Sulfonamides such as Sulfamethazine (SMT) are widely used as antibiotic in animal husbandry, fisheries aquaculture, fruit and vegetable in Taiwan. Although antibiotic has positive effects, once they are release will cause high risk to ecology or human body. In the research it has been pointed out that such bacteriostatic agents may cause allergies, pneumonia, and enhance the symptoms of asthma. The Ministry of Health in Taiwan has been clearly legislation about Sulfamethazine (SMT) residue.
This present study is to investigate the removal of SMT in aqueous solutions by electrochemical activation of sodium persulfate. In the part of SMT removal by the electrochemical activation system, various parameters such as electrode pair, current densities, electrolyte concentrations, oxidant concentrations, temperatures and initial pH were evaluated to characterize the removal of SMT efficiency. The effect of the current density on the special energy consumption were also investigated in this study, The optimum electrode pair, current densities, electrolyte concentrations, oxidant concentrations, temperatures and initial pH were found to be Fe/Fe, 16 mA/cm2, 4 mM, 0.6 mM, 298 K, pH 3, respectively.
Advanced oxidation processes(AOPs) are used as oxidation methods with highly reactive species such as hydroxyl radicals for the removal of organic pollutants having high chemical stability or low biodegradability in wastewater. However, the AOPs are not cost-effective and they need to combine with other treatment processes such as physicochemical or biological processes in order to achieve maximum economy efficiency. AOPs can be used as a pre-treatment or post-treatment method of biological process including activated sludge. In the pre-treatment AOPs will improve biodegradability of pollutants and in the post-treatment AOPs will remove the pollutants that cannot be completely degraded by biological systems. In the combination of AOPs as a pre-treatment with activated sludge treatment the chemical oxidation is specifically aimed to partially degrade the pollutants to more biodegradable components and the degree of partial degradation of pollutants will be important for the optimization of overall process efficiency.
In this study, ethylene glycol(EG) and Fenton reaction were employed as model synthetic organic component and AOP, respectively. The effects of operating conditions on biodegradability of EG by Fenton reaction were studied. The biodegradability rate, which is determined with the TOC before and after activated sludge treatment, increased from 3% to 39% by Fenton reaction for 2 min. From HPLC analysis EG was degraded into formic acid and methanol by this degradation reaction. The same analysis were conducted for glucose and poly ethylene glycol(PEG). The biodegradability rate of PEG increased from 0% to 13% by the Fenton reaction, while that of glucose decreased from 82% to 68%. From these observations showed that AOP as a pre-treatment of activated sludge process is useful for the treatment of poor biodegradable organic components.
The depletion of phosphate rocks has become problem, because the demand of fertilizer is increasing according to rapid population growth in developing country and an increase of biomass energy demands for CO2 problem. Sewage sludge ash is getting attention for recycling phosphorus resource because of containing phosphorus equivalent to low quality phosphate rocks. phosphorus recovering techniques from the ash have been studied. After using the ash as producing fertilizer, sewage sludge ash residue always remain and it is disposed even though there is a lack of landfill capacity. In this study, we propose a method of recycling sewage sludge ash residue as a marine fertilizer and evaluate the effects. In Japan, some of the coastal areas and bays currently suffer from a lack of phosphorus. Phosphorus could be supplied by sewage sludge ash residues with some treatments. Sewage sludge ash residue includes silicon and iron which are also important for ocean ecosystem to grow apart from phosphorus. For the control of solving rate of phosphorus, we add some carbon, calcium and sodium, and do thermal treatment. Since dissolution rate of heavy metals is much lower than the environment standards of Japan, we could control of solving rate of phosphorus supplied to the coastal areas and bays from 3 days to 3 years.
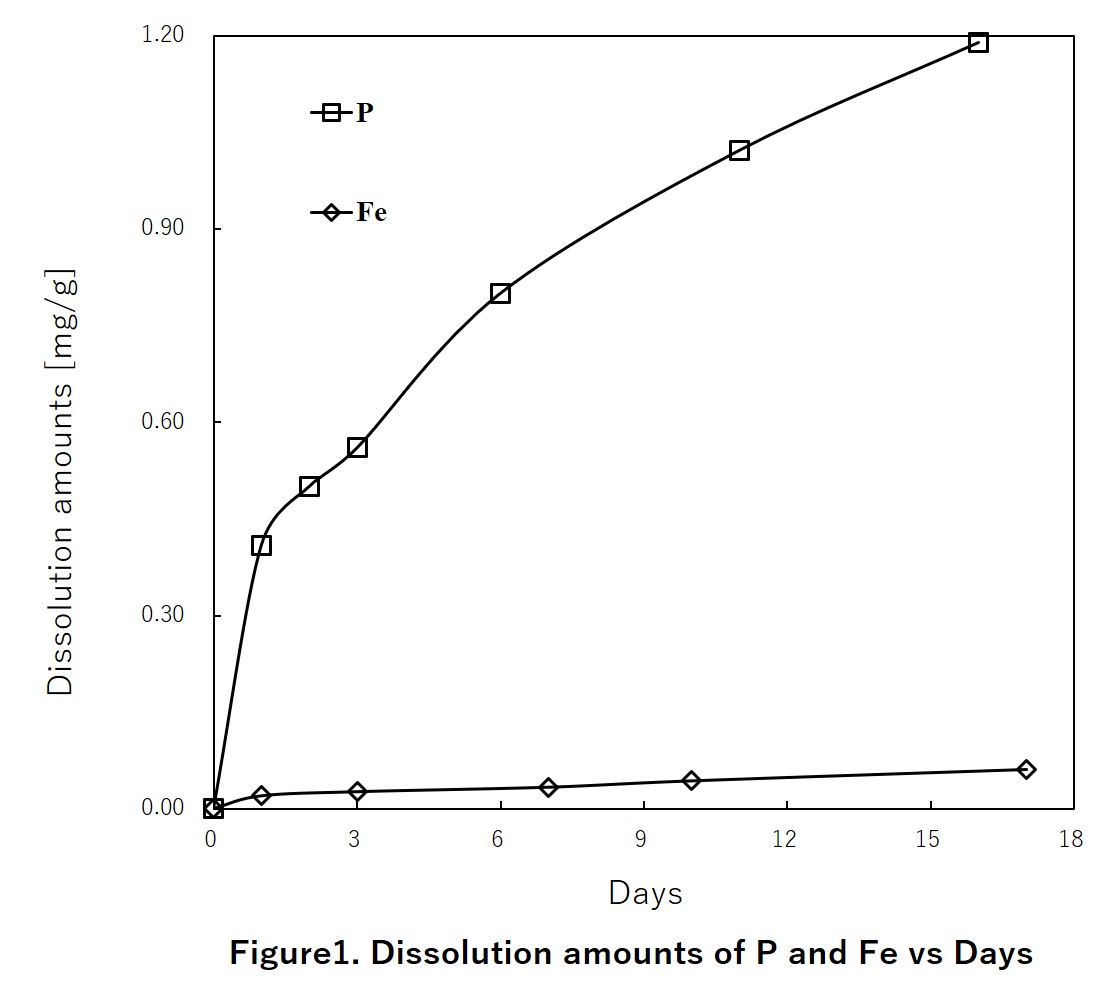
Phosphorous is main component of fertilize. Recently phosphate rock is one of the worldwide depleting resources, because demand of fertilize is increasing with increasing population and bioenergy. Furthermore we don't have phosphorous recourse in Japan. Fortunately, Sewage sludge incineration ash contains about 15 to 30% of phosphorous (P2O5 equivalent), and it is equivalent to low grade phosphorous rock. And the amount of phosphorous contained in sludge incineration ash generated from all over the country is equivalent to it which is imported from overseas as phosphate. Now the phosphorous recovery process from sewage sludge ash is already researched, so we can secure the phosphorous that are not concern for depletion. However, still much sewage sludge ash is landfilled because recovery rate of phosphorous in previous developed process is very low. So we have developed the method called two-step phosphorous recovering system, which is combined acid elution and alkali elution. In this system, recovery rate from sewage sludge ash is almost 80%.
