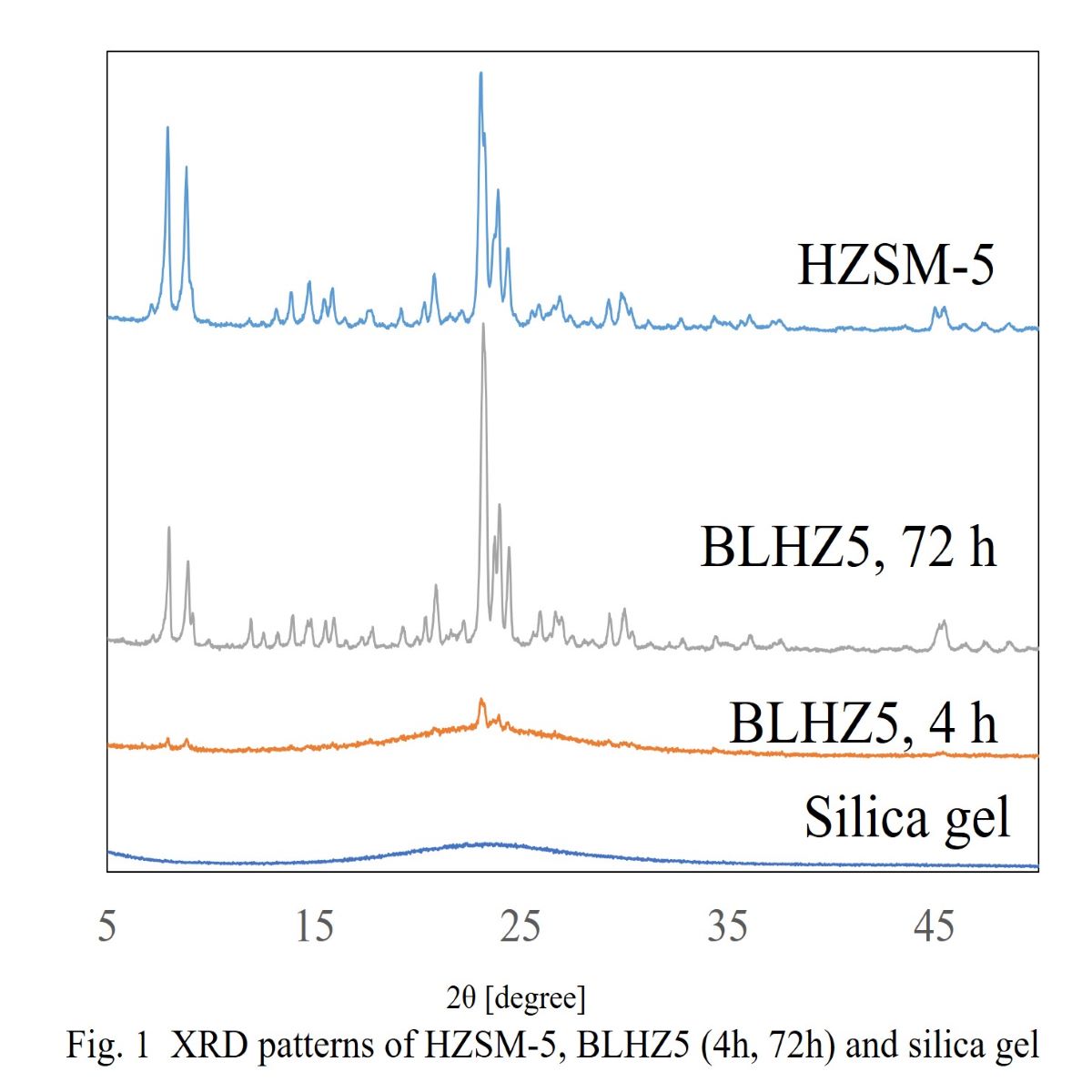
It was previously reported that the gas-phase one-step oxidation of benzene to phenol was carried out by using Cu/HZSM-5 modified with Ti as the catalyst. In this case, the almost same yield and selectivity of phenol was obtained by direct oxidation of benzene in comparison with the Cumene method. However, the morphology of Cu/HZSM-5 catalyst modified with Ti was the fine particle and the utilization of this morphology was not benefiting industrially. Cu/HZSM-5 was prepared by the Cu species impregnating on HZSM-5 which was one of zeolite. Although the Cu species was the active sites in this reaction, phenol di not produce when using supports other than HZSM-5. Therefore it was desire to prepare the forming of HZSM-5 zeolite, which had high thermal stability and did not use a binder. In this study, it is synthesized the binderless HZSM-5 (BLHZ5) following the preparation method of the patents from Nippon Shokubai and prepared the Cu catalyst doped on binderless HZSM-5 (Cu-BLHZ5) by an ion exchanged method.The structure and character of BLHZ5 supports which were prepared by hydrothermal synthesis method for different treatment times were investigation by XRD measurements and N2 adsorption-desorption measurements. From Fig. 1, it was cleared that XRD spectra of the BLHZ5 support indicated the formation of MFI structure for all hydrothermal synthesis time, even at 4h. On the other hand, the surface area and pore size of BLHZ5 were measured by N2 adsorption-desorption methods. No significant change of micro pore size for BLHZ surface area was observed for different synthesis time. However, the increasing of the BLHZ5 surface area was observed with increasing the synthesis time. Therefore, it is estimated that the optimum points of synthesis time is 72h.
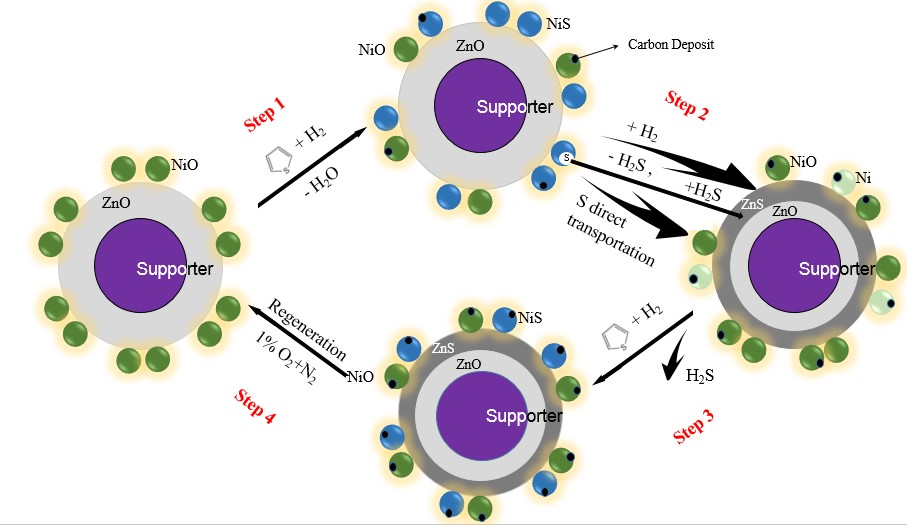
The reactive adsorption desulfurization (RADS) of a model gasoline n-hexane containing thiophene was conducted over a NiO/ZnO-Al2O3-SiO2 adsorbent in N2 and H2, respectively. A declining RADS trend has been observed in N2, without the presence of H2, indicating that NiO is sulfurized and exhibits activity for RADS. TPR and XPS results presented NiO in the adsorbent is difficult to be reduced because of the strong interaction between NiO and the support. The sulfurization of NiO into NiSx is a primary condition for RADS process the same as the presulfurization of hydrotreating catalyst, while metallic Ni is an intermediate reduction product of NiSx. The unreduced NiO/ZnO-Al2O3-SiO2 adsorbent performs a better desulfurization than the reduced adsorbent at the beginning of desulfurization process. NiO is assumed as the main active component and present a good desulfurization ability in RADS. Various pretreatments of the adsorbent showed the participation of hydrogen and n-hexane in pretreatment conducted at 420 °C contributed to the activation of adsorbent and the improvement of desulfurization performance under the reaction temperature of 300 °C. At last, some change of RADS mechanism is presented and discussed.
Methane steam reforming reaction is used to obtain hydrogen for use in fuel cells. In general, catalyst poisoning by the sulfur compounds is one of the main causes of catalyst performance degradation. Sulfur durability of alpha-alumina supported precious metal catalysts for methane steam reforming reaction were investigated. Methane conversion of all catalysts decreased in addition of tertiary-butylmercaptan (TBM) as a sulfur impurity in the reaction gas. Methane conversion of Ru catalyst was eventually deactivated almost completely in methane steam reforming with TBM. The time until catalyst deactivation depended on the amount of precious metal loading, and was further proportional to the sulfur adhesion amount and CO unit adsorption amount. In the TBM addition test of Rh catalyst, the methane conversion decreased to some extent, but thereafter the methane conversion reached a plateau and became a constant value. The methane conversion at the plateau was dependent on the loading of precious metal and the reaction temperature. There was a difference in adsorption characteristics with sulfur depending on the precious metal species. The performance degradation by sulfur poisoning in methane steam reforming reaction behaved differently depending on the precious metal species and their physical properties.
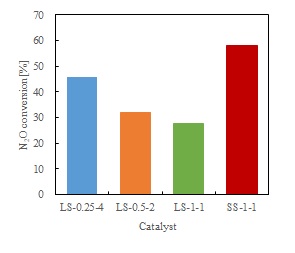
Nitrous oxide (N2O) is one of the greenhouse gases that lead to depletion of the ozone layer. Therefore, effective decomposition of N2O is imperative. In this study, MOR zeolite-supported Fe (Fe-MOR) catalysts were prepared by the various methods, and the effect of the preparation method on the N2O decomposition activity of the catalysts was investigated.
The Fe-MOR catalysts were prepared by the liquid and solid-state ion-exchange methods, using iron (III) nitrate enneahydrate (Fe(NO3)3·9H2O) and MOR zeolite as the support. In the liquid-state ion-exchange (LS) method, the MOR zeolite was immersed in Fe(NO3)3·9H2O aqueous solution and stirred at 80 °C for 3 h. After stirring, the ion-exchanged zeolite was filtered and washed, followed by drying at 110 °C for 12 h. This process was repeated arbitrarily. The dried sample was calcined at 500 °C for 3 h. In the solid-state ion-exchange (SS) method, the MOR zeolite was ground with Fe(NO3)3·9H2O in a mortar for 30 min. The mixture was dried at 110 °C above 12 h, after which, the dried sample was calcined under the same conditions as in the LS method. The Fe-MOR catalyst that was prepared by the LS process, which was repeated 4 times using Fe(NO3)3·9H2O aqueous solution (Fe/Al = 0.25), was denoted as LT-0.25-4. N2O decomposition was carried out using a fixed bed flow reactor at 500 °C.
The order of the N2O decomposition activity of catalysts used the same amount of Fe is as follows: SS-1-1 > LS-0.25-4 > LS-0.5-2 > LS-1-1. It is assumed that the Fe-MOR catalyst that was prepared by the SS method was not washed after grinding. Thus, much of the Fe species remained on the cation exchange sites, and this led to higher activity.
Incorporating covalently bound organic moiety to the surface of SiO2 is a general approach to tailor its surface properties for adsorbents and catalysts. Examples include tuning surface hydrophobicity/hydrophilicity by incorporating alkyl groups with various lengths, tailoring surface functionality by incorporating amine- and sulfonic acid-containing groups, and controlling interlayer spacing of layered silicates by grafting with alcohols. Functionalization of amorphous silica provides a cost-effective advantage. However, it involves a general challenge to achieve site-specificity and control a microscopic environment around functional groups. In contrast, connectivity defects (e.g. SiO–) contained in pure-silica zeolitic materials offer opportunities to achieve precise control of material properties. The location of connectivity defects in crystals is dictated largely by the local structure of zeolitic materials as well as the crystallographic location of charge-compensating structure-directing agents used in the synthesis of zeolitic materials. We chose lamellar precursors of a pure-silica MWW-type zeolitic material because these materials are known to possess abundant connectivity defects near the surface of zeolitic nanosheets and allow incorporation of bulky species between zeolitic nanohseets. Here, we report the site-specific functionalization of these materials with alcohols and alkanolamines and application of functionalized materials to the synthesis of metal-encapsulated catalysts.
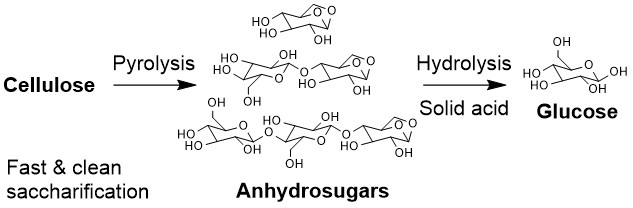
Saccharification is a key step for the conversion of a main component of lignocellulosic biomass, cellulose, to chemicals and fuels. Enzymatic hydrolysis is the most popular saccharification method but suffers from disadvantages from an economic perspective since it is expensive and time-consuming. A potential alternative method is herein proposed, where cellulose is first pyrolyzed to anhydrosugars such as levoglucosan, and then anhydrosugars are hydrolyzed to glucose. Pyrolysis is a very fast reaction and does not require any catalyst or solvent. The yield of anhydrosugars reaches more than 90%, though it depends on reaction conditions significantly. It has been known that anhydrosugars are readily hydrolyzed to glucose in an acidic aqueous solution. Sulfuric acid is often employed as the catalyst. However, such homogeneous catalyst generates undesirable waste stream and is difficult to be recycled to the process. The present study demonstrates the availability of a solid acid catalyst for this reaction. A type of strongly acidic cation exchange resin was employed as the catalyst, and a main study was performed with levoglucosan as anhydrosugar model compound. The catalysis was very selective, producing glucose with near-complete yield. When compared to sulfuric acid at the concentration equivalent to the proton concentration in the test with solid acid, the reaction rate was comparable, and, moreover, the selectivity was better. The reaction rate was not altered by the concentration of levoglucosan in the range between 0.1 M and 0.5 M. The catalyst was also active toward cleavage of glycosidic linkages, which was confirmed in the test with cellobiose. Finally, the catalytic test was carried out with biooil from cellulose pyrolysis and successfully produced glucose with sufficient yields, based on anhydrosugar concentration of the oil, even in the presence of various other organic compounds.
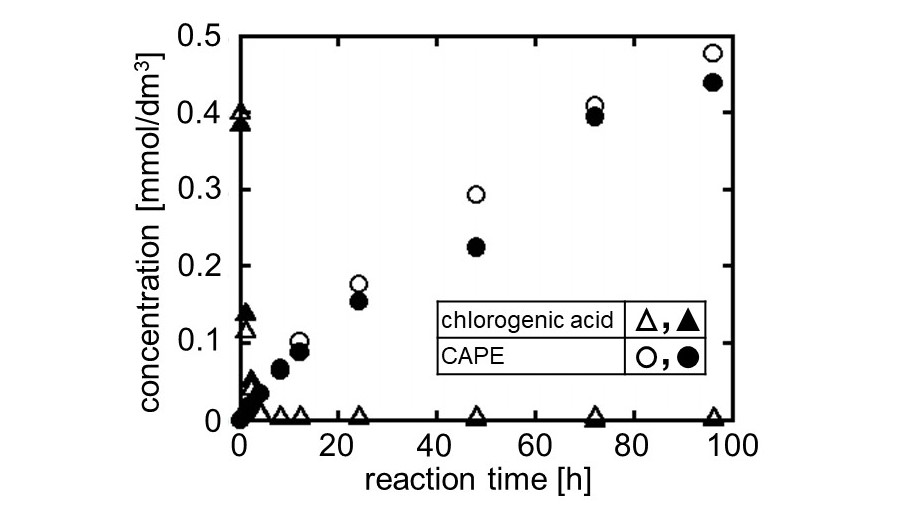
Caffeic acid phenethyl ester (CAPE) is a pharmaceutical ester with anti-cancer activity. It is recovered from propolis but is very expensive because of its low content (about 0.1%). On the other hand, esterification of caffeic acid and transesterification of chlorogenic acid (ester of caffeic acid and quinic acid) have been reported as chemical synthesis methods. Both caffeic acid and chlorogenic acid are contained in coffee beans at a relatively high concentration (approximately 8%) and these remain in spent coffee grounds (SCG). There is a possibility that CAPE might be synthesized from SCG. We have reported that ion-exchange resins show high catalytic activity of ester synthesis in non-aqueous system. In this study, we investigated whether CAPE was produced from SCG by ion-exchange resin catalysts.
First, extraction of caffeic acid and chlorogenic acid from SCG was performed using a conventional Soxhlet extractor. No caffeic acid was detected in the extract, but the chlorogenic acid content was about 0.6%, which was higher than that of propolis. Next, batch experiments of the CAPE production were performed using two kinds of ion-exchange resin catalysts. The resin catalyst was added to the reaction solution in which the extract was dissolved in phenethyl alcohol, and well shaken at 70°C. In case of using porous strong base anion-exchange resin, CAPE was not formed, which meant that the transesterification of chlorogenic acid did not proceed. In case of using porous strong acid cation-exchange resin, however, the CAPE concentration gradually increased as shown in Fig.1. Two sets of results under the same conditions overlapped each other, indicating a good reproducibility of the experiment. In addition, the CAPE concentration became higher than the initial value of chlorogenic acid. CAPE was considered to be formed from caffeic acid residues in other substances as well as in chlorogenic acid.
Undaria pinnatifida, brown macroalgae native to northwest Pacific areas, has been considered as a successful invasive species and is feared to cause a reduction in diversity and richness of native macroalgae. Though management of these invasive species is being investigated, it is often faced with failures which call for a resourceful utilization way of U. pinnatifida in a cheap and less energy-intensive way. In this study, U. pinnatifida is focused on as a feasible source for the production of solid acid catalyst. Solid acid catalyst from U. pinnatifida was prepared using sequential carbonization methods of pyrolysis and hydrothermal carbonization (HTC), and the influence of the combined carbonization methods on the characteristics of solid acid catalysts was investigated. The evaluation of catalytic performances of prepared catalysts was carried out using the esterification reaction of acetic acid and ethanol. Though catalysts prepared via HTC method without pyrolysis pretreatment showed better acidity, it had less porosity. Catalysts prepared with pyrolysis pretreatment showed higher specific surface area and coherently showed higher catalytic activity. When the catalytic performance was compared with conventional catalyst Amberlyst-15, U. pinnatifida derived solid acid catalyst showed higher conversion rate of acetic acid and ethyl acetate yield. Through this study, it is shown that marine biomass, U. pinnatifida can function as a solid acid catalyst with good catalytic performance and favorable reusability profile.
