Most of our ‘playing field’ is in liquids where chemical reactions and separations occur. For enhancing the production rate of the target substance, various methods have been developed and applied. The rate was frequently controlled by mass-transfer or accurate triggering of reactions. Ultrasound has a power of thinning the liquid boundary film, enhancing mixing in narrow spaces and generating radicals with high accuracy. Also, ultrasonic irradiation is highly controllable and is easy to adapt to existing equipment. Works of ultrasound will facilitate reactions as well as separations. The irradiation ensures nucleation from supersaturated solutions. Solid surfaces of catalysts and silicon wafers are successfully cleaned. Reaction path appears only under sonicated conditions. Radical reactions are initiated by sonolysis of liquid medium.
Since ultrasound is pressure wave, the unique phenomena, cavitation occurs due to the pressure oscillation. Cavitation is characterized by generating fine bubbles in liquids followed by oscillation of those bubbles and eventual violent collapse to emit energies. Onset of cavitation dramatically enhances interfacial area; the field for reactions and mass-transfer are in play.
Ultrasonic irradiation to liquids causes a lot of physical and chemical changes, which are strongly related to our ‘games’ in chemical processing. Examples will be presented in the lecture to show a high potential for ultrasound as a game changer.

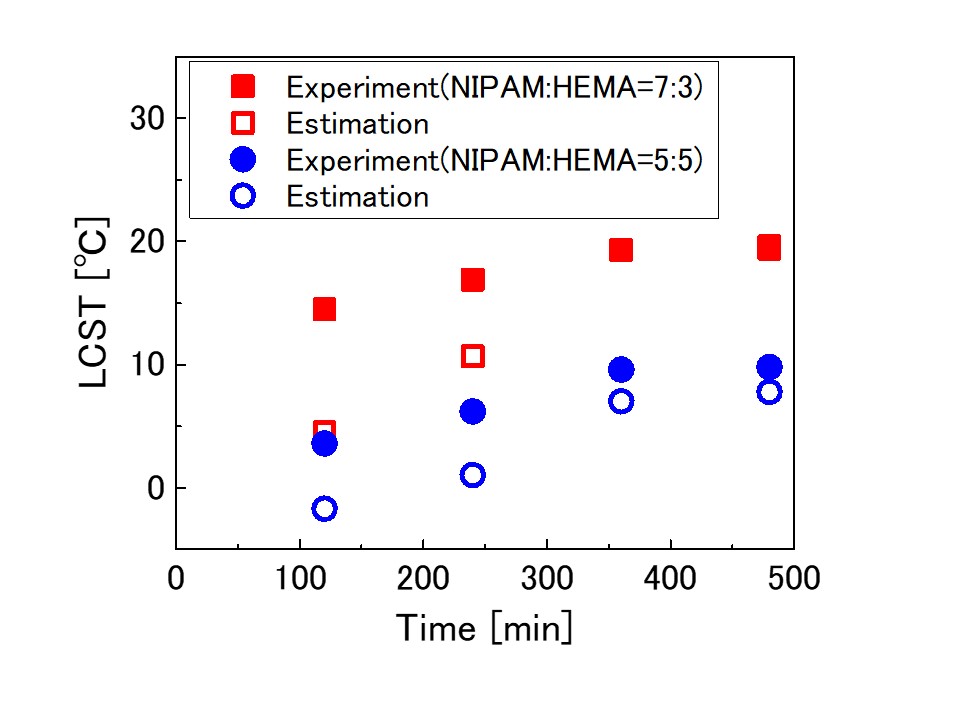
Poly(N-isopropylacrylamide) (PNIPAM) is a thermoresponsive polymer with a lower critical solution temperature (LCST). Poly(2-hydroxyethyl methacrylate) (PHEMA) has a high mechanical strength, so that a copolymer of poly(NIPAM-co-HEMA) is expected to be applied as advanced functional polymeric materials in various fields, such as sensors, actuators, and drug delivery system.