In this study, for more improving the recovery rate, we try to add the additive and control pH and discuss about the phosphorous recovery rate depending on additive amount and pH. Also, we discuss that alkali elution rate is changed by the structure of precipitation.
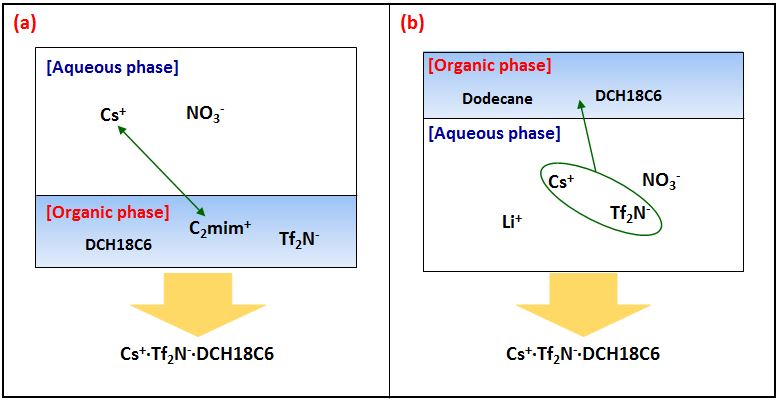
So far, much research has been done on the Cs+ extraction system using the 1-ethly-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (C2mimTf2N) as Ionic liquids (ILs) and dicyclohexano-18-crown-6 (DCH18C6) as a Cs+ selective extractant. In our laboratory, we found that when the amount of ILs was reduced, solid precipitate was formed, and it was confirmed through various analyzes that Cs+•Tf2N-•DCH18C6 was consisted. Formation of solid precipitate is useful for separation after waste treatment. In addition, despite a small amount of ILs, the Cs+ removal efficiency was about 90% when DCH18C6 was used in the same molar quantity as Cs+. Based on this, the study was conducted using LiTf2N as instead of expensive ILs under dodecane solvent (Figure 1). When the dodecane was reduced, solid precipitate was also formed, and confirmed it consists of Cs+•Tf2N-•DCH18C6 by analyzing scanning electron microscopy (SEM), Energy Dispersive Spectrometer (EDS) and Fourier transform infrared spectroscopy (FT-IR). Also, it was shown the high Cs+ removal efficiency of 90% in dodecane system.
In conclusion, the new facile method using dodecane and common anion salt (LiTf2N) can reduce the amount of waste, is easy to separate, and has a high Cs+ removal efficiency, so that it can be used for the treatment of radioactive liquid waste. Moreover, although dodecane is a volatile substance, it can be economically advantageous because it can eliminate danger by using Solid-liquid separation using a small amount and replace expensive ILs.
The removal ability of dibenzothiophene (DBT) in hydrocarbon liquid model fuel (MF) of Ni-loaded carbon (Ni/C) prepared from lignite, which is inexpensive and abundant with an ion-exchangeable capability, was investigated with a flow-type fixed-bed reactor. The Ni/C precursor (Ni/C_P) could only be prepared by adding lignite to a Ni2+ solution by impregnation method. Ni particles in the Ni/C_P existed as an amorphous form. Ni particles in Ni/C prepared by pyrolysis of Ni/C_P were well-dispersed into carbonaceous materials. The removal performance of DBT by Ni/C depended on the loaded Ni amount. The highest performance was observed at 11wt%-Ni content in Ni/C under a desulfurization temperature of 200 °C and H2 reduction time of 0.5h for Ni/C before desulfurization test. The H2 reduction time of Ni/C influenced the breakthrough curves, and it was found that the optimum time for DBT removal by Ni/C was 1.0h. When the treatment temperatures for desulfurization were varied at 25-200oC, the breakthrough and saturation points increased with increasing temperature, and the greatest performance was observed at 200 °C. As the initial S concentration in the MF was varied, the breakthrough curves depended on the concentration, and it was found that Ni/C could decrease the S amount in the MF to below 0.1ppmw. From the results of the identification of MF treated by Ni/C, it was suggested that adsorption desulfurization occurred due to the cleavage of the C-S bond in the DBT molecule to form an Ni-S bond and biphenyl. In addition, from the quantitative result of biphenyl in MF treated by Ni/C and carbonaceous material prepared by removal of Ni in Ni/C, it was suggested that competitive adsorption of biphenyl and DBT on the adsorption site on carbonaceous material in Ni/C prepared occurs in this study.
