
In some Western Australia's wheat production area, there is concern about the future decrease in yield due to water logging and salt accumulation. The land use change from forest to cropland in centuries age, is thought to be resulted a broken balance between water input by rainfall and output by evaporation and transpiration, due to limited water consumption of wheat.
Theoretically, re-planting of trees on farmland is countermeasures for this problem, however, this method is not economically feasible.
To solve this problem, our group now developing new technology, not afforestation conversion of farmland but afforestation conversion of abandoned agricultural land due to salt accumulation by using salt tolerant tree species. In this research, we focused on the following 4 issues
1: Afforestation demonstration test,
2: Optimal design of afforestation site,
3: Cost analysis,
4: Carbon credit, bio-energy production potential estimation.
In the presentation, outline of the research project will be explained and preliminary results on groundwater level measurements and water usage estimation of planted trees by SAP flow measurements rate will be discussed.
Nanocellulose derived from cellulosic biomass could become an option for replacing conventional materials such as steel and fossil-derived plastics due to its unique physical properties. There are difficulties to disintegrate celluloses in plant cell walls into nanocelluloses because they are tightly hooked each other by multiple hydrogen bonds. Among various nanocellulose production techniques, one of the most adopted techniques to facilitate disintegration is carboxylation of the 6-position hydroxyl group of cellulose to produce electrostatic repulsion between cellulose nanofibrils with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), called TEMPO oxidation. While it is said that the energy required for disintegration is small, it has hardly been assessed how the TEMPO oxidation could become environmentally benign technique to produce nanocellulose throughout its life cycle. Life cycle assessment (LCA) is a strong tool to quantify environmental impacts originating in product life cycle. However, LCA is originally based on process systems composed of existing technologies or services, although the technologies on nanocellulose are now developed and emerging for sustainability. In this study, we are tackling the LCA of emerging technologies for producing nanocellulose. The actual energy consumption in laboratory-scale processes for TEMPO-oxidized nanocellulose (TONC) was measured to assess the environmental impacts induced by the laboratory-scale and predict those through industrialization. The greenhouse gas (GHG) emissions from electricity for mechanical treatment is much larger than that from production of chemicals and energy for TEMPO-oxidation. In the experiment process, the amount of electricity to clean the fibrillation machine is also large, and the machine is cleaned much more carefully than the industrial process. Since the energy consumption of this cleaning process is significant part of the disintegration energy, it was found that the GHG emissions would be less than half if the careful cleaning were not required in continuous production.
The manure produced by intensive livestock farming is known to have significant impacts on the environment. The nitrogen and phosphorus compounds in the pig manure befoul the soil and water body. The microalgae-based piggery wastewater treatment system involved microalgae-based treatment, co-anaerobic digestion of pig manure and microalgal biomass, and biogas cogeneration. The organic residues from the anaerobic treatment can be applied as organic fertilizers to farmland replacing mineral fertilizers. Using anaerobic digestion of pig manure is a promising method of reducing greenhouse-gas (GHG) emissions. Cogeneration by burning biogas can produce the electricity power and usable heat to apply to the system. The environmental impacts of the system using high-rate algal ponds (HRAPs) were evaluated through mass, energy balances and life cycle assessment (LCA). LCA is usually used to evaluate the environmental performance of pig production. The performance of this microalgae-based treatment-scenario has been compared to the conventional application of pig manure (Activated sludge-treatment-scenario).
In recent year, the demand of fertilizers is increasing because of the increase of the food consumption by population growth in developing countries and the Bio-energy consumption. We developed the new recovery process of phosphorus, and the process is consisted of elution and precipitation steps, namely ‘Two-step Elution method'. The heavy metals are characterized by ICP-MS/ICP-AES spectrometer. The final phosphorus recovery rate is about 80%. The amount of metals and heavy metals in final precipitation of this method are much less than the fertilizer standards of Japan. In this study, we have started plant growing experiment by using the final precipitation as fertilizers. The purpose of this study is to investigate the usability of final precipitation as fertilizers.
With this phosphorus fertilizer, the small scale and large scale plant growing experiment was conducted as follows.
1) The plants used in this work are green soybeans and sweet potato.
2) A plant environmental control system (LPH-220N, Nippon Medical & Chemical Instruments Co. LTD, Japan) was used to cultivate plants from the seed.
3) After the cultivation of the plants, we transplanted them and fertilized. Ammonium sulfate and potassium sulfate was fertilized for all plants, but the phosphorus fertilizer was fertilized for commercial one, recovery one and none for all plants.
4) The phosphorus fertilizer was used the two types; phosphorus fertilizer derived from sludge ash and commercial one. Their molar ratio of the fertilizer is N:K:P=1:1:1.
We are also going to carry out more large scale experiment by cooperation of the Faculty of Agriculture.
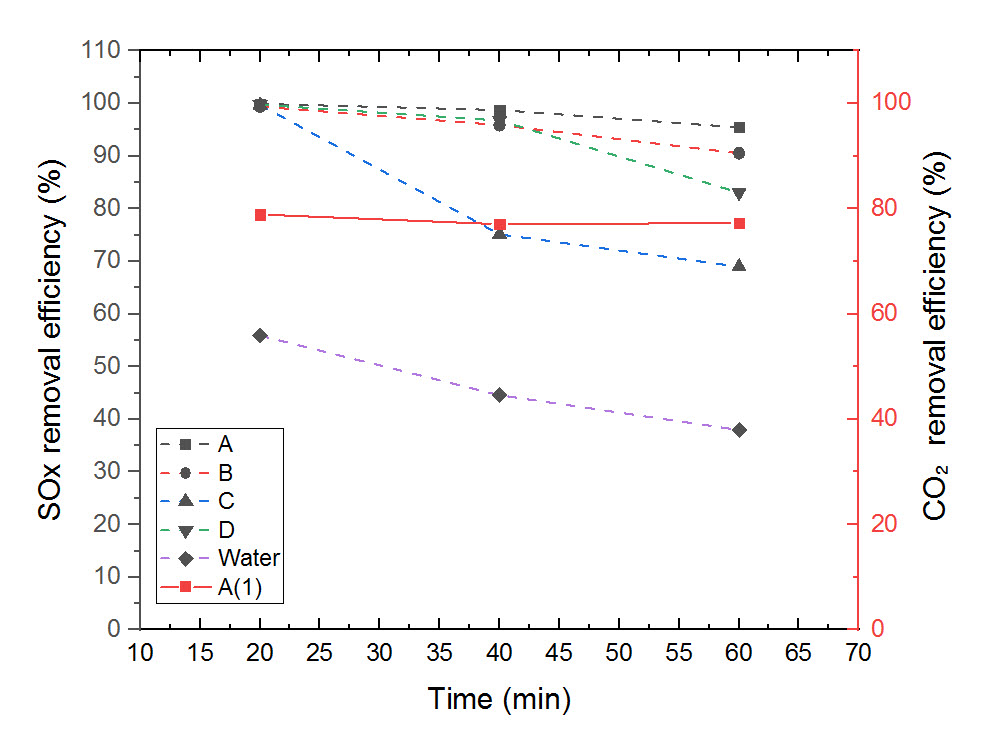
There exist several techniques to treat fossil fuel-combusted flue gas such as wet limestone-gypsum method and amine absorption. Although the techniques have been widely used, their gas-liquid contacting efficiencies are poor which makes the contactors bigger resulting in difficulties in installation. In this study, we proposed a novel gas scrubbing concept, which can effectively remove SO2 and CO2 from simulated flue gas by stages using two membrane contactors in series. The SO2 is captured and directly converted to aqueous ammonium sulphate which could be utilized as fertilizer. The CO2 is captured and recovered using aqueous amine solutions and temperature-swing desorption. Various absorbent solutions such as fresh water, NaOH, NH4OH, H2O2 are tested. Of the tested absorbents, aqueous ammonia solution showed the highest removal efficiencies (i.e. > 99 % for SO2, 80 % for CO2). Optimum condition, removal mechanism, and size reduction of gas-liquid contactor are alsso discussed.
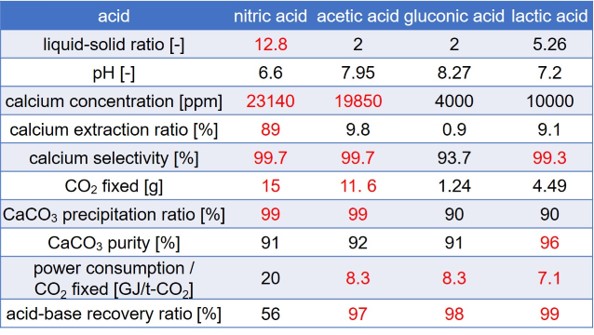
We have proposed a new type of mineral carbonation process, where CO2 is fixed as carbonates of alkaline earth metals. The process is composed of four steps: (1) Absorption of CO2 with an alkaline solution; (2) Extraction of calcium using an acid solution; (3) Precipitation of carbonates by mixing the calcium-leached solution and the alkaline carbonate solution; (4) Regeneration of an alkali-acid pair with the bipolar membrane electrodialysis. In this study, we examined optimum extraction conditions for the total process by using wollastonite as a calcium source and various kinds of acid in the view of the power consumption for the CO2 fixation. The acids used were nitric acid(NA), acetic acid(AA), lactic acid(LA), and gluconic acid(GA). The acid concentration and liquid-solid ratio were changed. The most important criterion of the extraction step is that the pH of the leached solution should be higher than 6.3, which is the acid dissociation constant of H2CO3: otherwise, a part of carbonate ions should be released as CO2 gas in the precipitation step. The table shows the optimum extraction conditions for the highest calcium concentration and the highest calcium extraction ratio of each acid after 24 h. The highest calcium concentration as well as the calcium extraction ratio was obtained when NA was used. In addition, the calcium selectivity in the extraction step was highest at 99.7%. When using AA, the calcium concentration was close to the value with NA, but the calcium extraction ratio was much lower. However, considering the electrodialysis process, the power consumption for the recovery of NA was much higher and the recovery ratio was much lower than those for the cases with other weak acids. The power consumption and the recovery ratio were almost equivalent for these three weak acids; acetic acid should be the optimum acid for the extraction step.
In recent years, the Asian monsoon region has a large business opportunity for the global food market for Japan. However, under severe conditions of high temperature and humidity, it is necessary to understand and control the cultivation environment in order to cause high-temperature injury in the process of crop growth. In this study, the authors evaluated the crop growth conditions and energy of greenhouse tomato production in several Asian monsoon areas. In these evaluations, a cultivation simulator considering growth conditions was used. This simulator is constructed from three processes of leaf photosynthesis, distribution of photosynthetic components, and fruit growth in order to analyze growth conditions in detail. In the calculation of the energy required to maintain the cultivation environment of the greenhouse, the photosynthesis rate of the plants and the transpiration rate were also calculated by simulation. In the evaluation of the growth condition, changes in the temperature of greenhouses at each region of Asia during planting and harvesting were investigated from weather data.
As a result, the difficulty of greenhouse tomato production under high temperature and humidity conditions in Asia and the differences in crop growth conditions among these regions could be clarified by this analysis.
It was suggested that the fruit yield was correlated with the seed number and days of the fruit development stage, and strongly affected by the temperature of the fruit-set stage.
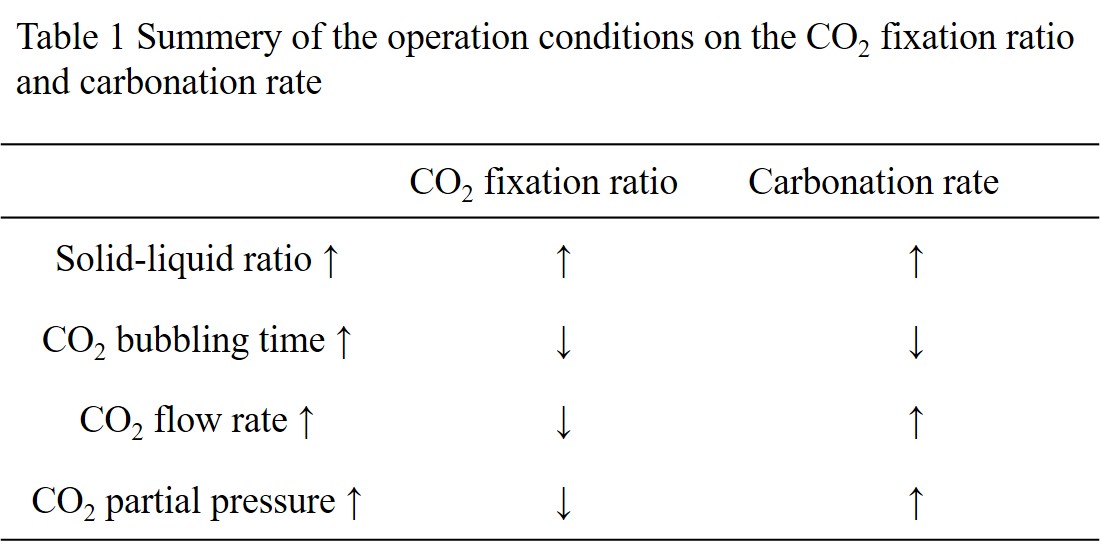
Mineral carbonation is a fixation method of CO2 by using alkaline earth metals to form carbonates. We focused on concrete sludge which is a waste fresh concrete, as an alkaline earth metal source for mineral carbonation. In this study, we examined the carbonation reactions of a model concrete sludge by CO2 bubbling under various operation conditions, and investigated the effects of these operation conditions on the CO2 fixation performances.