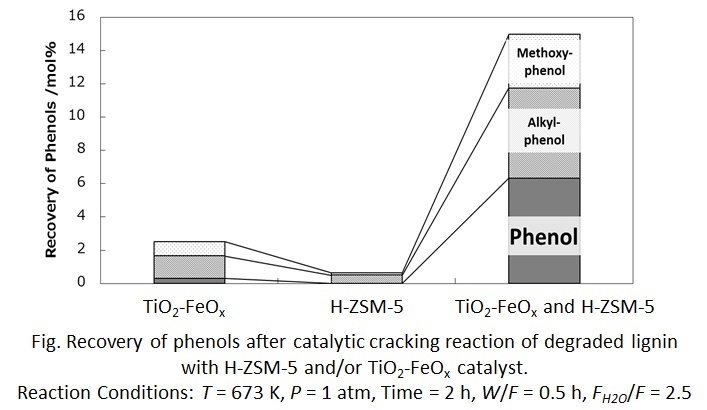
Lignin, a polymer with alkyl phenol units, has attracted attention as a resource for phenols. Our previous study has suggested a lignin conversion process to produce monomeric phenols from lignin. The degraded lignin that was depolymerized in water/1-butanol mixed solvent, was converted to monomeric phenols over TiO2-FeOx catalyst with high activity for oxidative decomposition and demethoxylation of lignin units. However, the catalyst was not effective for cleavage of C-C bond between the units of lignin structure. Thus, this study investigated the catalytic reaction of the lignin over a mixed catalyst of TiO2-FeOx and H-ZSM-5 for C-C bond cleavage.
3 wt% of degraded lignin in quinoline solvent was used as feedstock because quinoline was found to be inactive over the catalyst, and also had a good solubility against the degraded lignin, which was consistent with the investigation based on Hansen solubility parameters. The reaction was carried out using a fixed-bed flow reactor at 673 K for 2 h under atmospheric pressure.
As a result, TiO2-FeOx catalyst exhibited only 1.7 mol% of phenols from the lignin. H-ZSM-5 exhibited only 0.5 mol% of phenols, meaning that H-ZSM-5 was not effective for oxidative decomposition. Moreover, the degraded lignin molecular size was too large compared with pore size of H-ZSM-5 that the molecules could not diffuse into the pore and be decomposed. On the other hand, the physically mixed catalyst was high activity for recovering monomeric phenols from the lignin, achieving 11.7 mol% of phenols. Furthermore, GPC chromatogram indicated that molecular weight of products in the mixed catalyst was lower than that of products in only TiO2-FeOx or H-ZSM-5 catalyst individually. The mixed catalyst could dissociate C-C bond in the degraded lignin, leading to the improvement of the yield of phenols. In addition, the between the type of zeolite and reaction activity will be reported.
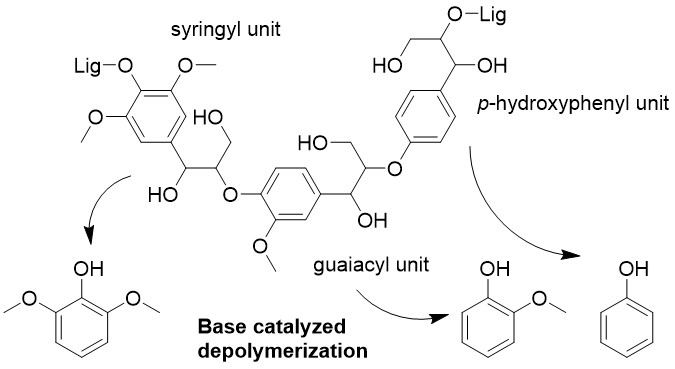
Lignin valorization is one of the biggest challenges for developing biomass-based chemical industry. Recent innovative works has enabled depolymerization of lignin to phenolic monomers with the yield close to the theoretical maximum. Hydrogenation in alcohol with heterogeneous catalysts is an example of the effective methods. The product from such effective depolymerization is a mixture of phenols derived mainly from the cleavage of β-O-4 linkages between phenylpropanoid units. However, the obtained highly functionalized phenols are not common in current industry and, therefore, needs further processing for utilization in the intended applications. The present study shows that simple phenols without functional groups at para-position form selectively in lignin depolymerization in hot compressed water with bases, such as sodium carbonate, as a type of catalyst. Alkali lignin derived from hard wood and bamboo released guaiacol and mixture of phenol, guaiacol, and syringol, respectively, as the main phenolic products. The selectivity to these phenols were more than 60% among identified phenols. The total phenols yield was not so high, up to 9%, but the selective formation of simple phenols was unusual when considering that the weakest and most abundant linkage was β-O-4. Although the reaction mechanism has not yet been clear, analysis of remaining lignin at different reaction times with several techniques showed that the lignin was condensed rather than depolymerized while releasing the phenols. Temporal change in the phenols composition indicated that the simple phenols were directly released from the lignin. On the other hand, utilizing this findings, the study also demonstrated a process that produced the simple phenols and high calorific value fuel gas along with near-complete recycle of the base catalyst.
Pyrolysis of cellulose has been studied for investigating combined effects of operating variables such as heating rate, type of reactor and a structural property of cellulose (crystallinity) on characteristics of its conversion into volatiles and char. A commercially available microcrystalline cellulose (MCC; crystallinity index (C.I.) = 87%) was subjected to ball-milling, which amorphized the MCC to C.I. of 60% and finally 0%. The MCC and amorphized celluloses were pyrolyzed at 445–764 °C with heating rates of around 103 °C/s in a Curie-point reactor where the secondary homogeneous and heterogeneous secondary reactions of volatiles were minimally involved. Results of the pyrolysis revealed two important characteristics. Firstly, the char yield was even below 1 wt% when the peak temperature was 590 °C or higher, and was much less than that from slow pyrolysis in a thermogravimetric analyzer, ca. 10 wt%. Suppression of the secondary reaction of the volatiles was thus effective for avoiding the char formation. Second, the char yield was almost independent of the crystallinity. The yield of the key product, H2O, formed by cross-linking, was hardly influenced by the crystallinity. The above-mentioned findings lead to a novel concept of char-free fast pyrolysis of any types of cellulose to produce mono- di- and oligo-anhydrosugars at high yield within seconds.
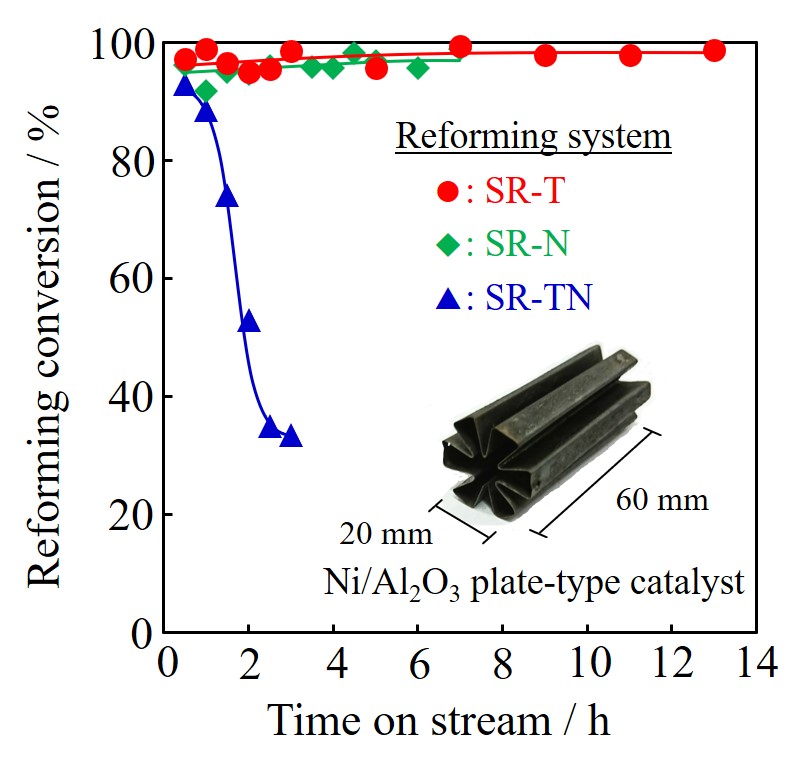
Biomass gasification process needs to be developed to ensure the future energy system. The important issue of the gasification system is to remove tar component generated in the gasification process. In order to achieve this purpose, a suitable catalyst should be developed for eliminating tar by steam reforming. Our group have already developed a powerful structured Ni/Al2O3 catalyst for steam reforming of toluene or naphthalene as the main components of tar [1][2]. In this study, we investigated the properties of the structured Ni/Al2O3 catalyst for simultaneous steam reforming of toluene and naphthalene mixture as to evaluate a viability for the practical application of the structured catalyst to tar reforming [3].
The structured Ni/Al2O3 catalyst was prepared by a sol-gel method and an electroless plating [3]. After H2 reduction at 800 °C for 1 h, the performance test was evaluated under the following conditions: setting temperature was 800 °C-830 °C; feed rate of toluene or mixtures (toluene/naphthalene=9/1) were 9.6×10–4 mol·min-1, and feed rate of naphthalene was 9.6×10–5 mol·min-1; steam to carbon ratio (S/C) was 1.5.
Figure shows reforming conversions for steam reforming of toluene (SR-T), naphthalene (SR-N) and mixtures of toluene and naphthalene (SR-TN) over the structured Ni/Al2O3 catalyst. In case of SR-T and SR-N, both reforming systems showed a high activity at 800 °C: each conversion was almost 100%. On the other hand, mixed reforming system showed deactivation in short time at 800 °C: the conversion was decreased from 92.9% to 33.6% during 3 h. Considering that reactivity of toluene is higher than naphthalene [4], naphthalene might not be sufficiently reformed and converted into a carbon precursor. Such serious problem was solved by setting reforming temperature at 820 °C or 830 °C. This result suggested a progressive advantage of Ni-based structured catalyst with high heat transfer ability.
Gasification of woody biomass can provide synthesis gases (CO, H2) at low temperature, however, byproducts such as tar, char, H2S, and NH3 are also generated at the same time. As these byproducts affect downstream equipment operation and reduce total energy efficiency, they should be treated appropriately before entering the downstream equipment. From this background, our research group have been proposed a new consecutive gasification and purification process that is consisted of biomass gasification, cyclone for char collection, metallic filter for volatile minerals, selective H2S decomposition, simultaneous decomposition of tar and NH3, and selective CO2 absorption. While the consecutive gasification and purification processes have been studied in our research group for a long time, developing CO2 selective absorbent material for produced gases is still remained as a research topic. Therefore, this study was started as a first step for developing selective absorbent material. At first CO2 absorption basic condition (material loading amount, pretreatment method, reactant flow rate, temperature, inner size of reactor, etc.) for obtaining the CO2 breakthrough profiles with all absorbent materials was determined. Then CO2 breakthrough profile was obtained with each sample under 10%CO2/Ar mixture flow, and the most excellent absorbent material was selected in terms of CO2 absorption capacity. In addition, both temperature and time needed for regenerating the used material was investigated using XRD and TG-DTA measurements. Finally, selected material was applied for CO2 absorption-regeneration cycle test to see the possibility of recycle usage.
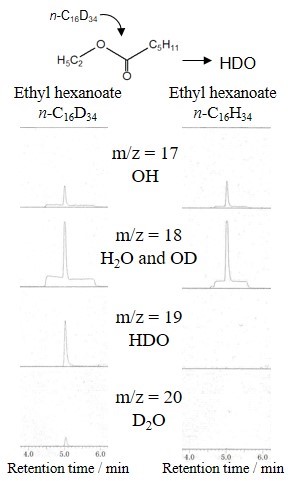
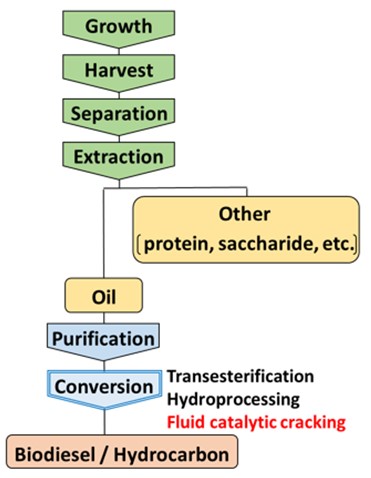
With the aim of efficient use of triglycerides as alternative fuels, deoxygenation of them and conversion to hydrocarbons are important for improving combustion heat. The basic approach of this conversion is a hydrodeoxygenation process, but using a pressurized hydrogen atmosphere incurs high process cost. Alternatively, we focused on a fluid catalytic cracking (FCC) process that can convert triglycerides to hydrocarbons producing CO, CO2, and H2O without using hydrogen atmosphere. Here, the deoxygenation pathway producing H2O is desirable for efficient conversion because the deoxygenation producing CO or CO2 cause a carbon loss. However, the detailed mechanism of H2O formation pathway has not been understood well. This study focused on the effect of a hydrogen transfer reaction that proceeds in the FCC process and investigated the reaction mechanism of ester deoxygenation using deuterated reagents as a hydrogen donor.
Ethyl hexanoate (ester model compound) and deuterated n-hexadecane (n-C16D34, hydrogen donor model compound) were mixed with an FCC equilibrium catalyst. The mixture of feedstocks and catalyst was instantaneously heated to 500°C using a Curie point pyrolyzer, and the reaction products were analyzed using a GC-MS. Figure 1 shows the mass chromatogram of the produced water in the catalytic cracking of ethyl hexanoate/n-C16D34 mixture compared with that of ethyl hexanoate/n-C16H34 mixture. In the reaction with n-C16D34, formation of HDO as well as H2O was observed whereas that of D2O was hardly observed. This result suggests that the ester received one hydrogen atom from the hydrogen donor and produced H2O. Thus, it was indicated that the hydrogen transfer reaction contributes to the deoxygenation of triglyceride, which can be useful information for a catalyst design with efficient conversion of triglycerides to hydrocarbon fuels.
Woody biomass has high oxygen content and requires an efficient deoxygenation process for upgrading to hydrocarbon fuel. Co-processing of bio-oil in the fluid catalytic cracking (FCC) of heavy oil is one of the effective methods for that purpose. Our recent study revealed that the hydrogen transfer reaction in the FCC can accelerate deoxygenation producing water rather than CO2 and CO evenunder a hydrogen-free atmosphere, which is an efficient deoxygenation pathway avoiding carbon loss. However, the effect of bio-oil property, which depends on the type and condition of the liquefaction pretreatment, on the deoxygenation pathway and the product composition in the co-processing FCC has not been understood well.
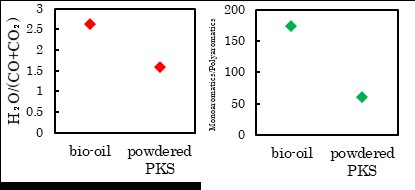
The previous study reported that the co-processing FCC of bio-oil prepared with fast pyrolysis resulted in a large amount of coke formation and low liquid yield. This may be due to the formation of condensed products during the pyrolysis that were difficult to upgrade in the FCC process. In this study, we focused on the solvolysis liquefaction that can produce bio-oil with high liquid yield (~90%) at a milder temperature than pyrolysis, thus avoiding the condensation reaction.
Bio-oil was prepared from the solvolysis of palm kernel shell (PKS). Catalytic cracking of the mixture of bio-oil and tetralin (heavy oil model compound) was investigated using a Curie point pyrolyzer connected directly to a GC-MS, and compared with the reaction of the mixture of powdered PKS and tetralin. The catalytic cracking of the bio-oil/tetralin mixture showed higher H2O/(CO2+CO) and Monoaromatics/Polyaromatics (3+ring aromatics) ratios than that of the powdered PKS/tetralin mixture. This result suggests that the pyrolytic reaction should be avoided through the whole process of liquefaction and catalytic cracking for the efficient conversion of the woody biomass, and the solvolysis pretreatment is adequate for that prosess.
Microalgal biomass has high productivity and expected as an alternative fuel resource. Euglena gracilis is one of such promising microalgae, whose mass cultivation in an open pond has been achieved recently. During the many steps of the fuel production from microalgae, oils are extracted from algal cells, purified and converted to transportation fuels. The most common conversion method is transesterification to biodiesel, but the biodiesel is an oxygen-containing fuels whose heat value and thermal stability are lower than that of hydrocarbon fuels. Conversion to hydrocarbons are usually conducted with hydroprocessing, but using pressurized hydrogen atmosphere requires high operation cost. Then, we focused on the fluid catalytic cracking (FCC) process, which is the major oil refinery process that can upgrade heavy oils to light fractions without using hydrogen atmosphere. In our previous study, we confirmed that the FCC process can convert wax esters derived from Euglena gracilis to hydrocarbons fuels with efficient deoxygenation. Furthermore, the FCC process is expected to be resistant against the impurities because it uses a circulating fluidized bed reactor and the catalyst is regenerated continuously in the regeneration tower. Therefore, in this study, we investigated the catalytic cracking of unrefined microalgae oil and compared the reaction with that of refined oil.
Polyisobutylene (PIB) manufactured by the living cationic polymerization exhibits desirable properties such as thermal stability and high barrier properties. Living polymerization often requires quite low reaction temperatures to avoid termination and chain transfer, for example, the polymerization of PIB is operated at around -80 to -70 °C in batch system. Living cationic polymerization of isobutylene (IB) utilizing TiCl4 in the batch system at low temperatures has been investigated [1-3]. It has been proposed that cationic reactive chain ends are generated in the equilibrium between dormant (tert-alkyl chloride chain ends) and active (ion-paired chain ends). However, the whole reaction mechanism remains to be elucidated due to the difficulty of direct evaluation of concentrations of the dormant and active species in the equilibrium. Moreover, understanding of the reaction mechanism is a key for process intensification.
Quantum chemical approaches have been used well to investigate polymerization reactions [4, 5]. These approaches have been showed the details of chemical reactions such as molecular geometries of reactants, products and transition states, as well as the changes of enthalpy, entropy and Gibbs free energy. The information provided by quantum chemical studies have helped not only to better understand reaction mechanisms, but also to design more efficient reaction processes.
In this work, we report the investigation of the reaction mechanism of the ionization equilibrium in the PIB polymerization with several catalysts using quantum chemical calculations. Experimental results of PIB polymerization with different catalysts are also discussed.
[1] Storey, R. F., Choate, K. R., Jr., Macromolecules, 30, 4799-4806 (1997)
[2] Fodor, Z., Bae, Y. C., Faust, R., Macromolecules, 31, 4439-4446 (1998).
[3] Tawada, M., Faust, R., Macromolecules, 38, 4989-4995. (2005)
[4] Dossi M., Storti G., Moscatelli D., Polym. Eng. Sci., 51, 2109 (2011)
[5] Deglmann P., Muller I., Becker F., SchaferA. , Hungenberg K.D., Weiß H., Macromol. React. Eng., 3, 496 (2009)
A kinetic model was developed for the synthesis of methyl acetate (MA) from dimethyl ether (DME) over ferrierite catalyst. Two types of active sites were considered; zeolite-exchange site that stabilizes acidic protons and methyl and acetyl groups, and CO-binding centers for non-competitive binding of DME and CO (Cheung et al., J. Catal., 245 (2007) 110–123). Pseudo-steady-state approximation was applied to develop the reaction rates, and kinetic parameters were estimated by fitting experimental data under a variety of temperature, pressure, space velocity, and feed composition. The developed model was used to evaluate the effects of operating conditions on the conversion and selectivity.