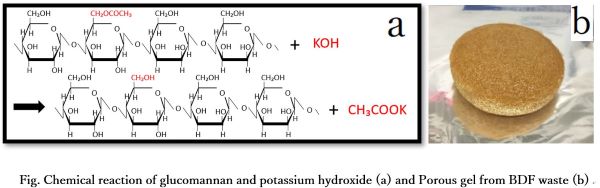
Bio diesel fuel (BDF) has an advantage of generating small amount of SOx during combustion. However, BDF production process discharges strong alkaline waste liquid including potassium hydroxide, methanol, fatty acids, and glycerin. Since this waste liquid is highly toxic and harmful to the environment, it is basically incinerated. The high waste disposal cost is one of the issues, and it is required to develop of the effective utilization method of BDF waste liquid. Its environmental hazard is mainly derived from the strong alkalinity of potassium hydroxide; however, potassium itself is an important element in plant growth. If the potassium hydroxide in BDF waste liquid is neutralized and solidified, BDF waste liquid can have a possibility to utilized as a potassium sources fertilizer for plants. In this study, we produced gel from BDF waste liquid by adding glucomannan. Glucomannan solidifies the waste liquid while convert potassium hydroxide to potassium acetate (Fig. a). The glucomannan was added to the diluted waste liquid, and a gel was formed (Fig. b). In order to evaluate the gel as potassium sources fertilizer, neutralization of potassium hydroxide with glucomannan and emission of potassium from the gel were confirmed by measuring the pH and potassium concentration in the solution. From the experimental results, it was suggested that BDF waste liquid has potential to use to agricultural fertilizer to supply potassium.
Acid mine drainage (AMD) containing toxic elements continues to be generated at about 100 abandoned mines throughout Japan, and It is urgent to be treated in the future.
There are various methods for AMD treatment, and it is necessary to select the best method for each mine. Passive treatment using constructed wetlands is attracting attention for some mines with low volume of wastewater and low concentration. Although the removal ability of the constructed wetland has been confirmed, quantitative modeling of its removal mechanism and removal capacity and its application to other mines have not been carried out. In this research, we model the contaminant removal process in the subsurface flow (SSF) of constructed wetlands in the H mine using geochemical modeling. We also evaluate the applicability of the model to other mines. As a result of field survey, it is suggested that δ-MnO2 is generated by microorganisms in the SSF. The experiment result confirms that cadmium (Cd) is removed by δ-MnO2. Therefore, we carried out removal experiments under several conditions to construct a surface complexation model (SCM) of δ-MnO2 for Cd. By adding the SCM and some reaction constructed in this study to the previous chemical reaction model and performing one-dimensional advection analysis, the reaction behavior in the wetland could be well reproduced. The result suggests that the toxic elements are removed by adsorption to iron, aluminum, manganese (hydro-) oxide or precipitation in the SSF. In addition, by applying this model to other mines, we calculated the area of wetland necessary for treatment. We compared the conventional treatment with the constructed wetland and examined the applicability of the constructed wetland from the aspect of economics and environmental impact.
Heavy metals are highly toxic substances contained in industrial wastewater or mining waste water. Among heavy metals, Pb(II) ions are difficult to remove under alkaline conditions. Generally, Pb(II) ions in aqueous solutions could be removed by pH adjustment, but could not be removed in some cases, such as leaching solution from incineration ash. In plating industry, if we remove Pb(II) ions at alkaline conditions, we can re-use the solution.
Generally, activated carbon is used for heavy metal removal, however it is expensive. Dolomite is one of the minerals, widely distributed all over the world, and very cheap comparing with activated carbon. Calcined dolomite has high adsorption capability to heavy metals. We found that As(III) ion adsorption capability at alkaline conditions is improved by phosphorus-doped in previous studies. Pb(II) ions are absorbed by phosphorus doped calcined dolomite adsorbent at alkaline conditions.
In this study, we investigate the effect of doped phosphorus amount, pH, and concentration of Pb(II) on adsorption. The structure of phosphorus doped dolomite and Pb(II) adsorbed dolomite with phosphorus are analyzed by XRD.
This experiment was conducted by changing parameters such as pH, concentration, temperature, doped phosphorus amount, and time. The result shows that above pH 11, the amount of Pb(II) ions by the phosphorus doped adsorbent increase than that of phosphorus non-doped dolomite. Adsorption amount is proportion to doped phosphorus amount.
The XRD analysis result show that phosphorus doped dolomite is Hydroxyapatite (HAp). This adsorption is both physical and chemical adsorption, and could be fitted to the Freundlich adsorption isotherm.
The present study shows that the doped phosphorus at high pH solution improves the Pb(II) ions adsorption capability of calcined dolomite.
Phosphorus (P) is essential for life, and phosphate rocks are main resource of P used in agriculture and industry. However, international price of phosphate rocks is soaring because of the recent concern about P scarcity. Furthermore, Japan is depend on import for almost phosphate resource. Therefore, we need to save and reuse P for sustainable use of P in Japan. Sewage is proposed as one of the intranational phosphate resources. It is estimated that P equivalent to about 50% in imported phosphate rocks flow into sewage treatment plants. P in sewage water is concentrated into sewage sludge, and sewage sludge is decreased its volume by anaerobic digestion. Wastewater after this treatment is called 窶彗naerobic digested liquor窶・and contains large amount of P. In this study, we investigate about the P recycling from actual anaerobic digested liquor using hydrotalcite by adsorption method. Hydrotalcite is known as inorganic ion exchanger, however, ability of P adsorption in actual anaerobic digested liquor has not been investigated. As a result, hydrotalcite can adsorb phosphate from anaerobic digested liquor, and adsorbed phosphate is desorbed rapidly by NaOH solution. Maximum adsorption amount is about 3.5 mmol/g-abs and desorption ratio is 70 ~ 80%. In continuous operation using fixed bed column, high concentration of phosphate eluate is obtained rapidly by high concentration of NaOH solution. Mass transfer coefficient of hydrotalcite is calculated by Cooper model and elusion curve, and the proportional relationship between mass transfer coefficient and NaOH concentration is revealed. Mass transfer coefficient of absorbent is essential parameter for adsorption method and important to determine the actual operate conditions.
