
In some Western Australia's wheat production area, there is concern about the future decrease in yield due to water logging and salt accumulation. The land use change from forest to cropland in centuries age, is thought to be resulted a broken balance between water input by rainfall and output by evaporation and transpiration, due to limited water consumption of wheat.
Theoretically, re-planting of trees on farmland is countermeasures for this problem, however, this method is not economically feasible.
To solve this problem, our group now developing new technology, not afforestation conversion of farmland but afforestation conversion of abandoned agricultural land due to salt accumulation by using salt tolerant tree species. In this research, we focused on the following 4 issues
1: Afforestation demonstration test,
2: Optimal design of afforestation site,
3: Cost analysis,
4: Carbon credit, bio-energy production potential estimation.
In the presentation, outline of the research project will be explained and preliminary results on groundwater level measurements and water usage estimation of planted trees by SAP flow measurements rate will be discussed.
Nanocellulose derived from cellulosic biomass could become an option for replacing conventional materials such as steel and fossil-derived plastics due to its unique physical properties. There are difficulties to disintegrate celluloses in plant cell walls into nanocelluloses because they are tightly hooked each other by multiple hydrogen bonds. Among various nanocellulose production techniques, one of the most adopted techniques to facilitate disintegration is carboxylation of the 6-position hydroxyl group of cellulose to produce electrostatic repulsion between cellulose nanofibrils with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), called TEMPO oxidation. While it is said that the energy required for disintegration is small, it has hardly been assessed how the TEMPO oxidation could become environmentally benign technique to produce nanocellulose throughout its life cycle. Life cycle assessment (LCA) is a strong tool to quantify environmental impacts originating in product life cycle. However, LCA is originally based on process systems composed of existing technologies or services, although the technologies on nanocellulose are now developed and emerging for sustainability. In this study, we are tackling the LCA of emerging technologies for producing nanocellulose. The actual energy consumption in laboratory-scale processes for TEMPO-oxidized nanocellulose (TONC) was measured to assess the environmental impacts induced by the laboratory-scale and predict those through industrialization. The greenhouse gas (GHG) emissions from electricity for mechanical treatment is much larger than that from production of chemicals and energy for TEMPO-oxidation. In the experiment process, the amount of electricity to clean the fibrillation machine is also large, and the machine is cleaned much more carefully than the industrial process. Since the energy consumption of this cleaning process is significant part of the disintegration energy, it was found that the GHG emissions would be less than half if the careful cleaning were not required in continuous production.
The manure produced by intensive livestock farming is known to have significant impacts on the environment. The nitrogen and phosphorus compounds in the pig manure befoul the soil and water body. The microalgae-based piggery wastewater treatment system involved microalgae-based treatment, co-anaerobic digestion of pig manure and microalgal biomass, and biogas cogeneration. The organic residues from the anaerobic treatment can be applied as organic fertilizers to farmland replacing mineral fertilizers. Using anaerobic digestion of pig manure is a promising method of reducing greenhouse-gas (GHG) emissions. Cogeneration by burning biogas can produce the electricity power and usable heat to apply to the system. The environmental impacts of the system using high-rate algal ponds (HRAPs) were evaluated through mass, energy balances and life cycle assessment (LCA). LCA is usually used to evaluate the environmental performance of pig production. The performance of this microalgae-based treatment-scenario has been compared to the conventional application of pig manure (Activated sludge-treatment-scenario).
In recent year, the demand of fertilizers is increasing because of the increase of the food consumption by population growth in developing countries and the Bio-energy consumption. We developed the new recovery process of phosphorus, and the process is consisted of elution and precipitation steps, namely 窶狼wo-step Elution method'. The heavy metals are characterized by ICP-MS/ICP-AES spectrometer. The final phosphorus recovery rate is about 80%. The amount of metals and heavy metals in final precipitation of this method are much less than the fertilizer standards of Japan. In this study, we have started plant growing experiment by using the final precipitation as fertilizers. The purpose of this study is to investigate the usability of final precipitation as fertilizers.
With this phosphorus fertilizer, the small scale and large scale plant growing experiment was conducted as follows.
1) The plants used in this work are green soybeans and sweet potato.
2) A plant environmental control system (LPH-220N, Nippon Medical & Chemical Instruments Co. LTD, Japan) was used to cultivate plants from the seed.
3) After the cultivation of the plants, we transplanted them and fertilized. Ammonium sulfate and potassium sulfate was fertilized for all plants, but the phosphorus fertilizer was fertilized for commercial one, recovery one and none for all plants.
4) The phosphorus fertilizer was used the two types; phosphorus fertilizer derived from sludge ash and commercial one. Their molar ratio of the fertilizer is N:K:P=1:1:1.