Clean and efficient utilization of low-rank coal is of great significance to the sustainable development of energy in China. “Regenerative Calcium Carbide Production Process” was proposed for clean and efficient utilization of low-rank coal. This technology takes pulverized medium to low rank raw coal and raw lime as raw materials that can form pellets. Then the pellets were sent into sealed calcium carbide furnace after pyrolysis in preheating furnace to obtain calcium carbide and pyrolysis oil and gas at the same time. The pyrolysis process of pellets is the key step of the technology. A comprehensive analysis of the influence of lime addition, pellet properties and operation conditions on pellet pyrolysis process plays an important role in comprehensively understanding pellet pyrolysis process and optimizing this new technology.
Firstly, the thermogravimetric experiments and basic experiments of pellet pyrolysis in fixed bed were carried out. The effects of calcium oxide, heating rate, pyrolysis temperature and pyrolysis time on the pyrolysis process of pulverized coal were investigated. The experimental data were provided for the establishment of pyrolysis kinetics. The results show that the addition of calcium oxide, the increase of heating rate, pyrolysis temperature and pyrolysis time can increase the volatile in the pyrolysis of pulverized coal. Then, the double distributed activation energy model based on genetic algorithm is chose to establish the pyrolysis kinetics. The results show that the double distributed activation energy model based on genetic algorithm can accurately describe the dynamic process of pellet pyrolysis. By comparing the pyrolysis kinetics, it is found that the addition of calcium oxide can reduce the activation energy of pulverized coal pyrolysis and increase the reaction rate of coal pyrolysis.
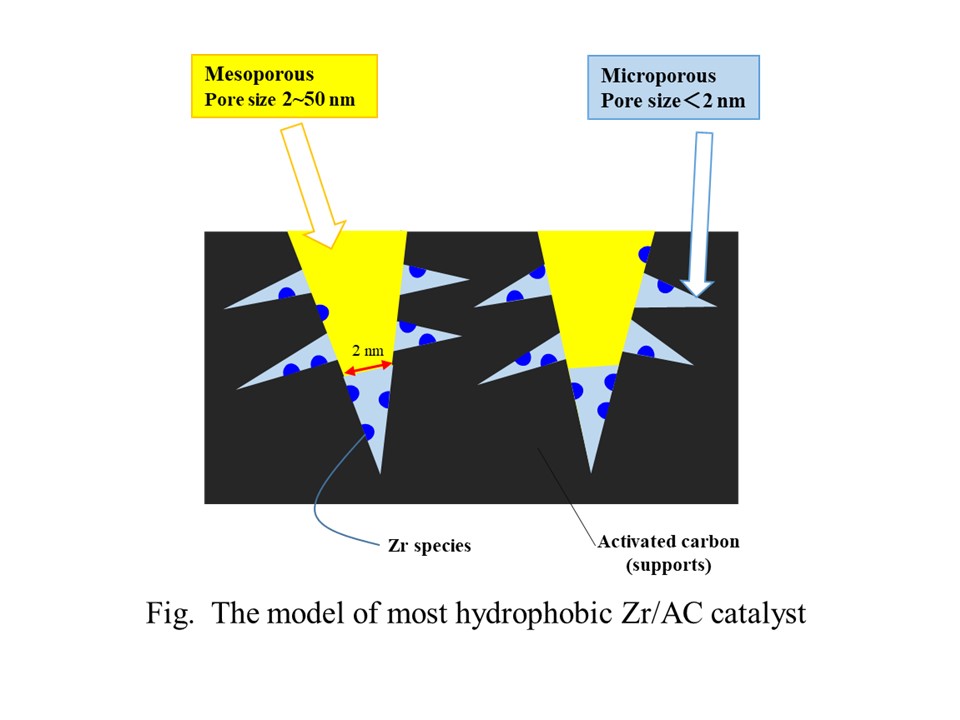
Meerwein-Ponndorf-Verley (MPV) reduction can selectively produce unsaturated alcohols from corresponding unsaturated aldehydes by using secondary alcohols as a hydrogen donor. Recently, bio-alcohols are expected to be used for MPV reduction. In bio-alcohols, large amount of water is included, and leads to decrease in the catalytic activity of Zr supported catalysts for MPV reduction due to the strong adsorption of water over Lewis acid sites on Zr species. In this study, MPV reduction of cinnamaldehyde to cinnamyl alcohol was carried out over Zr supported catalysts using activated carbon (AC) as supports possessing the hydrophobic properties.
Zr/AC catalysts were prepared by an impregnation methods. Zr loading was 10 wt%. In this study, 20 kinds of ACs were employed as supports of Zr/AC catalysts. Surface area of micropores and mesopores was determined by means of N2 adsorption isotherms. MPV reduction of cinnamaldehyde was carried out in an autoclave under 1 MPa (gauge) of N2 at 453 K for 1 h in 2-propanol with or without water (12.5 wt%).
To evaluate the hydrophobicity of each Zr/AC catalyst, water resistance was defined as cinnamyl alcohol yield in the presence of water divided by that without water. Over the Zr/AC catalysts possessing the mesopore surface area greater than 100 m2/g, the lower levels of water resistance were observed, suggesting that Zr species over the surface of mesopore was strongly poisoned by water. With respect to the Zr/AC catalyst indicating the highest water resistance, Zr species was selectively loaded in the micropore of activated carbon. These results suggest that the porous structures in AC strongly affect the water resistance of Zr supported AC catalysts.
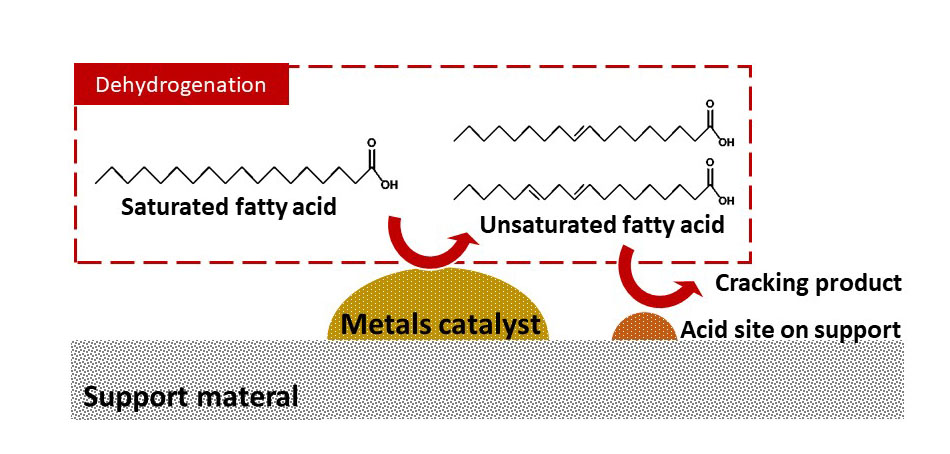
Short-chain fatty acids (SCFAs) have been used as raw materials in wide range of chemical and medical applications. One technique to produce SCFAs is oxidative cleavage of long-chain fatty acids (LCFAs). However, unless the LCFAs are unsaturated, the yield of SCFAs is often very low because the carboxylic group of the fatty acid is more active than other part of the molecule. This work explores the idea of introducing a double bond into saturated LCFA, i.e., stearic acid, via selective dehydrogenation using commercial heterogeneous catalysts. However, cracking of the LCFA is also catalyzed. Different type of metals was therefore investigated to study the effect of metals on the cracking and dehydrogenation. The experiments were conducted in an autoclave reactor under inert atmosphere. The temperature was in the range of 250-350 degree celsius. The products were analyzed by gas chromatography equipped with mass spectroscopy (GC/MS). The results reveal that the introduction of double bond in the aliphatic chain of the stearic acid is possible although the yields of the unsaturated LCFAs are low. Effects of various parameters, such as temperature, pressure, and reaction time, were also investigated and reported.
Short-chain fatty acids (SCFAs) and medium-chain fatty acid (MCFAs) are valuable raw materials in wide range of chemical and medical applications. They can be converted to other derivatives by known chemical reactions. Unfortunately, both SCFAs MCFAs are not as abundant in nature as long-chain fatty acid (LCFAs). In this work, the oxidative cleavage of oleic acid, which is one of the most abundant unsaturated LCFAs in nature, was studied. The oxidation was induced by hydrogen peroxide catalyzed by a commercial alumina-supported metal catalyst. The products were analyzed by gas chromatography equipped with mass spectroscopy (GC/MS). The results revealed that MCFAs and SCFAs were detected in a product. In addition, aldehydes were also found. In this work, effects of catalyst loading, oleic acid-to-hydrogen peroxide ratio, reaction time were investigated and reported.
Lactic acid is produced by the decomposition of sugars by lactic acid bacteria by fermentation, and is used as a raw material for biodegradable plastics.
Since the result of lactic acid fermentation depends on culture conditions such as sugar type and concentration, temperature and pH, it is necessary to set appropriate conditions depending on the bacteria used.
In this study, lactic acid fermentation using shochu lees without sterile condition at high temperatures was conducted to investigate effects of cultivation pH (5-7) and temperature (55,60 °C). Sludge collected from a pond on campus was used as inoculum.
The result was that most sugar was consumed and lactic acid was produced at the operating conditions of 55 °C and pH 6.5.
Many important chemical reactions, such as the water-gas shift reaction and ammonia synthesis via Haber-Bosch process, are reversible in nature and the conversion of product(s) limited by chemical equilibrium. Here, we have shown that by de-coupling the overall reaction into two or more sub-reactions via the use of a heterogeneous mediator (a reaction scheme known as “chemical looping”), it is possible to overcome the chemical equilibrium of the overall reaction and achieve almost full conversion of the products.
We demonstrate this possibility by performing the water-gas shift (WGS) reaction at 820 °C: If WGS is performed at this temperature using conventional reaction scheme with an equimolar feed of carbon monoxide and steam is used, the product stream will contain approximately equal amount of carbon monoxide, steam, hydrogen and carbon dioxide on a molar basis when equilibrium is achieved. On the other hand, by feeding the reactants counter-currently over a packed bed of solid mediator that can supply and uptake oxygen over an appropriate range of oxygen fugacities (rather than at a fixed oxygen fugacity), such as non-stoichiometric perovskites (and in this particular case, La0.6Sr0.4FeO3-δ), we are able to produce separate streams of high purity hydrogen and carbon dioxide separately, achieving average conversions over 80% for both carbon monoxide and steam.
The conversion that can be achieved depends on the oxygen fugacity – oxygen capacity relationship of the solid mediator and can be further increased by optimising the length of the bed and the cycle time (duration of each feed stream in the cyclic process). In theory it is possible to achieve full conversion of carbon monoxide to carbon dioxide in one half-cycle and steam to hydrogen in the other. In this way, not only have the equilibrium limitations been lifted, downstream separation units can be simplified or even eliminated.
A methanation processes using synthesis gas derived from naphtha, biomass or coal can generally be performed in both fixed and fluidized bed reactors. The fluidized bed methanation allows isothermal operation due to the high heat transfer coefficients, simple and easy control of the operation. Further advantage is that it is possible to easily remove, add and recycle catalyst continuously during operation. On the contrary, special attention should be paid to attrition and the entrainment of the catalyst particles. In the fixed bed methanation, the heat of reaction has to be considered because of the high amount of CO in the syngas. For controlling the temperature inside the reactor, several methanation reactors are connected in series with intermediate gas cooling or recycle of product gas.
However, the standards for natural gas pipeline network in Korea and Japan are calibrated in the range of 9,700 ~ 10,800 kcal/m3. In order to connect the SNG produced from coal to the pipe network, the calorific value should be adjusted by adding gaseous fuel such as LPG (Liquefied Petroleum Gas).
For this purpose, it is necessary to synthesize paraffinic hydrocarbons having C2 ~ C5 carbon number by designing a catalyst so that FT (Fischer-Tropsch synthesis) reaction occurs simultaneously with the methanation reaction. Therefore, in this study, the reaction characteristics to produce the higher calorific SNG (including C1 to C5 range hydrocarbon) over the hybrid catalyst were investigated in bench-scale fixed bed reactor (FBR), bubbling fluidized bed reactor (BFBR) and slurry bubble column reactor (SBCR).
Acknowledgement:
The authors are grateful to the financial support from Korea Institute of Energy Evaluation and Planning (KETEP, No. 20173010050110).
References:
Kang, S.H., Woo, K.J., Bae, J.W., Jun, K.W. and Kang, Y., Korean J. Chem. Eng., 26, 1533-1538(2009)
Among various methods to produce hydrogen from methane, catalytic direct decomposition of methane is the most environmental friendly process because COx is not generated. CH4 direct decomposition with the catalyst normally provides two hydrogen molecules and carbon deposited on the catalyst as an amorphous carbon or carbon nanotube or film-like carbon. After reaction, those carbonaceous species were separated from the catalyst and collected as functionalized carbon materials. In the most cases, a fixed bed reactor was used for this reaction, and the catalyst deactivation was observed after a short reaction time due to carbon deposition. Some research groups had reported that the usage of plasma or membrane reactor improved the catalytic performance for the direct decomposition of methane; however, catalyst deactivation was still observed, and the way for separating solid carbon from the catalyst and catalyst regeneration method are not clear. From these backgrounds, our research group recently proposed a newly circulating bed reactor which consists of cyclone type reactor for methane direct decomposition reaction and riser reactor for regenerating the carbon deposited catalyst with steam to produce synthesis gas. As a novel circulating bed reactor is under construction, methane direct decomposition with Fe-based or Co-based catalysts and steam gasification of the carbon deposited on the catalysts were performed in a fixed bed reactor in the present study. It was found from the obtained results that the catalytic activity for methane direct decomposition depended on metal loading amount and type of metal and support. Moreover, the kind of carbon deposited on the catalyst was changed with the aforementioned parameters. Since it could be predicted that the reactivity of those carbonaceous species with steam might be changed, the results obtained from steam gasification of carbon deposited on the catalysts were also presented.
Over last decades, electrochemical advanced oxidation processes based on Fenton reaction (EAOP-Fenton) have been paid great attentions because they are capable of continuous generation of strongly oxidizing radicals that can completely oxidize organic pollutants into CO2 and H2O. Among the EAOP-Fenton processes, Fered-Fenton (FF) process, which relies on the electrochemical reduction of Fe(Ⅲ), has demonstrated great effectiveness and has potential for practical application. In FF process, a variety of factors have an impact on the efficiency, although the cathode potential that is especially important because not only in-situ generation of Fenton reagent but competition reactions can occur on the cathode. Furthermore, many researchers have shown the utility of FF process, but most of them have referred to a batch reactor. It is required to develop a flow-type reactor, which achieve continuous treatment, for the application of FF as an industrial method. We have previously presented a flow-type reactor with macro porous carbon electrode as cathode, which is water-permeable. The electrodes were prepared through the carbonization of carbon particles molded with binder.
In this study, effect of reaction conditions such as flow rate, current, on the degradation ratio was examined to understand the electrochemical reaction in the electrodes. It was found that ionic transfer in the electrode is a key factor. Therefore, we measured the liquid potential in up and downstream of the cathode to elucidate the ionic transfer in the cathode. Anode electrode was placed in the downstream of the cathode. Liquid potential in the downstream side was raised with the increase of current, whereas that in upstream side was not changed, indicating that reaction does not occur on the electrode in the upstream side. Change of liquid potential in the cathode was also estimated using electrodes with different thickness.
As photocatalysis is one of useful methods for the cleanup of polluted water, various photocatalyst powders have been reported. Once photocatalyst powder was dispersed into the polluted water, clean water without any organic compounds could be obtained under light irradiation; however, the separation process is necessary to remove the powders from clean water, which imposes high cost and reduces the recovery rate. To solve these problems, our research group proposed a novel catalyst system that photocatalyst powder is encapsulated in alginate shell, and both reactants and products could be permeated through the shell by the concentration gradient between inside and outside. Usage of this system makes catalyst recovery easy, and catalyst-loaded capsules could be successively used several times if the capsules are not broken. In our previous study, two kinds of photocatalyst-loaded alginate capsules were successfully prepared and applied for the degradation of methyl orange under light irradiation. It was found that this new system operated smoothly, and encapsulated nitrogen doped TiO2 powder prepared by original sol-gel method showed higher activity than commercial TiO2-loaded capsules under the irradiation of ultraviolet and/or visible light. Characterization results obtained by various analytical methods suggested that catalyst surface area, crystallinity of anatase TiO2 phase, light absorption property, and powder dispersibility inside the capsule might be key factors to determine its activity. In this study, high surface area TiO2 powders were prepared by original sol-gel method and loaded into the alginate capsules. These capsules were also tested, and the performance was compared with the results obtained in a previous study. Finally, it is indicated which are significant factors to exhibit excellent performance for the degradation of methyl orange under light irradiation.
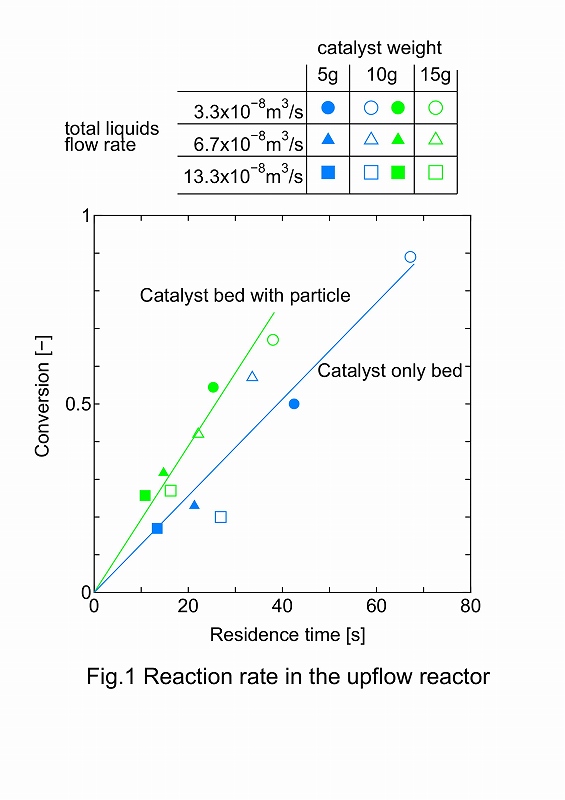
The reactant (carbobenzoxy phenylalanine) is soluble in an organic solvent and hydrogenated to the product (phenylalanine) using solid catalyst. Phenylalanine is not soluble in the organic solvent, so it needed to be dissolved in an aqueous phase. This was the gas-liquid-liquid-solid four-phase system. Mass transfer becomes more complicated in comparison to three-phase reactors as the catalyst needs to efficiently contact a gas phase as well as two immiscible liquid phases. The catalyst surface properties (hydrophilic versus hydrophobic) also play an important role in the reaction related to the wetting efficiency of the liquids at the solid interface. Gas-liquid-liquid cocurrent upflow reator was used. Column inner diameter was 2.0 cm. Gas flow rate was 1.7x10-6m3/s. Liqud flow rate ratio of water to organic solution was 3. 1-octanol was used as organic solvent. Reaction temperature was 323 K.
To improve Pd/Al2O3 (3 mm in diameter, 3.2 mm inlength) reaction rate, 0.3 mm glass beads were inserted to the catalyst bed. Object of glass bead was calming the gas and liquid flow patern and incresing the gas and liquid velocity.