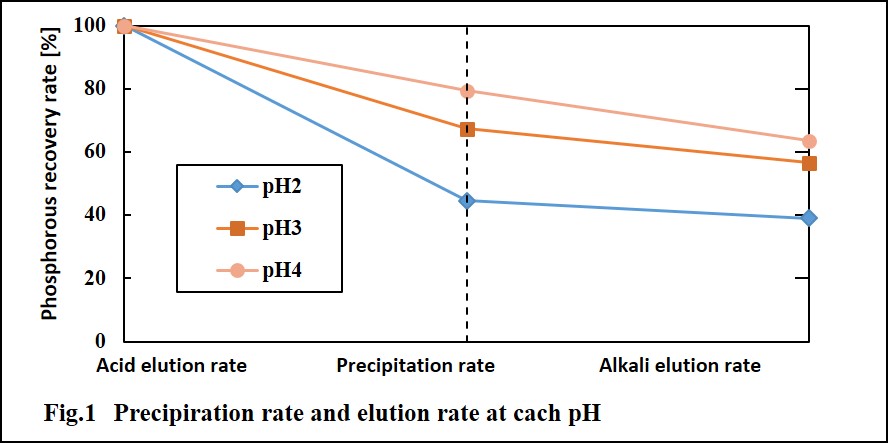
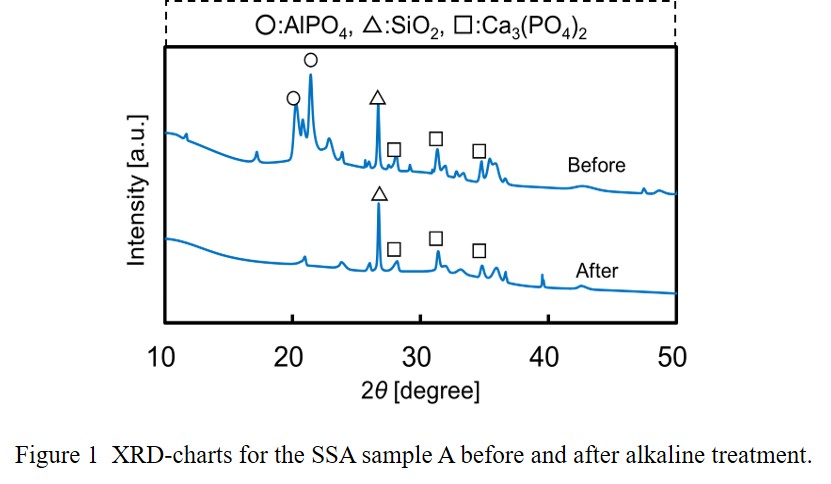
Sewage Sludge Ash (SSA) is generated by the combustion process of sewage sludge in an incinerator. Treatments of SSA with alkaline solution would lead a variety of recycling methods such as building materials, phosphorous recovery. In this study, we examined three types of alkaline treatment processes for real SSA samples collected having a wide range of chemical compositions: the method 1 is the phosphorous extraction with a dilute alkaline solution (0.8 to 1.0 M) with low solid/liquid (S/L ~ 0.1) ratio; the method 2 is preparation of materials for heavy-metal removal treated with a dilute alkaline solution (~1.0 M) adding silica as a silicon source for building geopolymer-like structures; the method 3 is solidification of SSA with a concentrated alkaline solution (~10 M) with high S/L = 0.8 to 1.0.
The phosphorous extraction performances with the method 1 strongly depended on the chemical composition of the raw SSA samples. The SSA samples with higher aluminum content and lower calcium content showed higher phosphorous extraction ratio. The disappearance of aluminum phosphate peaks in XRD charts by the alkaline treatment indicates that phosphorus in SSA is extracted mainly from aluminum phosphate, AlPO4 as shown in Figure 1.
All the solid samples prepared by the method 2 showed an excellent lead removal performance in water, especially the solidified sample after washing with pure water. In this case, formation of lead exchanged hydroxyapatite, Pb10(PO4)6(OH)2, was observed after the lead removal. This result suggests that the geopolymer-like solid (amorphous) was formed by the method 2, which has a cation-exchange capability.
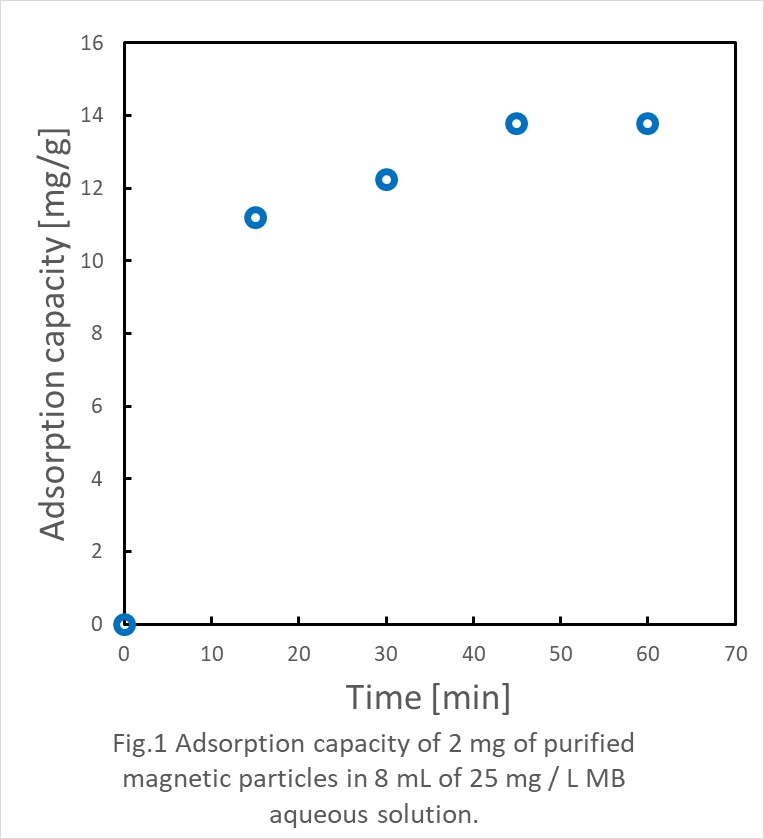
In this research, we consider a process for mass production of magnetic particles consisting of metal and carbon inexpensively using a diesel engine. The carbon material can be easily recovered by a magnet being complexed with a ferromagnetic metals and its application to water purification process is being considered although it is still disadvantageously expensive. By a diesel engine for chemical synthesis, the production cost can be reduced by the cogeneration of the chemical product and the power. In this study, iron-carbon fine particles are synthesized from diesel oil in which ferrocene is dissolved, and the characteristics as an adsorbent are evaluated. With ferrocene and JIS No. 1 diesel oil as raw materials, diesel oils with ferrocene concentrations of 0, 1000 and 10000 ppm were prepared. After the particulates were synthesized in the diesel engine, they were collected together with the exhaust gas in a gas collection bag. The engine used was a 4-stroke engine with a rated output of 2.5 kW and 3000 rpm and a displacement of 211 mL. The synthesized particles were collected when operated at an engine load of 1.65 kW. For evaluation of water purification, collected particles have been purified by magnetic separation in ethanol three times. Then 2 mg of purified magnetic particles were dispersed into 8 mL of 25 mg / L methylene blue (MB) aqueous solution. After rapid stirring, the solution was placed next to a neodymium magnet, and the absorbance at 611 nm of the solution supernatant was measured every 15 minutes. After the absorbance measurement, the solution was stirred and placed next to a magnet again. The particles were quickly and clearly attracted to the magnet. It was also confirmed that the concentration of MB decreased by the synthesized magnetic particles.
