
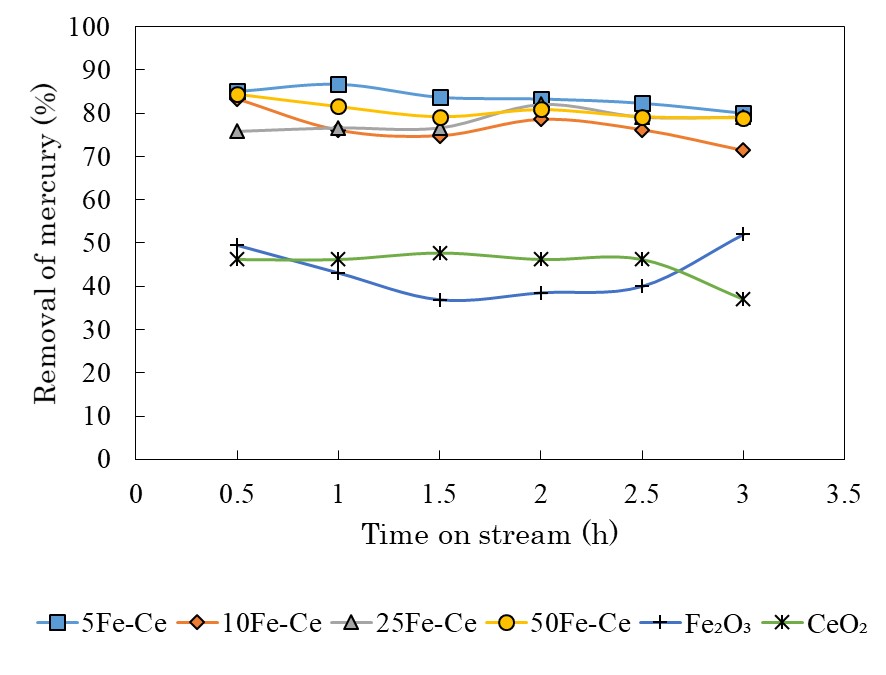
At present, coal is widely used as a fuel for thermal power generation. When coal is used for thermal power generation, there is a problem that mercury contained in coal is emitted to the atmosphere as exhaust gas. The forms of mercury emitted by thermal power generation include mercury oxide, particulate mercury, and elemental mercury. Of the three, the former two can be removed with existing equipment, but elemental mercury is difficult to remove with the current technology due to its high volatility and low water solubility. The aim of this study is development of sorbents suitable for the removal of elemental mercury from coal gasification gas. Fe2O3 and CeO2 were chosen as sorbents because they are inexpensive and stable in the mercury removal reaction. The effects of combination of Fe2O3 and CeO2 at various ratio were investigated.
The simulated coal gasification gas was used for the mercury removal experiment. It was found that the inclusion of Fe2O3 in CeO2 improves the mercury removal performance and also improves the durability in repeated use. After the mercury removal experiment, TPDD experiment was performed to examine the adsorption state of mercury by the sorbents from the desorbed mercury peak. Since the peaks derived from HgS appeared in all the sorbents, it was suggested that the mercury was captured in the form of HgS. When the experiment was performed in the absence of H2S and HCl, the mercury removal rate decreased. Both H2S and HCl are believed to contribute to the mercury removal reaction.
Generally, zinc contained in zinc-plating wastewater is removed as zinc hydroxide precipitate by adjusting the pH from 9.5 to 10.5. Zinc hydroxide sludge generated from this process is discharged about 20000 t/yr in Japan, and the greater part of them are disposed in landfills because of high recycling costs. However, in recent years, industrial waste disposal costs have risen due to the shortage of land fill disposal sites. Therefore, development of volume reduction and effective usage method for zinc sludge is required.
As a solution to this problem, we aimed at the effective usage of zinc sludge by producing Zn-Al LDH from zinc-plating wastewater. Zn-Al LDH can be obtained by adding polyaluminum chloride (PAC) generally used as an inorganic flocculant to zinc-plating wastewater. LDH is well known to have anion exchange property and then can be applied as an anion adsorbent. In fact, Zn-Al LDH also has excellent phosphate ion adsorption capacity. Accordingly, in this study, we investigated whether Zn-Al LDH obtained from could be used as an anion (especially boron and fluorine) adsorbent.