We are also going to carry out more large scale experiment by cooperation of the Faculty of Agriculture.
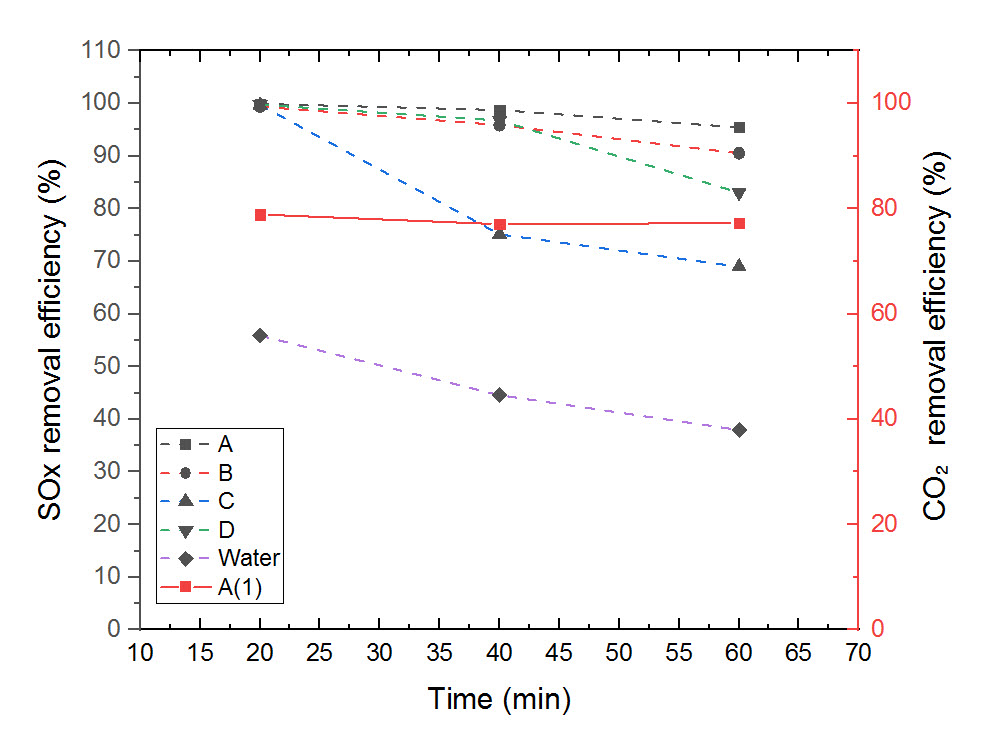
There exist several techniques to treat fossil fuel-combusted flue gas such as wet limestone-gypsum method and amine absorption. Although the techniques have been widely used, their gas-liquid contacting efficiencies are poor which makes the contactors bigger resulting in difficulties in installation. In this study, we proposed a novel gas scrubbing concept, which can effectively remove SO2 and CO2 from simulated flue gas by stages using two membrane contactors in series. The SO2 is captured and directly converted to aqueous ammonium sulphate which could be utilized as fertilizer. The CO2 is captured and recovered using aqueous amine solutions and temperature-swing desorption. Various absorbent solutions such as fresh water, NaOH, NH4OH, H2O2 are tested. Of the tested absorbents, aqueous ammonia solution showed the highest removal efficiencies (i.e. > 99 % for SO2, 80 % for CO2). Optimum condition, removal mechanism, and size reduction of gas-liquid contactor are alsso discussed.
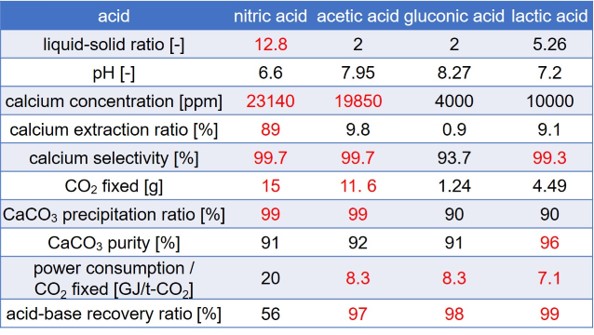
We have proposed a new type of mineral carbonation process, where CO2 is fixed as carbonates of alkaline earth metals. The process is composed of four steps: (1) Absorption of CO2 with an alkaline solution; (2) Extraction of calcium using an acid solution; (3) Precipitation of carbonates by mixing the calcium-leached solution and the alkaline carbonate solution; (4) Regeneration of an alkali-acid pair with the bipolar membrane electrodialysis. In this study, we examined optimum extraction conditions for the total process by using wollastonite as a calcium source and various kinds of acid in the view of the power consumption for the CO2 fixation. The acids used were nitric acid(NA), acetic acid(AA), lactic acid(LA), and gluconic acid(GA). The acid concentration and liquid-solid ratio were changed. The most important criterion of the extraction step is that the pH of the leached solution should be higher than 6.3, which is the acid dissociation constant of H2CO3: otherwise, a part of carbonate ions should be released as CO2 gas in the precipitation step. The table shows the optimum extraction conditions for the highest calcium concentration and the highest calcium extraction ratio of each acid after 24 h. The highest calcium concentration as well as the calcium extraction ratio was obtained when NA was used. In addition, the calcium selectivity in the extraction step was highest at 99.7%. When using AA, the calcium concentration was close to the value with NA, but the calcium extraction ratio was much lower. However, considering the electrodialysis process, the power consumption for the recovery of NA was much higher and the recovery ratio was much lower than those for the cases with other weak acids. The power consumption and the recovery ratio were almost equivalent for these three weak acids; acetic acid should be the optimum acid for the extraction step.
