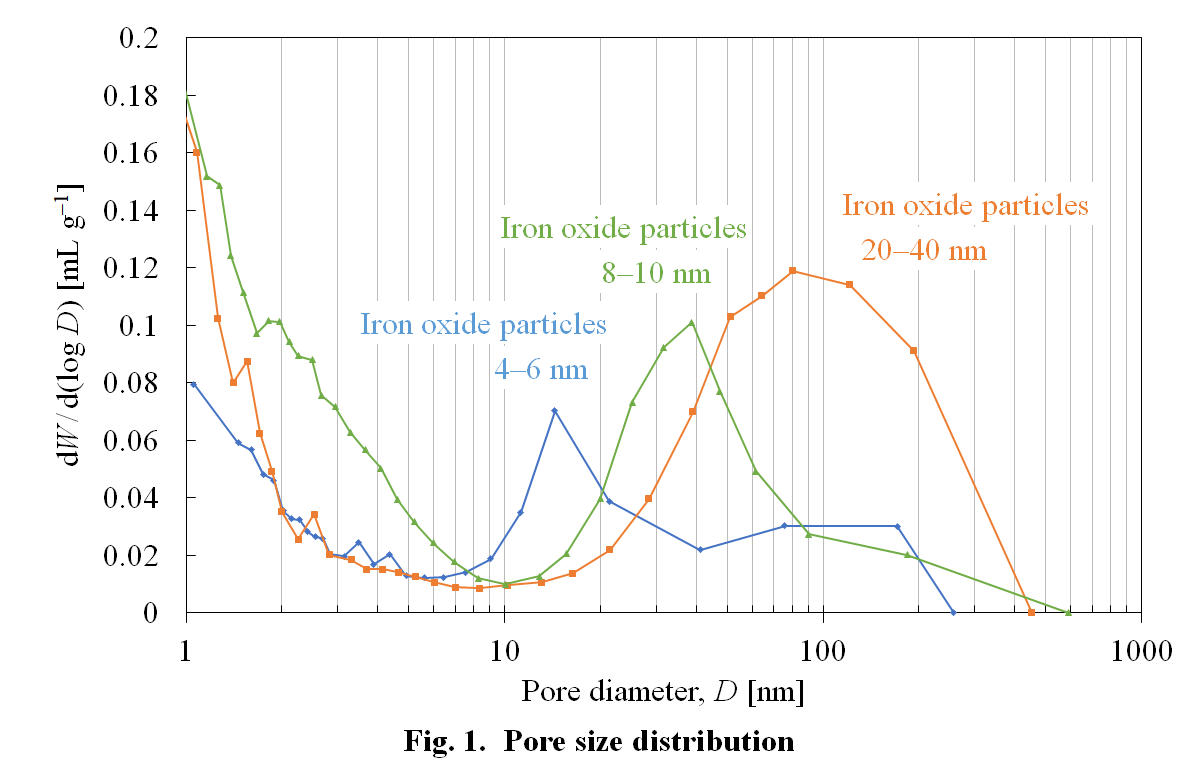
Mesoporous carbon that is a porous carbon material with regular meso-scale (2-50 nm) pores has large surface area and electrical conductivity, so it is used for various applications such as to catalyst support and for electrodes of electric double layer capacitors. Presently, mesoporous carbon is mainly produced by the template method. By the template method, however, it is difficult to form pores of over 20 nm, and the production cost is high due to the complexity of the process. In this study we have developed a method to produce mesoporous carbon by solid-solid reaction between carbon and iron oxide nanoparticles in dilute oxygen atmosphere. In the proposed method, the pore size of the mesoporous carbon can be arbitrarily controled by changing the size of the metal catalyst particles, and it may be advantageous in terms of time and cost for production because the process is simpler than the template method. Three composites were prepared by supporting iron oxide particles of 20-40 nm, 8-10 nm, and 4-6 nm respectively on carbon derived from coal tar pitch, and pore formation was conducted by heating each composite at 490 °C in the presence of oxygen. Nitrogen adsorption measurement of the prepared samples showed that pores of larger size were formed as the size of iron oxide particles became larger, suggesting the possibility of manipulating the pore diameter by selecting the size of iron oxide particles. In addition, by using iron oxide with a particle size of 10 nm or less, we succeeded in producing mesoporous carbon having pores of 7 to 50 nm by the proposed method.