Selenium is commonly contained in wastewater generated from the process glass coloring agent, red pigment, and so on. An efficient method to remove selenium is required. Selenium is generally removed by co-precipitation with ferric hydroxide. However, this method requires a large amount of ferric salts due to low removal efficiency of selenium by ferric hydroxide. This research investigates the possibility of another method using magnesium oxide as a selenium remover from aqueous solution. Although magnesium oxide has a disadvantage because of its low reactivity, it can be overcome using low crystalline magnesium oxide obtained by calcining magnesium carbonate at low temperature. In this research, we tried to understand the selenium removal performance and its mechanism using this low crystalline magnesium oxide. To investigate the amount of selenium removed, the low crystalline magnesium oxide was added to simulated wastewater containing selenium. The concentration of selenium in simulated wastewater was measured by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). In addition, to clarify the removal mechanism of selenium, the solid after removal was analyzed using X-Ray Diffraction (XRD). Selenate was removed within 20 minutes after the addition of low crystalline magnesium oxide. The XRD result showed the shifted peak of (001) surface of the magnesium precipitate after the addition of low crystalline magnesium oxide in 10 to 30 minutes. This result indicated selenate entered the surface between the layers of magnesium hydroxide's (001). On the other hand, selenite was removed within 30 minutes after the addition of low crystalline magnesium oxide. A new peak was formed near the (001) surface of the magnesium hydroxide when the selenite was in high concentration. A precipitate containing selenite and magnesium besides magnesium hydroxide was formed when selenite was removed.
Flotation methods were designed for the efficient removal of fluoroquinolone antibiotics from wastewater. The removal of four fluoroquinolones including norfloxacin, ciprofloxacin, levofloxacin, and enrofloxacin were conducted by adding electrically equivalent amounts of sodium dodecyl sulfate (SDS) and poly (allylamine hydrochloride) [PAH] followed by feeding air bubbles. The air bubbles vigorously mixed water and induced the coagulation of SDS-PAH complex to from agglutinative coagulation on water surface. In the presence of Al (III) ions, fluoroquinolones in water were collected to the coagulum as a hydrophobic ion-pair of the Al (III)-chelate with a dodecyl sulfate ion. Experimental conditions including the concentration of Al (III) ions, SDS, PAH, alcohols, salts as well as solution pH, vessel diameter, water height, amount of air babbles, and flotation time were examined. Combined use of 1.0 mg/L Al (III), 20 mg/L SDS, 6.5 mg/L PAH, allowed more than 99% removal of four antibiotics within 5 min. Practical applicability was examined by using synthesized municipal and hospital wastewaters. Effects of dissolved organic and inorganic substances were studied in detail.
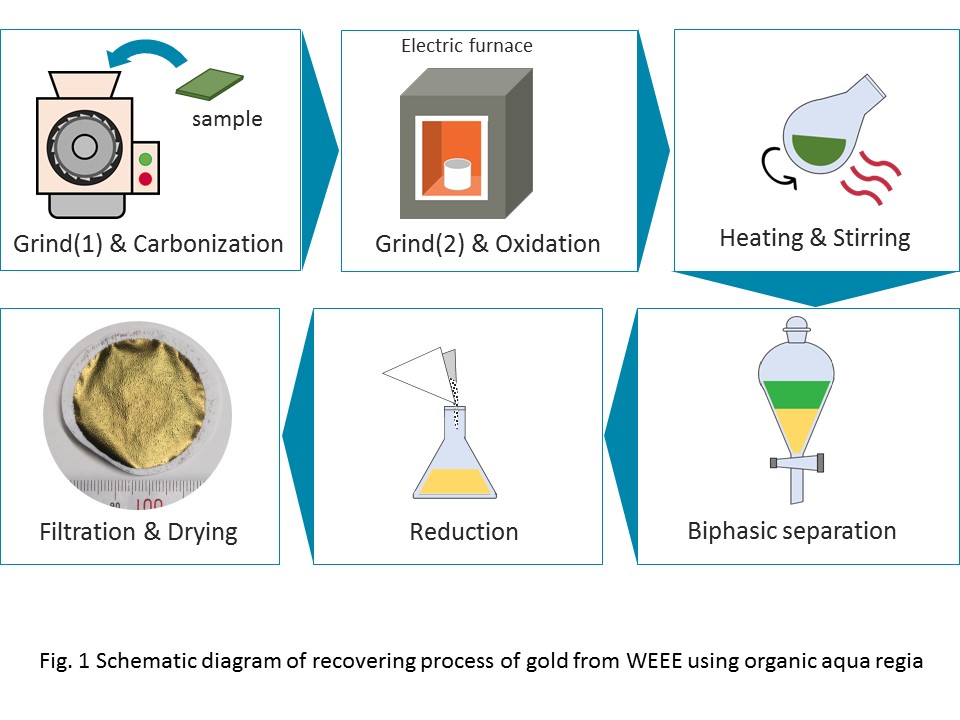
We have developed a novel process for recycling gold from secondary sources. The process consists of the leaching of gold using Organic Aqua Regia, propylene carbonate (PC) solutions containing copper bromide (CuBr2) and potassium bromide (KBr), In this study, we applied this process to recover gold from Waste Electric and Electronic Equipment (WEEE). First, the WEEE samples (memory card, mobile phone board and small appliances) were coarsely grinded and carbonated. Then, the samples were finely grinded and oxidized. Next, the leaching of gold from the oxidized samples were conducted in a PC solution with 0.2 M of CuBr2 and 0.2 M of KBr, at 353-373 K, followed by biphasic separation with sulfuric acid. The dissolved Au in the PC phase was recovered by reduction of ascorbic acid. The maximum recovery ratio of Au was 88%, 74%, 69%, 80% and 94% from memory card, mobile phone board, digital camera board, game board, TV board, respectively. Our developed process could offer a number of advantages, including eco-friendliness and simple operation.