In recent years, the Asian monsoon region has a large business opportunity for the global food market for Japan. However, under severe conditions of high temperature and humidity, it is necessary to understand and control the cultivation environment in order to cause high-temperature injury in the process of crop growth. In this study, the authors evaluated the crop growth conditions and energy of greenhouse tomato production in several Asian monsoon areas. In these evaluations, a cultivation simulator considering growth conditions was used. This simulator is constructed from three processes of leaf photosynthesis, distribution of photosynthetic components, and fruit growth in order to analyze growth conditions in detail. In the calculation of the energy required to maintain the cultivation environment of the greenhouse, the photosynthesis rate of the plants and the transpiration rate were also calculated by simulation. In the evaluation of the growth condition, changes in the temperature of greenhouses at each region of Asia during planting and harvesting were investigated from weather data.
As a result, the difficulty of greenhouse tomato production under high temperature and humidity conditions in Asia and the differences in crop growth conditions among these regions could be clarified by this analysis.
It was suggested that the fruit yield was correlated with the seed number and days of the fruit development stage, and strongly affected by the temperature of the fruit-set stage.
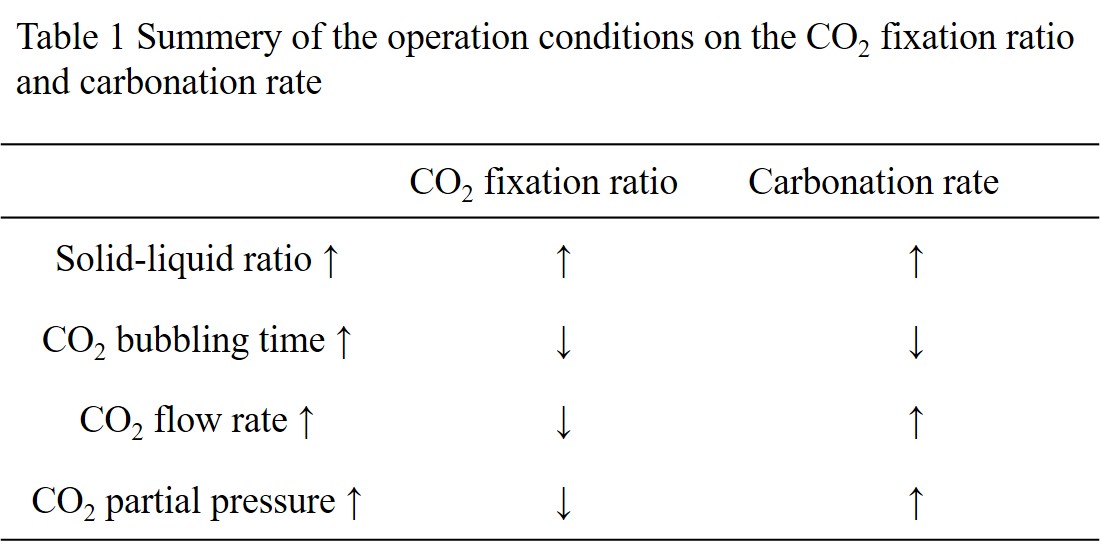
Mineral carbonation is a fixation method of CO2 by using alkaline earth metals to form carbonates. We focused on concrete sludge which is a waste fresh concrete, as an alkaline earth metal source for mineral carbonation. In this study, we examined the carbonation reactions of a model concrete sludge by CO2 bubbling under various operation conditions, and investigated the effects of these operation conditions on the CO2 fixation performances.
A model concrete sludge was prepared by mixing a commercial Portland cement with water for 60 minutes. After hydration, CO2 was bubbled through the model concrete sludge to start the carbonation reaction. The dissolved calcium concentration was measured with ICP-AES and the pH was measured with a pH meter. After carbonation, the model concrete sludge was filtered and the solid residue was dried in an oven at 100 °C for 24 h. The solid residue was then measured by a thermogravimetric method to determine the conversion of CO2 to calcium carbonate.