Gas and liquids hold up was measured to determined the residence time. Water was the continuous phase in this reaction system. Each liquid residence time was calculated from holdup and flow rate. Total liquids flow rate were varied and conversion was measured in each liquid flow rate. Figure 1 shows the effects of the organic solvent residence time on the conversion. Two type of catalyst bed were used. Diluted bed is shown in green mark and catalyst only bed is shown in blue mark. Conversions were linealy increased with increase of the residence time. Slopes of the lines show the reaction rate. The raction rate was increased 1.6 times by using glass beads.
Zeolites are microporous crystalline aluminosilicates, and are industrially used as adsorbents and catalysts because of their very specific physicochemical properties such as ion exchange, adsorption properties.and solid acidity. CHA type (CHA) zeolite, which is one of the most important zeolite, has a three-dimensional pore system with ellipsoidal large cages (6.7x10 â) that are accessible via eight-membered ring windows (3.8x3.8 â). High silica CHA zeolite SSZ-13 and silicoaluminophosphate zeotype SAPO-34 with CHA topology are of great interest as they exhibit a particular shape selectivity for converting methanol or bioethanol to light olefins such as ethylene and propylene. Pure silica CHA also has potential for industrial applications such as adsorption and separation of organic molecules and gas storage because of a larger void space due to the absence of counter cations in the pores and the hydrophobicity. Recently, the hydrothermal conversion of one zeolite into another, i.e., interzeolite transformation has attractive interest from the standpoint of an alternative strategy for zeolite synthesis. Zones and van Nordstand have already reported the interzeolite conversions of cubic P and FAU zeolites used as only aluminum source to high-silica CHA zeolite SSZ-13 with Si/Al ratio of 13. In this study, we investigated influence of Si/Al ratio of FAU zeolite as silica and aluminum sources in the interzeolite conversion to high-silica CHA zeolite. As a result of detailed investigation, by using FAU type zeolite as a silica source and an aluminum source, the Si / Al ratio of the obtained SSZ-13 was successfully extended to 10 to 200. In addition, Moreover, highly crystalline CHA zeolite (Si / Al =100) with a particle size of 1.0 μm was obtained using FAU type zeolite having a Si / Al ratio of 100 as a raw material at 160 °C. for 4 days.
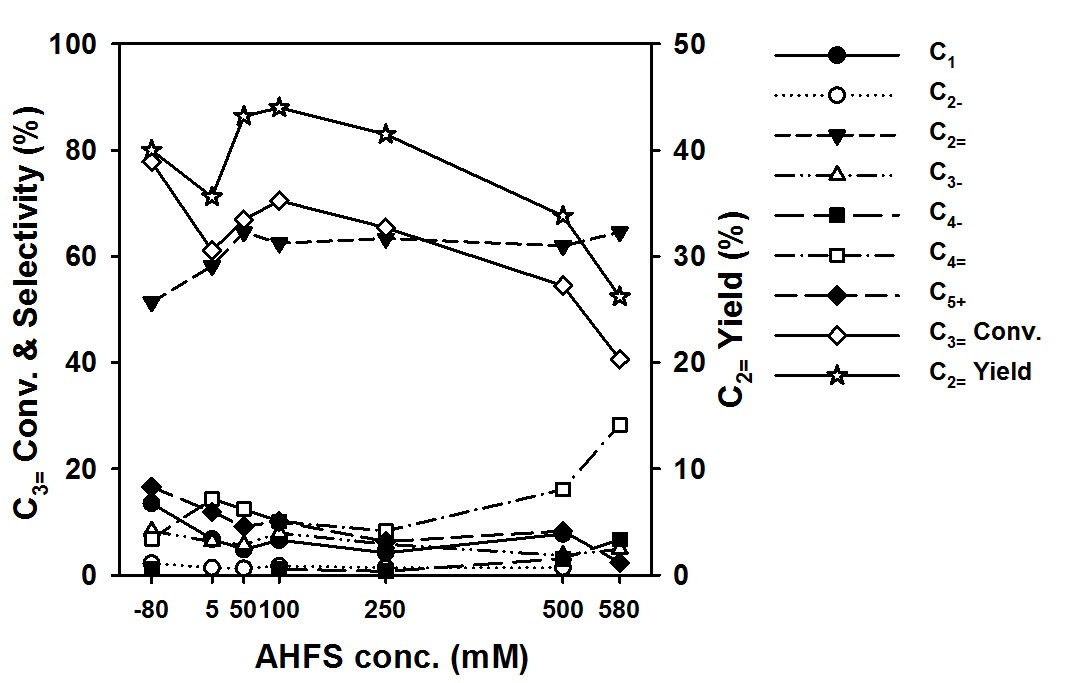
In recent years, the light olefin manufacturers have often confronted an unpredictable regional supply-demand imbalance between light olefins. Therefore, the olefin plant requires an olefin interconversion technology as a simple and optional process to respond quickly to the sudden imbalance of light olefins. The olefin interconversion process is typically divided into two processes: the ethylene-to-propylene (ETP) process and propylene-to-ethylene (PTE) process. The ZSM-5 zeolite was known to exhibit the highest ethylene selectivity in zeolite-catalyzed PTE reaction, and the post-modification with phosphorus could further increase the selectivity. However, the P modification was accompanied by lowering propylene conversion due to the decrease of the strong acid site, which leads to a decrease of the yield of ethylene. Herein, the post-treatment of ZSM-5 zeolite with ammonium hexafluorosilicate (AHFS) was carried out in order to improve the yield of ethylene in PTE reaction. The AHFS-treated ZSM-5 showed a considerably increased yield of ethylene than the pristine (H-Z50) and P-modified ZSM-5 (P-Z50) as shown in the figure. AHFS-treated ZSM-5 exhibits high ethylene selectivity and propylene conversion simultaneously, thus results in the improved ethylene yield. The origin of increased catalytic performance was investigated using ammonia temperature-programmed desorption (TPD) method and solid-state 27Al MAS NMR spectroscopy. From the TPD measurement, it was observed that the number of medium acid sites was increased and the number of weak acid sites was decreased. The NMR spectra indicated that the extra-framework aluminum species (EFALs), which known as having weaker acid strength than framework Al species, increased after AHFS treatment. Therefore, the AHFS treatment causes the generation of EFALs on the external surface of the zeolite and the selective increases of the medium acid site, so that thus improve ethylene selectivity and yield. The synergetic effect of Brønsted acid sites and EFALs would be suitable for acid-catalyzed PTE reaction.
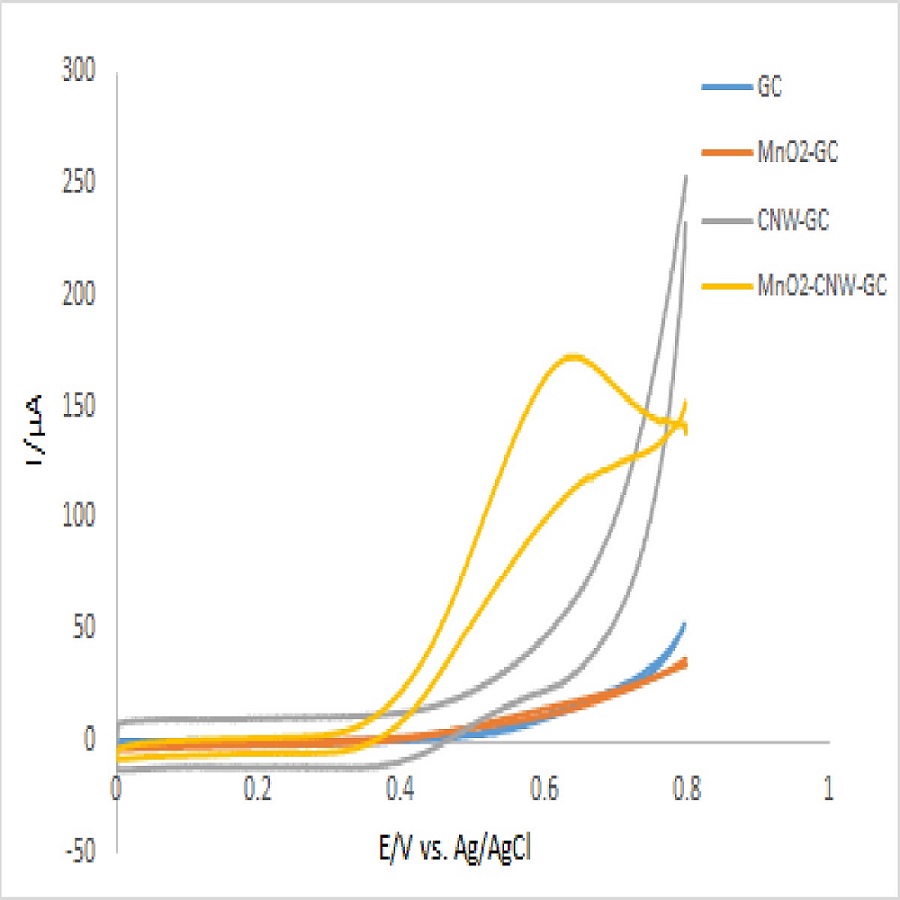
Recently, in electrochemical sensing of H2O2, manganese dioxide has drawn attention as a promising electrode material due to its excellent catalytic activity which can promote H2O2 decomposition. However, many challenges are encountered with its practical application due to the poor electronic conductivity, chemical and mechanical stabilities. Considering this, in our study, we applied carbon nanowall (CNW) as the base nanoporous structure for depositing MnO2 films on it to improve the electrochemical sensor performance of MnO2.
CNWs template was synthesized by plasma enhanced chemical vapor deposition in a CO/H2 microwave discharge system. Glassy carbon with an area of 1cm2 was used as the substrate. The applied parameters of the CNWs deposition process were as follows: CO flow rate: 23sccm, H2 flow rate: 2sccm, pressure: 250Pa, microwave power: 80W, and deposition time: 60 seconds. After synthesizing CNW, MnO2 coating was anodically electrodeposited on CNWs using a three-electrode system consisting of the CNW/GC as a working electrode, an Ag/AgCl electrode as a reference electrode, and a platinum wire as a counter electrode at a constant potential of 1.0V vs. Ag/AgCl in the 0.25M Mn(CH3COO)2 solution.
The morphological and elemental characterization of the fabricated electrodes were carried out by SEM and TEM-EDX. Cyclic voltammetry and amperometric tests were applied for evaluating the sensor performance of the fabricated electrode.
In addition, new challenges of applying anodic deposition technique for synthesizing MnO2 films on porous CNW structure will be introduced and potential biosensor application will be discussed.
1. Liqiang Luo, Electrochimica Acta 77 (2012) 179– 183.
2. Chunyan Guo, Sensors and Actuators B 206 (2015) 407–414.
The utilization of surplus electricity from renewable energy resources is one of the social problems; accordingly, non-thermal plasma is drawn attention as its utilization methods.
In this study direct synthesis of liquid hydrocarbons from methane and carbon dioxide using pressure-swing plasma is studied.
In traditional Gas to Liquids (GTL) process, low-pressure conditions are preferred for the reforming reaction of methane to form syngas while high-pressure conditions are preferable for the synthesis of higher-hydrocarbons from syngas (Fischer-Tropsch Synthesis).
In addition to conventional plasma system, in this study, unprecedented pressure-swing of CH4-CO2 plasma is applied for the solution of trade-off pressure conditions.
It is expected that dissociative reaction by plasma and synthesis reaction by pressure-swing will proceed concertedly and liquid hydrocarbon will be synthesized directly.
As pressure-swing plasma system, we utilized a uniquely modified diaphragm pump as a pressure-swing reactor with creeping discharge which develops inside the chamber of reactor.
Electrode has structure that quartz plate as dielectric put between cupper tape as ground electrode and carbon tape as high-voltage electrode and plasma forms near ground electrode. We can also put other metal (e.g. Co foil, Fe foil, and so on) on cupper tape.
Pressure-swing range is approximately 1 – 3 bar, frequency of swing is ~25 Hz. Typical applied voltage of plasma is 9 kV, power consumption of plasma is approximately 4.5 W. Timing of plasma discharge is pulsed and manipulated by micro-controller, typical pulsed frequency is 100 Hz.
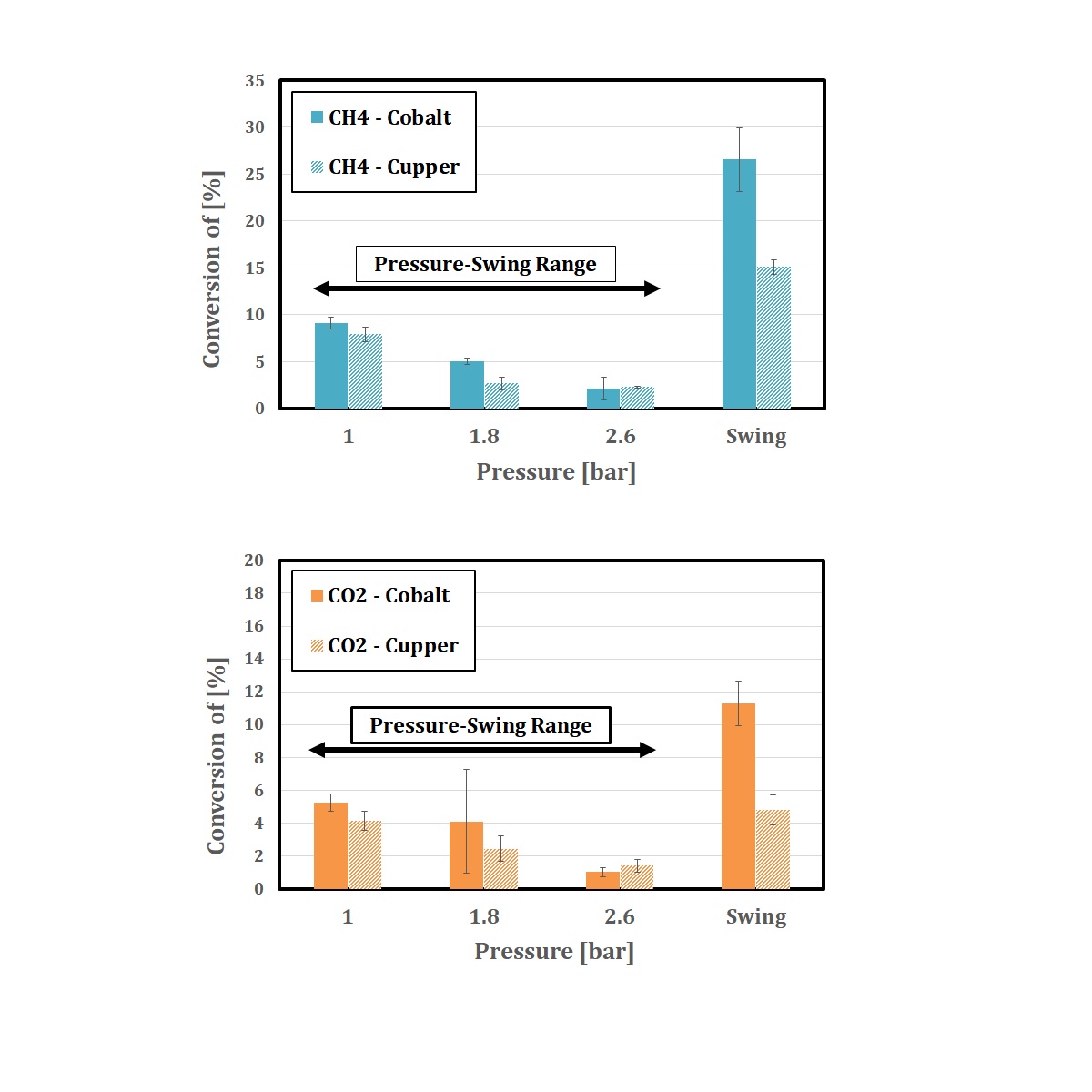
The conversion of methane and carbon dioxide and the selectivity of product hydrocarbon are investigated by GC.
From the experiments, we observed that the conversion is improved and the selectivity of product hydrocarbons is shifted to heavier hydrocarbons in pressure-swing condition.
Moreover, we discussed and considered the relation between discharge timing and the selectivity.
Lithium air battery is one of the most promising energy storages due to its high theoretical energy conversion comparing to conventional lithium ion battery. However, development of lithium air battery to practically use in actual application has been faced many problems such as dendrite on lithium anode, decomposition of electrolyte and pore clogging on cathode. Focusing on cathode, many researches applied highly porous and functional carbon materials such as graphene or carbon nanotube to fabricate cathode by evaporation method. Despite the good properties, those materials too expensive to practically use. Moreover, evaporation causes aggregation of carbon material due to high surface tension, decrease surface area for cathodic reaction.
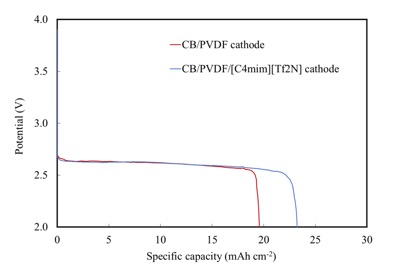
In this work, we applied carbon black (CB), which is extremely cheap material comparing to graphene or carbon nanotube, as the active material for cathodic reaction and Polyvinylidene fluoride (PVDF) as the binder. Moreover, [C4mim][Tf2N] ionic liquid was impregnated in PVDF to improve ionic conductivity, electrical conductivity and reaction gases selectivity. Those explained materials were dispersed in N-Methyl-2-pyrrolidone (NMP) and dried by supercritical carbon dioxide at 20 MPa, 40 °C. Cathodes were assembled into meshed coin cell with 1 M LiTFSI/TEGDME electrolyte. The capacity was tested with air, pure O2, O2/CO2 mixing gases at 0.075 mA cm-2.
CB/PVDF cathode dried by supercritical CO2 has 92% of porosity, while CB/PVDF/[C4mim][Tf2N] cathode has porosity around 84%. Despite the lower porosity, Li-air battery applied CB/PVDF/[C4mim][Tf2N] cathode provides capacity at 23.2 mAh cm-2 (42.4% efficiency), which is larger than CB/PVDF cathode at 19.6 mAh cm-2 (35.7% efficiency). The discharge products were confirmed as Li2O2 by XRD in both cases. The improvement of specific capacity is considered that ionic liquid gel enhances ionic conductivity and improves selectivity and solubility of reaction gas on surface of cathode.