A model concrete sludge was prepared by mixing a commercial Portland cement with water for 60 minutes. After hydration, CO2 was bubbled through the model concrete sludge to start the carbonation reaction. The dissolved calcium concentration was measured with ICP-AES and the pH was measured with a pH meter. After carbonation, the model concrete sludge was filtered and the solid residue was dried in an oven at 100 °C for 24 h. The solid residue was then measured by a thermogravimetric method to determine the conversion of CO2 to calcium carbonate.
The calcium concentration decreased by CO2 bubbling, and reached 250 ppm after 80 min and almost unchanged up to 200 min, which is close to the equilibrium concentration. The calcium concentration increased after 200 min to be leveled off at about 600 ppm after 300 min. The pH of the solution decreased by CO2 bubbling, and almost unchanged at 6.5 after 250 min, which is close to the equilibrium value. The effects of the operation conditions are summarized in Table 1; the carbonation rate is defined as the amount of calcium carbonate divided by the bubbling time, and the CO2 fixation ratio is defined as the mole fraction of CO2 fixed as carbonate to that the total amount bubbled through. From these results, the carbonation process can be designed.
The behavioral studies of fission product aerosols are extremely important in the context of probable environmental hazard in the event of a severe nuclear reactor accident. Fission Product aerosols are generated in such a case are expected to travel from the primary heat transport system to the containment and may get released to the environment. Presence of steam also affects their dynamic behavior and fate. Interaction of aerosol particles with water vapor in the supersaturation domain affects their physical as well as chemical characteristics. Radioactive compounds of cesium (CsI and CsOH) forms a major part of fission product aerosols, which may be released into the air in huge amounts by nuclear power plants during nuclear accidents and also at the time of nuclear weapons testing. Severe nuclear reactor accidents that involve considerable core melting generate large source terms of fission product aerosols to the environment. Cesium, with a half-life of 30 years is highly reactive and combines readily with other elements, especially oxygen, other gases, and nonmetals. Cesium is treated as a hazardous material because it reacts violently with water. Cesium also reacts violently with sulfur, phosphorous, acids, and halogens (fluorine, chlorine, bromine, iodine, and astatine). The lifetime, along with its reactive nature makes it a concern for human health. Radioactive isotopes of cesium are produced in nuclear power plants by the fission of uranium in fuel rods and also by the explosion of nuclear weapons. In this study, the CCN properties at different supersaturations for different size of laboratory generated single salt CsI (Cesium Iodide) and CsOH (cesium hydroxide) aerosols were investigated. CCN spectra of CsI and CsOH particles were measured at room temperature (298K) with the help of DMT CCN counter. A comparison of experimental data with theoretical values is also done for both the salts.
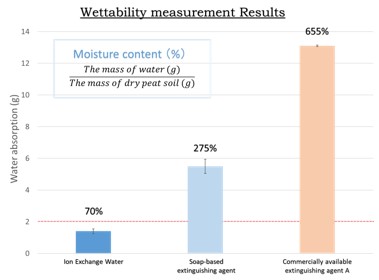
Peat fire in Indonesia not only releases a large amount of carbon into the atmosphere, but also causes significant damage to peatland ecology and the landscape. Smoldering is the dominant combustion process in peat fire, and the combustion is the slow, low-temperature, flameless burning of porous fuels, and the most persistent type of combustion phenomena. To suppress the peat fire, it should be very important to increase the water content of dry peat and deliver water toward the combustion zone to remove heat efficiently. We have been developing a novel soap-based firefighting agent for peat fire with significantly lower environmental risk. Soaps possess very high surface activity, very high biodegradability and very low toxicity. In this study, it was evaluated by focusing on permeability and wettability in peat soil as the physical properties of the soap-based firefighting agent. The peat moss in Russia was used as the peat model for permeability and wettability test. Permeability and wettability in peat moss of the synthetic-surfactant-based firefighting agent and soap-based firefighting agents was much better than ion-exchanged water. It was clear that the firefighting agent solution is very effective for firefighting peat fire compared to water alone. Among the soap components, the potassium myristate showed the best results in both permeability and wettability test.
Firefighting agents including fluorochemical surfactant for oil is widely used in Japan.
However, fluorochemical surfactants have much low biodegradable, and
perfluorooctane sulfonic acid was prohibited by the law in 2011. Regulations will be
tightened in the future, therefore, the fluorochemical surfactant-free firefighting agents
are eagerly anticipated. We have been developing a fluorochemical surfactant-free
firefighting agent with very low biotoxicity (LC50 ≥ 100 ppm) for oil fires. In this study,
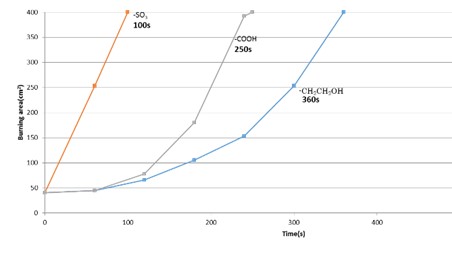
three surfactants containing different types of functional groups (-SO3,-CH2CH2OH,-
COOH) were used. After preparing firefighting agents, it was diluted to the required
concentration. Japanese-killifish, Himedaka, was exposed to the firefighting agent
solution, and the survival number after 96 h was measured. Firefighting tests were
conducted according to the scale of the national verification standard. The surfactant
containing -CH2CH2OH functional group showed the best firefighting performance among the three types of surfactant.
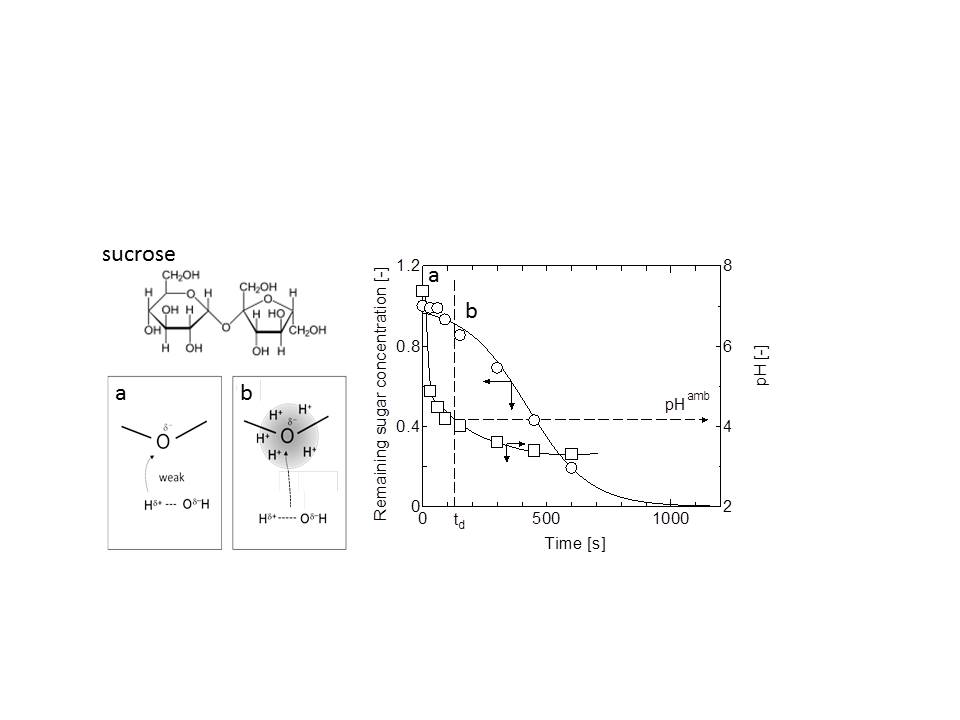
The hydrloysis of disaccharides is an important issue for the design of the conversion of cellulose biomass. In this study, the decomposition of disaccharide, sucrose, under the hydrothermal conditions (160~190 oC at 10 MPa). was examined in the microcapillary, as shown in the Figure (right) [1].
The kinetic model taking into consideration of induced period (td) was adopted to analyse the hydrolysis process of disaccharides The present analysis could give the pH value to start the hydrolysis of disaccharides (pHamb) as shown in Figure. Estimated the activation energy, the hydrolysis of disaccharides under the hydrothermal conditions were conducted in an ionic reaction manner. Therefore, possible parameters relating to the ionic reaction manner is (i) hydration structure of disaccharides and (ii) electron charge of oxygen in glycosidic bond. The hydration structure was discussed with the dielectric measurement. It was considered that the sucrose, turanose, meribiose were well hydrated relative to other disaccharides (see Figure a and b of left). Also, the electron environment at oxygen atom in glycosidic bond related to the hydrolysis of disaccharides (Q value) was calculated using a MOPAC. Then, pHamb value was roughly correlated to the Q value. Therefore, the method only requires knowledge of the electron charge of oxygen atom in glycosidic bond of disaccharides at the calculation to predict the pHamb of the target disaccharide.
To predict pHamb would lead to avoid the use of unnecessary quantity of acids to adjust the initial pH condition, which is the significance in environmental impact. Besides, it was a use of microcapillary system that could monitor the time-course of pH to obtain the pHamb value for hydrothermal hydrolysis of disaccharides. This would be just a significance of the microcapillary in this study.
Reference
[1] T. Shimanouchi et al., J. Chem., Article ID 3985915 (2019)
Over the past decades, the use of carbon fiber reinforced plastics (CFRPs) has been steadily increasing across a wide range of sporting and industrial applications. This growing production and use of CFRPs in applications today will inevitably lead to larger volumes of CFRP waste in the future. Since fiber reinforced composites are difficult to separate into their elemental components, composite waste is principally disposed in landfills or incinerated without any recycling approaches. Disposing a large amount of CFRP waste on reclaimed land is not feasible for small countries; moreover, burning expensive long carbon fibers also causes environment pollution. Use of biodegradable plastic is an effective way to solve the disposal problem of large CFRPs. APEXA© is a biodegradable polyester consisting of terephthalic acid (TPA), ethylene glycol (EG), and an organic acid. Its heat resistance and durability are far higher than those of other biodegradable polyesters and are comparable to those of polyethylene terephthalate (PET). Although the decomposition of APEXA© in compost has been studied, little is known about its degradation in an aqueous environment. In this study, APEXA© fiber was hydrolyzed at pH 3.0–10.5 for up to 4 weeks. The molecular weight of the APEXA© fiber decreased remarkably in dilute sulfuric acid (pH 3.0); specifically, it decreased to 22.9% of the original molecular weight after 1 week. The sharp decline in the molecular weight of APEXA© suggests at composite recycling combined with mild hydrolysis and biodegradation. The APEXA© composite enables to collect maintained long-carbon-fiber in hydrolysis and return the separated resin to the earth.
Background
Polyurethane (PU) was originally synthetized by Otto Bayer in 1937 in Germany. This material is very stable on a lot of different applications, such as, transportation, packaging, footwear, paint, and insulation component for refrigerators and buildings. In 2016, approximately 18 million tons of PU will be produced and 3/4 of them were in foam format. Asian countries used most of them.
Methods
A new study on polyurethane degradation, catalyzed by the different catalyst was reported. NaOH, KOH, sodium acetate and an alkaline metal oxide catalyst were used under the presence of ethyl glycol at the designated reaction condition was proposed. The resulting glycolysate was analyzed to determine the physical and chemical properties. The will be used as the source to reproduce polyurethane for recycling purpose.
Results
With the addition of sodium hydroxide and sodium acetate as the catalyst in the glycolysis, more wasted PU can be dissolved than that without the presence of the catalyst. The reaction time to reach the equilibrium condition is almost the same. More waste PU can be dissolved into the glycolysate with a shorter reaction time if the reaction was catalyzed by the proposed heterogeneous catalyst. The spent catalyst can be removed from the mother glycolysate solution easily upon the completion of the reaction. The glycolysate of the by different catalysts were also re-synthesized to form the recycled polyurethane. The thermalgrametric analysis of the recycled polyurethane from the heterogeneous catalyst showed very similar to the original waste PU. The results showed a potential route to reproduced the polyurethane from the recycled method. The PU foam as also reproduced in this reaction process.
Financial support: Ministry of Science and Technology in Taiwan, ROC (MOST 107-2218-E-035-017 – and MOST 106-2221-E-035-079 -).
I. Introduction
Recycling catalyst is needed for biomass conversion (e.g., producing bio-oil) in order to reduce cost and protect the environment. However, most catalysts used for producing bio-oil from the pyrolysis of biomass cannot be recyclable use, leading to catalyst pollution and waste. Based on previous research results (shown at this conference) it was shown that inexpensive Ni, Fe catalyst clusters can improve the quantity or quality of bio-oil produced from the biomass, but to our knowledge, very few studies have actually investigated the reusability of catalyst, especially from experiments when mixing cellulose (biomass model compound) and catalyst directly together. In this study, Ni2Fe3 catalyst was mixed with cellulose in a pyrolysis batch reactor to investigate its reusability and its impact on the bio-oil yield and content.