The calcium concentration decreased by CO2 bubbling, and reached 250 ppm after 80 min and almost unchanged up to 200 min, which is close to the equilibrium concentration. The calcium concentration increased after 200 min to be leveled off at about 600 ppm after 300 min. The pH of the solution decreased by CO2 bubbling, and almost unchanged at 6.5 after 250 min, which is close to the equilibrium value. The effects of the operation conditions are summarized in Table 1; the carbonation rate is defined as the amount of calcium carbonate divided by the bubbling time, and the CO2 fixation ratio is defined as the mole fraction of CO2 fixed as carbonate to that the total amount bubbled through. From these results, the carbonation process can be designed.
The behavioral studies of fission product aerosols are extremely important in the context of probable environmental hazard in the event of a severe nuclear reactor accident. Fission Product aerosols are generated in such a case are expected to travel from the primary heat transport system to the containment and may get released to the environment. Presence of steam also affects their dynamic behavior and fate. Interaction of aerosol particles with water vapor in the supersaturation domain affects their physical as well as chemical characteristics. Radioactive compounds of cesium (CsI and CsOH) forms a major part of fission product aerosols, which may be released into the air in huge amounts by nuclear power plants during nuclear accidents and also at the time of nuclear weapons testing. Severe nuclear reactor accidents that involve considerable core melting generate large source terms of fission product aerosols to the environment. Cesium, with a half-life of 30 years is highly reactive and combines readily with other elements, especially oxygen, other gases, and nonmetals. Cesium is treated as a hazardous material because it reacts violently with water. Cesium also reacts violently with sulfur, phosphorous, acids, and halogens (fluorine, chlorine, bromine, iodine, and astatine). The lifetime, along with its reactive nature makes it a concern for human health. Radioactive isotopes of cesium are produced in nuclear power plants by the fission of uranium in fuel rods and also by the explosion of nuclear weapons. In this study, the CCN properties at different supersaturations for different size of laboratory generated single salt CsI (Cesium Iodide) and CsOH (cesium hydroxide) aerosols were investigated. CCN spectra of CsI and CsOH particles were measured at room temperature (298K) with the help of DMT CCN counter. A comparison of experimental data with theoretical values is also done for both the salts.
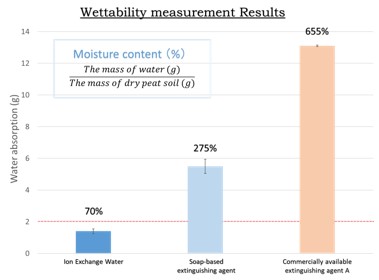
Peat fire in Indonesia not only releases a large amount of carbon into the atmosphere, but also causes significant damage to peatland ecology and the landscape. Smoldering is the dominant combustion process in peat fire, and the combustion is the slow, low-temperature, flameless burning of porous fuels, and the most persistent type of combustion phenomena. To suppress the peat fire, it should be very important to increase the water content of dry peat and deliver water toward the combustion zone to remove heat efficiently. We have been developing a novel soap-based firefighting agent for peat fire with significantly lower environmental risk. Soaps possess very high surface activity, very high biodegradability and very low toxicity. In this study, it was evaluated by focusing on permeability and wettability in peat soil as the physical properties of the soap-based firefighting agent. The peat moss in Russia was used as the peat model for permeability and wettability test. Permeability and wettability in peat moss of the synthetic-surfactant-based firefighting agent and soap-based firefighting agents was much better than ion-exchanged water. It was clear that the firefighting agent solution is very effective for firefighting peat fire compared to water alone. Among the soap components, the potassium myristate showed the best results in both permeability and wettability test.
Firefighting agents including fluorochemical surfactant for oil is widely used in Japan.
However, fluorochemical surfactants have much low biodegradable, and
perfluorooctane sulfonic acid was prohibited by the law in 2011. Regulations will be
tightened in the future, therefore, the fluorochemical surfactant-free firefighting agents
are eagerly anticipated. We have been developing a fluorochemical surfactant-free
firefighting agent with very low biotoxicity (LC50 ≥ 100 ppm) for oil fires. In this study,
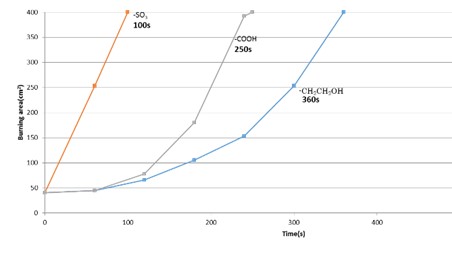
three surfactants containing different types of functional groups (-SO3,-CH2CH2OH,-
COOH) were used. After preparing firefighting agents, it was diluted to the required
concentration. Japanese-killifish, Himedaka, was exposed to the firefighting agent
solution, and the survival number after 96 h was measured. Firefighting tests were
conducted according to the scale of the national verification standard. The surfactant
containing -CH2CH2OH functional group showed the best firefighting performance among the three types of surfactant.