When ultrasound is irradiated to a solution containing vinyl monomers, the radical species are generated due to thermal decomposition of the monomers and then radical polymerization takes place without any chemical initiator. The synthesized polymer decomposes due to the high shear stress. Therefore, the polymer with a narrow molecular weight distribution can be obtained. It is expected to control the properties of the copolymer by controlling the condition of ultrasonic irradiation.
In this research, poly(NIPAM-co-HEMA) was synthesized using ultrasonic irradiation in a mixed solvent of water and ethanol without any chemical initiator. The effects of the ultrasonic power intensity, the ultrasonic irradiation time and the monomer molar ratio on the responsive temperature of synthesized polymers were investigated.
The responsive temperature of the copolymer obtained at the completion of the reaction linearly increased with the copolymer composition. The experimental results were in agreement with the estimated values by copolymer composition, which was formulated according to the results obtained by using chemical initiator (Shen et al., Colloid Polym. Sci., 2006, 284, 1001). However, the responsive temperature of the copolymer obtained in the early stage of the reaction was higher than that estimated by the copolymer composition. These results suggested that some parts of the polymer chain have higher NIPAM composition than overall composition because of the difference in the reactivity of the monomers. In conclusion, the composition and responsive temperature of the copolymer can be controlled by changing the ultrasonic irradiation time.
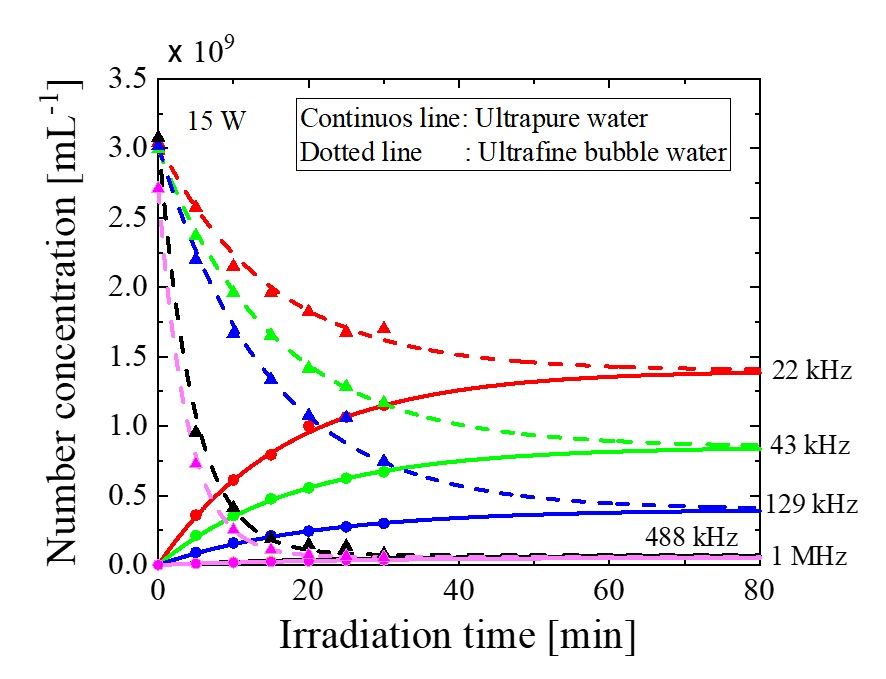
When ultrasound was irradiated to water, ultrafine bubbles were generated. Effects of ultrasonic power and frequency on ultrafine bubble concentration and diameter were investigated. Number concentration of ultrafine bubbles increased with time and approached asymptotically to an equilibrium value. Increase rate of ultrafine bubble concentration became higher as ultrasonic power increased. The equilibrium number concentration of ultrafine bubbles increased with decreasing frequency. The mode diameter of ultrafine bubbles was within 90 -100 nm. Effects of ultrasonic frequency and irradiation time on the mode diameter was small.
On the other hand, when ultrasound was irradiated to water containing high concentration of ultrafine bubbles (ultrafine bubble water), number concentration of ultrafine bubbles decreased with time and approached asymptotically to the equilibrium value. Reduction rate of ultrafine bubble concentration became higher as ultrasonic power decreased.