Experiment
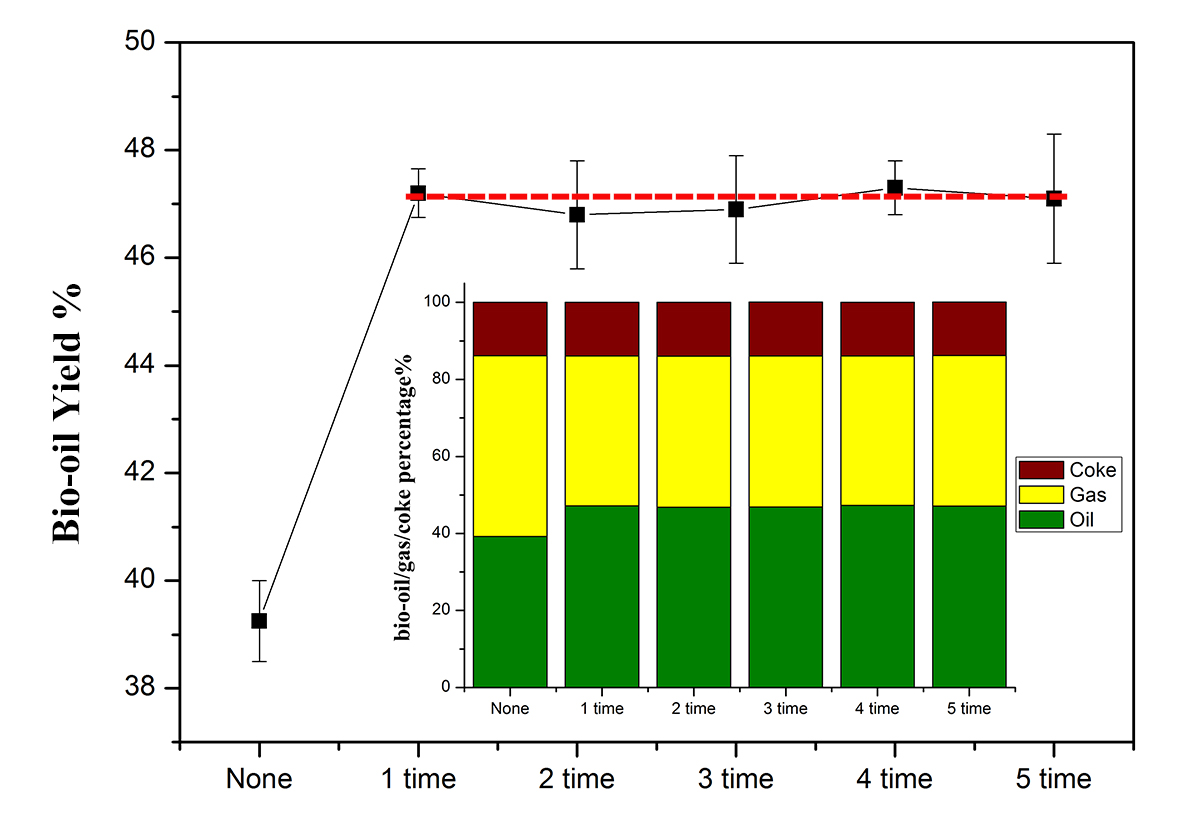
The catalyst was prepared by sol-gel method and heat-treated in flowing H2/N2 gas. Pyrolysis experiments were conducted using a fixed bed reactor, flowing 100ml/min N2 gas, at 450 °C with the catalyst repeating five times. The amount of pyrolyzed bio-oil and coke were weighed, and then the amount of gas was calculated by subtracting the amount of bio-oil and coke from the initial feed.
II. Results & Discussion
Figure 1 shows the bio-oil/gas/coke mass yields from each pyrolysis experiment, and the bio-oil yield is plotted versus a number of experiments. The Ni2Fe3 metal catalyst can increase the bio-oil yield from 39.5% to 47.5%. Moreover, the activity of the catalyst is relatively constant based upon bio-oil yield. Furthermore, the gas/coke yield also shows a similar trend. What can we say here is that the NiFe cluster metal catalyst is recyclable for bio-oil production by pyrolysis. Research work is continuing on the evaluation of the sampled bio-oil content and understands why the catalyst can be used repeatedly.
In this study, montmorillonite-supported nanoscaled zero-valent iron (Mt-nZVI) composites were fabricated using a facile liquid-phase reduction method. The morphology, crystal structure, functional groups, and magnetic properties of as-prepared composites were explored using scanning and transmission electron microscope, X-ray diffractometer, Fourier-transform infrared spectroscope, X-ray photoelectron spectroscope, zeta potential analyzer, and superconducting quantum interference device. The ZVI nanoparticles (particle size, 60-100 nm) were well dispersed on Mt support (particle size, 25 μm), which effectively decreased the tendency of agglomeration behavior. The optimal weight ratio of Mt particles to ZVI nanoparticles was equal to 2, which was denoted to be 2Mt-nZVI composite. The synthesized 2Mt-nZVI was then used to eliminate oxytetracycline antibiotic with a variety of operating parameters such as Mt particle size, weight ratio of Mt to ZVI, solution pH, and initial oxytetracycline concentration. Experimental results showed that 99% of 100-mg/L oxytetracycline at pH 5.0 could be removed by 0.6 g/L of 2Mt-nZVI composite and the mineralization could reach 70% after 20 min of reaction. Moreover, six possible intermediates were detected and identified by an ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTof/MS, Waters) during the degradation of oxytetracycline over 2Mt-nZVI composite. This study demonstrated that the prepared Mt-nZVI composites possessed a promising potential for removing and mineralizing oxytetracycline from polluted water.
Photocatalysts represented by titanium oxide are important material in utilization of light energy. In recent year, a new use of silver orthophosphate (Ag3PO4) semiconductor as an active photocatalyst has reported which can harness visible light to oxidize water as well as decompose organic contaminants in aqueous solution. Previously, silver orthophosphate fine particles were mainly synthesized by the liquid phase reaction, which required multi-step operation. Furthermore, the only report of silver phosphate synthesis by solid-solid reaction has also proposed time-consuming methods. There are several important technologies to realize the practical use of materials, one of which is method to rapid and facile synthesis. In this study, we developed a rapid synthesis method for silver orthophosphate using a vibration mill. Furthermore, the obtained silver orthophosphate was characterized in different ways. Silver orthophosphate was easily obtained by preparing a tube containing silver nitrate, one of several phosphates (Na3PO4, Na2HPO4, NaH2PO4) and zirconia beads, followed by vibrating with a vibration mill. The unreacted raw materials were removed by washing to obtain a yellow powder. The powder X-ray diffraction pattern of the sample obtained is in accordance with that of the reported silver orthophosphate. Furthermore, the results of energy dispersive X-ray analysis show the consistent values with the stoichiometric ratio of silver orthophosphate. The yield of silver orthophosphate was 48% when disodium hydrogenphosphate was used as the phosphate source. Furthermore, the yield depends on the shaking time, but it saturates in only 10 seconds at a shaking speed of 4000 rpm. The yield was also dependent on the shaking rate with constant shaking time. The photocatalytic activity of obtained silver orthophosphate was evaluated by degradation of methylene blue in suspension. The supernatant of the suspension was discolored by the irradiation of blue LED light, indicating the photocatalytic activity of the obtained sample.
A simple and efficient method was developed to remove dyes from wastewaters. The method was conducted by adding alcohols followed by feeding air bubbles. In this study, cationic dyes (rhodamine B (RB), crystal violet (CV), malachite green (MG)) and anionic dye (erythrosine B (EB)) were selected because of their extensive use in staining and textiles dyeing processing. Removal of these dyes increased significantly by adding small amount of alcohols (0.01% ~ 0.5% v/v). The effect of alcohol (ethanol < 2-propanol < 1-butanol) increased with increasing in its carbon number. Cationic dyes (RB, CV, MG) were removed in the range of pH 3 to 10, while EB was removed in the range of pH 1 to 3. Effects of instrumental parameters including vessel diameter and water height were also studied. Under the optimum conditions, nearly complete (> 98%) removal of these dyes was achieved by the flotation within 5 min. Influences of co-existing organic (humic acid, starch, etc.) and inorganic substances (NaCl, KCl, etc) omnipresent in wastewaters were investigated in detail. Practical applicability was examined by using synthesized dye wastewaters.
The water treatment using photocatalytic titania nanoparticles for removing organic contaminations is one of the advanced water purification systems, but the immobilization method for nanoparticles should be developed. For overcoming this problem, the degradation of methylene blue (MB) in aqueous solution by a titania particle film was studied using the photocatalytic system. The electrophoretic deposition technique applied to preparing titania thin films on stainless steel screens out of titania particle suspension. After annealing in air at 773 K for 10 minutes, the crack-free, porous titania particle film coated on the screen was dipped in 50 mL MB aqueous solution to carry out the MB degradation test, in which ultraviolet light (254-nm wavelength) was illuminated to the surface of the titania film while the MB solution was passed through the meshes of the screen. The rate of MB degradation was estimated from the variation of MB absorbance measured with a spectrophotometer. The degradation rate was found to increase with the thickness of titania particle film and to reach an asymptotic maximum range, even for different meshes of the screen. This trend indicates that a certain thickness of the titania porous film was required for the photocatalytic reaction with the surface area of the coated screen being a dominant parameter for the MB degradation rate. As the circulation rate of the MB solution was increased, its degradation rate increased; this could be explained as that increasing flow rate resulted in rendering the diffusion boundary layer thinner with less resistance to MB's contacting to the surface of titania particles.
A photo-assisted Ce(IV) mediated electrochemical oxidation (MEO) system is developed to treat the phenol-containing water in comparison to Ce(IV) MEO and photocatalytic oxidation systems. Operation conditions of electrical current, nitric acid concentration, stirring speed of magnetic stirrer, and electrode area ratio of anode to cathode for Ce(IV) electrogeneration are optimized in terms of its production yield and energy consumption. According to a kinetic model incorporating the Ce(III)/Ce(IV) redox reaction, the forward rate constant of Ce(III) oxidation is found to be twice as large as that for the reverse reaction. Regarding the wastwwater treatment, Ce(IV) MEO and photocatalytic oxidation systems both have favorable capability in degrading phenol under an acidic environment rather than the DEO system. More importantly, the photo-assisted MEO system offers excellent removal efficiency of phenol up to 98% in only 40 mins, benefiting from the synergistic effect of the individual electrocatalytic and photocatalytic systems. Moreover, a pseudo-first order kinetic model is applied to well describe the phenol degradation in the photoelectrocatalytic oxidation system, resulting in a rate constant of 0.083 min-1.
Sulfonamides such as Sulfamethazine (SMT) are widely used as antibiotic in animal husbandry, fisheries aquaculture, fruit and vegetable in Taiwan. Although antibiotic has positive effects, once they are release will cause high risk to ecology or human body. In the research it has been pointed out that such bacteriostatic agents may cause allergies, pneumonia, and enhance the symptoms of asthma. The Ministry of Health in Taiwan has been clearly legislation about Sulfamethazine (SMT) residue.
This present study is to investigate the removal of SMT in aqueous solutions by electrochemical activation of sodium persulfate. In the part of SMT removal by the electrochemical activation system, various parameters such as electrode pair, current densities, electrolyte concentrations, oxidant concentrations, temperatures and initial pH were evaluated to characterize the removal of SMT efficiency. The effect of the current density on the special energy consumption were also investigated in this study, The optimum electrode pair, current densities, electrolyte concentrations, oxidant concentrations, temperatures and initial pH were found to be Fe/Fe, 16 mA/cm2, 4 mM, 0.6 mM, 298 K, pH 3, respectively.
Advanced oxidation processes(AOPs) are used as oxidation methods with highly reactive species such as hydroxyl radicals for the removal of organic pollutants having high chemical stability or low biodegradability in wastewater. However, the AOPs are not cost-effective and they need to combine with other treatment processes such as physicochemical or biological processes in order to achieve maximum economy efficiency. AOPs can be used as a pre-treatment or post-treatment method of biological process including activated sludge. In the pre-treatment AOPs will improve biodegradability of pollutants and in the post-treatment AOPs will remove the pollutants that cannot be completely degraded by biological systems. In the combination of AOPs as a pre-treatment with activated sludge treatment the chemical oxidation is specifically aimed to partially degrade the pollutants to more biodegradable components and the degree of partial degradation of pollutants will be important for the optimization of overall process efficiency.
In this study, ethylene glycol(EG) and Fenton reaction were employed as model synthetic organic component and AOP, respectively. The effects of operating conditions on biodegradability of EG by Fenton reaction were studied. The biodegradability rate, which is determined with the TOC before and after activated sludge treatment, increased from 3% to 39% by Fenton reaction for 2 min. From HPLC analysis EG was degraded into formic acid and methanol by this degradation reaction. The same analysis were conducted for glucose and poly ethylene glycol(PEG). The biodegradability rate of PEG increased from 0% to 13% by the Fenton reaction, while that of glucose decreased from 82% to 68%. From these observations showed that AOP as a pre-treatment of activated sludge process is useful for the treatment of poor biodegradable organic components.