Nitrogen-doped carbons have attracted significant attention as catalysts for the oxygen reduction reaction (ORR) for fuel cell applications and H2O2 synthesis. Many research works have been devoted to the identification of the type of active nitrogen species doped in carbon framework to improve catalyst performance. For example, Nakamura et al.1 synthesized nitrogen-doped carbon catalysts possessing exclusively pyridinic or graphitic nitrogen species and have reported that pyridinic nitrogen present at graphene edge forms an active site. Our goal was to investigate the effects of the degree of crystallinity of carbon framework near active nitrogen sites on the ORR performance because it was anticipated to affect the binding strength of reactive oxygen species at active sites and potentially tune the selectivity to 2e– vs. 4e– reduction of O2. Tuning the degree of crystallinity of carbon materials may be accomplished through annealing in a conventional high-temperature furnace (>1200 °C). However, high-temperature annealing of nitrogen-doped carbons reduces their nitrogen concentrations. To minimize the loss of the nitrogen content of catalysts, high-temperature annealing may be conducted in a flow of NH3. However, it causes the decomposition of NH3, which generates radicals that gasify carbon materials. To address these challenges, we chose a microwave (MW)-assisted approach, which enables fast annealing of carbon materials.2,3 Here, we report that MW-assisted annealing of nitrogen-doped carbons in NH3 increases the degree of crystallinity while retaining porosity and active nitrogen species. We demonstrate that the annealed catalysts exhibit significantly higher ORR activity per pyridinic nitrogen site in an acidic electrolyte than a catalyst before MW-assisted annealing.
1. Guo, D., et al., Science 351, 361 (2016).
2. Mukai, S.R. et al., JP Patent 2015–23070; Kamatari, S. M.Eng. thesis (2015).
3. Ogino, I. et al., J. Energy Chem., 27, 1468 (2018).
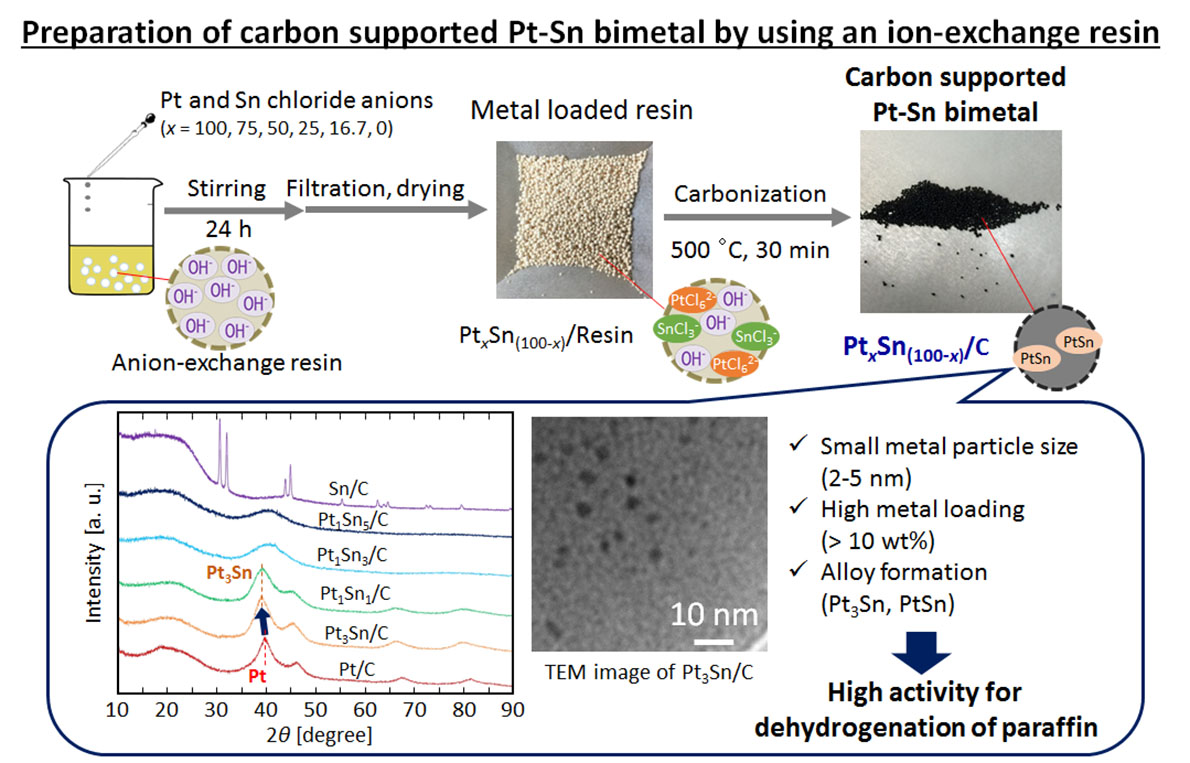