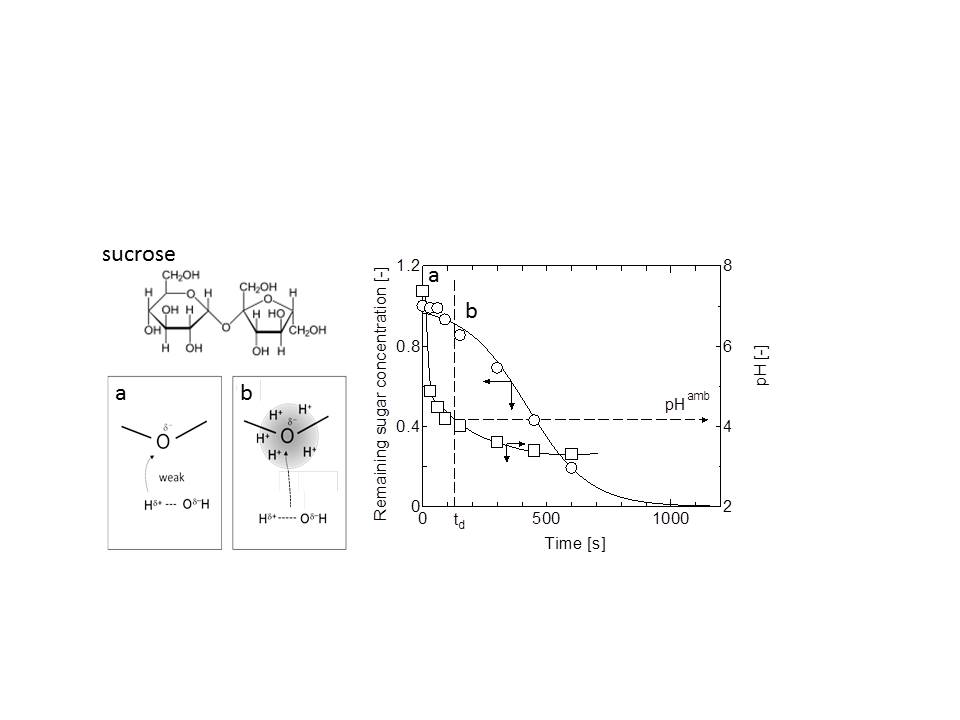
The hydrloysis of disaccharides is an important issue for the design of the conversion of cellulose biomass. In this study, the decomposition of disaccharide, sucrose, under the hydrothermal conditions (160~190 oC at 10 MPa). was examined in the microcapillary, as shown in the Figure (right) [1].
The kinetic model taking into consideration of induced period (td) was adopted to analyse the hydrolysis process of disaccharides The present analysis could give the pH value to start the hydrolysis of disaccharides (pHamb) as shown in Figure. Estimated the activation energy, the hydrolysis of disaccharides under the hydrothermal conditions were conducted in an ionic reaction manner. Therefore, possible parameters relating to the ionic reaction manner is (i) hydration structure of disaccharides and (ii) electron charge of oxygen in glycosidic bond. The hydration structure was discussed with the dielectric measurement. It was considered that the sucrose, turanose, meribiose were well hydrated relative to other disaccharides (see Figure a and b of left). Also, the electron environment at oxygen atom in glycosidic bond related to the hydrolysis of disaccharides (Q value) was calculated using a MOPAC. Then, pHamb value was roughly correlated to the Q value. Therefore, the method only requires knowledge of the electron charge of oxygen atom in glycosidic bond of disaccharides at the calculation to predict the pHamb of the target disaccharide.
To predict pHamb would lead to avoid the use of unnecessary quantity of acids to adjust the initial pH condition, which is the significance in environmental impact. Besides, it was a use of microcapillary system that could monitor the time-course of pH to obtain the pHamb value for hydrothermal hydrolysis of disaccharides. This would be just a significance of the microcapillary in this study.