The depletion of phosphate rocks has become problem, because the demand of fertilizer is increasing according to rapid population growth in developing country and an increase of biomass energy demands for CO2 problem. Sewage sludge ash is getting attention for recycling phosphorus resource because of containing phosphorus equivalent to low quality phosphate rocks. phosphorus recovering techniques from the ash have been studied. After using the ash as producing fertilizer, sewage sludge ash residue always remain and it is disposed even though there is a lack of landfill capacity. In this study, we propose a method of recycling sewage sludge ash residue as a marine fertilizer and evaluate the effects. In Japan, some of the coastal areas and bays currently suffer from a lack of phosphorus. Phosphorus could be supplied by sewage sludge ash residues with some treatments. Sewage sludge ash residue includes silicon and iron which are also important for ocean ecosystem to grow apart from phosphorus. For the control of solving rate of phosphorus, we add some carbon, calcium and sodium, and do thermal treatment. Since dissolution rate of heavy metals is much lower than the environment standards of Japan, we could control of solving rate of phosphorus supplied to the coastal areas and bays from 3 days to 3 years.
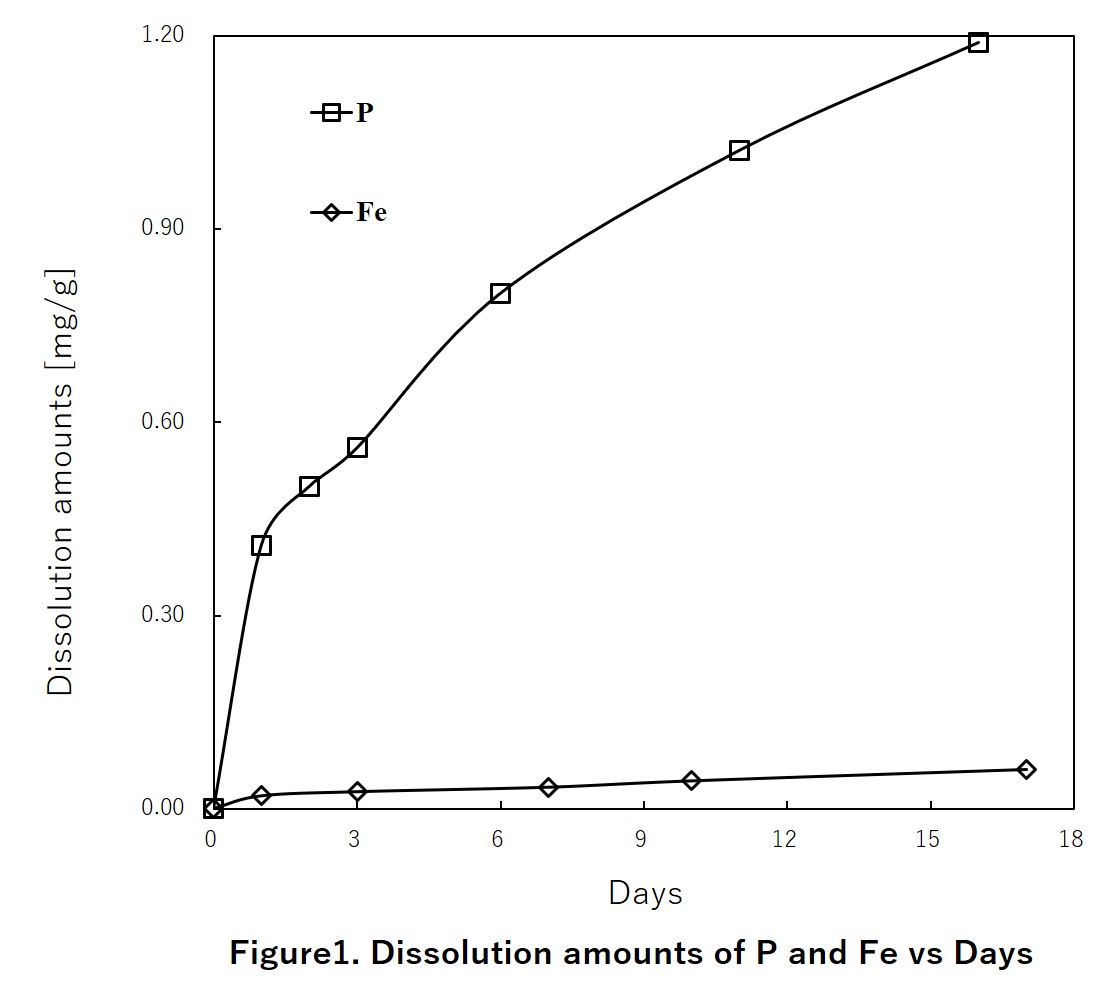
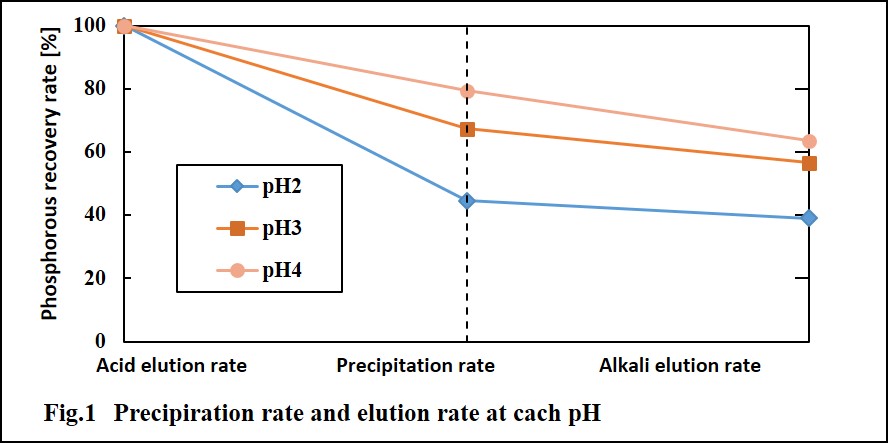
Phosphorous is main component of fertilize. Recently phosphate rock is one of the worldwide depleting resources, because demand of fertilize is increasing with increasing population and bioenergy. Furthermore we don't have phosphorous recourse in Japan. Fortunately, Sewage sludge incineration ash contains about 15 to 30% of phosphorous (P2O5 equivalent), and it is equivalent to low grade phosphorous rock. And the amount of phosphorous contained in sludge incineration ash generated from all over the country is equivalent to it which is imported from overseas as phosphate. Now the phosphorous recovery process from sewage sludge ash is already researched, so we can secure the phosphorous that are not concern for depletion. However, still much sewage sludge ash is landfilled because recovery rate of phosphorous in previous developed process is very low. So we have developed the method called two-step phosphorous recovering system, which is combined acid elution and alkali elution. In this system, recovery rate from sewage sludge ash is almost 80%.
In this study, for more improving the recovery rate, we try to add the additive and control pH and discuss about the phosphorous recovery rate depending on additive amount and pH. Also, we discuss that alkali elution rate is changed by the structure of precipitation.
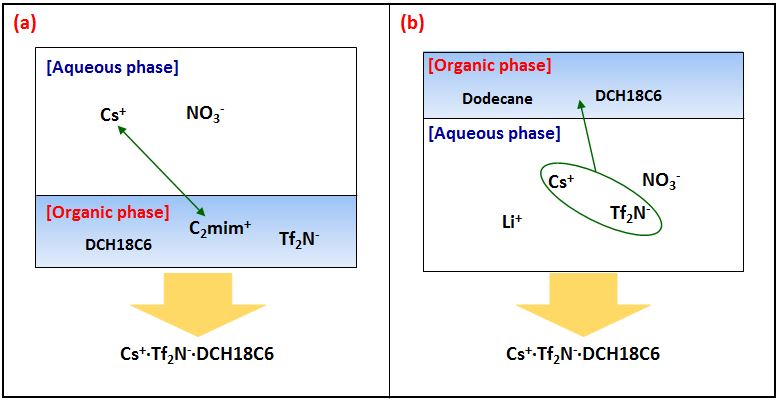
So far, much research has been done on the Cs+ extraction system using the 1-ethly-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (C2mimTf2N) as Ionic liquids (ILs) and dicyclohexano-18-crown-6 (DCH18C6) as a Cs+ selective extractant. In our laboratory, we found that when the amount of ILs was reduced, solid precipitate was formed, and it was confirmed through various analyzes that Cs+•Tf2N-•DCH18C6 was consisted. Formation of solid precipitate is useful for separation after waste treatment. In addition, despite a small amount of ILs, the Cs+ removal efficiency was about 90% when DCH18C6 was used in the same molar quantity as Cs+. Based on this, the study was conducted using LiTf2N as instead of expensive ILs under dodecane solvent (Figure 1). When the dodecane was reduced, solid precipitate was also formed, and confirmed it consists of Cs+•Tf2N-•DCH18C6 by analyzing scanning electron microscopy (SEM), Energy Dispersive Spectrometer (EDS) and Fourier transform infrared spectroscopy (FT-IR). Also, it was shown the high Cs+ removal efficiency of 90% in dodecane system.
In conclusion, the new facile method using dodecane and common anion salt (LiTf2N) can reduce the amount of waste, is easy to separate, and has a high Cs+ removal efficiency, so that it can be used for the treatment of radioactive liquid waste. Moreover, although dodecane is a volatile substance, it can be economically advantageous because it can eliminate danger by using Solid-liquid separation using a small amount and replace expensive ILs.
The removal ability of dibenzothiophene (DBT) in hydrocarbon liquid model fuel (MF) of Ni-loaded carbon (Ni/C) prepared from lignite, which is inexpensive and abundant with an ion-exchangeable capability, was investigated with a flow-type fixed-bed reactor. The Ni/C precursor (Ni/C_P) could only be prepared by adding lignite to a Ni2+ solution by impregnation method. Ni particles in the Ni/C_P existed as an amorphous form. Ni particles in Ni/C prepared by pyrolysis of Ni/C_P were well-dispersed into carbonaceous materials. The removal performance of DBT by Ni/C depended on the loaded Ni amount. The highest performance was observed at 11wt%-Ni content in Ni/C under a desulfurization temperature of 200 °C and H2<.sub> reduction time of 0.5h for Ni/C before desulfurization test. The H2 reduction time of Ni/C influenced the breakthrough curves, and it was found that the optimum time for DBT removal by Ni/C was 1.0h. When the treatment temperatures for desulfurization were varied at 25-200oC, the breakthrough and saturation points increased with increasing temperature, and the greatest performance was observed at 200 °C. As the initial S concentration in the MF was varied, the breakthrough curves depended on the concentration, and it was found that Ni/C could decrease the S amount in the MF to below 0.1ppmw. From the results of the identification of MF treated by Ni/C, it was suggested that adsorption desulfurization occurred due to the cleavage of the C-S bond in the DBT molecule to form an Ni-S bond and biphenyl. In addition, from the quantitative result of biphenyl in MF treated by Ni/C and carbonaceous material prepared by removal of Ni in Ni/C, it was suggested that competitive adsorption of biphenyl and DBT on the adsorption site on carbonaceous material in Ni/C prepared occurs in this study.
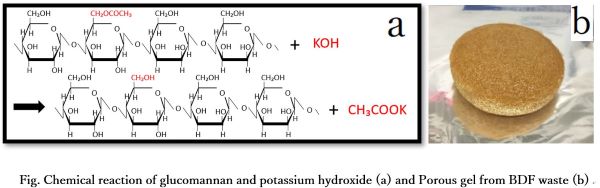
Bio diesel fuel (BDF) has an advantage of generating small amount of SOx during combustion. However, BDF production process discharges strong alkaline waste liquid including potassium hydroxide, methanol, fatty acids, and glycerin. Since this waste liquid is highly toxic and harmful to the environment, it is basically incinerated. The high waste disposal cost is one of the issues, and it is required to develop of the effective utilization method of BDF waste liquid. Its environmental hazard is mainly derived from the strong alkalinity of potassium hydroxide; however, potassium itself is an important element in plant growth. If the potassium hydroxide in BDF waste liquid is neutralized and solidified, BDF waste liquid can have a possibility to utilized as a potassium sources fertilizer for plants. In this study, we produced gel from BDF waste liquid by adding glucomannan. Glucomannan solidifies the waste liquid while convert potassium hydroxide to potassium acetate (Fig. a). The glucomannan was added to the diluted waste liquid, and a gel was formed (Fig. b). In order to evaluate the gel as potassium sources fertilizer, neutralization of potassium hydroxide with glucomannan and emission of potassium from the gel were confirmed by measuring the pH and potassium concentration in the solution. From the experimental results, it was suggested that BDF waste liquid has potential to use to agricultural fertilizer to supply potassium.
Acid mine drainage (AMD) containing toxic elements continues to be generated at about 100 abandoned mines throughout Japan, and It is urgent to be treated in the future.