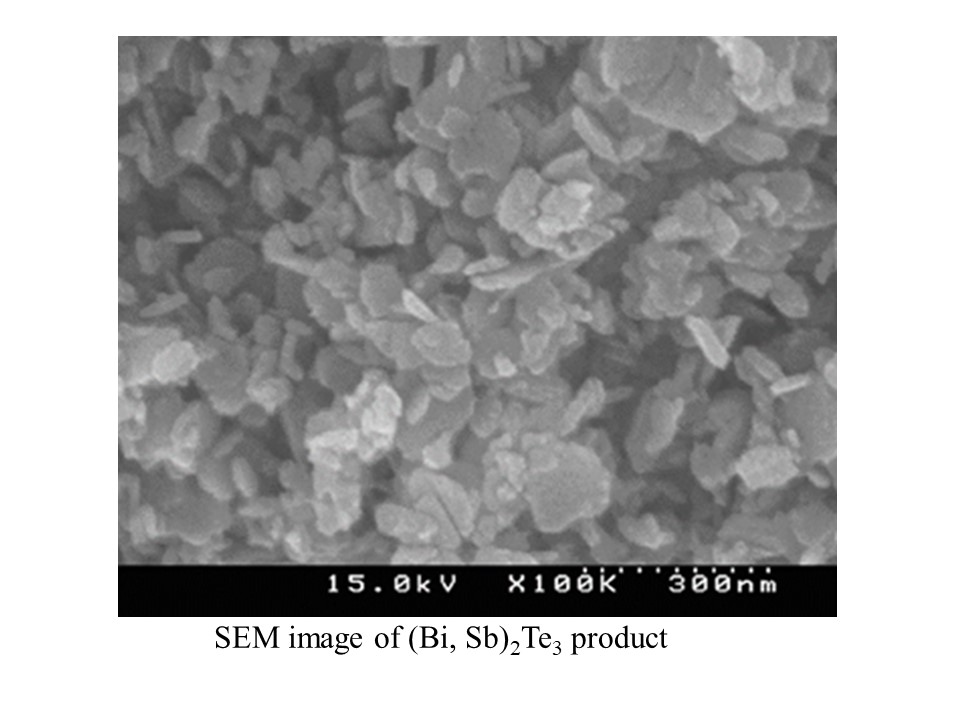
Bi2Te3 type semiconductor is well known as a thermoelectric conversion material generally used in a temperature range around room temperature, and is also put to practical use as a Peltier element for cooling. The Bi2Te3 type semiconductor is generally produced from depositing fine metal powder by a melt under high vacuum or inert atmosphere, and sintering from a ground alloy or mechanical alloying method, but these synthesis process are complicated and the cost benefits are low. Therefore, direct synthesis of single phase fine particles was attempted by preparing a neutralized co-precipitate from each metal chloride mixed aqueous solution, adding a reducing agent and subjecting it to hydrothermal treatment. An aqueous solution of chloride of Bi, Te, Sb, and/or Se was mixed in an appropriate ratio, and the aqueous solution of NaOH was added dropwise with stirring vigorously to prepare a co-precipitate of each composition. HCOOH or NaBH4 as a reducing agent is added to the aqueous solution, hydrothermal treatment is performed at 473 K. for 24 h in an autoclave equipped with a Teflon bottle, the product is filtered off with suction with a 0.45 μm filter, and after distilled water washing, and dried at 333 K or less. As a product, a plate-like fine crystal having a diameter of about 100 nm and a thickness of about 20 nm was obtained, in which the chemical composition was almost uniform in all of Bi2Te3, (Bi, Sb) 2Te3 and Bi2 (Se, Te) 3 ). The electrical resistance and Hall effect for representative product samples were measured. As the results, both products show relatively low efficiency, and it is found that Bi2Te3 and Bi2 (Se, Te) 3 show n-type, and (Bi, Sb) 2Te3 shows p-type semiconductors.
Examination of fouling mechanism in heat exchanger is demanded to minimize fouling trouble. This study analyzed fouling deposits recovered from heat exchanger to reveal the chemical structure of these deposits. The basic structure of the deposits was determined by nuclear magnetic resonance, pyrolysis and X-ray diffraction. The deposits consisted of diene and iron oxide. The CHNS elemental analysis showed that H/C atomic ratio was approximately 1.4 and the deposits had oxygen. The infrared spectroscopy analysis indicated that the deposits had carbonyl groups.
Acknowledgment: This study was supported by the Advanced Production System Project Committee of the Society of Chemical Engineers, Japan.
Introduction
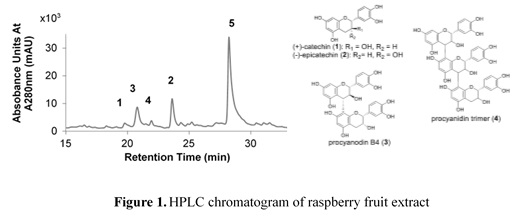
Berry fruits are well known to contain large amounts of polyphenol compounds. Among them, flavan-3-ol and anthocyanidin derivatives are a group of secondary metabolism compounds currently attracting a great deal of attention owing to their health benefits. Berry fruits are often consumed as processed products such as juices and jams in addition to fresh fruits. Most processing methods are heat-drying, but there is little detailed research on how polyphenol compounds contained in fruits change as a result of processing. Therefore, we wanted to chemically track how the polyphenol compounds contained in the fruit change with processing. Therefore, we selected Red Ruspberry (Rubus idaeus L.) as an experimental model plant because it is widely known for edible fruits and is rich in useful polyphenols such as flavan-3-ol.
Results and discussion
The HPLC chromatogram of the fresh raspberry-MeOH extract is shown in Figure 1. The peaks in the chromatogram were identified as catechin (1), epicatechin (2), procyanidin B4 (3), procyanidin trimer (4) and hydrolyzable tannin (5), respectively. In dried fruits, it was confirmed that hydrolyzable tannin was decreased. Measurement of DPPH radical scavenging activity, which is an indicator of antioxidant activity, showed that the activity was reduced in dried fruits. In this report, we will discuss in detail the chemical changes of polyphenol components by drying and heating.
Reactive dyes, which are most widely used for dyeing cotton, have reactors capable of chemical reaction with the hydroxyl groups of cotton fiber. Depending on the type of reactor, the dyes vary greatly in the size of the reactivity and the dyeing temperature. Therefore, the optimum dyeing temperature depends on the reactor.
The dye molecules adsorbed by the addition of the initial neutral salts are markedly less substantivity between the fibers and the dye when the alkali is added. At this time, dye molecules and fibers are covalently bonded by chemical reaction.
The alkali catalyzes the reaction when the dye and the fiber react with each other because the concentration of the OH group in the fiber is increased by the addition of the alkali to increase the reaction. At the same time, however, the OH group concentration of water is also increased, facilitating the hydrolysis, and the pH of the bath is determined by the kind and amount of alkali. The reaction proceeds rapidly as the pH of the bath is high.
In this study, we used the regression analysis to determine the optimum alkali injection timing at the time of the exhaustion equilibrium. The point in time when the substantivity between the fiber and the dye was significantly lowered was calculated and it was decided that the optimum alkali injection time point.
The main factors affecting the primary exhaustion equilibrium during the dyeing process with reactive dyes are neutral salts, that is, in general, the neutral salt, which inhibits the electrostatic barrier(Donnan potential) between fibers and dyes, Eliminate the repulsive force on the fibers.
It also facilitates the action of the Van der Waals force, facilitating the access of the dye to the fiber surface, thereby enhancing the adsorption of the dye.
The amount of neutral salt used is determined by the amount of dye. The more dyed dyes require more neutral salts, the more the amount of neutral salts added, the higher the adsorption of dye in the dyes. This is the basic condition for the next fixing reaction step. In order to increase the adsorption of the reactive dye which is lower than the direct dye, the use of a large amount of neutral salt is required, but the adsorption rate is not improved.
In this study, the basic method of determining the optimum injection timing of alkali additives was analyzed by analyzing the input method and the input amount of the manganese which are the main factors of the dyeing equilibrium of reactive dyes.
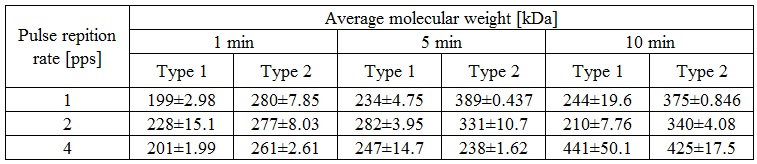
Poly(N-Isopropylacrylamide)(PNIPAM), a thermo-sensitive polymer, is well known for undergoing coil-to-globule transition at lower critical solution temperature (LCST) of 32 °C. As this LCST is close to the temperature of the human body, considerable research efforts have been directed towards its potential use in drug delivery systems, functional materials, and humectant absorbents. Conventionally, PNIPAM is produced in aqueous system through the addition of an initiator, however these methods require continuous stirring and lengthy treatment times (more than 6 h). Recently, plasma initiated polymerization methods have been attracting attention because of the generation of free radicals in the process. To generate plasma, pulsed power is commonly used. It is an instantaneous form of energy that, when temporally compressed, can produce a tremendous amount of electricity. This study presents a novel, more efficient PNIPAM polymerization method that uses pulsed arc discharge. An optimal raw material conversion, over 90%, is obtained with an initiator concentration of 0.1mol/L at 4 pps for 1 min in the presence of 2,2´Azobis(2-Methylpropionamidine) Dihydrochloride as an initiator. Furthermore, the effect of the initiator concentration and the distance between the cathode and the aqueous solution are studied. When the cathode is submerged in the aqueous solution, these conditions produce a yield over 80% and an average molecular weight of 261±2.61 (see Table 1).
Plasma technology has great potential for converting excess renewable energy into value-added chemicals. In the previous research in our laboratory, we were able to synthesize ammonia efficiently by pressure swing of N2-H2 plasma. In this research, we will consider the process of synthesizing ammonia by pressure swing of N2-H2O plasma.
Nitrogen gas and water vapor were used as feed gas, electrodes were inserted inside the pressure swing reactor to generate plasma, and then the product gas was analyzed. Nitrogen gas was bubbled into pure water and water vapor, which is a hydrogen source of ammonia, was introduced. The pressure-swing was performed by using a modified diaphragm pump. The product gas was trapped by bubbling into pure water, and the obtained aqueous solution was analyzed by HPLC.
The amount of ammonia synthetic efficiency when the reaction is carried out under a constant pressure and under pressure swing is shown in Figure. By performing pressure swing, the efficiency of ammonia synthesis increased from under a constant pressure.
Moreover, when the metal electrode was changed from copper to platinum, an increase in the synthesis efficiency of ammonia was confirmed under both constant pressure and swing conditions. This indicates that the rate-determining step of ammonia synthesis in this study is a surface reaction, and pressure swing enhances the surface reaction.
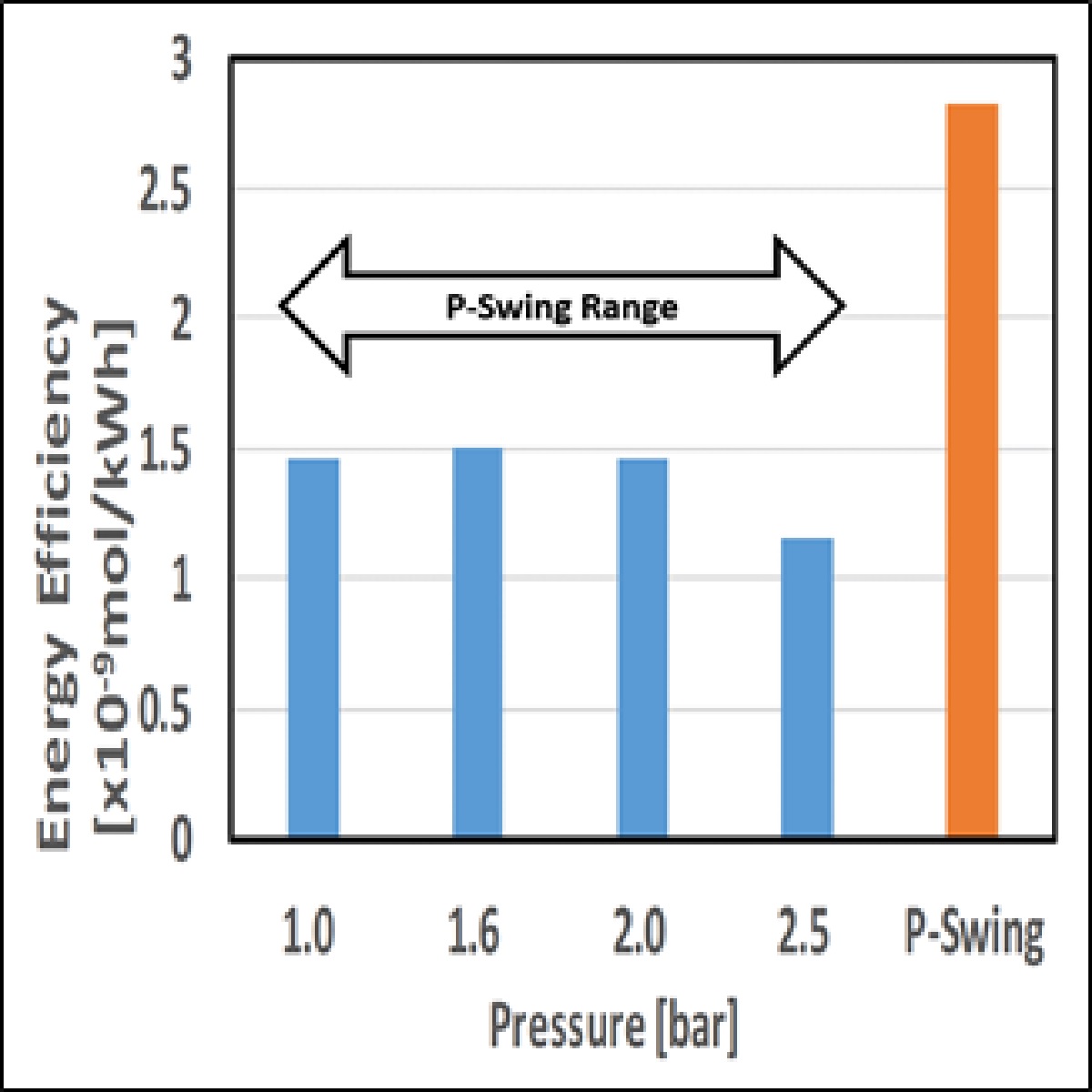
It has been well known that Ammonia is a very important chemical substance for the life of humankind. Simultaneously, conventional ammonia production process needs nitrogen gas and also hydrogen gas which has been produced from either LNG or coal. This study is regarding ammonia production from nitrogen gas and water (Do not hydrogen) by plasma/liquid interface (P/L reaction). We have developed the unique reaction which produces ammonia at the plasma gas phase/liquid (water) phase interface (P/L reaction locus). Although the outermost of surface water molecules on the water phase show a unique orientation, the hydrogen (H) of water molecule on a water surface is possibly oriented toward the gas-phase side. Activated nitrogen extracts H from H2O at water phase surface to produce ammonia, which dissolves in the water.
In excited nitrogen gas (N2 plasma) phase, several excited state have been reported. These include atomic nitrogen (Natom), excited nitrogen molecules (N2*), nitrogen ions (N2+), and the like, which have different lifetimes and reactivities. We have already shown that atomic nitrogen is highly reactive by quantitative analysis of N(4S) nitrogen atoms. Furthermore, it has been reported that increased the amount of ammonia production starting from low reactivity nitrogen species (N2* and N2+) pathways by activating the water phase. Upon UV irradiation onto the water phase, the activated nitrogen with low reactivity also promotes a reduction reaction system using water molecules.
In this study, we examined the P/L reaction pathways that changed the conditions of the water phase. The reaction pathway of ammonia production was examined by changing the pH, changing ionic strength, and changing the amount of water.
This poster focuses on the utilization of the synergy of microwave irradiation and graphene-based catalysis as applied to various reactions related to biomass conversion processes including cellulose depolymerization, glucose conversion into platform chemicals, and transesterification/esterification/etherification of oil fractions and products in biomass. The result are part of the research contributions of the Japan team to the recently concluded JST e-ASIA Joint Research Program on “Development of Functional Nanocarbon-Based Catalysts for Biomass Conversion Processes.”
Conversion and utilization of inedible lignocellulosic biomass, which mainly consists of cellulose, hemicellulose and lignin, have greatly attracted attention to reduce carbon dioxide emission and to establish the sustainable society. Cellulose and hemicellulose are polysaccharides, which can be converted into valuable products and fuels. Lignin is a three-dimensional polymer of aromatic compounds with cross-linking via C-O-C ether bonds and C-C bonds, which can be converted into aromatic products by depolymerization; however, the lignin conversion to aromatic monomers is still challenging. We reported the bond cleavage reactions between aromatic monomers using lignin model compounds in supercritical water at 673 K in the presence of supported palladium, platinum, and rhodium catalysts without added hydrogen gas and without causing hydrogenation of the aromatic rings in the previous paper. Thus, we applied this technique to the lignin into aromatic compounds. In this paper, we found that lignin could be converted into aromatic monomers in supercritical water at 673 K over supported metal catalysts. We investigated the effect of supported metal catalysts on lignin deporimarization into aromatic monomer in supercritical water.
Acid-catalyzed condensation between alcohols and carboxylic acids to produce ester compounds (i.e., Fischer esterification) plays an important role in chemical industries. Since Fischer esterification is an equilibrium reaction, it is mandatory to shift the equilibrium to obtain the ester products with high yields. Increasing reaction temperatures more than 100 °C to remove water by-product from reaction systems is a typical procedure to shift the equilibrium. However, undesirable side reactions might occur at higher temperatures (e.g., degradation of reactants/products, etherification of alcohols, and condensation between alcohols and acid catalysts). In addition, separation/purification of the products generally requires distillation process, as well as neutralization process of acid catalysts, which severely raises energy costs.
In the present study, we have prepared ionic liquid catalysts (ILCs) that undergo phase separation with ester products, while water by-product is selectively absorbed in the ILC phases [1,2]. The phase separation of esters and water efficiently shifted the equilibrium at moderate temperature, and high conversion values over 90% was obtained for the synthesis of wax esters from long-chain fatty acids/alcohols. The ILCs were not contaminated in the ester phases. On the other hand, the partition of unreacted acids/alcohols and ester products was strongly dependent on the structure of ILCs. Details on the relation between the partition behavior and reactivity will be discussed. Moreover, our preliminary studies to utilize flow reactors will also be presented.
References
[1] Y. Kohno, T. Makino, M. Kanakubo, React. Chem. Eng., 2019, 4, 627-633.
[2] Y. Kohno, T. Makino, M. Kanakubo, Fluid Phase Equilib., 2019, 490, 107-113.
When ultrasound is irradiated to water, degassing, that is, decrease of dissolved gas concentration occurs [1]. However, the effect of ultrasonic frequency on degassing behavior is few reported. In this study, the change in dissolved oxygen with ultrasonic irradiation time was investigated for a wide range of ultrasonic frequencies.
Experiments were carried out using a cylindrical vessel with a 56 mm in inside diameter. Transducers (Honda Electronics) attached to the vessel were a Langevin type multi-frequency transducer with 45 mm in diameter at frequencies of 22, 43, and 129 kHz, and disk type transducers with 50 mm in diameter at 209, 308, 400, 514, 1018, and 1960 kHz. Transducers were driven by a power amplifier. Ultrasound at 15 W of the ultrasonic power was directly irradiated to a 100 mL sample in the vessel. The ultrasonic power which was the energy applied to the sample per unit time was obtained by calorimetry. Ultrapure water was used as the sample. The degassing behavior was measured by the fluorescence dissolved oxygen meter.