We have previously reported on the adsorption properties of Zn-Al LDH obtained from zinc-plating wastewater. In this poster presentation, we will report on the evaluation of environmental burden of Zn-Al LDH adsorbents produced from zinc-plating wastewater. The conventional methods of boron and fluorine wastewater treatment are two-stage coagulation sedimentation methods, and a lot of sludge is generated in these processes. Therefore, the environmental burden of these methods will be high, so boron and fluorine wastewater treatment with Zn-Al LDH adsorbents produced from zinc-plating wastewater may be superior to the conventional methods. We evaluated the environmental burden in each wastewater treatment method with life cycle assessment (LCA) and compared them to confirm the advantages of the Zn-Al LDH adsorbents.
A core-shell structured Fe-ZSM-5@CeO2 catalyst was originally fabricated via dopamine polymerization for selective catalytic reduction of NOx by NH3. Fe-ZSM-5@CeO2 possess a better catalytic activity with higher N2 selectivity, more extensive operating-temperature window, and better water resistance compared with Fe-ZSM-5/CeO2 via physical mixing. The synergistic effect of Fe-ZSM-5 and CeO2 over Fe-ZSM-5@CeO2 facilitated the formation of redox-cycle between Ce3+/Ce4+ redox-couple and high rate active oxygen species (Oβ and Oγ), leading to over 90% conversation of NOx in the temperature range of 250-400 °C. The feature of hierarchical structure with CeO2 outer shell provides a pathway to convert NO to NO2, facilitating the fast SCR. Meanwhile, the formed NO2 can activate Fe2+/Fe3+ redox-couple, offering an extra redox cycle which can further contribute to fast SCR. The CeO2 shell outside can also improve the water resistance of Fe-ZSM-5, resulting in a higher catalytic stability of Fe-ZSM-5@CeO2. To be concluded, Fe-ZSM-5@CeO2 was proved to be a promising candidate for NH3-SCR as the core-shell structure, uniform distribution of active sites, as well as the synergistic effect of Fe-ZSM-5 and CeO2 species.
Industrial wastewater of electroplating contains various toxic substance such as metals, degreasing agents and oil. Metals such as zinc are harmful to the environment. Therefore, discharge of zinc wastewater in the electroplating industry is regulated. In previous study, it is presumed that plating chemicals, particularly degreasing agents disturb the zinc wastewater treatment. Thus, low environmental impact cleaning technology is required to replace degreasing agents. Recently, fine bubble, which is a bubble of 100 ホシm or less, is attracting attention. Fine bubbles exhibit unique properties, such as surface charge, and cleaning technology utilizing the properties is expected. To comply with zinc effluent regulations, we investigated the effect of fine bubble cleaning on oil deposited on metal surfaces.
The samples for cleaning test were prepared by applying each oil to metal surfaces. The cleaning test was performed for 15 minutes using fine bubble or ultrafine bubble generators with different performance. The surface cleanliness were measured by ultraviolet and visible spectrophotometer.
The results of cleanliness measurement for metal surface show that the oil removal capability by fine bubble depends on oil type, shape of metal, gas type, and the concentrations of micro bubbles classified as bubble size 1 to 100 ホシm. In particular, the concentration of micro bubble and gas type exert a beneficent influence. These results indicate that the shifts of removal capability of fine bubble are attributed to changes in the fine bubble structure. Furthermore, to investigate the interfering effect of fine bubble in zinc wastewater treatment, we conducted experiments using zinc wastewater containing fine bubbles. The results of experiments show that the contamination of fine bubble does not affect zinc wastewater treatment. From these results, we will discuss the cleaning effects of the fine bubble.
In Japan, laws on effluent standard such as Water Pollution Prevention Act and Sewerage Act were established to protect the water environment. Recently, since tougher standard value has been imposed, more conscious from the electroplating industry to the environment are demanded.
For this reason, we are studying on the improvement of wastewater treatment process being applied in the plating factories. According to previous research, it is shown that pretreatment reagent such as degreasing agents inhibited zinc treatment. Therefore, in order to reduce the use of degreasing agents, the fine bubble washing is studied.
However, the effect of fine bubble washing still have many unclear points. And it may not be evaluated by the current method definitely. So in this study, evaluation method for cleanliness of products contaminated by machine oil and washed by fine bubble is examined.