There are various methods for AMD treatment, and it is necessary to select the best method for each mine. Passive treatment using constructed wetlands is attracting attention for some mines with low volume of wastewater and low concentration. Although the removal ability of the constructed wetland has been confirmed, quantitative modeling of its removal mechanism and removal capacity and its application to other mines have not been carried out. In this research, we model the contaminant removal process in the subsurface flow (SSF) of constructed wetlands in the H mine using geochemical modeling. We also evaluate the applicability of the model to other mines. As a result of field survey, it is suggested that δ-MnO2 is generated by microorganisms in the SSF. The experiment result confirms that cadmium (Cd) is removed by δ-MnO2. Therefore, we carried out removal experiments under several conditions to construct a surface complexation model (SCM) of δ-MnO2 for Cd. By adding the SCM and some reaction constructed in this study to the previous chemical reaction model and performing one-dimensional advection analysis, the reaction behavior in the wetland could be well reproduced. The result suggests that the toxic elements are removed by adsorption to iron, aluminum, manganese (hydro-) oxide or precipitation in the SSF. In addition, by applying this model to other mines, we calculated the area of wetland necessary for treatment. We compared the conventional treatment with the constructed wetland and examined the applicability of the constructed wetland from the aspect of economics and environmental impact.
Heavy metals are highly toxic substances contained in industrial wastewater or mining waste water. Among heavy metals, Pb(II) ions are difficult to remove under alkaline conditions. Generally, Pb(II) ions in aqueous solutions could be removed by pH adjustment, but could not be removed in some cases, such as leaching solution from incineration ash. In plating industry, if we remove Pb(II) ions at alkaline conditions, we can re-use the solution.
Generally, activated carbon is used for heavy metal removal, however it is expensive. Dolomite is one of the minerals, widely distributed all over the world, and very cheap comparing with activated carbon. Calcined dolomite has high adsorption capability to heavy metals. We found that As(III) ion adsorption capability at alkaline conditions is improved by phosphorus-doped in previous studies. Pb(II) ions are absorbed by phosphorus doped calcined dolomite adsorbent at alkaline conditions.
In this study, we investigate the effect of doped phosphorus amount, pH, and concentration of Pb(II) on adsorption. The structure of phosphorus doped dolomite and Pb(II) adsorbed dolomite with phosphorus are analyzed by XRD.
This experiment was conducted by changing parameters such as pH, concentration, temperature, doped phosphorus amount, and time. The result shows that above pH 11, the amount of Pb(II) ions by the phosphorus doped adsorbent increase than that of phosphorus non-doped dolomite. Adsorption amount is proportion to doped phosphorus amount.
The XRD analysis result show that phosphorus doped dolomite is Hydroxyapatite (HAp). This adsorption is both physical and chemical adsorption, and could be fitted to the Freundlich adsorption isotherm.
The present study shows that the doped phosphorus at high pH solution improves the Pb(II) ions adsorption capability of calcined dolomite.
Phosphorus (P) is essential for life, and phosphate rocks are main resource of P used in agriculture and industry. However, international price of phosphate rocks is soaring because of the recent concern about P scarcity. Furthermore, Japan is depend on import for almost phosphate resource. Therefore, we need to save and reuse P for sustainable use of P in Japan. Sewage is proposed as one of the intranational phosphate resources. It is estimated that P equivalent to about 50% in imported phosphate rocks flow into sewage treatment plants. P in sewage water is concentrated into sewage sludge, and sewage sludge is decreased its volume by anaerobic digestion. Wastewater after this treatment is called “anaerobic digested liquor” and contains large amount of P. In this study, we investigate about the P recycling from actual anaerobic digested liquor using hydrotalcite by adsorption method. Hydrotalcite is known as inorganic ion exchanger, however, ability of P adsorption in actual anaerobic digested liquor has not been investigated. As a result, hydrotalcite can adsorb phosphate from anaerobic digested liquor, and adsorbed phosphate is desorbed rapidly by NaOH solution. Maximum adsorption amount is about 3.5 mmol/g-abs and desorption ratio is 70 ~ 80%. In continuous operation using fixed bed column, high concentration of phosphate eluate is obtained rapidly by high concentration of NaOH solution. Mass transfer coefficient of hydrotalcite is calculated by Cooper model and elusion curve, and the proportional relationship between mass transfer coefficient and NaOH concentration is revealed. Mass transfer coefficient of absorbent is essential parameter for adsorption method and important to determine the actual operate conditions.
Sewage Sludge Ash (SSA) is generated by the combustion process of sewage sludge in an incinerator. Treatments of SSA with alkaline solution would lead a variety of recycling methods such as building materials, phosphorous recovery. In this study, we examined three types of alkaline treatment processes for real SSA samples collected having a wide range of chemical compositions: the method 1 is the phosphorous extraction with a dilute alkaline solution (0.8 to 1.0 M) with low solid/liquid (S/L ~ 0.1) ratio; the method 2 is preparation of materials for heavy-metal removal treated with a dilute alkaline solution (~1.0 M) adding silica as a silicon source for building geopolymer-like structures; the method 3 is solidification of SSA with a concentrated alkaline solution (~10 M) with high S/L = 0.8 to 1.0.
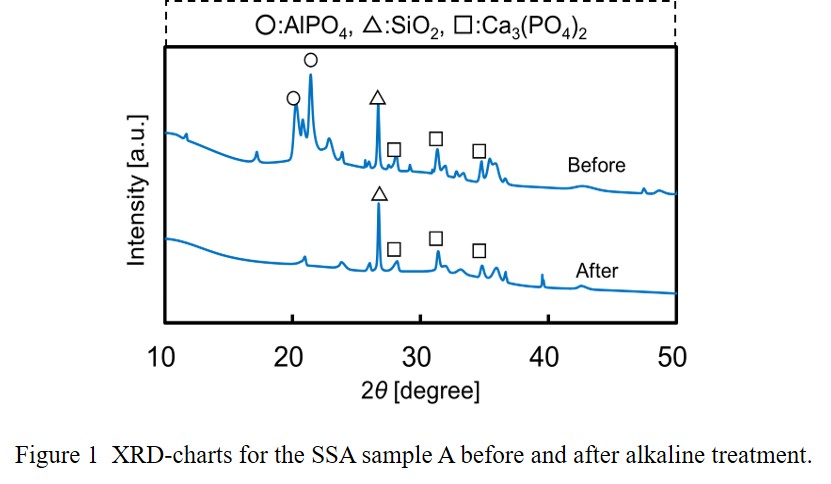
The phosphorous extraction performances with the method 1 strongly depended on the chemical composition of the raw SSA samples. The SSA samples with higher aluminum content and lower calcium content showed higher phosphorous extraction ratio. The disappearance of aluminum phosphate peaks in XRD charts by the alkaline treatment indicates that phosphorus in SSA is extracted mainly from aluminum phosphate, AlPO4 as shown in Figure 1.
All the solid samples prepared by the method 2 showed an excellent lead removal performance in water, especially the solidified sample after washing with pure water. In this case, formation of lead exchanged hydroxyapatite, Pb10(PO4)6(OH)2, was observed after the lead removal. This result suggests that the geopolymer-like solid (amorphous) was formed by the method 2, which has a cation-exchange capability.
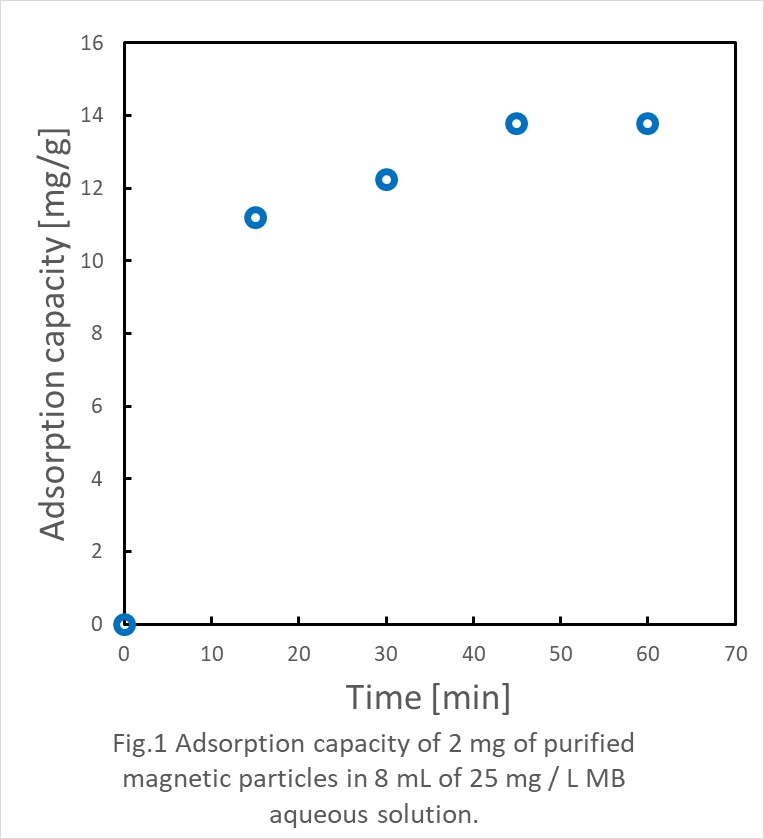
In this research, we consider a process for mass production of magnetic particles consisting of metal and carbon inexpensively using a diesel engine. The carbon material can be easily recovered by a magnet being complexed with a ferromagnetic metals and its application to water purification process is being considered although it is still disadvantageously expensive. By a diesel engine for chemical synthesis, the production cost can be reduced by the cogeneration of the chemical product and the power. In this study, iron-carbon fine particles are synthesized from diesel oil in which ferrocene is dissolved, and the characteristics as an adsorbent are evaluated. With ferrocene and JIS No. 1 diesel oil as raw materials, diesel oils with ferrocene concentrations of 0, 1000 and 10000 ppm were prepared. After the particulates were synthesized in the diesel engine, they were collected together with the exhaust gas in a gas collection bag. The engine used was a 4-stroke engine with a rated output of 2.5 kW and 3000 rpm and a displacement of 211 mL. The synthesized particles were collected when operated at an engine load of 1.65 kW. For evaluation of water purification, collected particles have been purified by magnetic separation in ethanol three times. Then 2 mg of purified magnetic particles were dispersed into 8 mL of 25 mg / L methylene blue (MB) aqueous solution. After rapid stirring, the solution was placed next to a neodymium magnet, and the absorbance at 611 nm of the solution supernatant was measured every 15 minutes. After the absorbance measurement, the solution was stirred and placed next to a magnet again. The particles were quickly and clearly attracted to the magnet. It was also confirmed that the concentration of MB decreased by the synthesized magnetic particles.
Selenium is commonly contained in wastewater generated from the process glass coloring agent, red pigment, and so on. An efficient method to remove selenium is required. Selenium is generally removed by co-precipitation with ferric hydroxide. However, this method requires a large amount of ferric salts due to low removal efficiency of selenium by ferric hydroxide. This research investigates the possibility of another method using magnesium oxide as a selenium remover from aqueous solution. Although magnesium oxide has a disadvantage because of its low reactivity, it can be overcome using low crystalline magnesium oxide obtained by calcining magnesium carbonate at low temperature. In this research, we tried to understand the selenium removal performance and its mechanism using this low crystalline magnesium oxide. To investigate the amount of selenium removed, the low crystalline magnesium oxide was added to simulated wastewater containing selenium. The concentration of selenium in simulated wastewater was measured by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). In addition, to clarify the removal mechanism of selenium, the solid after removal was analyzed using X-Ray Diffraction (XRD). Selenate was removed within 20 minutes after the addition of low crystalline magnesium oxide. The XRD result showed the shifted peak of (001) surface of the magnesium precipitate after the addition of low crystalline magnesium oxide in 10 to 30 minutes. This result indicated selenate entered the surface between the layers of magnesium hydroxide's (001). On the other hand, selenite was removed within 30 minutes after the addition of low crystalline magnesium oxide. A new peak was formed near the (001) surface of the magnesium hydroxide when the selenite was in high concentration. A precipitate containing selenite and magnesium besides magnesium hydroxide was formed when selenite was removed.
Flotation methods were designed for the efficient removal of fluoroquinolone antibiotics from wastewater. The removal of four fluoroquinolones including norfloxacin, ciprofloxacin, levofloxacin, and enrofloxacin were conducted by adding electrically equivalent amounts of sodium dodecyl sulfate (SDS) and poly (allylamine hydrochloride) [PAH] followed by feeding air bubbles. The air bubbles vigorously mixed water and induced the coagulation of SDS-PAH complex to from agglutinative coagulation on water surface. In the presence of Al (III) ions, fluoroquinolones in water were collected to the coagulum as a hydrophobic ion-pair of the Al (III)-chelate with a dodecyl sulfate ion. Experimental conditions including the concentration of Al (III) ions, SDS, PAH, alcohols, salts as well as solution pH, vessel diameter, water height, amount of air babbles, and flotation time were examined. Combined use of 1.0 mg/L Al (III), 20 mg/L SDS, 6.5 mg/L PAH, allowed more than 99% removal of four antibiotics within 5 min. Practical applicability was examined by using synthesized municipal and hospital wastewaters. Effects of dissolved organic and inorganic substances were studied in detail.