Reference
[1] T. Shimanouchi et al., J. Chem., Article ID 3985915 (2019)
Over the past decades, the use of carbon fiber reinforced plastics (CFRPs) has been steadily increasing across a wide range of sporting and industrial applications. This growing production and use of CFRPs in applications today will inevitably lead to larger volumes of CFRP waste in the future. Since fiber reinforced composites are difficult to separate into their elemental components, composite waste is principally disposed in landfills or incinerated without any recycling approaches. Disposing a large amount of CFRP waste on reclaimed land is not feasible for small countries; moreover, burning expensive long carbon fibers also causes environment pollution. Use of biodegradable plastic is an effective way to solve the disposal problem of large CFRPs. APEXA© is a biodegradable polyester consisting of terephthalic acid (TPA), ethylene glycol (EG), and an organic acid. Its heat resistance and durability are far higher than those of other biodegradable polyesters and are comparable to those of polyethylene terephthalate (PET). Although the decomposition of APEXA© in compost has been studied, little is known about its degradation in an aqueous environment. In this study, APEXA© fiber was hydrolyzed at pH 3.0–10.5 for up to 4 weeks. The molecular weight of the APEXA© fiber decreased remarkably in dilute sulfuric acid (pH 3.0); specifically, it decreased to 22.9% of the original molecular weight after 1 week. The sharp decline in the molecular weight of APEXA© suggests at composite recycling combined with mild hydrolysis and biodegradation. The APEXA© composite enables to collect maintained long-carbon-fiber in hydrolysis and return the separated resin to the earth.
Background
Polyurethane (PU) was originally synthetized by Otto Bayer in 1937 in Germany. This material is very stable on a lot of different applications, such as, transportation, packaging, footwear, paint, and insulation component for refrigerators and buildings. In 2016, approximately 18 million tons of PU will be produced and 3/4 of them were in foam format. Asian countries used most of them.
Methods
A new study on polyurethane degradation, catalyzed by the different catalyst was reported. NaOH, KOH, sodium acetate and an alkaline metal oxide catalyst were used under the presence of ethyl glycol at the designated reaction condition was proposed. The resulting glycolysate was analyzed to determine the physical and chemical properties. The will be used as the source to reproduce polyurethane for recycling purpose.
Results
With the addition of sodium hydroxide and sodium acetate as the catalyst in the glycolysis, more wasted PU can be dissolved than that without the presence of the catalyst. The reaction time to reach the equilibrium condition is almost the same. More waste PU can be dissolved into the glycolysate with a shorter reaction time if the reaction was catalyzed by the proposed heterogeneous catalyst. The spent catalyst can be removed from the mother glycolysate solution easily upon the completion of the reaction. The glycolysate of the by different catalysts were also re-synthesized to form the recycled polyurethane. The thermalgrametric analysis of the recycled polyurethane from the heterogeneous catalyst showed very similar to the original waste PU. The results showed a potential route to reproduced the polyurethane from the recycled method. The PU foam as also reproduced in this reaction process.
Financial support: Ministry of Science and Technology in Taiwan, ROC (MOST 107-2218-E-035-017 – and MOST 106-2221-E-035-079 -).
I. Introduction
Recycling catalyst is needed for biomass conversion (e.g., producing bio-oil) in order to reduce cost and protect the environment. However, most catalysts used for producing bio-oil from the pyrolysis of biomass cannot be recyclable use, leading to catalyst pollution and waste. Based on previous research results (shown at this conference) it was shown that inexpensive Ni, Fe catalyst clusters can improve the quantity or quality of bio-oil produced from the biomass, but to our knowledge, very few studies have actually investigated the reusability of catalyst, especially from experiments when mixing cellulose (biomass model compound) and catalyst directly together. In this study, Ni2Fe3 catalyst was mixed with cellulose in a pyrolysis batch reactor to investigate its reusability and its impact on the bio-oil yield and content.
Experiment
The catalyst was prepared by sol-gel method and heat-treated in flowing H2/N2 gas. Pyrolysis experiments were conducted using a fixed bed reactor, flowing 100ml/min N2 gas, at 450 °C with the catalyst repeating five times. The amount of pyrolyzed bio-oil and coke were weighed, and then the amount of gas was calculated by subtracting the amount of bio-oil and coke from the initial feed.