Base oil, cutting oil and hydraulic oil were used as machine oil. An infrared spectrometer, an ultraviolet and visible spectrophotometer, and a gas chromatograph mass spectrometer were used for the evaluation. Metal plates were soaked into each oil and washed by the fine bubble. The residual oil was extracted using the 50 mL of hexane by ultrasonic extraction. Then these extraction liquids and standard liquid were measured and quantified by comparison of peak strength or peak area.
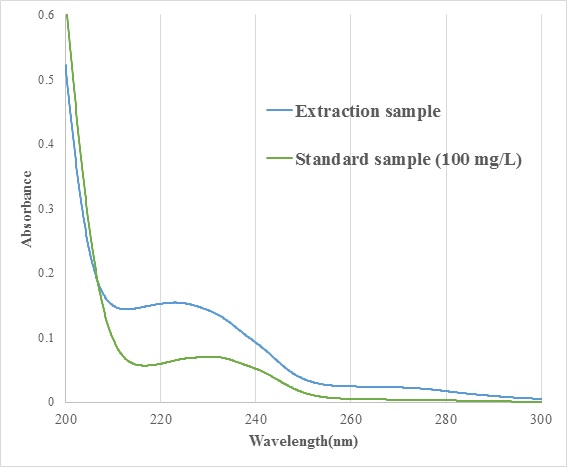
Fig.1 shows UV-vis spectra of the cutting oil. It was suggested that the cutting oil were changed by the fine bubble. Since the cutting oil was mainly composed of fats, it was impossible to quantitate the fats by the UV-vis spectroscopic analysis in this study. On the other hand, the quantifiability for the hydraulic oil was particularly good. Moreover, the deviations of the measurements were less than the measurement by gravimetry. This indicated that some analysis methods were unsuitable for some types of oil.
Soil contamination caused by oil spills is a global problem, and bioaugmentation using oil-degrading bacteria is an effective treatment to clean up the contaminated soil. However, various environmental conditions, such as soil composition, water content, and temperature, influence its efficiency. In the present study, an effective bioaugmentation under low temperature condition was developed with mixture of two Rhodococcus oil-degraders, strain A and C, which are officially permitted to be used in bioaugmentation in Japan. A rotational slurry bioreactor was used with a model slurry prepared with 3.0 × 108 cells/g-slurry of strain A and C as inocula. A-fuel oil was added to a final concentration of 2,500 and 5,000 mg/kg-slurry. The decomposition test was carried out by rotating under 15 °C, the residue of A-fuel oil and the number of bacteria were measured every two days. After 4-day treatment, 90% of degradation was achieved, which was 3-fold faster than previous degradation experiment without rotation (90% of A-fuel oil was degraded after 12 days of incubation 1)). For practical application, we conducted a semi-continuous treatment by removing 90% of treated slurry, then added the same amount of contaminated slurry into the system without additional degraders. Ninety-four % of A-fuel oil was degraded after 6 days by this repeated treatment. Our study provides an efficient and cost-effective removal of A-fuel oil by the semi-continuous system.
[1] Shintani et al., 2019, J. Biosci. Bioeng., 127:197-200
The dust monitor is a device that measures the dust concentration continuously in a flue, a chimney, a duct, etc. It is widely used in thermal power plants, waste incinerators, etc. because it can always grasp the operation status of soot and smoke generating facilities.
In Europe, a certification system has been established by TüV of Germany and MCERT of the United Kingdom, and if formal certification is obtained, it will be legally recognized as a continuous monitoring system (CMSs) of dust concentration from the facilities. In Japan, with regard to the emission of dust from the facilities, emission regulations have been introduced under the Air Pollution Control Act. However, although it is not possible to use a dust monitor for dust concentration measurement at present, the related JIS standards are being developed for introduction.
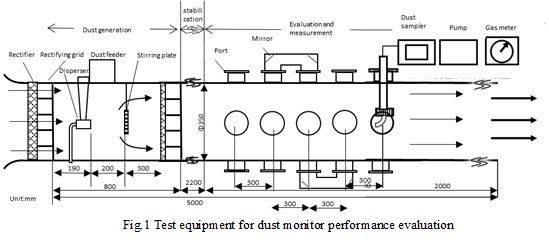
In this work, we developed a flue test equipment aiming at the construction of the performance evaluation system of various dust monitors and the establishment of official legislation. In this maintenance, the diameter of the flue was enlarged, rectification, optimization of dust generation, improvement of the evaluation part, and it became possible to respond to the evaluation of the main method of dust monitor. This device was developed to be able to generate gas containing dust at any dust concentration in a constant velocity flow. Since the measurement unit is provided with a plurality of measurement ports, it is able to evaluate the correlation with the analysis value of JIS Z 8808 for various dust monitors (light scattering type, light transmission type, electrostatic detection type). On the other hand, since the general exhaust gas velocity of large-scale thermal power plants is 10 to 20 m / s, it is necessary to work on increasing the flow velocity of the test equipment.