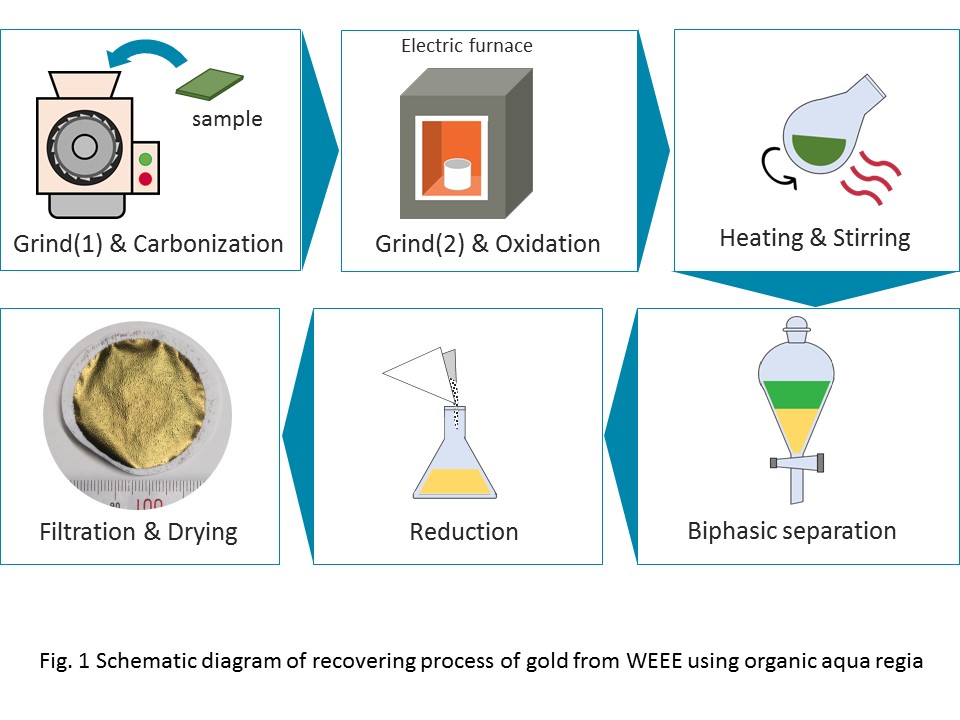
We have developed a novel process for recycling gold from secondary sources. The process consists of the leaching of gold using Organic Aqua Regia, propylene carbonate (PC) solutions containing copper bromide (CuBr2) and potassium bromide (KBr), In this study, we applied this process to recover gold from Waste Electric and Electronic Equipment (WEEE). First, the WEEE samples (memory card, mobile phone board and small appliances) were coarsely grinded and carbonated. Then, the samples were finely grinded and oxidized. Next, the leaching of gold from the oxidized samples were conducted in a PC solution with 0.2 M of CuBr2 and 0.2 M of KBr, at 353-373 K, followed by biphasic separation with sulfuric acid. The dissolved Au in the PC phase was recovered by reduction of ascorbic acid. The maximum recovery ratio of Au was 88%, 74%, 69%, 80% and 94% from memory card, mobile phone board, digital camera board, game board, TV board, respectively. Our developed process could offer a number of advantages, including eco-friendliness and simple operation.
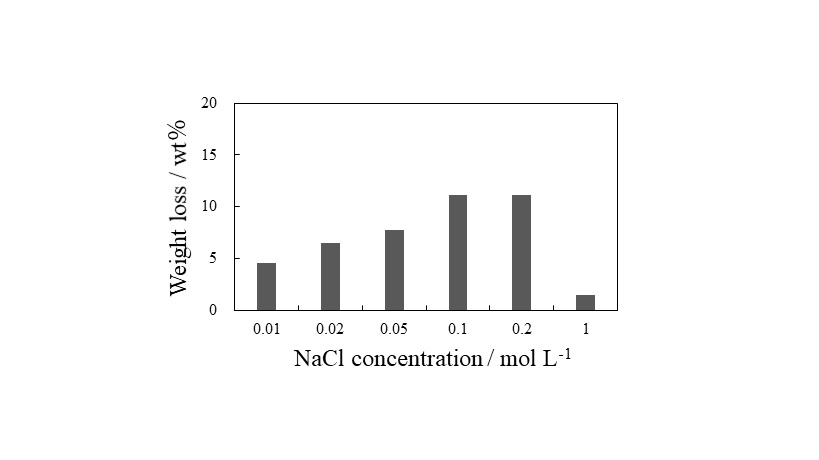
Carbon fiber reinforced plastic (CFRP) is a composite material consisted of carbon fiber and resin. Since the demand for CFRP is expected to increase at approximately 140,000 tons a year by 2020, it is necessary to consider the disposal method of a large amount of the CFRP waste. Recycling the CFRP waste to produce inexpensive carbon fiber can contribute to the expanded demand. The main recycling methods are a thermal decomposition and a sub-/super-critical dissolution method. Although these methods have some advantages, they have not yet reached a sufficient recycling method for the commercial scale due to remaining issues. Therefore, we focused on a voltage application method for the CFRP recycling. In this study, we investigated the effect of electrolyte solution, its concentration for the method, in order to optimize the conditions. The experiment was performed using a two-electrode cell, in which cross-ply CFRP laminate of carbon fiber and epoxy resin as an anode. The damage of carbon fiber after the experiment was assessed using a digital microscope. Figure shows the dependence of the weight loss of the CFPR laminate on the electrolyte (NaCl) concentration. The weight loss increased, as the NaCl concentration increased until 0.1 mol L-1. From the result, as the concentration was higher, the CFRP recycling by the voltage application method was effective, because the current showed high value. On the other hand, the experiment in 1.0 mol L-1 electrolyte showed the small weight loss despite the high current value. From the observation by the digital microscope, NaCl crystal was observed on the surface of the CFRP laminate after the experiment. From these results, we conclude that the suitable concentration of NaCl electrolyte was approximately 0.1 mol L-1 for the CFRP recycling by the voltage application method.
Sustainable development require human being well manage the pollutants in water and air. Removing the pollutants by microbes or catalytic electrochemical process in fuel cell type reactors present energy-saving options and electricity generation features. The electrodes and catalysts are crucial for such design of reactor and process , in pollutants removal efficiency, electricity generation level and operation costs. Through more than 5 years of study, we developed series catalysts, electrodes and fuel cell reactors , that favorably increased pollution control efficiency for both water and air. Significant catalysts such as Fe-N-C, Bi-V-O, BiOBr, Ag/AgBr, Pt/TiO2/ZnO, and their composites were loaded on substrate electrodes ( carbon cloth or stainless steel or Ni foam), as anode or cathode. These electrodes then were tested in Photocatalytic fuel cell or microbial fuel cell, that can integrate electrochemical effect with membrane filtration, or utilize the synergetic effect of microbes with (photo-)electro-chemical functions. Thus, pollutants such as PFOS, aromatic pollutants in real coking wastewater, antibiotics and dyes in water, HCHO and ethyl acetate, ammonia and trimethylamine, and toluene can be removed through electricity generation processes. The energy cost of operation is lowered through solar powered, bio-powered or self-biased and self-driven catalytic processes. Highest obtained electricity generation can reach 87Wm-2h-1,assisted by self-sustained degradation of pollutants.
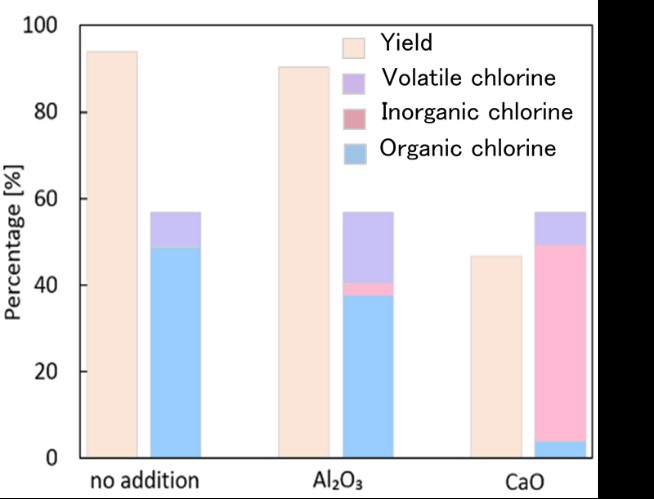
In this study, the chlorine removal efficiency of waste plastic by superheated steam was investigated using adsorbents. 5 g of PVC and 5 g of adsorbent were physically mixed and compressed into pellet using a hydraulic press. The adsorbents in this study were CaO, Al2O3, SiO2 and Fe3O4. The sample was placed in a chamber where the superheated steam was introduced at a flow rate of 10 kg/h. The reaction time and chamber temperature were kept constant at 1 h and 473 K, respectively. The experiment of nitrogen heating was also compared with the superheated steam heating. The dechlorination rate was in the descending order of CaO> Al2O3> no addition, and the PVC yield was in the descending order of no addition> Al2O3> CaO. Although the decrease in the yield of PVC seemed to result from the increase in the volatile matter such as hydrogen chloride and hydrocarbons, addition of CaO increased the dechlorination rate as organic chlorine and it was higher than the others. This is due to the formation of the inorganic chlorine compounds such as CaClOH or CaCl2 6H2O with decomposed chlorine and CaO, according to the XRD analysis. The neutralization reaction of CaO, which is an alkaline earth metal oxide, with the volatile HCl occurred in the sample. Therefore, the CaO addition is more effective for the PVC decomposition and dechlorination in the superheated steam atmosphere. The dechlorination rate of the CaO addition became 92% in the superheated steam, whereas it remained 32% in the nitrogen atmosphere. As a conclusion, the PVC dechlorination can be efficiently achieved in the superheated steam in the presence of adsorbent, such as CaO.
Biological removal of dichloromethane (DCM) from pharmaceutical industry is limited by its recalcitrance. In this study, an airlift packing reactor (ALPR), which combined the suspended and fixed-film microbial growth system, was set up to remove DCM and co-existed toluene. The removal performance of the ALPR for DCM was greater than traditional airlift reactor (ALR). The maximum elimination capacity (ECmax) of the ALPR for DCM reached 108 g m-3 h-1 with removal efficiency (RE) of 41%, increased by 145% if compared to the ALR. The ECmax for toluene was 172 g m-3 h-1 with RE of 70%, decreased by 25% than the ALR, which was mainly due to the higher liquid-phase biomass in the ALR. The results of high-throughput sequencing showed that the microbial composition on the packings of the ALPR had a large difference from its liquid-phase or the liquid-phase of the ALR. Gemmobacter, Rhizomicrobium, Chitinophaga, Vampirovibrio, and Fodinicurvata were genera with great abundance fixed on the packings and Rhizomicrobium, Chitinophaga, Vampirovibrio, and Fodinicurvata are first to be reported in VOCs biological removal. This study indicated that the ALPR can augment the microbial community and effectively improve the removal of recalcitrant VOCs.
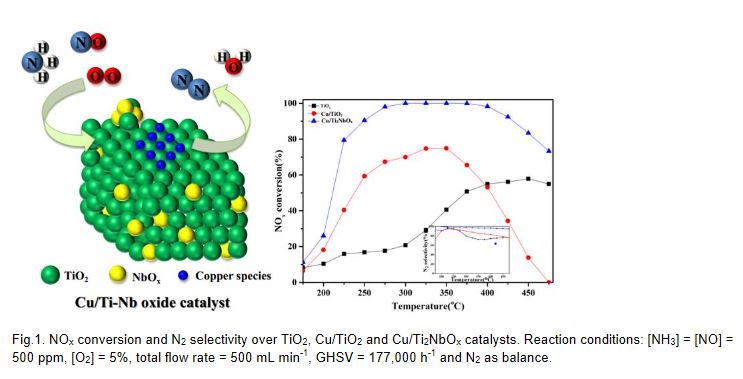
As the traditional VW(Mo)Ti catalyst existed some drawbacks using in NH3-SCR reaction, it is necessary to develop new TiO2-based alternative catalysts for NOx abatement. 0.8%Cu/Ti2NbOx as a novel catalyst was developed and investigated. The NOx conversion and N2 selectivity maintained upon 90% and 97% respectively in the temperature range of 250 °C-425 °C under a high gas hourly space vel °City (GHSV) of 177,000 h-1. Water vapor or SO2 would not pose any negative effect on the SCR activity. Also, 0.8%Cu/Ti2NbOx exhibited an excellent hydrothermal stability and alkali mental resistance. The performance characterization indicated 0.8%Cu/Ti2NbOx was a promising candidate for the NH3-SCR catalyst in the future practical application. Besides, an array of analytical techniques were employed to elucidate the correlations among the performance-structure-property and the reaction mechanism over 0.8%Cu/Ti2NbOx catalyst. The introduction of niobium oxide increased the surface area of TiO2 and decreased the crystallite size, promoting the high dispersion of copper species. Meanwhile, the total acidity and the amount of surface active oxygen over the catalyst were increased by the addition of niobium oxide. Copper species mainly existing in the state of isolated Cu2+ and non-isolated Cu+ enhanced the redox capability of the catalyst, resulting in an excellent catalytic performance. There is a promotional synergistic effect between copper and niobium in 0.8%Cu/Ti2NbOx catalyst during the catalytic reaction. Furthermore, the reaction pathway over 0.8%Cu/Ti2NbOx catalyst followed both Eley-Rideal mechanism and Langmuir-Hinshelwood mechanism at 225 °C.