The effect of ultrasonic frequency on time change of the dissolved oxygen by ultrasonic irradiation is shown in the figure. The dissolved oxygen decreases with ultrasonic irradiation time, and the decrease rate is maximum at 300 kHz. Dependence of sonochemical reaction on the ultrasonic frequency at 15 W was investigated by the KI method. In this method, triiodide ion is generated from iodine ion by ultrasonic cavitation. Sonochemical efficiency was also maximum at 300 kHz. Because a lot of cavitation bubbles generated in the sample at 300 kHz, the dissolved oxygen decreased significantly. It was proved that frequency dependences of sonochemical efficiency and degassing rate correlate each other.
[1] N. Gondrexon, V. Renaudin, P. Boldo, Y. Gonthier, C. Pettier, Chem. Eng. J., 66 (1997) 21-26.
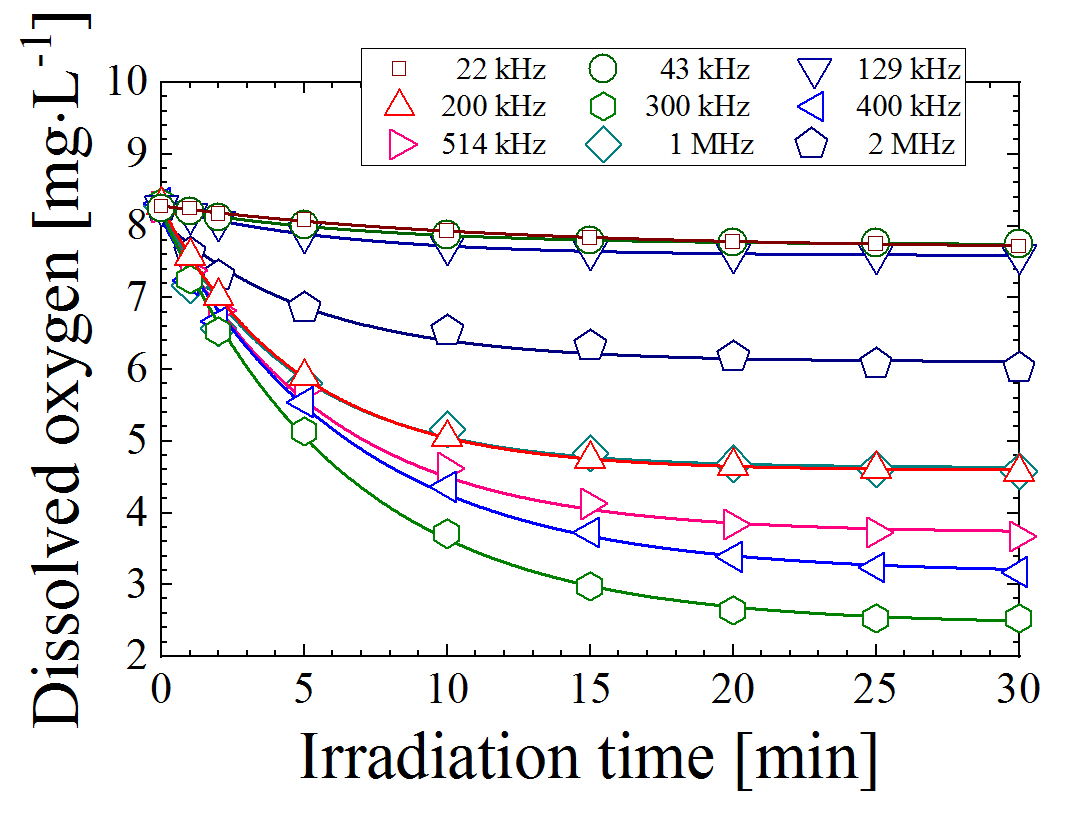
Recently, attention about phenomena and effects caused by supply of fine bubbles to aqueous solution is increasing. With regard to fine bubble species, the dissolution phenomena of hydrogen has not been clarified. Reduction of the concentration of oxygen active species owing to the dissolution of hydrogen fine bubbles is expected for application of food, substance synthesis, and environmental conservation fields. In this study, effects of molar fraction and bubble size of hydrogen/nitrogen fine bubbles on dissolution rate, solubility and reaction characteristics with oxygen species active species were investigated.
We have previously reported that OH-radical formation was enhanced by the existence of microbubbles under the specific frequency (45 kHz) of ultrasound irradiation. In the present work we have performed the experiment of ultrasonic degradation of azo dyes with various frequencies with and without microbubbles. It was found that for 45 kHz ultrasonic irradiation microbubbles enhances the degradation of azo dyes. To confirm that the effect was due to the cavitation bubbles formed by the irradiation ultrasound, we have introduced microbubbles with various gases and it was found that Kr and Ar gases have much larger formation of OH radicals, while He gas was very few effect on the enhancement of OH radicals. It was concluded that the cavitation bubbles were formed due to the ultrasonic irradiation and OH radicals were formed from these cavitation bubbles. Thus microbubbles have some effects on the enhancement of cavitation bubbles only in the case of 45 kHz ultrasonic irradiation.
In recent years, a great interest has been taken in microwave enhanced chemical reaction and many research have been conducted using microwave as a heat source. It is said that microwave heating has many advantages such as generating a uniform temperature distribution within a irradiated medium, quick heating, enhancing a reaction rate and improvement of selectivity on reaction products.
On the other hand, ultrasonic are also utilized for chemical reaction and it is reffered to as sonochemistry. There arises some effects by ultrasonic such as macroscale and microscale mixing due to the moving or oscillation of bubbles generated by cavitation and hot or high pressure spot derived from the collapse of bubbles.
In this study, experiments of esterification were carried out where irradiation intensity of microwave and ultrasonic were changed, and the effect of both irradiation on the conversion or reaction rate of esterification was investigated. The irradiation of microwave and/or ultrasonic was conducted until the reagent temperature reaches 333K.
As a result, when the irradiation of microwave and ultrasonic were introduced simultaneously where microwave power was varied with constant ultrasonic power, apparent reaction rate constant became larger than in the case that only microwave was irradiated. As for conversion, which is the result determined by reaction rate and reaction time, it decreased as microwave power become larger under constant ultrasonic power since the reaction time shortened because of quick ascent of temperature due to large microwave power , whereas it increased as ultrasonic power become larger under constant microwave power.
In this work, a spiral microchannel reactor was fabricated, and then applied to a selective synthesis of monoglycerides (MON) from palm fatty acid distillate (PFAD) and glycerol via esterification in the presence of sulfuric acid. An angle-less stainless-steel microchannel reactor with 200-cm length and 1×1 mm cross-sectional square-shape channel was designed to overcome the pressure drop related to a high viscosity of PFAD and glycerol. The flow patterns of both reactants were determined by using different fluid mechanic equations. The effects of reaction temperature and residence time on the PFAD conversion and the MON selectivity were investigated. Moreover, the results were compared with those obtained from stirred-tank batch reactor. It was found that the esterification of PFAD with glycerol under batch conditions gave the highest PFAD conversion (>95 wt.%) but the MON selectivity was only 40% due to the formation of di- and tri-glycerides. At a similar conversion, the spiral microchannel reactor gave higher MON selectivity than the batch reactor. The suitable condition for MON synthesis in the spiral microchannel reactor was 2 wt% sulfuric acid (based on the PFAD weight) at 200 °C and 2 h residence time under which the PFAD conversion and the MON selectivity were 81 and 74 %. The selective MON formation was explained by counter rotating vortices flow and solubility parameter of reactants and products.
To enhance the thermal controllability of the reaction system, a compact reactor was introduced, where heat transfer with both cooling media and cool feed gas was utilized. A computational fluid dynamics model was developed to analyze detailed profiles of mass, momentum and heat, and it was shown that the compact reactor could efficiently remove the generated heat. The space velocity and feed temperature were manipulated to increase the production rate, and the proposed reactor could produce twice of methanol with its peak temperature maintained below that of the conventional reactor. When the diameter of the catalytic bed in the compact reactor was increased, the reaction stayed in the kinetic regime, resulting in high methanol productivity as well as the maximum utilization of the bed.
Extraction with slug flow (alternating flow of different phases) gives fast mass transfer rates and rapid phase separation than the conventional extraction operations. Therefore, there are many advantages such as a reduction in volume of the device and a drastic decrease of the solvent used in the process. The circulating flow in each liquid segment and the interface area per the unit liquid volume largely depend on the geometry of the liquid segment. When the extraction by the slug flow is performed in a narrow diameter tube, the pulse flow (pulsation) of the pump that supplies the liquid gives a great influence on the flow condition in the tube. When the movement of each liquid segment is not kept uniform, the liquid flow disturbance cannot ignore the influence on the extraction rate. So, this unstable change of the flow is not desirable in analysis of extraction rate and device design. Therefore, a syringe pump with little pulsation has been used in many studies on the slug flow. The syringe pump is suitable for extraction by the slug flow since it shows no pulsation. However, it is not possible to supply continuously more liquid than the volume filled of the syringe. This indicates that it is not difficult to handle more than the volume of the syringe. The development of a stable flow liquid pump that supplies liquid without pulsation is expected.
In this study, two diaphragm pumps were interlocked to control the flow rate and the reciprocation frequency of the membrane. We succeeded in generating a liquid-liquid slug flow with a fixed length of about 1-6 mm. It was also confirmed that the generated slug flow was a stable flow of about 1 hour. We have found the possibility of becoming a new slug flow generation device.
Extraction by slug flow gives high mass transfer rate and rapid phase separation than the conventional extraction operation. Therefore, there are many advantages such as the volume of the device becomes small and the solvent drastically reduced. The slug flow extraction is expected as a sophisticated separation process. Especially, these advantages become more prominent in the multistage extraction. In this study, the extraction by the slug flow was applied to mutual separation of lithium, cobalt and nickel ions in the mixed aqueous solution. By combining forward and backward extractions, cobalt ions could be recovered with concentrating about 1000 times. We succeeded in developing a process with lithium, cobalt, nickel ion purity of 85% or more and recovery rate of 90-95%. Furthermore, it was also confirmed that the flow state of the liquid is influenced by the hydrophobicity (PTFE tube) and hydrophilicity (glass tube) of the extraction tube. The overall mass transfer volumetric coefficient is affected by (1) the interface area of the oil phase and aqueous phase, (2) the strength of the circulating flow in each segment of the liquid, (3) whether the mass transfer resistance is in the aqueous phase or the oil phase, and (4) the volume flow ratio of the oil phase and the aqueous phase, and so on. In general, the total mass transfer volumetric coefficient increased when the liquid with low mass transfer resistance wetted the wall of the extraction tube. This suggests that the wetting of the pipe has a great influence on the extraction rate in the slug flow extraction. It is necessary to select the tube material for operation properly. Furthermore, we confirmed that the overall mass transfer volumetric coefficient can be easily calculated from the experiments and the extraction rate can be simulated by a numerical method.
Quenching flow method using flow microreactors is known to be suitable for kinetic analysis of chemical reactions. This is because the characteristics of the flow microreactors such as fast mixing, precise residence time control, and precise temperature control makes it possible to control precise reaction conditions. The product can be accurately analyzed by various analytical methods such as gas chromatography. We have already reported kinetic analysis of organolithium reaction by quenching flow method using flow microreactors. Herein, we apply this method to the Schotten-Baumann reaction, which is the reaction of amine and acid chloride in the presence of basic aqueous solution. In this presentation, we report the kinetic analysis of the amidation and the comparison of the kinetic difference between various amines.
Among multiphase reactors in the chemical and pharmaceutical industries, packed bed compact reactors with internal diameters on the order of millimeters or micrometers are of interest. However, the design and operation method of packed bed compact reactors has not been established because it is difficult to analyze the flow pattern, which greatly influences heat and mass transfer performance, by visualization. In this study, an experimental system consisting of a packed bed compact reactor was constructed, and a method of estimating the flow pattern in the reactor was developed through numerical simulation as well as experimental analysis of the measurement results of voltage or differential pressure. The results show that when gas and liquid were supplied to the reactor, the developed method made it possible to distinguish the flow patterns of trickle flow and pulse flow from the shapes of the measured voltage or differential pressure and to estimate the pulse frequency of pulse flow (i.e., peak frequency of power spectral density), which is useful in grasping the degree of mixing of gas and liquid. It was suggested that the design of the mixer capable of producing shorter gas and liquid slug as well as the design of the reactor inlet should be developed in order to realize the process with higher gas and liquid mixing.
Introduction
Recent medical/orthodontic curable compositions have acquired high physical properties by using the newly designed long-chain silane coupling agent[1]. However, since the synthesis process required hydrosilylation as shown in Figure 1, it was essential to remove the platinum group complex from the synthesized material. In this study, we synthesized a long-chain silane coupling agent from a both-terminal-equivalent compound[2] such as ethylene glycol as a substrate using a micro flow reactor, and evaluated the influence of various synthesis conditions on the yields, etc. of the resultant silane coupling agent.
Results and Discussion
In comparison to the batch method, the flow method more efficiently synthesized mono-substituted products. Since the mixed solutions of 2-isocyanatoethyl methacrylate and ethylene glycol were heterogeneous, the reactions may have occurred at the liquid-liquid interface. The flow method provided larger surface area of the liquid-liquid interface than the batch method because the flow method produced fine emulsions using a chip (static mixer) for mixing the solutions. This resulted in more efficient reactions compared to the batch method.
Reference
[1] Fuchigami K, Fujimura H, Teramae M, Nakatsuka T. Precision synthesis of a long-chain silane coupling agent using micro flow reactors and its application in dentistry. Journal of Encapsulation and Adsorption Sciences, 2016, 6(1), 35-46.
[2] Huang X, Liu Y, Wang S, Zhou S, Zhu D. Synthesis and self-assembly of 2,9,16-tri(tert-butyl)-23-(10-mercaptodecyloxy)phthalocyanine and the application of its self-assembled monolayers in organic light-emitting diodes. Chemistry - A European Journal, 2002, 8(18), 4179-4184.
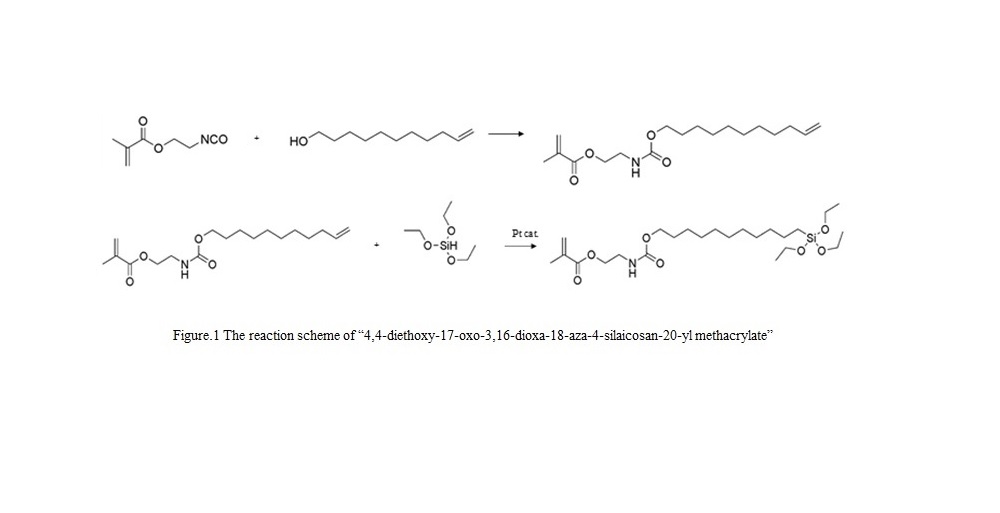
The main objective of this study is to investigate the flow pattern with gel reaction inside a continuous circular flow pipe and to identify the governing dimensionless number of flow patterns with gel reaction. The flow channel consists of a transparent main flow pipe and a transparent branch flow pipe which is connected to the main flow pipe. 10 mass% PVA and 3 mass% borax solutions are used as working fluids. 10 mass% PVA solution flows in the main flow pipe with steady-state conditions. Reynolds number (Rem) based on the mean velocity and the diameter of the main flow pipe is less than 30. The borax solution are injected from the branch flow pipe to the main flow pipe. The duration of the injection time and injection volume flow rate from branch flow are varied correspondingly to deliver a fixed volume of borax solution. At first, three flow patterns are observed inside of flow pipe, Capsule (C), Capsule + Stretched (CS) and Capsule + Stretched + Fingering (CSF) flow pattern. To identify the dimensionless number of three flow patterns with gel reaction quantitatively, we introduced Rem and β which is the volume flow ratio of the main flow to that of the branch flow. The results show that when β is less than unity, C flow forms and β is the main parameter that determines the flow pattern with gel reaction. When β exceeds unity, Rem is the main parameter that determines the flow pattern with gel reaction. When Rem exceeds 10.0, CSF flow forms. These results indicate that β and Rem play key dimensionless number in the gel formation and flow stability in a flow channel.
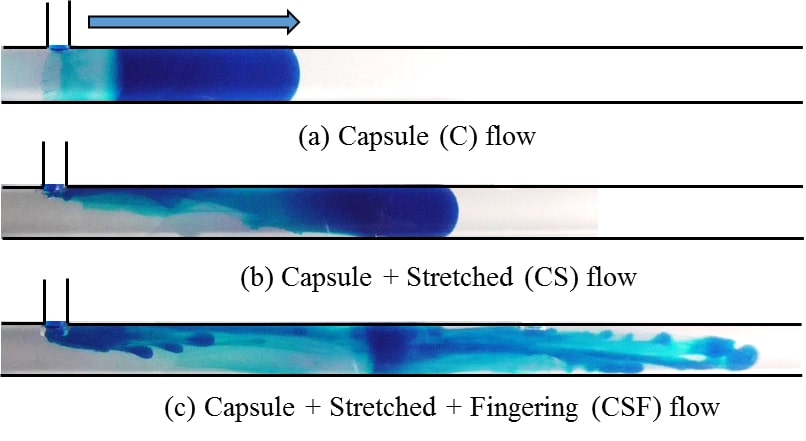
Hydrogenation is an important chemical reaction process in the production of petrochemical products, perfumes, and pharmaceutical intermediates. A gas-liquid-solid three phase reaction was carried out in a slug flow reactor in order to improve the apparent reaction rate by enhancing mass transfer rate of gas to the solid catalyst surface through the liquid. Hydrogenation of alpha-methyl styrene (AMS) using hydrogen gas was employed as the model three-phase reaction. The reactor was an aluminum tube of length 300 mm and inner diameter 4 mm. A thin alumina layer was formed on the inner wall and contained palladium as a catalyst. The hydrogen and AMS were introduced into a T-joint installed at the upstream of the reactor to form the gas-liquid slug flow. The AMS conversion obtained by the slug flow was much higher than that without slug flow. In order to examine the effect of the hydrogen absorption promotion by the circulating flow in the AMS slug, the total flow rate of the reactants was varied while the lengths of the gas and liquid slug were fixed to be constant. Although the absorption must be increased due to the faster surface renewal, only slight improvement could be observed. When the total flow rate was fixed and the ratio of the gas slug length to the liquid slug length was increased, the apparent reaction rate was improved significantly. These results indicate that the diffusion in the thin liquid film directly toward the solid catalyst was dominant for the reaction rate enhancement. Furthermore, the influence of the specific surface area of the liquid film layer was investigated from the mass transfer model by conducting the reaction experiments using the tubular reactors with different inner diameters.