The adverse environmental impact caused by eutrophication has recently prompted the Philippine government to issue stringent regulatory standards for wastewater effluent quality. The involved stakeholders and industries are assessing the integration of biological nutrient removal (BNR) technologies in the current sewage treatment plant (STP) scenario. Moreover, efforts are being done to utilize wastewater as a resource such us recovery of nutrients as struvite fertilizer from the wastewater sludge. Since BNR and nutrient recovery systems are not yet integrated in STPs, the magnitude of the environmental impacts are yet to be evaluated in the Philippine setting. This study covers the holistic evaluation of the overall environmental performance scores of the following scenarios using a consequential Life Cycle Assessment (LCA) framework integrated with Analytic Hierarchy Process (AHP) in the context of agriculture, food consumption and wastewater: 1) current STP scenario; 2) BNR technology; and 3) nutrient recovery system. The environmental impact assessment was done using IMPACT 2002+ methodology in terms of the following impact indicators: human health, ecosystem quality, climate change, resources, aquatic acidification, and aquatic eutrophication. Value judgments from relevant stakeholders were elicited to rank the relative importance of the impact indicators in the evaluation of the overall environmental performance score. The LCA-AHP results show that the integration of a nutrient recovery system is the most preferred scenario. Sensitivity analysis was also done to evaluate the effects of changes in diet and utilization of alternative energy.
Bauxite residue, commonly known as red mud, is a slurry waste generated in huge quantities in the extraction of alumina from bauxite by the Bayer process. It is highly alkaline and caustic with pH greater than 12. The treatment and management of red mud have posed environmental challenges to the alumina industry worldwide. In this work, a systematic study of the neutralization of RM by carbonation with CO2 over a range of different operating conditions was conducted with the aim of establishing the optimal conditions for the carbonation process. The carbonation was performed at room temperature and atmospheric pressure using a stirred tank reactor operating at different conditions such as total gas flow rate (TF), CO2 gas concentrations, stirring speeds and solids concentrations.
The carbonation process was observed to be significantly dependent on CO2 concentration, TF and stirring speeds, whereas solids concentrations seemed to have a little effect based in the range of concentrations studied. In the carbonation of red mud slurry, it took from 30-75 minutes to lower the pH to 6.6 – 7.5 with CO2 gas concentrations ranging from 10%-100%. In contrast, in the carbonation of the red mud liquor, at stable pH of 6.3 – 7.0 was reached within 15-30 minutes at the same CO2 levels. After carbonation, the pH of carbonated red mud slurry was found to rebound and reached 9.7 after a period of 25 days. The carbonated liquor, however, showed a slower rate of pH recovery, and took a month to equilibrate to the same pH. The amount of CO2 captured was evaluated to be 45.6 gCO2/kg at 30% CO2 concentration, with the alkalinity decreasing from 11,610mg/L to 2,104mg/L as CaCO3. This represents a promising potential of CO2 capture by using bauxite residue.
In an industrial painting, an adherence rate of paint to product is about 20%, so large amount of unpainted paint waste - paint sludge - is discharged. Since disposal of a large amount of sludge is high cost and high environmental impact, an appropriate disposal method is necessary. Paint is a resin and it is combustible, so energy recycling is one of a possible method. However, paint sludge contains incombustible inorganic components as pigments, it may inhibit the energy recycling. Therefore, the influences of inorganic components on energy recycling was examined.
The paint sludge used for this study was obtained from several paint factories. The ash content, the contained elements and the calorific value of the paint sludge were analyzed. Endothermic energy amount in combustion and consumption of energy in transportation were calculated from the analysis results.