Dehydrogenation reaction has attracted much attention for hydrogen production from hydrogen carrier such as methylcyclohexane and co-production of hydrogen and olefin or aromatic compounds from paraffin. Noble metals, such as Pt and Pd, are reported to exhibit high activity for dehydrogenation. Carbon is one of promising catalyst support because they have no acid/base sites, which causes undesired reaction such as cracking and isomerization. On the other hand, the metal fine particles on carbon support are easily aggregated during the catalyst preparation and the reaction, leading to deactivation of the catalyst, and Pt is active for C-C cleavage, causing low hydrogen selectivity. To maintain the metal particle size small, we employed a novel preparation method for carbon supported Pt fine particles by using an anion-exchange resin and Pt chloride anion as initial materials of carbon support and Pt, respectively. In addition, it has been reported that C-C cleavage reaction is suppressed by the addition of transition metal, such as Sn, Mn, and Fe, to Pt. Because Sn forms chloride anion in HCl solution, we considered that carbon supported Pt-Sn bimetal catalyst could be obtained by adding Sn chloride anion to Pt precursor solution in the preparation of Pt/C from the anion-exchange resin. In this study, the carbon supported Pt-Sn bimetalcatalysts with various metal compositions were prepared by using the anion-exchange resin and Pt and Sn chloride anions. Although Sn composition was smaller than the predetermined one, which was because Sn chloride melted and leached during the carbonization at 500 °C, Pt-Sn bimetal catalysts possessing 2-3 nm of metal particles were successfully obtained. Moreover, the metal loading of the obtained catalysts exceeded 10 wt% and hence they could exhibit high activity based on unit volume. We applied the prepared catalysts to dehydrogenation of paraffin to evaluate hydrogen production activity.
Polyoxymethylene dimethyl ether (POME) has gained much attention due to its unique properties such as high cetane number, better intermiscibility, and low soot and NOx formation. Therefore, POME has become a potential candidate for direct fuel or fuel additive in diesel engines. Conventionally, POME is produced through acetalization of formaldehyde with methanol by using acidic catalysts. Klankermayer and co-workers reported the homogeneous Ru and Co triphos-based catalysts with Lewis/Brønsted acidic co-catalysts for dimethoxy methane (DMM) or OME1 production from CO2 hydrogenation in methanol. To our best knowledge, POME synthesis from heterogeneous catalytic COx hydrogenation have not reported in the literature.