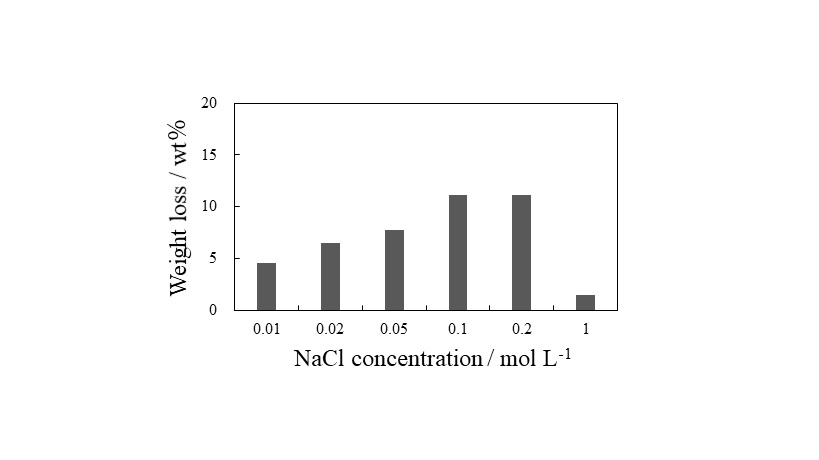
Carbon fiber reinforced plastic (CFRP) is a composite material consisted of carbon fiber and resin. Since the demand for CFRP is expected to increase at approximately 140,000 tons a year by 2020, it is necessary to consider the disposal method of a large amount of the CFRP waste. Recycling the CFRP waste to produce inexpensive carbon fiber can contribute to the expanded demand. The main recycling methods are a thermal decomposition and a sub-/super-critical dissolution method. Although these methods have some advantages, they have not yet reached a sufficient recycling method for the commercial scale due to remaining issues. Therefore, we focused on a voltage application method for the CFRP recycling. In this study, we investigated the effect of electrolyte solution, its concentration for the method, in order to optimize the conditions. The experiment was performed using a two-electrode cell, in which cross-ply CFRP laminate of carbon fiber and epoxy resin as an anode. The damage of carbon fiber after the experiment was assessed using a digital microscope. Figure shows the dependence of the weight loss of the CFPR laminate on the electrolyte (NaCl) concentration. The weight loss increased, as the NaCl concentration increased until 0.1 mol L-1. From the result, as the concentration was higher, the CFRP recycling by the voltage application method was effective, because the current showed high value. On the other hand, the experiment in 1.0 mol L-1 electrolyte showed the small weight loss despite the high current value. From the observation by the digital microscope, NaCl crystal was observed on the surface of the CFRP laminate after the experiment. From these results, we conclude that the suitable concentration of NaCl electrolyte was approximately 0.1 mol L-1 for the CFRP recycling by the voltage application method.
Sustainable development require human being well manage the pollutants in water and air. Removing the pollutants by microbes or catalytic electrochemical process in fuel cell type reactors present energy-saving options and electricity generation features. The electrodes and catalysts are crucial for such design of reactor and process , in pollutants removal efficiency, electricity generation level and operation costs. Through more than 5 years of study, we developed series catalysts, electrodes and fuel cell reactors , that favorably increased pollution control efficiency for both water and air. Significant catalysts such as Fe-N-C, Bi-V-O, BiOBr, Ag/AgBr, Pt/TiO2/ZnO, and their composites were loaded on substrate electrodes ( carbon cloth or stainless steel or Ni foam), as anode or cathode. These electrodes then were tested in Photocatalytic fuel cell or microbial fuel cell, that can integrate electrochemical effect with membrane filtration, or utilize the synergetic effect of microbes with (photo-)electro-chemical functions. Thus, pollutants such as PFOS, aromatic pollutants in real coking wastewater, antibiotics and dyes in water, HCHO and ethyl acetate, ammonia and trimethylamine, and toluene can be removed through electricity generation processes. The energy cost of operation is lowered through solar powered, bio-powered or self-biased and self-driven catalytic processes. Highest obtained electricity generation can reach 87Wm-2h-1,assisted by self-sustained degradation of pollutants.
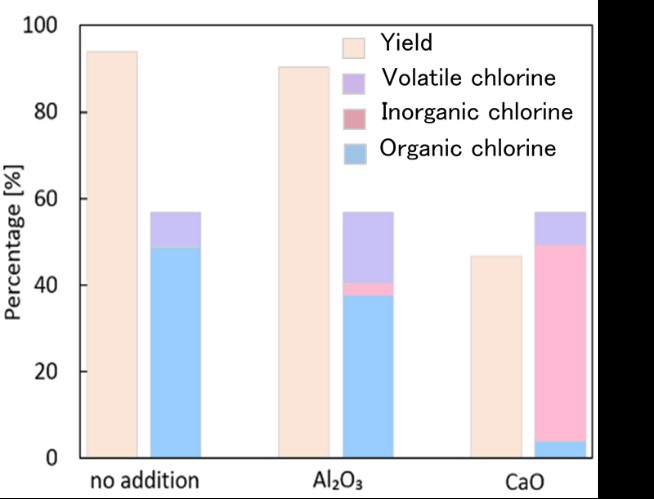
In this study, the chlorine removal efficiency of waste plastic by superheated steam was investigated using adsorbents. 5 g of PVC and 5 g of adsorbent were physically mixed and compressed into pellet using a hydraulic press. The adsorbents in this study were CaO, Al2O3, SiO2 and Fe3O4. The sample was placed in a chamber where the superheated steam was introduced at a flow rate of 10 kg/h. The reaction time and chamber temperature were kept constant at 1 h and 473 K, respectively. The experiment of nitrogen heating was also compared with the superheated steam heating. The dechlorination rate was in the descending order of CaO> Al2O3> no addition, and the PVC yield was in the descending order of no addition> Al2O3> CaO. Although the decrease in the yield of PVC seemed to result from the increase in the volatile matter such as hydrogen chloride and hydrocarbons, addition of CaO increased the dechlorination rate as organic chlorine and it was higher than the others. This is due to the formation of the inorganic chlorine compounds such as CaClOH or CaCl2 6H2O with decomposed chlorine and CaO, according to the XRD analysis. The neutralization reaction of CaO, which is an alkaline earth metal oxide, with the volatile HCl occurred in the sample. Therefore, the CaO addition is more effective for the PVC decomposition and dechlorination in the superheated steam atmosphere. The dechlorination rate of the CaO addition became 92% in the superheated steam, whereas it remained 32% in the nitrogen atmosphere. As a conclusion, the PVC dechlorination can be efficiently achieved in the superheated steam in the presence of adsorbent, such as CaO.