The ash, which is the noncombustible fractions was more than 50% for any of the sludge. Main substances of the ash were analyzed by X-ray fluorescence analysis. And they were iron, titanium and calcium. The lower heating value of the sludge was 9900 kJ/kg on average, and the moisture content was 38 % on average. Heat absorption amounts of the ash were calculated based on the contents of it, and it was about 2.6%. It means that the influence of the noncombustible fractions on energy recovery is small. In addition, the effect of the ash on sludge transportation is calculated. Assuming a transportation distance of 50 km, the energy required for transportation of paint sludge is about 4.3% and for transportation of ash is about 3.1% of the energy to be recycled. From these results, it is shown that the influence of the inorganic component is small in the whole recycling system.
A variety of technologies such as ion exchange, membrane filtration, precipitation, and biological degradation, have been developed for the removal of environmental pollutants such as bisphenol A and heavy metals from waste effluents. However, many of those methods have weaknesses such as low efficiency, high maintenance cost, and inflexible process. Some methods have relatively limited application for the effluent including high concentration of pollutants. The peptide, oligomer of amino acids, which is the simplest biological recognition element, has been known to have high selectivity toward target component. By using biopanning protocol, various peptide sequences with high affinity to target components have been found. For example, TNTLSNN exhibiting high selectivity to Pb2+ against other heavy metal ions and KSLENSY having specific affinity to bisphenol A were screened previously. In the present work, the application of those peptides is suggested for the removal of environmental pollutants. The peptide-magnetic bead system is first constructed and tested as a reusable adsorbent to remove metal or bisphenol A from aqueous solution. Compared to the bare bead without the linked peptides, the peptide-linked adsorbent showed higher removal capacity. Since the magnetic bead-based adsorbents are easily separated and reused from aqueous system, the process cost can be lowered.
Traditionally, there are several of chemical and physical methods to remove heavy metal ions from wastewater. However, the traditional method of removing heavy metal ions usually had higher cost and limited effectiveness. Therefore, many studies have used low-cost and reusable biological adsorbent to remove the pollutants in wastewater. The wasabi is a common edible plant. However, when people used wasabi, the part of leaves often was discarded. The main purpose of this study is to use the leaves of wasabi powder for heavy metal chromium, copper, nickel and lead adsorption. The adsorption experiment was to investigate the effect of reaction time, pH, initial concentration, different wasabi powder, the dose of wasabi powder and the particle size of wasabi powder.
According to the results the adsorption capacity of heavy metal ion in the order was : Pb (II) > Cu (II) > Cr (II) > Ni (II). The adsorption data of these metal ions were fitted well to the Pseudo second order rate law. Using NaOH pretreatment the wasabi powder for these metal ions had greater than 90% removal efficiency. The pH value has a significant impact on the adsorption. The adsorption capacity of these metal ions will increase to a certain value with the increase of pH value. Because the higher pH value, metal ions, will be accompanied by precipitation. The adsorption isotherms of these metal ions were all fitted well to the Langmuir isotherm. The adsorption capacity decreased with the dose of wasabi powder increased. The wasabi powder agglomerates, thus the surface area of adsorption decreased. The particle size of wasabi powder have no a significant effect on the adsorption.
The air pollution has become a serious issue in Korea with the quick advancement of urbanization and industrialization. Besides, the concerns about air contamination are increasing more since it influences both human health and economic development. However, there are limits to the temporal and spatial resolution in the air quality forecast, due to the low observation density of the traditional fine dust monitoring network. To solve these problems, the objectives of this research are to construct effective air quality big data based on a high-resolution monitoring system and to develop an improved air quality forecasting methodology using deep learning approach. The mobile sensors which were developed to be attached to the vehicle were applied to construct the big data about air quality. The pollutant considered for this research was Particulate Matter 10 (PM10). The PM10 concentration was monitored at intervals of 1 second by the sensors. Also, its spatial resolution was from tens to hundreds of meters. Recurrent Neural Network (RNN), which is widely used deep learning model, was used for forecasting based on the constructed PM10 time series data. To figure out the trend of data and improve the accuracy of the model, noises of the monitored data with mobile sensors were removed by a filter. The Exponentially Weighted Moving Average Filter (EWMAF) was applied in this research. It is observed from this study that the model performance for predicting PM10 was comparatively well (R2>0.9). Finally, this study may play an important role as a beginning step to improve the air quality monitoring and forecasting system. Furthermore, the suggested methodology in this research can help to develop an air pollutant emission reduction process in response to increased fine dust.