II. Results & Discussion
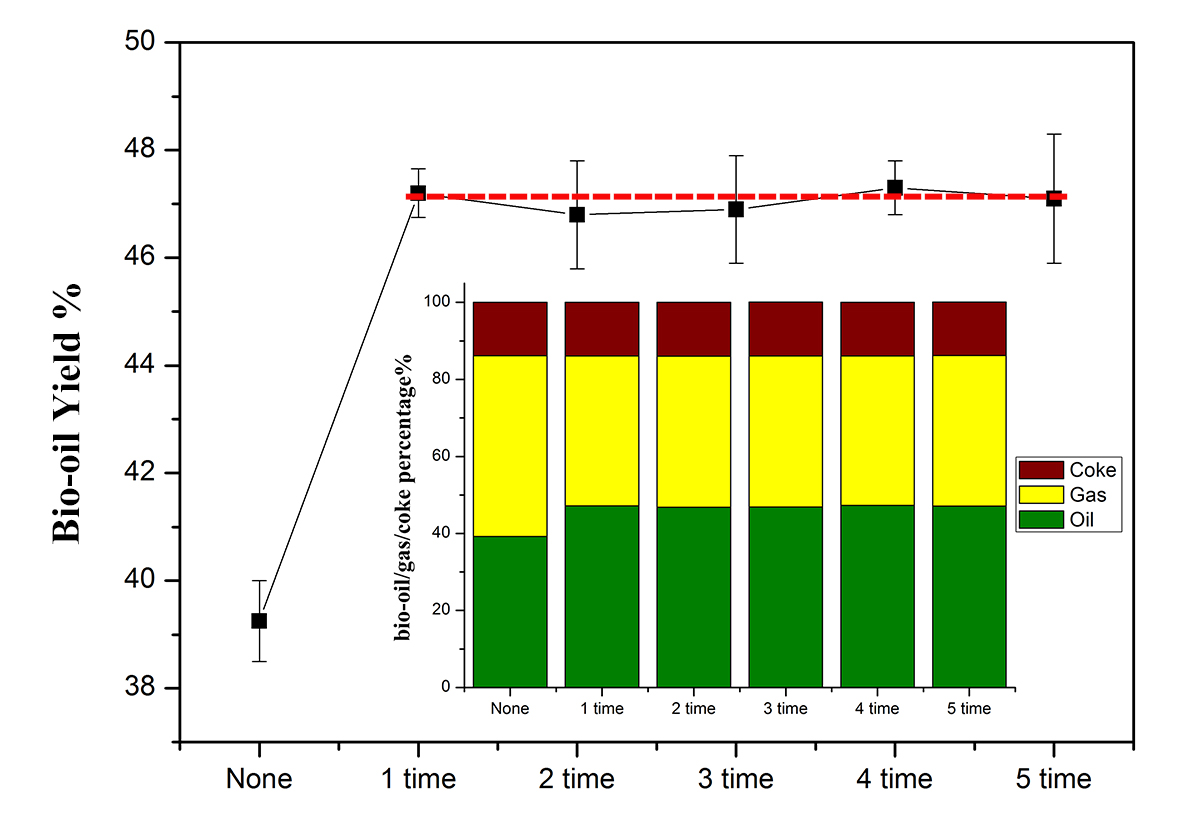
Figure 1 shows the bio-oil/gas/coke mass yields from each pyrolysis experiment, and the bio-oil yield is plotted versus a number of experiments. The Ni2Fe3 metal catalyst can increase the bio-oil yield from 39.5% to 47.5%. Moreover, the activity of the catalyst is relatively constant based upon bio-oil yield. Furthermore, the gas/coke yield also shows a similar trend. What can we say here is that the NiFe cluster metal catalyst is recyclable for bio-oil production by pyrolysis. Research work is continuing on the evaluation of the sampled bio-oil content and understands why the catalyst can be used repeatedly.
In this study, montmorillonite-supported nanoscaled zero-valent iron (Mt-nZVI) composites were fabricated using a facile liquid-phase reduction method. The morphology, crystal structure, functional groups, and magnetic properties of as-prepared composites were explored using scanning and transmission electron microscope, X-ray diffractometer, Fourier-transform infrared spectroscope, X-ray photoelectron spectroscope, zeta potential analyzer, and superconducting quantum interference device. The ZVI nanoparticles (particle size, 60-100 nm) were well dispersed on Mt support (particle size, 25 ホシm), which effectively decreased the tendency of agglomeration behavior. The optimal weight ratio of Mt particles to ZVI nanoparticles was equal to 2, which was denoted to be 2Mt-nZVI composite. The synthesized 2Mt-nZVI was then used to eliminate oxytetracycline antibiotic with a variety of operating parameters such as Mt particle size, weight ratio of Mt to ZVI, solution pH, and initial oxytetracycline concentration. Experimental results showed that 99% of 100-mg/L oxytetracycline at pH 5.0 could be removed by 0.6 g/L of 2Mt-nZVI composite and the mineralization could reach 70% after 20 min of reaction. Moreover, six possible intermediates were detected and identified by an ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTof/MS, Waters) during the degradation of oxytetracycline over 2Mt-nZVI composite. This study demonstrated that the prepared Mt-nZVI composites possessed a promising potential for removing and mineralizing oxytetracycline from polluted water.