Biological removal of dichloromethane (DCM) from pharmaceutical industry is limited by its recalcitrance. In this study, an airlift packing reactor (ALPR), which combined the suspended and fixed-film microbial growth system, was set up to remove DCM and co-existed toluene. The removal performance of the ALPR for DCM was greater than traditional airlift reactor (ALR). The maximum elimination capacity (ECmax) of the ALPR for DCM reached 108 g m-3 h-1 with removal efficiency (RE) of 41%, increased by 145% if compared to the ALR. The ECmax for toluene was 172 g m-3 h-1 with RE of 70%, decreased by 25% than the ALR, which was mainly due to the higher liquid-phase biomass in the ALR. The results of high-throughput sequencing showed that the microbial composition on the packings of the ALPR had a large difference from its liquid-phase or the liquid-phase of the ALR. Gemmobacter, Rhizomicrobium, Chitinophaga, Vampirovibrio, and Fodinicurvata were genera with great abundance fixed on the packings and Rhizomicrobium, Chitinophaga, Vampirovibrio, and Fodinicurvata are first to be reported in VOCs biological removal. This study indicated that the ALPR can augment the microbial community and effectively improve the removal of recalcitrant VOCs.
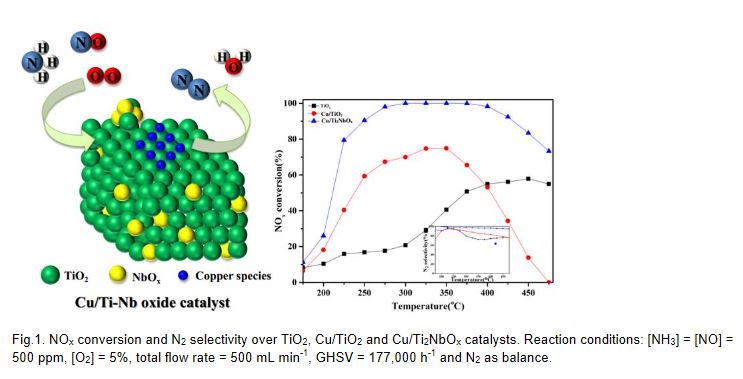
As the traditional VW(Mo)Ti catalyst existed some drawbacks using in NH3-SCR reaction, it is necessary to develop new TiO2-based alternative catalysts for NOx abatement. 0.8%Cu/Ti2NbOx as a novel catalyst was developed and investigated. The NOx conversion and N2 selectivity maintained upon 90% and 97% respectively in the temperature range of 250 °C-425 °C under a high gas hourly space vel °City (GHSV) of 177,000 h-1. Water vapor or SO2 would not pose any negative effect on the SCR activity. Also, 0.8%Cu/Ti2NbOx exhibited an excellent hydrothermal stability and alkali mental resistance. The performance characterization indicated 0.8%Cu/Ti2NbOx was a promising candidate for the NH3-SCR catalyst in the future practical application. Besides, an array of analytical techniques were employed to elucidate the correlations among the performance-structure-property and the reaction mechanism over 0.8%Cu/Ti2NbOx catalyst. The introduction of niobium oxide increased the surface area of TiO2 and decreased the crystallite size, promoting the high dispersion of copper species. Meanwhile, the total acidity and the amount of surface active oxygen over the catalyst were increased by the addition of niobium oxide. Copper species mainly existing in the state of isolated Cu2+ and non-isolated Cu+ enhanced the redox capability of the catalyst, resulting in an excellent catalytic performance. There is a promotional synergistic effect between copper and niobium in 0.8%Cu/Ti2NbOx catalyst during the catalytic reaction. Furthermore, the reaction pathway over 0.8%Cu/Ti2NbOx catalyst followed both Eley-Rideal mechanism and Langmuir-Hinshelwood mechanism at 225 °C.