Wet impregnation method was used in current study for synthesis of X%Ru/BEA (X = 1, 3, 5) catalysts to produce POME from COx hydrogenation. POME synthesis experiments were conducted in autoclave batch reactors at 75 bar, 50 mL methanol and 100 rpm impeller speed. The prepared catalysts were characterized to check the catalyst structure, BET surface area and pore size distribution, Ru dispersion over BEA zeolite support and particle size, Ru reduction properties and metal-support interaction. Experimentally, 3%Ru/BEA revealed the best POME yield for COx hydrogenation at 125 °C under mentioned reaction condition. The optimized temperature range for CO2 hydrogenation over 3%Ru/BEA was reported as 150-175 °C. The adverse effects of water in product mixture and plausible reaction pathway were also investigated in presented study. The maximum OME1 and OME2 yields were 7.42 and 0.48 mmol/gcat.LMeOH, respectively at 150 °C, H2/CO2 ratio 3 after 2 h of reaction under same reaction conditions.
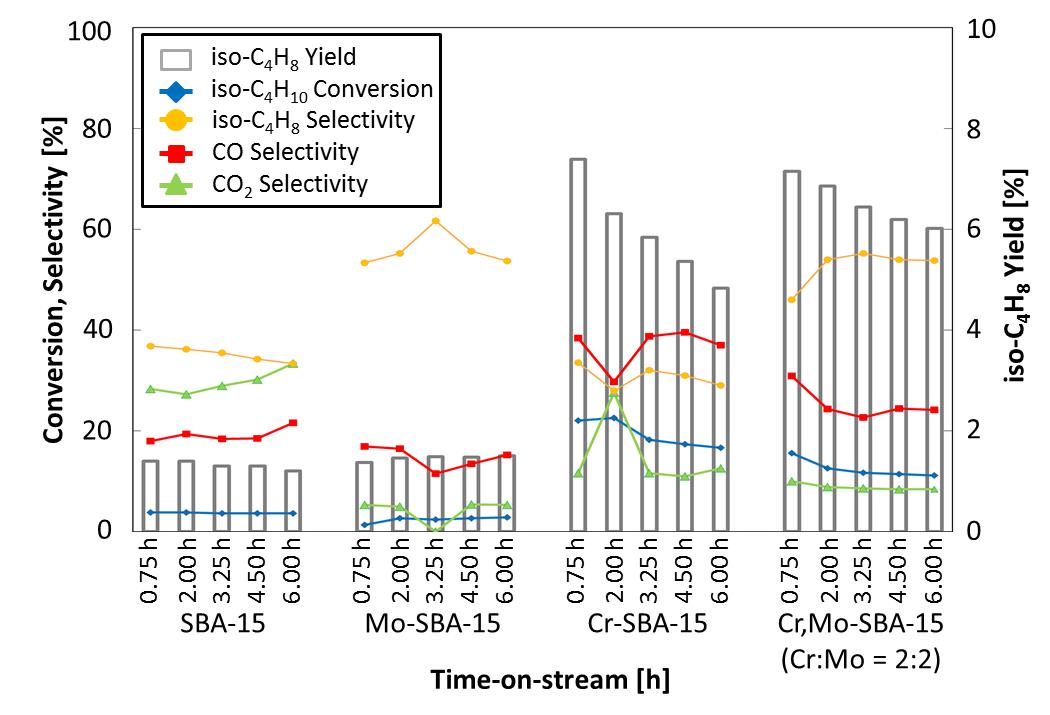
Isobutene is widely used as a starting material for alkyl t-butyl ether, isooctane, p-xylene, methyl methacrylate (MMA), butyl rubber and so on. Therefore, it is very important to establish an alternative route for isobutene production. Especially for us, it is preferable to obtain cheaply available isobutene for MMA production. Considering one of the routes, we have been studying the oxidative dehydrogenation (ODH) reaction of isobutane to isobutene as our aim. Our team has investigated various metal doped mesoporous silica catalysts and confirmed that SBA-15 modified with Cr and Mo (Cr, Mo-SBA-15) showed high performances for ODH reaction of isobutane. According to our investigation, the conversion of isobutane for SBA-15 and Mo-SBA-15 were quite low but Cr-SBA-15 showed the highest conversion of isobutane. However, in the case of Cr-SBA-15, it was quickly deactivated. On the other hand, in the case of Cr, Mo-SBA-15, the conversion of isobutene and the selectivity of isobutene were much higher than those of other catalysts. In addition, deactivation of the catalyst was remarkably suppressed than others. The binary metal system, Cr, Mo-SBA-15, had excellent functions for ODH reaction of isobutene.