Key words: Cu/Nb-Ti mixed oxide catalyst; Synergistic effect; Reaction pathway
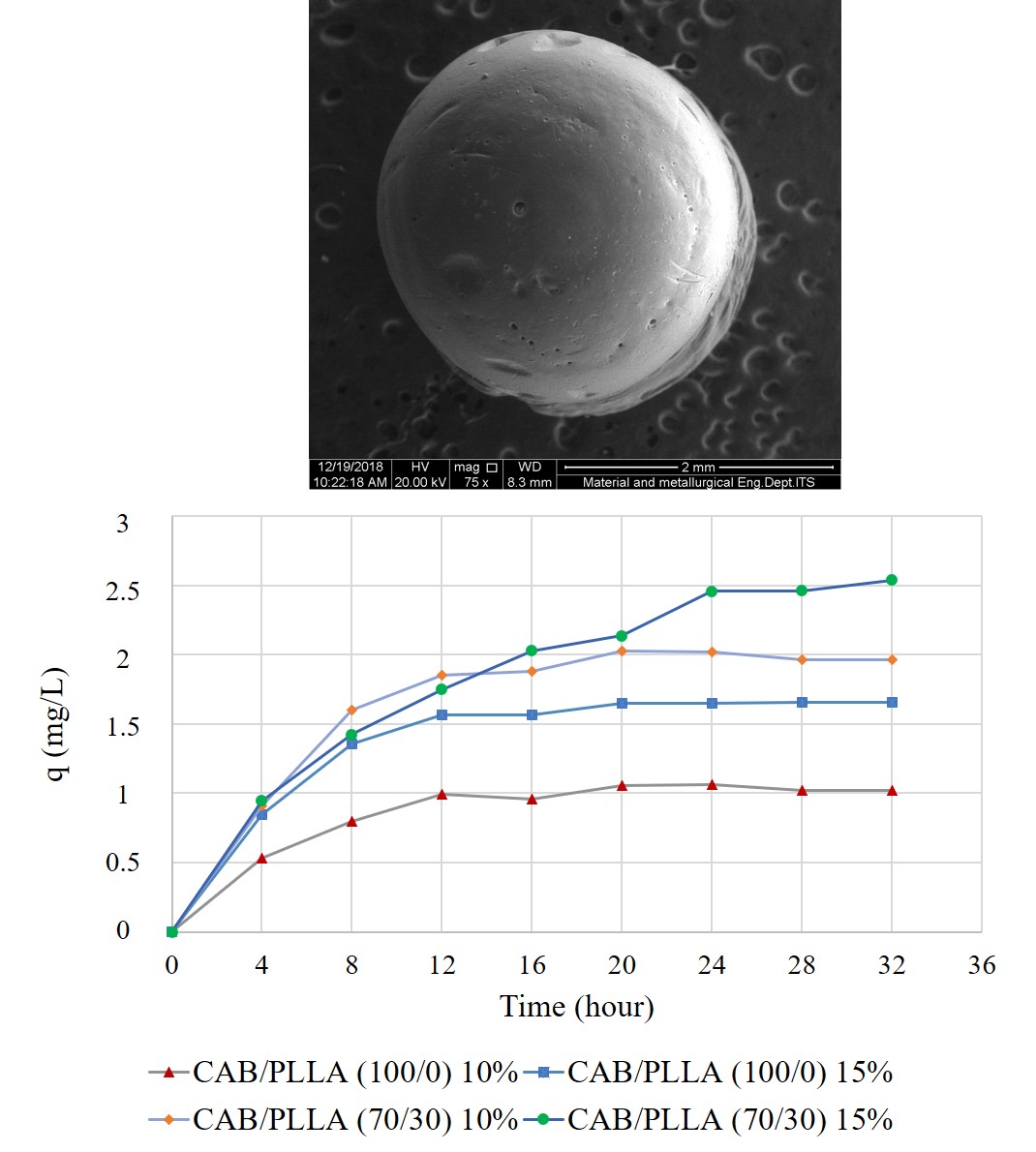
The disposal of untreated synthetic dye wastes from various industries causes serious environmental problems. Adsorption has been reported as promising method in industrial wastewater treatment especially for dye removal. Cellulose-based beads have been reported to show great potential as dye adsorbents. Some modification of cellulose-based adsorbent beads can be done to increase their adsorption capacity. One of the modification techniques is by blending with other materials to form composite beads. In this present study, cellulose acetate butyrate (CAB) and poly(L-lactic acid) (PLLA) were blended through solvent blending method followed by solution dropping technique to fabricate composite CAB/PLLA beads and applied as adsorbent for methylene blue removal from aqueous solution. It has been reported that cellulose beads can be prepared from some cellulose derivates sources such as CAB via solution dropping method. The weight ratio of CAB/PLLA was varied, which are 100/0, 90/10, 80/20, and 70/30, to see the effect of PLLA addition to the adsorption performance of the composite beads. The concentration of polymers in the solution was also varied, which are 10 wt% and 15 wt%. Batch equilibrium adsorption was carried out to study the adsorption kinetics. The adsorption results showed that the increasing of PLLA content generally increases the absorption capacity (Q) and dye removal percentage (% R). The highest adsorption capacity and % dye removal was obtained by CAB/PLLA (70/30) 15 wt%, which are 2.54 mg/g and 25.35% respectively at the adsorption condition of pH 7, temperature 30 °C, initial dye concentration of 20 mg/L, and beads dose of 2 g/L. The % dye removal increased to 69.85% by increasing the beads dose to 20 g/L. The kinetics data obtained from the experiment fitted well with Lagergren's pseudo-first-order model, showing the characteristic of physically adsorption.
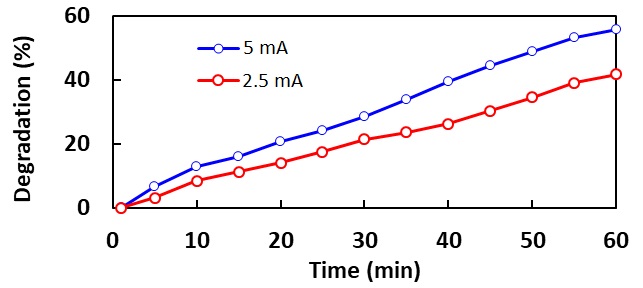
Degradation of Methylene Blue (MB) in aqueous solution is investigated using a pulsed DC discharge on liquid surface synchronized by droplet formation between two electrodes. The high voltage electrode is located in the return tube while a ground electrode is placed inside the bulk solution. Plasma discharge is ignited between the two electrodes when the aqueous solution runs to the lower electrode. The gap between the top electrode and the water surface is about 15 mm and the discharge is ignited when droplet forms. The concentration and total amount of the MB solution was 10 ppm and 100 ml respectively. The solution is circulated at a rate of 20 ml/min by a pump through the lower electrode, spectrophotometer and return tube (upper electrode). UV-vis spectroscopy is used to evaluate the degradation rate by the absorbance at a wavelength of 615 nm. The efficacy of discharge currents is studied at a voltage -10 kV. It is found out that higher current has more degradation efficiency for the same initial concentration of dye. The maximum degradation efficiency is determined about 56% for the 5mA current, and 42% for the 2.5 mA current. However, the more stable discharge is obtained for lower current despite the fact that the higher current has more efficiency of degradation. When plasma turned off after 60 min, the degradation is continued to cease down after 10 min. The latter phenomenon is attributed to the free radical which is elaborated within the solution and reacts with MB.
Figure 1. Effect of current on the degradation rate of MB.
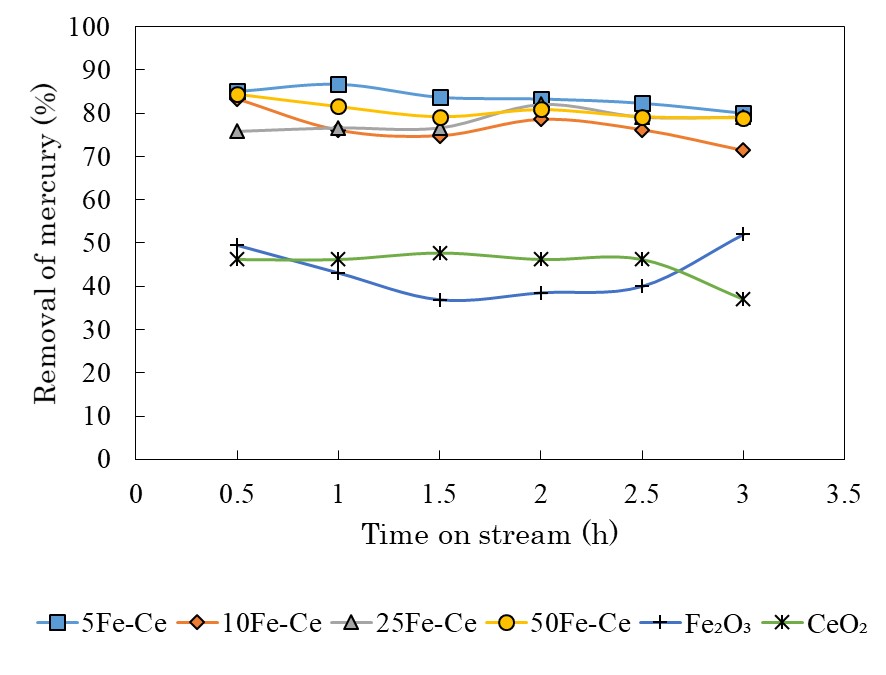
At present, coal is widely used as a fuel for thermal power generation. When coal is used for thermal power generation, there is a problem that mercury contained in coal is emitted to the atmosphere as exhaust gas. The forms of mercury emitted by thermal power generation include mercury oxide, particulate mercury, and elemental mercury. Of the three, the former two can be removed with existing equipment, but elemental mercury is difficult to remove with the current technology due to its high volatility and low water solubility. The aim of this study is development of sorbents suitable for the removal of elemental mercury from coal gasification gas. Fe2O3 and CeO2 were chosen as sorbents because they are inexpensive and stable in the mercury removal reaction. The effects of combination of Fe2O3 and CeO2 at various ratio were investigated.
The simulated coal gasification gas was used for the mercury removal experiment. It was found that the inclusion of Fe2O3 in CeO2 improves the mercury removal performance and also improves the durability in repeated use. After the mercury removal experiment, TPDD experiment was performed to examine the adsorption state of mercury by the sorbents from the desorbed mercury peak. Since the peaks derived from HgS appeared in all the sorbents, it was suggested that the mercury was captured in the form of HgS. When the experiment was performed in the absence of H2S and HCl, the mercury removal rate decreased. Both H2S and HCl are believed to contribute to the mercury removal reaction.
Generally, zinc contained in zinc-plating wastewater is removed as zinc hydroxide precipitate by adjusting the pH from 9.5 to 10.5. Zinc hydroxide sludge generated from this process is discharged about 20000 t/yr in Japan, and the greater part of them are disposed in landfills because of high recycling costs. However, in recent years, industrial waste disposal costs have risen due to the shortage of land fill disposal sites. Therefore, development of volume reduction and effective usage method for zinc sludge is required.
As a solution to this problem, we aimed at the effective usage of zinc sludge by producing Zn-Al LDH from zinc-plating wastewater. Zn-Al LDH can be obtained by adding polyaluminum chloride (PAC) generally used as an inorganic flocculant to zinc-plating wastewater. LDH is well known to have anion exchange property and then can be applied as an anion adsorbent. In fact, Zn-Al LDH also has excellent phosphate ion adsorption capacity. Accordingly, in this study, we investigated whether Zn-Al LDH obtained from could be used as an anion (especially boron and fluorine) adsorbent.
We have previously reported on the adsorption properties of Zn-Al LDH obtained from zinc-plating wastewater. In this poster presentation, we will report on the evaluation of environmental burden of Zn-Al LDH adsorbents produced from zinc-plating wastewater. The conventional methods of boron and fluorine wastewater treatment are two-stage coagulation sedimentation methods, and a lot of sludge is generated in these processes. Therefore, the environmental burden of these methods will be high, so boron and fluorine wastewater treatment with Zn-Al LDH adsorbents produced from zinc-plating wastewater may be superior to the conventional methods. We evaluated the environmental burden in each wastewater treatment method with life cycle assessment (LCA) and compared them to confirm the advantages of the Zn-Al LDH adsorbents.