Photocatalysts represented by titanium oxide are important material in utilization of light energy. In recent year, a new use of silver orthophosphate (Ag3PO4) semiconductor as an active photocatalyst has reported which can harness visible light to oxidize water as well as decompose organic contaminants in aqueous solution. Previously, silver orthophosphate fine particles were mainly synthesized by the liquid phase reaction, which required multi-step operation. Furthermore, the only report of silver phosphate synthesis by solid-solid reaction has also proposed time-consuming methods. There are several important technologies to realize the practical use of materials, one of which is method to rapid and facile synthesis. In this study, we developed a rapid synthesis method for silver orthophosphate using a vibration mill. Furthermore, the obtained silver orthophosphate was characterized in different ways. Silver orthophosphate was easily obtained by preparing a tube containing silver nitrate, one of several phosphates (Na3PO4, Na2HPO4, NaH2PO4) and zirconia beads, followed by vibrating with a vibration mill. The unreacted raw materials were removed by washing to obtain a yellow powder. The powder X-ray diffraction pattern of the sample obtained is in accordance with that of the reported silver orthophosphate. Furthermore, the results of energy dispersive X-ray analysis show the consistent values with the stoichiometric ratio of silver orthophosphate. The yield of silver orthophosphate was 48% when disodium hydrogenphosphate was used as the phosphate source. Furthermore, the yield depends on the shaking time, but it saturates in only 10 seconds at a shaking speed of 4000 rpm. The yield was also dependent on the shaking rate with constant shaking time. The photocatalytic activity of obtained silver orthophosphate was evaluated by degradation of methylene blue in suspension. The supernatant of the suspension was discolored by the irradiation of blue LED light, indicating the photocatalytic activity of the obtained sample.
A simple and efficient method was developed to remove dyes from wastewaters. The method was conducted by adding alcohols followed by feeding air bubbles. In this study, cationic dyes (rhodamine B (RB), crystal violet (CV), malachite green (MG)) and anionic dye (erythrosine B (EB)) were selected because of their extensive use in staining and textiles dyeing processing. Removal of these dyes increased significantly by adding small amount of alcohols (0.01% ~ 0.5% v/v). The effect of alcohol (ethanol < 2-propanol < 1-butanol) increased with increasing in its carbon number. Cationic dyes (RB, CV, MG) were removed in the range of pH 3 to 10, while EB was removed in the range of pH 1 to 3. Effects of instrumental parameters including vessel diameter and water height were also studied. Under the optimum conditions, nearly complete (> 98%) removal of these dyes was achieved by the flotation within 5 min. Influences of co-existing organic (humic acid, starch, etc.) and inorganic substances (NaCl, KCl, etc) omnipresent in wastewaters were investigated in detail. Practical applicability was examined by using synthesized dye wastewaters.
The water treatment using photocatalytic titania nanoparticles for removing organic contaminations is one of the advanced water purification systems, but the immobilization method for nanoparticles should be developed. For overcoming this problem, the degradation of methylene blue (MB) in aqueous solution by a titania particle film was studied using the photocatalytic system. The electrophoretic deposition technique applied to preparing titania thin films on stainless steel screens out of titania particle suspension. After annealing in air at 773 K for 10 minutes, the crack-free, porous titania particle film coated on the screen was dipped in 50 mL MB aqueous solution to carry out the MB degradation test, in which ultraviolet light (254-nm wavelength) was illuminated to the surface of the titania film while the MB solution was passed through the meshes of the screen. The rate of MB degradation was estimated from the variation of MB absorbance measured with a spectrophotometer. The degradation rate was found to increase with the thickness of titania particle film and to reach an asymptotic maximum range, even for different meshes of the screen. This trend indicates that a certain thickness of the titania porous film was required for the photocatalytic reaction with the surface area of the coated screen being a dominant parameter for the MB degradation rate. As the circulation rate of the MB solution was increased, its degradation rate increased; this could be explained as that increasing flow rate resulted in rendering the diffusion boundary layer thinner with less resistance to MB's contacting to the surface of titania particles.
A photo-assisted Ce(IV) mediated electrochemical oxidation (MEO) system is developed to treat the phenol-containing water in comparison to Ce(IV) MEO and photocatalytic oxidation systems. Operation conditions of electrical current, nitric acid concentration, stirring speed of magnetic stirrer, and electrode area ratio of anode to cathode for Ce(IV) electrogeneration are optimized in terms of its production yield and energy consumption. According to a kinetic model incorporating the Ce(III)/Ce(IV) redox reaction, the forward rate constant of Ce(III) oxidation is found to be twice as large as that for the reverse reaction. Regarding the wastwwater treatment, Ce(IV) MEO and photocatalytic oxidation systems both have favorable capability in degrading phenol under an acidic environment rather than the DEO system. More importantly, the photo-assisted MEO system offers excellent removal efficiency of phenol up to 98% in only 40 mins, benefiting from the synergistic effect of the individual electrocatalytic and photocatalytic systems. Moreover, a pseudo-first order kinetic model is applied to well describe the phenol degradation in the photoelectrocatalytic oxidation system, resulting in a rate constant of 0.083 min-1.