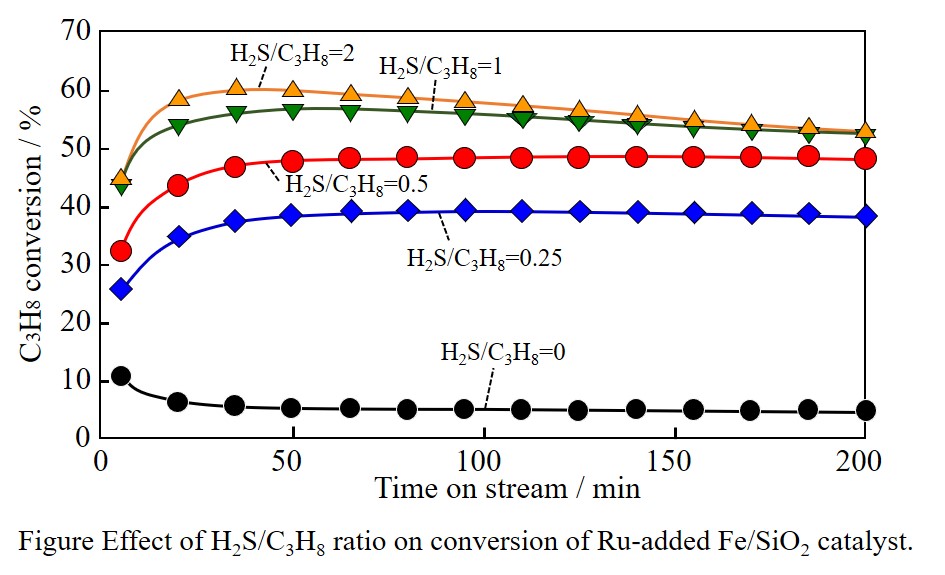
Propane dehydrogenation (PDH, C3H8 → C3H6 + H2) has gained much attention due to a direct production of valuable propylene. A metal sulfide is known to be a highly-active catalyst for PDH, however the loss of sulfide ion (S2-) from the surface frame structure by reduction with H2 leads to decrease of catalytic activity. In a previous study, we investigated a performance of SiO2 supported transition metal catalysts (Fe, Co, Ni, Mn, Cu) for PDH in the presence of H2S, expecting for replenishing S2- in the the surface frame structure of metal sulfide continuously. Among transition metal catalysts, a Fe/SiO2 catalyst showed a relatively stable performance with highly-active and selective for PDH with co-feeding H2S. In this study, we examined the effect of noble metal (Ru, Pd, Pt) addition to the Fe/SiO2 catalyst on its catalytic performance, in order to further enhance the dehydrogenation performance.
By the addition of noble metals, the catalytic activity of the Fe/SiO2 catalyst was largely enhanced. In particular, the Ru-added Fe/SiO2 catalyst showed a stable performance with high conversion and selectivity, comparing to that of the Pd- and Pt-added Fe/SiO2 catalysts. Figure shows the effect of the ratio of co-feeding H2S to propane (H2S/C3H8) on conversion over the Ru-added Fe/SiO2 catalyst. By increasing H2S/C3H8 ratio, a high conversion was obtained at the initial stage of the reaction, but the catalytic activity was decreased gradually. While, under low H2S/C3H8 condition, high stable performance was obtained. Almost no deterioration was observed for 3 h-dehydrogenation under 0.25 and 0.5 of H2S/C3H8. According to Fe K-edge XANES spectra, a high oxidation number of Fe species was confirmed. Such cation species could accept electron from intermediate species, which might promote the PDH performance.