Key words: Cu/Nb-Ti mixed oxide catalyst; Synergistic effect; Reaction pathway
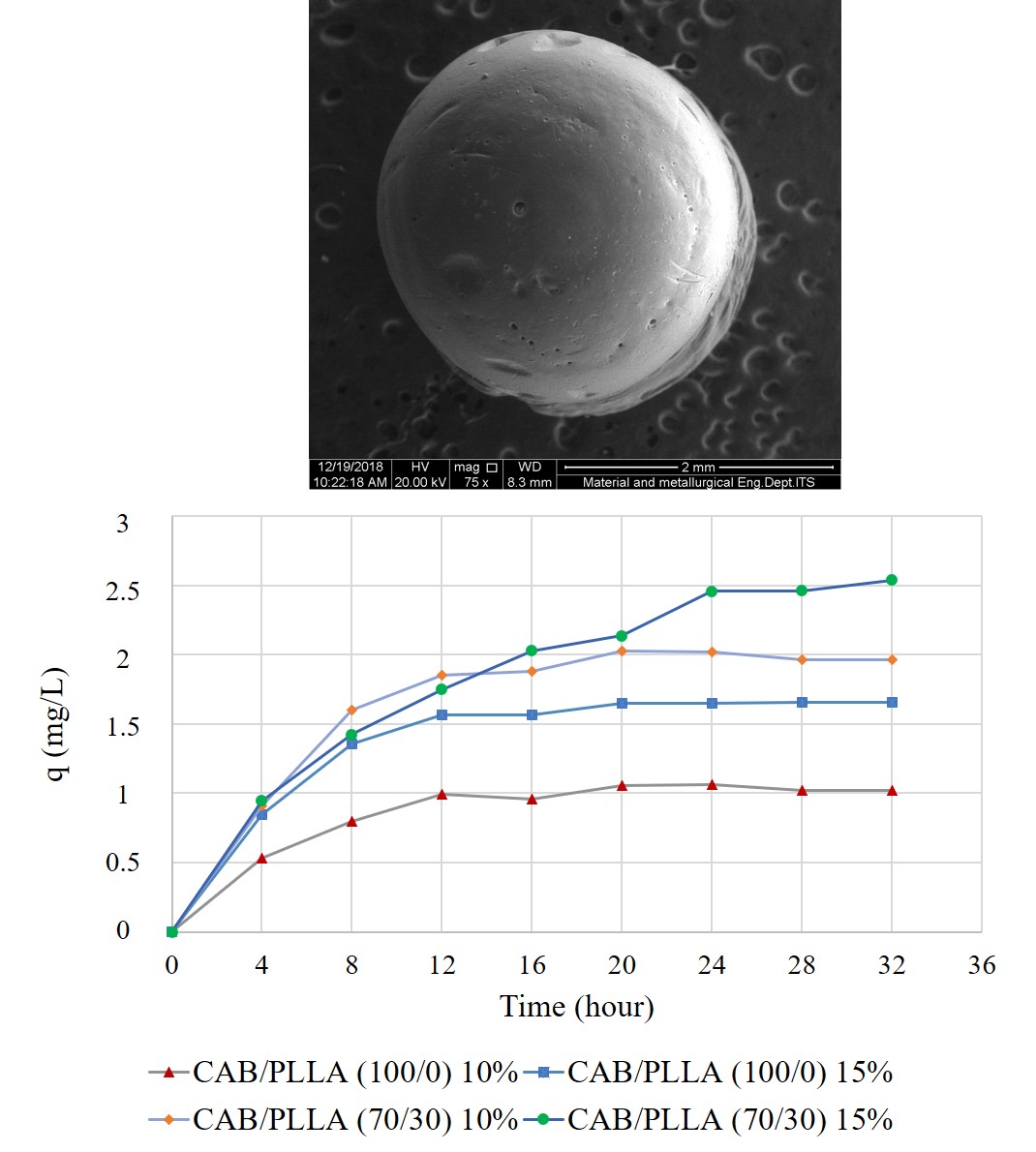
The disposal of untreated synthetic dye wastes from various industries causes serious environmental problems. Adsorption has been reported as promising method in industrial wastewater treatment especially for dye removal. Cellulose-based beads have been reported to show great potential as dye adsorbents. Some modification of cellulose-based adsorbent beads can be done to increase their adsorption capacity. One of the modification techniques is by blending with other materials to form composite beads. In this present study, cellulose acetate butyrate (CAB) and poly(L-lactic acid) (PLLA) were blended through solvent blending method followed by solution dropping technique to fabricate composite CAB/PLLA beads and applied as adsorbent for methylene blue removal from aqueous solution. It has been reported that cellulose beads can be prepared from some cellulose derivates sources such as CAB via solution dropping method. The weight ratio of CAB/PLLA was varied, which are 100/0, 90/10, 80/20, and 70/30, to see the effect of PLLA addition to the adsorption performance of the composite beads. The concentration of polymers in the solution was also varied, which are 10 wt% and 15 wt%. Batch equilibrium adsorption was carried out to study the adsorption kinetics. The adsorption results showed that the increasing of PLLA content generally increases the absorption capacity (Q) and dye removal percentage (% R). The highest adsorption capacity and % dye removal was obtained by CAB/PLLA (70/30) 15 wt%, which are 2.54 mg/g and 25.35% respectively at the adsorption condition of pH 7, temperature 30 °C, initial dye concentration of 20 mg/L, and beads dose of 2 g/L. The % dye removal increased to 69.85% by increasing the beads dose to 20 g/L. The kinetics data obtained from the experiment fitted well with Lagergren's pseudo-first-order model, showing the characteristic of physically adsorption.
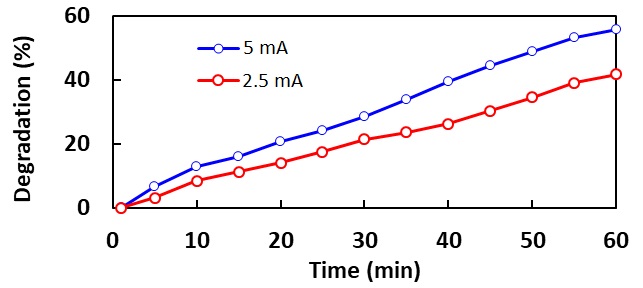
Degradation of Methylene Blue (MB) in aqueous solution is investigated using a pulsed DC discharge on liquid surface synchronized by droplet formation between two electrodes. The high voltage electrode is located in the return tube while a ground electrode is placed inside the bulk solution. Plasma discharge is ignited between the two electrodes when the aqueous solution runs to the lower electrode. The gap between the top electrode and the water surface is about 15 mm and the discharge is ignited when droplet forms. The concentration and total amount of the MB solution was 10 ppm and 100 ml respectively. The solution is circulated at a rate of 20 ml/min by a pump through the lower electrode, spectrophotometer and return tube (upper electrode). UV-vis spectroscopy is used to evaluate the degradation rate by the absorbance at a wavelength of 615 nm. The efficacy of discharge currents is studied at a voltage -10 kV. It is found out that higher current has more degradation efficiency for the same initial concentration of dye. The maximum degradation efficiency is determined about 56% for the 5mA current, and 42% for the 2.5 mA current. However, the more stable discharge is obtained for lower current despite the fact that the higher current has more efficiency of degradation. When plasma turned off after 60 min, the degradation is continued to cease down after 10 min. The latter phenomenon is attributed to the free radical which is elaborated within the solution and reacts with MB.
Figure 1. Effect of current on the degradation rate of MB.