In recent years, with the increase of global energy demand and adaptation to a sustainable society, there is a demand for higher efficiency of energy use in chemical plants. As one of the methods to improve the efficiency and energy saving of the chemical reaction process, this study examined the electric heating catalytic reaction process. The electric heating is a system in which Joule heat is supplied to a catalyst carrier having electric resistance. In this system, thermal energy is directly supplied to the reaction site. The etched aluminum foil was used for a catalyst carrier. This material has through holes at a density of about 10,000 holes / mm2 and its diameter can be controlled to 1 to 4 μm. A reaction space such as a microreactor can be realized by using the through holes as reaction channels. Further, the catalyst metal can be supported by making the wall surface of the flow path porous, and it also has a function as a structured catalyst. With regard to electric heating, thermal energy is generated by supplying current to the base aluminum. In the evaluation of the electric heating performance, the model reaction was the methanol steam reforming. Evaluation factors were temperature rising rate, temperature reached, power consumption, and hydrogen generation rate. As a result of conducting the activity test, energy consumption was reduced by 40% in the electric heating as compared with the power consumption of the process by the external heating. In the future, we will study the temperature non-stationary operation in order to evaluate the startup performance of electric heating.
Multiphase reaction systems for organic synthesis are encountered in many chemical industries producing petrochemical derivatives, fine chemicals and pharmaceuticals.
Operating multiphase flows in the microscale has proved to be effective owing to its enhanced mass transfer. Additionally, microreactors bring the advantage of safety, easy control and flexible scalability by numbering up compared to the large chemical reactors. In microstructured devices where the diameter falls into the range of 100 to 1000 microns, time scales are small and molecular diffusion is the dominant driving force for mass and heat transfer. Enhancing the performance of microreactors requires improving advective mass and heat transport which can be realized by implementing helical structures. The flow in the helical structures is known to generate secondary flow vortices in the radial and tangential directions, which are believed to enhance the convective transport.
In order to provide an insight into the transport phenomena taking place, this research work aims to simulate multiphase flows with a focus on the effect of helical structures on mass transfer. A new numerical model is suggested to reduce the computational domain from a full-scale helical structure to one straight 3D slug. The numerical model is built using the Volume of Fluid (VOF) method and simulations are run using the open source OpenFOAM software. A multiphase simulation of water-air segmented flow showed that the diffusion of oxygen from air to water is faster in a helically coiled microcapillary compared to a straight microcapillary even at low Reynolds numbers.
Mixing characteristics under laminar flow conditions was examined. A thin tube is co-axially inserted into the channel through which the solution of reactant A flows. The solution of reactant B was co-currently fed from the thin tube into the A solution. The mixing characteristics of B into A in the channel were evaluated under the condition of flow ratio A : B = 100 : 1. An orifice was installed downstream of the junction of the two solutions for promoting mixing. The influence of the flow rate ratio of the two solutions, the installation position and the geometrical shape of an orifice on the mixing characteristics was evaluated by Dushman reaction. As a result, in the co-axial double tube type mixer, the mixing characteristics of the two solutions became better as the total flow rate increased, even under co-current and laminar flow conditions. Moreover, it was found that the double tube type mixer was much better compared with mixing characteristics in a beaker stirred by a magnetic stirrer. The mixing characteristics when the orifice was placed downstream of the confluence point were significantly better than those without the orifice under the same Reynolds number condition. These results are considered to be due to the promotion of mass transfer in the greatly stretched thinner flow from the inner thin tube because the flow rate of the solution in the annular region is greater than that from the inner thin tube. When the orifice is inserted, it is thought that the contraction flow when passing the orifice and the expansion from there have developed the same effect. These flow characteristics of the flow from the inner thin tube were also confirmed by numerical simulation.
We describe the stability of liquid-liquid slug flow in this study. Knowledge of slug stability is necessary to utilize the liquid-liquid slug flow for unit operation. Fragmentation or coalescence of slugs causes unexpected reaction performance and extraction efficiency, and affect the productivity or product purity. Establishment of the stable operation guideline for the liquid-liquid slug flow is necessary.
We made the liquid-liquid slug flow by using dodecane as an organic phase and water colored with rhodamine B as an aqueous phase. The stability of slug flow based on two control systems was examined: active slug formation with solenoid valves and passive slug formation without solenoid valves. The optical sensor was set in the flow path to measure the slug velocity. The experiment was conducted with different aqueous slug lengths.
We depicted the map for the stable slug operation, in order to the capillary number and valve frequency. Then, the behavior of slugs at various flow rates and slug lengths was investigated. When aqueous slugs of different lengths were alternately formed, a long-length slug caught up with a short-length slug. Optical measurement suggested that the velocity of the aqueous slugs was faster than the flow velocity assuming the plug-flow, and the long-length slug flowed faster than the short-length slug.
In conclusion, the stability of the liquid-liquid slug flow depended on the capillary number and the valve frequency. The uniform length of the dispersed slug contributed to the steady operation.
Hydrogen obtained by water electrolysis using electric power derived from renewable energy is attracting attention as a clean next-generation energy. The difficulty of storage and transportation of hydrogen can be solved by converting hydrogen to chemical hydride. The production of chemical hydride with hydrogen derived from water is important technology for hydrogen energy society. The methylcyclohexane (MCH) is one of useful hydrogen carrier due to about 6 wt% of its high hydrogen content and low melting point. The system combining hydrogen formation by electrolysis of water and hydrogenation of toluene to form MCH will be useful to produce chemical hydride from water. The hydrogenation process can be expected as a new energy storage method in a decentralized society because the device can be miniaturized while using electric power derived from individual renewable energy. Hydrogenation of toluene to produce MCH proceeds in the presence of catalytic Pt/Al2O3 powder at the permeation side of Pd-Ag membrane electrode in our previous study. In this case, it is difficult to fill up a lot of catalyst powder in small space. Structured catalyst will solve the structured problem and give lower pressure drop than that of catalyst bed.
In this study, the electrolysis of water with hydrogen permeable Pd-Ag membrane electrode and hydrogenation of toluene from hydrogen permeated through the membrane in catalytic layer made of washcoated Pt/Al2O3 catalyst on stainless steel mesh. The potassium aqueous solution was supplied to reaction cell with HPLC pump at 0.8 MPa and toluene was supplied by bubbling with nitrogen at 0.1 MPa. The hydrogenation was conducted around 373 K. The stability of hydrogenation-electrolysis cell was stable for several hours. The effect of temperature and flow rate of toluene on hydrogenation rate and hydrogen utilization efficiency was evaluated, which revealed that the hydrogenation was enhanced in higher temperature region.
Syngas production had been great attention among the researcher especially from non-conventional feedstocks. Syngas is a basic feedstock for many commercial important chemicals and fuels and can be produced as a result by dry reforming of methane (DRM). However, conventional reactors involved high reaction temperature due to the high endothermicity of DRM. Membrane reactors are expected to decrease the reaction temperature by shifting the thermodynamic equilibrium of DRM. Several researchers have explored the performance of membrane reactors using metal and silica membranes in DRM, and confirmed hydrogen yield was improved. However, this reveals insufficient activity of conventional catalysts in low reaction temperature. Previously, we have developed Ru/Y2O3-ZrO2catalysts to beapplied to the low temperature DRM. In this study, we aimed to evaluate the effect of membrane performance on membrane reactor performance using our developed catalyst in DRM. In the case of the membrane reactor using a Pd membrane at 500 °C, CH4 and CO2 conversions were 32.5% and 25% respectively, which were a half-more higher than the conventional reactor. The hydrogen yield was found to be 33.9% that was three times higher than its conventional reactor, and interestingly,H2/CO ratio counted as 1.08 for the Pd membrane reactor and 0.70 for the conventional reactor. This indicates H2 separation gave a significant impact on not only the H2 yield but also H2/CO ratio. To compare with the Pd membrane reactor, Ni-doped silica membrane was also prepared by sol-gel method and its gas permeances were 0.53 × 10-6 mol m–2 s–1 Pa–1 for N2 and 3.20 × 10-6 mol m–2 s–1 Pa–1 for H2. The performance of the membrane reactor using the silica membranes will be presented as a comparison.
Key words : DRM, membrane reactor, syngas.
A great interest has concentrated to liquefied ammonia as a chemical hydrogen carrier in Japan because of its higher hydrogen content, i.e., 17.6wt%-H2 per unit weight of NH3. As far as ammonia is used as an energy carrier, its decomposition for obtaining H2 should be carried out at as low temperature as possible. However, the decomposition rate is known to remain very low at lower temperatures below 500 °C although the equilibrium conversion in the range 0.1-0.5 MPa at 300 °C reaches more than 80%. In practice, according to our data, the conversion remained around 10% at 300°C when 2wt% Ru/Al2O3 catalyst was used. Therefore, it is required to propose an innovative technology that can not only realize a higher conversion even at lower temperature below 400 °C but also obtain pure hydrogen.
As a way of improving such a situation, in this study, we have focussed on adoption of more active catalyst other than conventional Ru/Al2O3 and composite thin palladium membrane. A packed-bed palladium membrane reactor incorporating these catalyst and membrane is set up and then used for testing the ammonia decomposition at low temperatures.
First, it was found that Ru/CeO2 had a higher activity for ammonia decomposition when compared with Ru/Al2O3 catalyst at 350 °C. This is considered to be related to the basic property of CeO2 support itself, which may promote the recombinative desorption of N species. About 0.01 mm-thick palladium-silver alloy membrane supported on porous YSZ tube (11 mm in outer diameter) prepared with electroless-plating was used. The decomposition was carried out using the membrane reactors packed with either Ru/Al2O3 or Ru/CeO2. When comparing the performances between the conventional and membrane reactors at 300 °C, it becomes obvious that the conversion was increased by employing the Ru/CeO2 catalyst-packed palladium membrane reactor.
As an application of zeolite membranes to chemical reactions, various esterification reactions have already been examined mostly in liquid-phase perfectly mixed reactor to aim an equilibrium shift by selective water separation. Differently from such a batch operation, a vapor-phase flow one that makes efficient and continuous production possible, is considered to be advantageous. Dehydration reaction of alcohols, limited thermodynamically, is also industrially important and operated at higher temperatures, producing ethers together with water. This study focuses on vapor-phase dehydration reaction of ethanol to diethyl-ether, that is, 2EtOH → (Et)2O + H2O, which is equilibrium-limited. A membrane reactor incorporating chabazite (CHA) membrane and solid-acid catalyst is employed for removing water vapor selectively from the reacting mixture, thereby obtaining higher conversion.
CHA zeolite membrane was synthesized on a porous alumina tubular support (2.6mm in outer diameter and 105mm in length). A double tube membrane reactor, where around CHA zeolite membrane tube either granulated H-ZSM-5 or Amberlyst 15 (acidic ion exchange resin) as catalyst was packed, was built. Ethanol dehydration to diethyl ether was examined in the temperature range of 130 - 160 °C.
Prior to reaction, vapor permeation performance of the CHA membrane using an 80wt% ethyl alcohol-water mixture was evaluated at 150 °C. It was observed that the water content in the permeate was nearly 100wt% and the permeance was in the order of 10-7 mol/ (m2 s Pa). As the feed rate of ethanol decreases, i.e., the residence time increases, the reaction can proceed more. The yield of diethyl-ether in the membrane reactor is increased by 7% on the basis of the conventional reactor, where granular H-ZSM5 is used as catalyst. This is simply because more water vapour can be separated. More conversion is expected to be obtained when the more active Amberlyst instead of H-ZSM5 is used.
To decrease the pumping power for transporting thermal energy, a latent heat transportation system, which uses phase change material (PCM) slurries as a heat media has been attracted. Especially, the micro-encapsulation of PCMs is one of the promising techniques for preventing the pipe blockage due to the particle growth. The authors successfully fabricated silica hard-shell microcapsules containing PCMs. The microcapsules consisted of an inner hollow cavity and an outer SiO2 shell with porous structures. PCMs can be charged into the inner hollow cavity through the pores in the SiO2 shell. However, the charged PCMs are readily leaked out through the pore in the SiO2 shell during the utilization for the latent heat transportation system. To prevent leak of PCMs, the pores in the SiO2 shell are required to be covered with materials possessing micropores. In this study, the modification of ZIF-8 (Zeolitic Imidasolate Framework-8) film over an outer surface of spherical SiO2 was investigated.
Spherical SiO2 (CHROMATOREX) was obtained from Fuji Silysia Chemical Ltd. Japan. To facilitate the nucleation of ZIF-8 over the SiO2 surface, 3-(2-imidazolin-1-yl)propyltriethoxysilane (IPTES) was modified over the SiO2 in an aqueous solution of HCl at 333 K. The IPTES-modified sample was dispersed in a mixture of Zn(NO3)2 and 2-methylimidazole (Hmim) followed by still standing at room temperature for 24 h to form ZIF-8 film. The obtained sample was denoted as ZIF-8/SiO2.
From the result of XRD measurement, ZIF-8 structure was formed at the Hmim/Zn molar ratio of 30 during the preparation process of ZIF-8/SiO2. The roughness surface was observed over the ZIF-8/SiO2 by SEM. From the mapping of Zn obtained by SEM-EDX analysis, Zn species existed over the outer surface of SiO2. These results suggest that the ZIF-8 film can be formed over the outer surface of SiO2.
Acknowledgements: This study was supported by JST-Mirai-project: #JPMJMI17EK.

In order to obtain the value-added cheicals ethyl cynnamate, this produce should be synthesized with high stereoselectivity. The Horner-Wadsworth-Emmons (HWE) reaction is a promising reaction to obtain the ethyl cynnamate. The high selectivity of HWE reaction required the sever low temperature or basic conditions. To overcome these severe reaction condition, the effect of liposome membranes as a reaction field was examined because liposome that is a closed lipid bilayer exhibits the enhanced-effect of reactivity for some reactions [1].
In this study, ethyl diethylphosphonoacetate and ethyl diphenylphosphonoacetate were used as E-prefer and Z-prefer substrates for of HWE reaction, respectively. The HWE reaction was, at 25 oC for 8 hours, performed to mix the substrate with benzaldehyde in the presece or absence of K2CO3, sodium iodide, 1,8-diazabicyclo[5,4,0] undec-7-ene (DBU), and liposomes. The obtained products were analyzed by a 1H-NMR for a quantification of E- and Z-isomers.
The effect of liposomes on the stereoselectivity of two substrates was tested. Use of the liposomes existing in the gel phase resulted in the enhanced activity of the substrates and furnished the products with same E/Z stereoselectivity as in the liposome-free system. The membrane environment in the gel phase most likely assisted the formation of adducts that induced selective generation of the E-isomer. It is noted that the energy barrier for the Z-isomer could be reduced by the addition of liposomes. Therefore, the observed effects of liposomes were likely to result from the use of their voids for the stable formation of intermediate adducts and their activity similar to the basicity of DBU. Figure shows the case of ethyl diethylphosphonoacetate. The possible role of liposomes is to assist the proton removal from the reactant, rather than providing the basic interfacial environment.
[1] T. Shimanouchi et al., J. Biosci. Bioeng., in press.
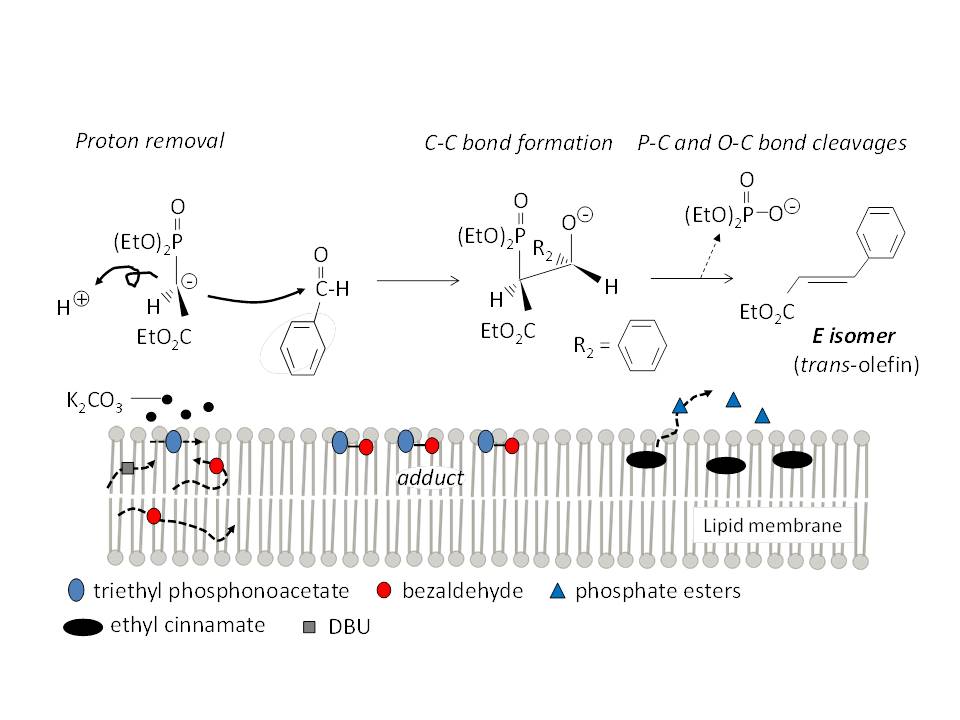
The transparent conductive film is a thin film having conductivity with an average transmittance of 80% or more in the visible light region and a resistivity of 10 -3 Ωcm or less, is used for flat panel displays and the like. At present, indium tin oxide is mainly used as a transparent conductive film, but since the price is so high due to concern about depletion of indium, development of alternative materials is required. Zinc oxide thin films are known as transparent conductive films, are reported to be further lowered in specific resistance by adding aluminum to form aluminum-doped zinc oxide (AZO), and thus are expected as alternative materials. Thermal CVD is a surface coating method in which substances in the gas phase are deposited on the substrate in solid form using chemical reactions on the surface of the substrate, and heat is applied to promote the chemical reaction to raise the temperature of the substrate. And, it is mainly used for oxide film formation. Further, by reducing the pressure in the apparatus, the mean free path of the reaction gas becomes longer, and the film thickness uniformity is improved. Therefore, an AZO thin film was prepared by a low pressure thermal CVD method, the average transmittance in the visible light region and the resistivity were measured, and the relationship with the film forming conditions was studied.
Silicon nano particle is a promising material as an electrode of Li ion rechargeable battery. Productivity of the silicon nano particle is low, so improvement of the productivity is required. We have investigated combination of plasma enhanced Chemical vapor deposition (PE-CVD) and high speed jet as a gas supply method in order to obtain high deposition rate of silicon material. In this study aggregated silicon nano particles were rapidly deposited on cupper substrates using Plasma Enhanced CVD in SiH4/H2 system to improve productivity of silicon nano particles.