Generation and elimination of ultrafine bubbles by ultrasound were modeled to analyze experimental data. The generation rate coefficient of ultrafine bubbles increased with decreasing frequency because cavitation collapse became stronger. The reduction rate constant of ultrafine bubbles at 488 kHz and 1 MHz in ultrafine bubble water became much higher than those in ultrapure water due to aggregation and floatation of ultrafine bubbles. The equilibrium number concentration increased with decreasing frequency as shown in Figure 1. The results calculated by our model were in good agreement with the experimental data. The ultrafine bubble generator or eliminator using ultrasound is compact, simple in operation and produce or eliminate ultrafine bubbles in a short time. This method is contamination-free because the liquid pump and mixer are unnecessary.
Figure 1 Change in number concentration of ultrafine bubbles by ultrasonic irradiation to ultrapure water and ultrafine bubble water for different ultrasonic frequency at 15 W of ultrasonic power.
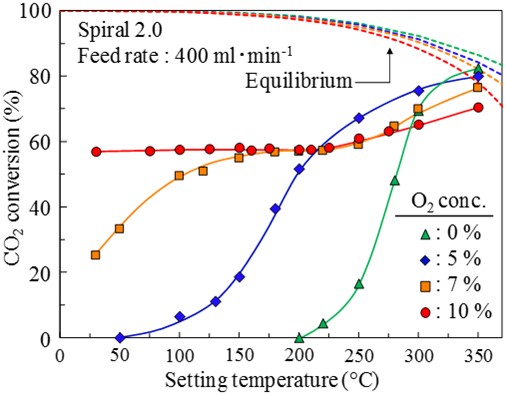
CO2 methanation (CO2 + 4H2 → CH4 + 2H2O, ΔH0298K = -165 kJ/mol) is one of the promising routes for converting CO2 into a useful resource of CH4. In a previous study, we developed a spiral-type Ni/CeO2 structured catalyst for enhancing a catalytic performance by promoting mass transfer diffusion. The developed spiral-type structured catalyst showed a high processing capability for CO2 methanation. This study focuses on the addition of O2 in the methanation feed gas, expecting for direct heat supply to the reaction field by H2 combustion. Therefore, in this study, the methanation performance of the spiral-type Ni/CeO2 structured catalyst was evaluated with changed O2 concentrations in the feed gas in the range from 0% to 10%.
The structured catalyst was prepared by an impregnation method and a wash-coating method. The catalyst component of 75 mg was coated on each spiral substrate. The methanation performance of the catalyst was evaluated by plug-flow type reactor. Four spiral catalysts were set in reactor tube. The reaction gas of 400 ml/min (CO2: 10vol %, H2: 40-60 vol%, O2: 0-10 vol%, N2: balance) was fed to the reactor.
Fig.1 shows the effects of O2 concentrations on CO2 conversions over the spiral-type Ni/CeO2 structured catalyst. In the CO2 methanation system without O2, the spiral-type structured catalyst showed a high activity at relatively low temperatures. By the addition of O2 in the methanation feed, the catalyst displayed a high performance at lower setting temperature. The increase in O2 concentration had a remarkable effect on the improvement of the catalytic performance. In case of 10% O2 in the methanation feed, 57% of CO2 conversion was obtained at room temperature without external heating. The result indicated that the generated high heat energy due to H2 combustion accelerated CO2 methanation on the spiral-type structured catalyst.