In our experiments input power and frequency of the power supply was 50 W and100 MHz respectively, and pressure of an experimental vacuum chamber was 800 Pa. The deposited particles was evaluated by SEM image. Deposited mass was measured by an electronic balance. Crystallinity was analyzed by Raman spectroscopy. Deposition thickness and surface roughness was measured by a laser 3-dimensional measuring instrument.
Figure shows SEM image of deposited silicon particles. Diameter of the silicon particles are several tens of nm. Thickness of silicon materials by 1 min deposition is several tens of micrometer. Raman analysis show the deposited particles are mixture of amorphous and nanocrystalline silicon.
Relation between deposited mass of silicon and both distance of two electrodes and mass flow rate of the source gas was investigated in order to obtain better productivity of the nano particles.
Velocity of the source gas introduced into a vacuum chamber was several hundreds of m/s in our experimental setup. The experimental result shows that the nano particles were generated during convection in the vacuum chamber after and transported to substrate by the jet of source gas.
Atmospheric-pressure plasma jets (APPJ) have recently been a topic of great interest. Especially, dielectric barrier discharge (DBD) jets, which can be driven at room temperature come to play an increasing role on process of organic materials which are sensitive to heat and biomedical applications. Cancer treatment, sterilization and dental treatment are example.
For less invasive APPJ optimization, we tried to develop a new type of jet which can process objects at low temperature and high speeds. This APPJ can generate stable, arc-free discharge with only air plasma gas. High voltage electrode was Ti wire and coaxially inserted into nozzle. Material of the nozzle was quartz tube. Inner diameter of the quartz tube was 4mm, and outer diameter was 6mm. Ground electrode was Ta foil with irradiation hole and placed at the tip of the nozzle. Size of the irradiation hole was 1mm. Voltage applied was 4kVp-p, and the frequency was 25kHz. Feed gas was dry air, and the flow rate was 5, 7.5, 10slm. The polymer film based on poly-methyl methacrylate (PMMA) was used to etch, placed downstream from the jet nozzle. Irradiation time was 60s. At each flow rates, etching was performed at a distance such that the temperature of the irradiated object was about 40°C(Jet to object distance was; 7mm at 5slm, 4mm at 7.5slm, 3mm at 10slm). As shown in the figure, the results were 0.05μg/s/W at 5slm, 0.11μg/s/W at 7.5slm and 0.13μg/s/W at 10slm. From this result, temperature may not significantly affect etching. The effects of thickness and hole size of the ground electrode were also evaluated.
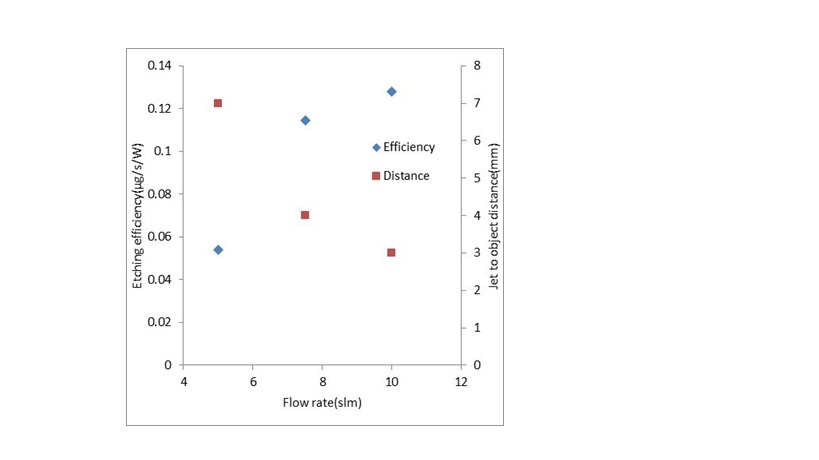
Carbon nanotubes (CNTs) have various excellent properties and potential applications. When synthesizing CNTs, metals such as Fe are normally used as catalyst, which need be removed for many applications. Wet process using acid solution is widely used as removal, which causes difficulty in redispersion of CNTs due to strong agglomeration upon drying. In addition, oxidation is also often combined to the acid etching to remove the carbon shell covering the catalyst particles, which causes damage to CNTs. To solve these problems, dry process has been developed in which halogen gas is flowed through CNT powder at high temperature and remove catalyst metals as metal chlorides. However, halogen gas is highly dangerous and require special facilities, thus challenges remain in industrialization. Here, we propose a highly safe process using FeCl3, which is solid and has negligibly small vapor pressure at normal temperature and pressure. CNT powder by floating catalyst chemical vapor deposition (CVD) and FeCl3 power were set apart in a quartz glass tube, the tube was filled with Ar and heated using a furnace. FeCl3 evaporates and diffuses through the CNT powder, removing Fe catalyst by the reaction of Fe (s) + 2FeCl3 (g) → 3FeCl2 (g). Over 80 % of Fe was removed from CNT by scanning electron microscope-energy dispersive X-ray spectroscopy measurement (Fig. 1). Raman spectra showed the retained intensity ratio of G-band to D-band peaks, showing CNT was not damaged (Fig. 1). From TEM images, CNTs had metal particles covered with carbon shells before purification (Fig. 1) while had empty carbon shells after purification (Fig. 1). Furthermore, this method proved effective for various CNTs, removing Fe catalyst from multi-wall CNTs synthesized by supported catalyst CVD and removing Ni-Y catalyst from single-wall CNTs synthesized by arc discharge method.
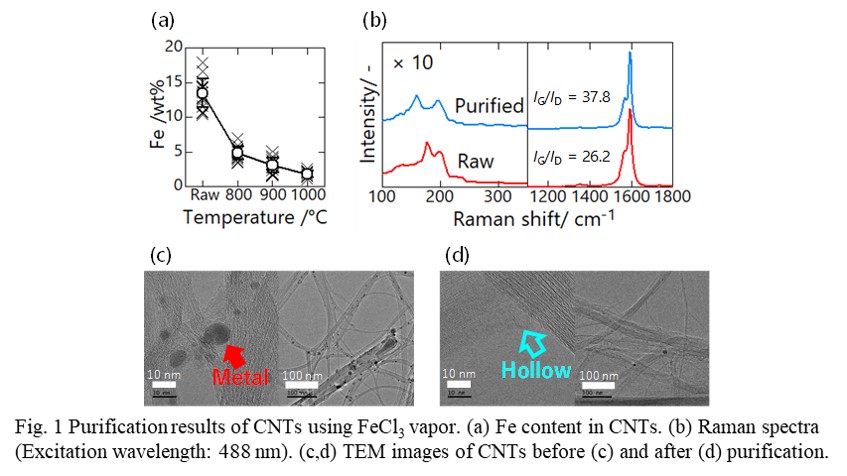
Carbon nanotubes (CNTs) have been extensively researched owing to their unique one-dimensional nanostructure and properties. Mass production has been established for multi-wall CNTs (MWCNTs) instead of single-wall CNTs (SWCNTs). Floating catalyst chemical vapor deposition (FCCVD) yields high-quality SWCNTs [1,2] but the productivity is small because individual SWCNTs are much lighter (~1/10,000 of 150-nm-diameter MWCNTs of the same length). Generally, catalyst precursors are fed to externally heated reactors, and heated in >1 s, and catalyst particles gradually nucleate at low density. We have previously proposed and developed the flame-assisted CVD (FACVD) method [3], in which a premixed flame is used to heat and mix the gas quickly in ~1 ms. The FACVD method yielded 1 nm-diameter, high-quality SWCNTs. However, the flame requires oxygen at high concentration, causing narrow growth window and small productivity (~0.3 mg-CNT/min).
In this work, we report the FCCVD method with a micro-nozzle for preheating catalyst precursors. We used ferrocene and sulfur vapors as catalyst precursors, preheated and mixed them with CH4/H2/Ar, and synthesized SWCNTs at 1200 C. Product is yielded as black smog continuously from the reactor (Fig. 1a). The product was clean ropes of SWCNTs with few particles (SEM, Fig. 1b) with a Raman spectrum characteristic for high-quality SWCNTs (sharp G-band peak, high G-band to D-band peak intensity ratio of 102, and strong RBM peaks, Fig. 1c). The thermogravimetry analysis in air showed the ash content of 6.66 wt%, corresponding to carbon purity of >95 wt%. A productivity of 8.0 mg-CNT/min and carbon yield of 4.2% were achieved using a lab-scale reactor (inner diameter of 40 mm).
[1] A.G. Nasibulin, et al., Chem. Phys. Lett. 402, 227 (2005).
[2] T. Saito, et al., J. Phys. Chem. B 110, 5849 (2006).
[3] S. Okada, et al., Carbon 138, 1 (2018).
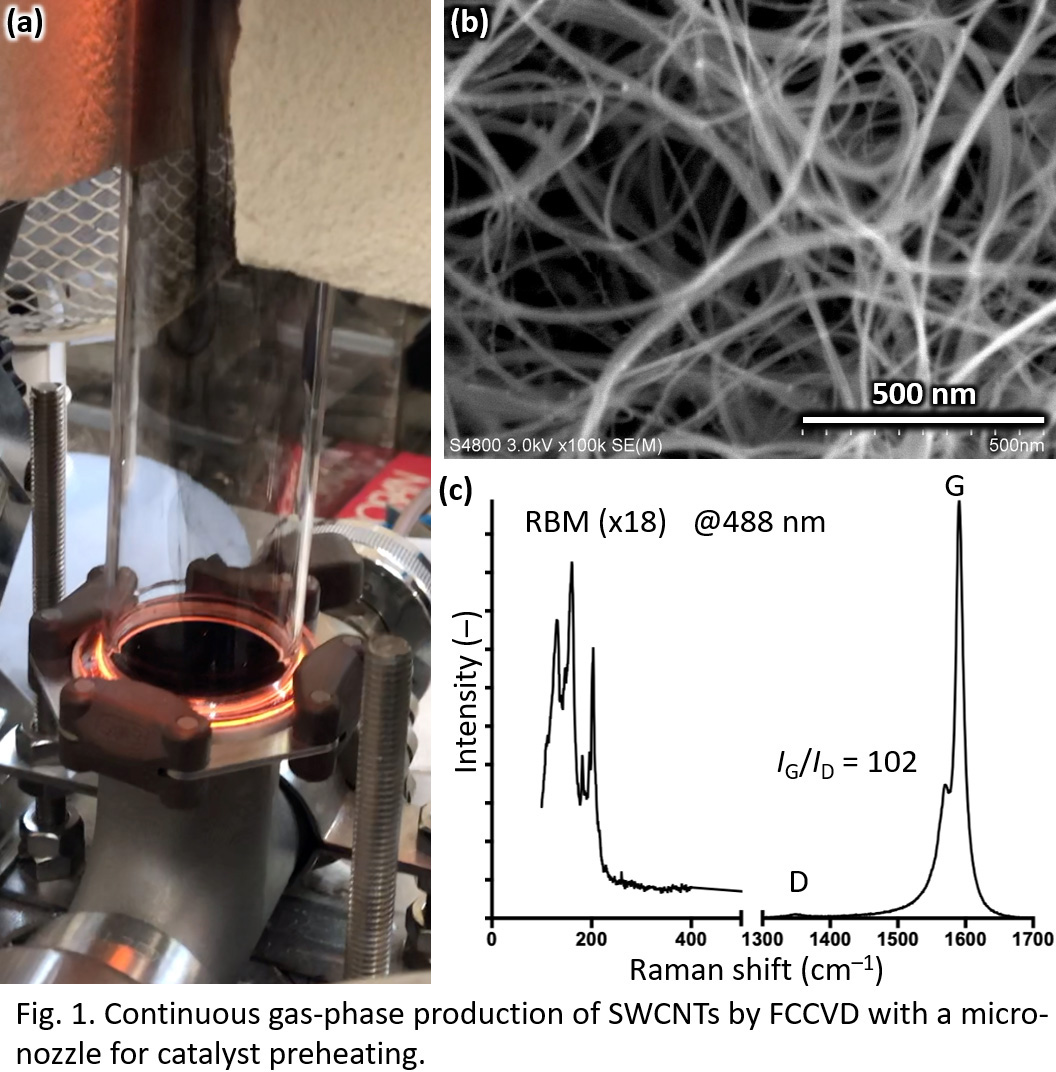
Carbon Nanotubes (CNTs) are attractive with high hopes in many different fields for their excellent properties. However, the practical applications of CNTs has been a challenge due to their high price and/or insufficient quality. Floating catalyst chemical vapor deposition (CVD) enables the synthesis of high quality CNTs, but it has had an issue with its low catalyst usage. To combat this situation, many groups have conducted research to improve the productivity of this process, and as a result it has been reported that the implementation of sulfur can increase the overall yield of floating catalyst CVD [1]. Many groups have proposed positive roles of sulfur; Fe-S to be the active site for catalytic reaction or reduced surface energy of Fe-S for nucleation of small Fe particles. However, these models are not supported by clear evidences and are not applicable to supported catalysts.
Here, we propose an opposite model; sulfur deactivates the Fe catalyst, small amount of sulfur forms an inactive region on a Fe particle, and the inactive region works as an entrance for the carbon source without being covered with graphitic carbon. The analysis of this is difficult for floating catalysts, therefore for this research, supported catalysts were used by implementing graphite sheets as substrates to replicate the floating catalysts of spherical structure. Scanning electron microscope (SEM) shows that Fe on graphite grew no CNT without sulfur but many CNTs with sulfur (Fig. 1a). To confirm the importance of having inactive region within the catalyst particle for CNT synthesis, thin Al2O3 layer were deposited on top of the Fe catalysts. The thin Al2O3 layer significantly enhanced the CNT growth (Fig. 1b) in a similar manner as sulfur. Our new concept enhancing catalyst activity by making inactive region on catalyst particles enables new catalyst design.
[1] H.M. Cheng, et al., Appl. Phys. Lett. 72, 3282 (1998).
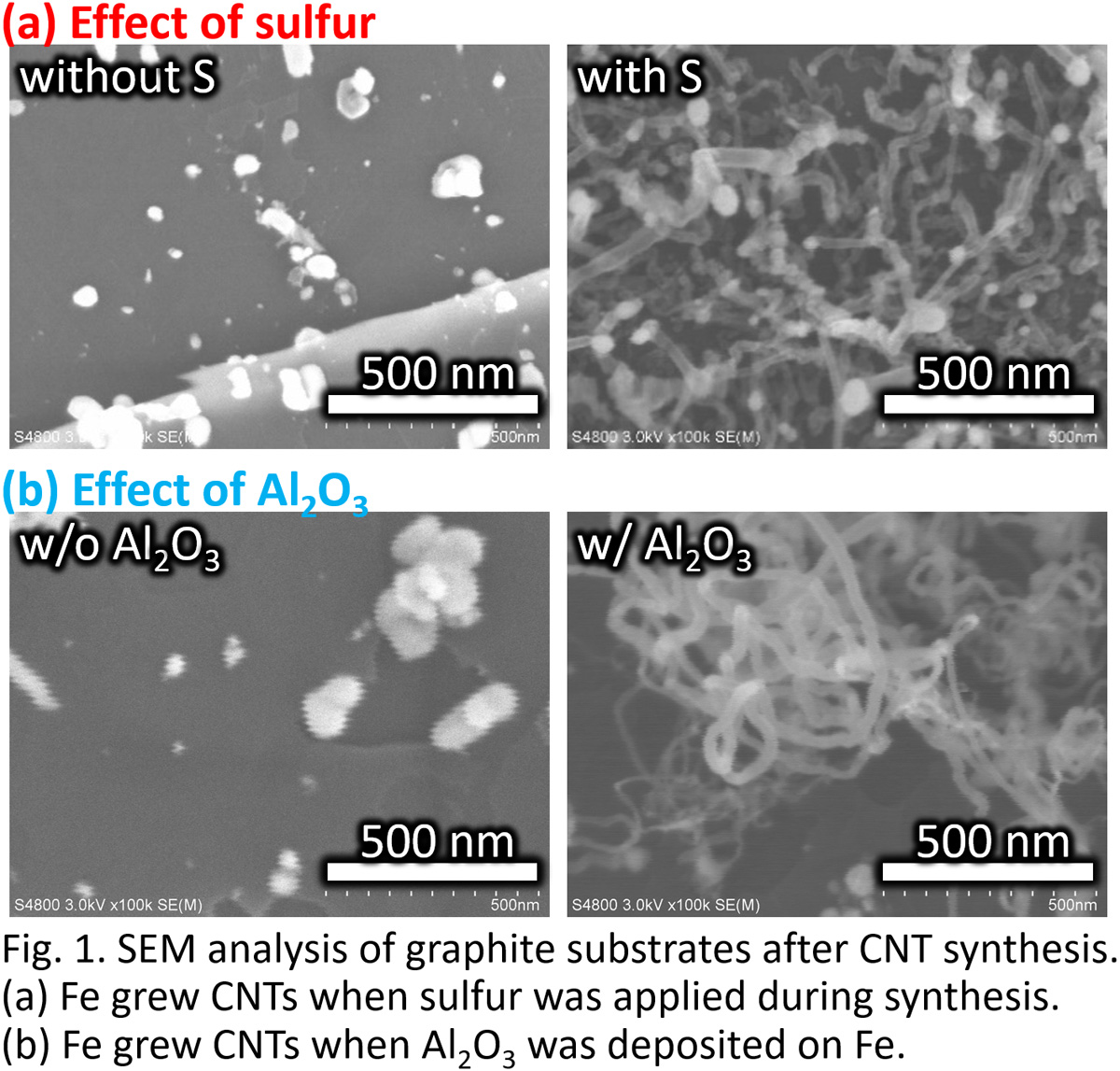
Chemical vapor deposition (CVD) has been widely used for fabrication of functional films. Solving mass transfer of relevant chemical species and their chemical reactions to make deposition during CVD is essential to predict the film thickness profile thereby to design an optimal reactor without resorting to empirical approach. As the figure shows, sometimes the substrates for CVD have microstructures on the surface, but solving the equations in a single geometry that includes both reactor and microstructures is unfeasible due to scales difference. Accordingly, microstructures have been separated from the reactor, where equations were solved individually and iterated until boundary condition of their interface becomes identical. However, it always imposes repeated calculation, which cannot be implemented in the commercial software, thus troublesome. Here, we propose a concise low-cost multiscale simulation method by using COMSOL, a software basing on finite element method. Microstructures and reactor were separated likewise iteration method, while the equations were concurrently solved by coupling the geometries with boundary conditions expressed by equations. The boundary conditions are concentration and flux of each species. The concentration was set to be continuous. The flux was defined as integral of deposition rate in the microstructures because total consumption rate of the precursor in the microstructures equals to the precursor flux across the interface. Hence, automatic low-cost multiscale simulation was enabled. Then, we imparted time-dependent simulation capability to cope with shape change of the microstructures by the deposited film. Growth rate profile in the microstructures obtained above was used to update the shape, which will be used for simulation in next time step, by level set method. By repeating this at each time step, time-dependent multi-scale simulation was enabled. We applied this methodology on W deposition to get profile evolution successfully and the result showed the importance of feedback by microscale topography.