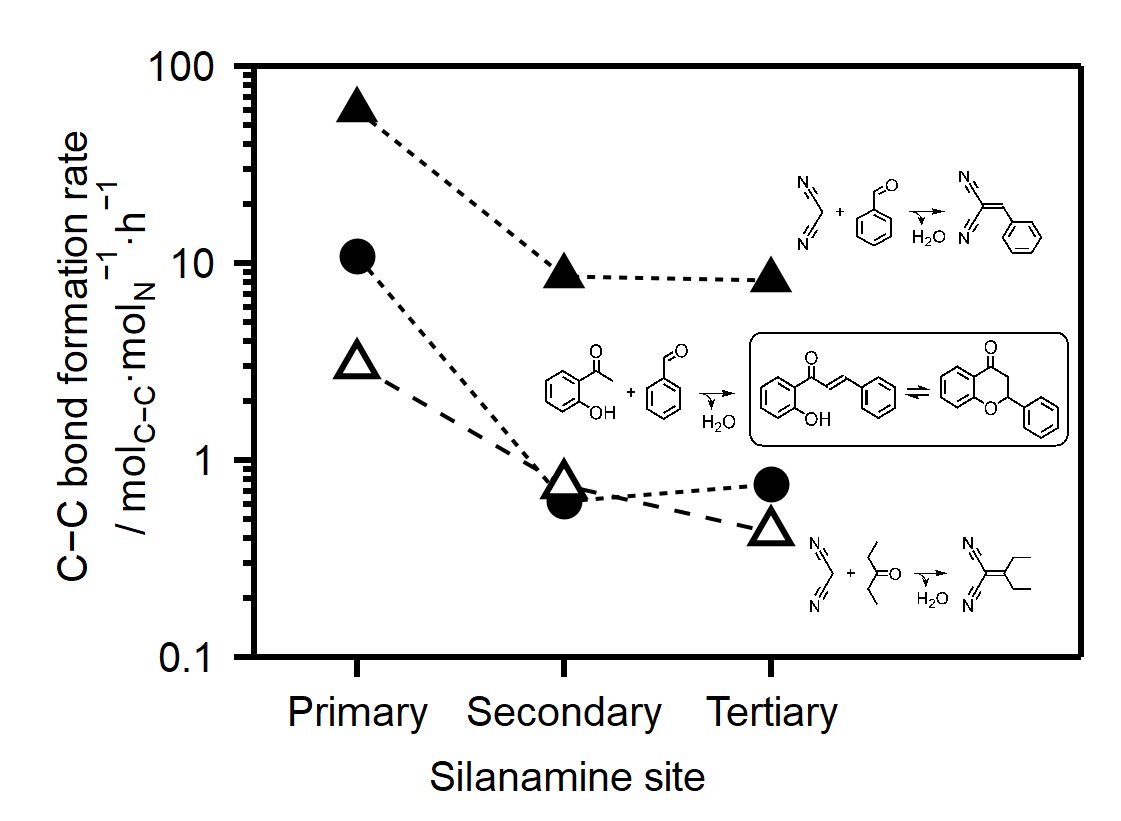
Nitridation on mesoporous silica by ammonia gas forms framework substituted nitrogen, and it has been known to work as base catalytic sites. Since the first attempt to use them as catalysts, several reactions have been catalyzed and further functionalization broadened applicable reactions. However, the detailed quantitative analysis of each silanamine site and their contribution toward catalytic reaction have been insufficient, and further study has been required for the future design of the catalyst
In this study, a series of nitrided mesoporous silica SBA-15 samples was synthesized at different nitridation temperatures. Quantitative analysis of formed silanamine species was conducted by both bulk and surface analyses (i.e., chemical analysis and XPS, respectively). For the quantitative analysis of each silanamine species, we used narrow scan spectra of XPS. The careful deconvolution of the spectra of N 1s level revealed the sequential formation of primary to tertiary silanamine species. The prepared samples were applied to several Knoevenagel and aldol condensation reactions as catalysts. The contribution of each silanamine species to the reaction was deconvolved by mathematical approach. Based on the result, the catalytic activity was in the order of primary > secondary ~ tertiary silanamine species. The order was different from the general textbook-order of the basicity of amine. This would be explained by more influential steric effect due to the formation of secondary and tertiary silanamine site inside the mesopore wall. Furthermore, primary silanamine showed significantly high activity for the reaction involving aldehydes, which would be explained by the formation of imine species. The formation of imine species was confirmed by FT-IR measurement.