A core-shell structured Fe-ZSM-5@CeO2 catalyst was originally fabricated via dopamine polymerization for selective catalytic reduction of NOx by NH3. Fe-ZSM-5@CeO2 possess a better catalytic activity with higher N2 selectivity, more extensive operating-temperature window, and better water resistance compared with Fe-ZSM-5/CeO2 via physical mixing. The synergistic effect of Fe-ZSM-5 and CeO2 over Fe-ZSM-5@CeO2 facilitated the formation of redox-cycle between Ce3+/Ce4+ redox-couple and high rate active oxygen species (Oβ and Oγ), leading to over 90% conversation of NOx in the temperature range of 250-400 °C. The feature of hierarchical structure with CeO2 outer shell provides a pathway to convert NO to NO2, facilitating the fast SCR. Meanwhile, the formed NO2 can activate Fe2+/Fe3+ redox-couple, offering an extra redox cycle which can further contribute to fast SCR. The CeO2 shell outside can also improve the water resistance of Fe-ZSM-5, resulting in a higher catalytic stability of Fe-ZSM-5@CeO2. To be concluded, Fe-ZSM-5@CeO2 was proved to be a promising candidate for NH3-SCR as the core-shell structure, uniform distribution of active sites, as well as the synergistic effect of Fe-ZSM-5 and CeO2 species.
Industrial wastewater of electroplating contains various toxic substance such as metals, degreasing agents and oil. Metals such as zinc are harmful to the environment. Therefore, discharge of zinc wastewater in the electroplating industry is regulated. In previous study, it is presumed that plating chemicals, particularly degreasing agents disturb the zinc wastewater treatment. Thus, low environmental impact cleaning technology is required to replace degreasing agents. Recently, fine bubble, which is a bubble of 100 μm or less, is attracting attention. Fine bubbles exhibit unique properties, such as surface charge, and cleaning technology utilizing the properties is expected. To comply with zinc effluent regulations, we investigated the effect of fine bubble cleaning on oil deposited on metal surfaces.
The samples for cleaning test were prepared by applying each oil to metal surfaces. The cleaning test was performed for 15 minutes using fine bubble or ultrafine bubble generators with different performance. The surface cleanliness were measured by ultraviolet and visible spectrophotometer.
The results of cleanliness measurement for metal surface show that the oil removal capability by fine bubble depends on oil type, shape of metal, gas type, and the concentrations of micro bubbles classified as bubble size 1 to 100 μm. In particular, the concentration of micro bubble and gas type exert a beneficent influence. These results indicate that the shifts of removal capability of fine bubble are attributed to changes in the fine bubble structure. Furthermore, to investigate the interfering effect of fine bubble in zinc wastewater treatment, we conducted experiments using zinc wastewater containing fine bubbles. The results of experiments show that the contamination of fine bubble does not affect zinc wastewater treatment. From these results, we will discuss the cleaning effects of the fine bubble.
In Japan, laws on effluent standard such as Water Pollution Prevention Act and Sewerage Act were established to protect the water environment. Recently, since tougher standard value has been imposed, more conscious from the electroplating industry to the environment are demanded.
For this reason, we are studying on the improvement of wastewater treatment process being applied in the plating factories. According to previous research, it is shown that pretreatment reagent such as degreasing agents inhibited zinc treatment. Therefore, in order to reduce the use of degreasing agents, the fine bubble washing is studied.
However, the effect of fine bubble washing still have many unclear points. And it may not be evaluated by the current method definitely. So in this study, evaluation method for cleanliness of products contaminated by machine oil and washed by fine bubble is examined.
Base oil, cutting oil and hydraulic oil were used as machine oil. An infrared spectrometer, an ultraviolet and visible spectrophotometer, and a gas chromatograph mass spectrometer were used for the evaluation. Metal plates were soaked into each oil and washed by the fine bubble. The residual oil was extracted using the 50 mL of hexane by ultrasonic extraction. Then these extraction liquids and standard liquid were measured and quantified by comparison of peak strength or peak area.
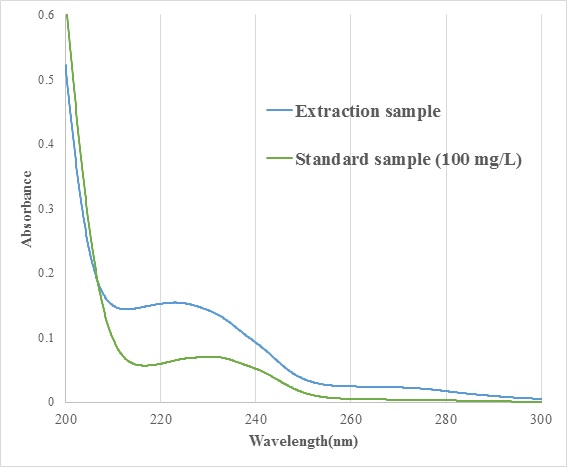
Fig.1 shows UV-vis spectra of the cutting oil. It was suggested that the cutting oil were changed by the fine bubble. Since the cutting oil was mainly composed of fats, it was impossible to quantitate the fats by the UV-vis spectroscopic analysis in this study. On the other hand, the quantifiability for the hydraulic oil was particularly good. Moreover, the deviations of the measurements were less than the measurement by gravimetry. This indicated that some analysis methods were unsuitable for some types of oil.
Soil contamination caused by oil spills is a global problem, and bioaugmentation using oil-degrading bacteria is an effective treatment to clean up the contaminated soil. However, various environmental conditions, such as soil composition, water content, and temperature, influence its efficiency. In the present study, an effective bioaugmentation under low temperature condition was developed with mixture of two Rhodococcus oil-degraders, strain A and C, which are officially permitted to be used in bioaugmentation in Japan. A rotational slurry bioreactor was used with a model slurry prepared with 3.0 × 108 cells/g-slurry of strain A and C as inocula. A-fuel oil was added to a final concentration of 2,500 and 5,000 mg/kg-slurry. The decomposition test was carried out by rotating under 15 °C, the residue of A-fuel oil and the number of bacteria were measured every two days. After 4-day treatment, 90% of degradation was achieved, which was 3-fold faster than previous degradation experiment without rotation (90% of A-fuel oil was degraded after 12 days of incubation 1)). For practical application, we conducted a semi-continuous treatment by removing 90% of treated slurry, then added the same amount of contaminated slurry into the system without additional degraders. Ninety-four % of A-fuel oil was degraded after 6 days by this repeated treatment. Our study provides an efficient and cost-effective removal of A-fuel oil by the semi-continuous system.
[1] Shintani et al., 2019, J. Biosci. Bioeng., 127:197-200
The dust monitor is a device that measures the dust concentration continuously in a flue, a chimney, a duct, etc. It is widely used in thermal power plants, waste incinerators, etc. because it can always grasp the operation status of soot and smoke generating facilities.
In Europe, a certification system has been established by TüV of Germany and MCERT of the United Kingdom, and if formal certification is obtained, it will be legally recognized as a continuous monitoring system (CMSs) of dust concentration from the facilities. In Japan, with regard to the emission of dust from the facilities, emission regulations have been introduced under the Air Pollution Control Act. However, although it is not possible to use a dust monitor for dust concentration measurement at present, the related JIS standards are being developed for introduction.
In this work, we developed a flue test equipment aiming at the construction of the performance evaluation system of various dust monitors and the establishment of official legislation. In this maintenance, the diameter of the flue was enlarged, rectification, optimization of dust generation, improvement of the evaluation part, and it became possible to respond to the evaluation of the main method of dust monitor. This device was developed to be able to generate gas containing dust at any dust concentration in a constant velocity flow. Since the measurement unit is provided with a plurality of measurement ports, it is able to evaluate the correlation with the analysis value of JIS Z 8808 for various dust monitors (light scattering type, light transmission type, electrostatic detection type). On the other hand, since the general exhaust gas velocity of large-scale thermal power plants is 10 to 20 m / s, it is necessary to work on increasing the flow velocity of the test equipment.
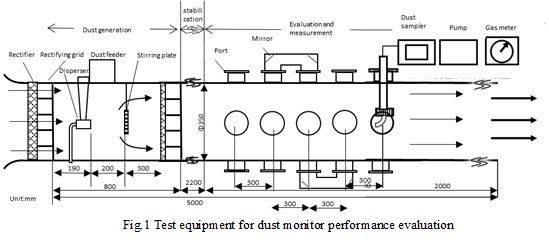
The adverse environmental impact caused by eutrophication has recently prompted the Philippine government to issue stringent regulatory standards for wastewater effluent quality. The involved stakeholders and industries are assessing the integration of biological nutrient removal (BNR) technologies in the current sewage treatment plant (STP) scenario. Moreover, efforts are being done to utilize wastewater as a resource such us recovery of nutrients as struvite fertilizer from the wastewater sludge. Since BNR and nutrient recovery systems are not yet integrated in STPs, the magnitude of the environmental impacts are yet to be evaluated in the Philippine setting. This study covers the holistic evaluation of the overall environmental performance scores of the following scenarios using a consequential Life Cycle Assessment (LCA) framework integrated with Analytic Hierarchy Process (AHP) in the context of agriculture, food consumption and wastewater: 1) current STP scenario; 2) BNR technology; and 3) nutrient recovery system. The environmental impact assessment was done using IMPACT 2002+ methodology in terms of the following impact indicators: human health, ecosystem quality, climate change, resources, aquatic acidification, and aquatic eutrophication. Value judgments from relevant stakeholders were elicited to rank the relative importance of the impact indicators in the evaluation of the overall environmental performance score. The LCA-AHP results show that the integration of a nutrient recovery system is the most preferred scenario. Sensitivity analysis was also done to evaluate the effects of changes in diet and utilization of alternative energy.
Bauxite residue, commonly known as red mud, is a slurry waste generated in huge quantities in the extraction of alumina from bauxite by the Bayer process. It is highly alkaline and caustic with pH greater than 12. The treatment and management of red mud have posed environmental challenges to the alumina industry worldwide. In this work, a systematic study of the neutralization of RM by carbonation with CO2 over a range of different operating conditions was conducted with the aim of establishing the optimal conditions for the carbonation process. The carbonation was performed at room temperature and atmospheric pressure using a stirred tank reactor operating at different conditions such as total gas flow rate (TF), CO2 gas concentrations, stirring speeds and solids concentrations.
The carbonation process was observed to be significantly dependent on CO2 concentration, TF and stirring speeds, whereas solids concentrations seemed to have a little effect based in the range of concentrations studied. In the carbonation of red mud slurry, it took from 30-75 minutes to lower the pH to 6.6 – 7.5 with CO2 gas concentrations ranging from 10%-100%. In contrast, in the carbonation of the red mud liquor, at stable pH of 6.3 – 7.0 was reached within 15-30 minutes at the same CO2 levels. After carbonation, the pH of carbonated red mud slurry was found to rebound and reached 9.7 after a period of 25 days. The carbonated liquor, however, showed a slower rate of pH recovery, and took a month to equilibrate to the same pH. The amount of CO2 captured was evaluated to be 45.6 gCO2/kg at 30% CO2 concentration, with the alkalinity decreasing from 11,610mg/L to 2,104mg/L as CaCO3. This represents a promising potential of CO2 capture by using bauxite residue.
In an industrial painting, an adherence rate of paint to product is about 20%, so large amount of unpainted paint waste - paint sludge - is discharged. Since disposal of a large amount of sludge is high cost and high environmental impact, an appropriate disposal method is necessary. Paint is a resin and it is combustible, so energy recycling is one of a possible method. However, paint sludge contains incombustible inorganic components as pigments, it may inhibit the energy recycling. Therefore, the influences of inorganic components on energy recycling was examined.
The paint sludge used for this study was obtained from several paint factories. The ash content, the contained elements and the calorific value of the paint sludge were analyzed. Endothermic energy amount in combustion and consumption of energy in transportation were calculated from the analysis results.
The ash, which is the noncombustible fractions was more than 50% for any of the sludge. Main substances of the ash were analyzed by X-ray fluorescence analysis. And they were iron, titanium and calcium. The lower heating value of the sludge was 9900 kJ/kg on average, and the moisture content was 38 % on average. Heat absorption amounts of the ash were calculated based on the contents of it, and it was about 2.6%. It means that the influence of the noncombustible fractions on energy recovery is small. In addition, the effect of the ash on sludge transportation is calculated. Assuming a transportation distance of 50 km, the energy required for transportation of paint sludge is about 4.3% and for transportation of ash is about 3.1% of the energy to be recycled. From these results, it is shown that the influence of the inorganic component is small in the whole recycling system.
A variety of technologies such as ion exchange, membrane filtration, precipitation, and biological degradation, have been developed for the removal of environmental pollutants such as bisphenol A and heavy metals from waste effluents. However, many of those methods have weaknesses such as low efficiency, high maintenance cost, and inflexible process. Some methods have relatively limited application for the effluent including high concentration of pollutants. The peptide, oligomer of amino acids, which is the simplest biological recognition element, has been known to have high selectivity toward target component. By using biopanning protocol, various peptide sequences with high affinity to target components have been found. For example, TNTLSNN exhibiting high selectivity to Pb2+ against other heavy metal ions and KSLENSY having specific affinity to bisphenol A were screened previously. In the present work, the application of those peptides is suggested for the removal of environmental pollutants. The peptide-magnetic bead system is first constructed and tested as a reusable adsorbent to remove metal or bisphenol A from aqueous solution. Compared to the bare bead without the linked peptides, the peptide-linked adsorbent showed higher removal capacity. Since the magnetic bead-based adsorbents are easily separated and reused from aqueous system, the process cost can be lowered.
Traditionally, there are several of chemical and physical methods to remove heavy metal ions from wastewater. However, the traditional method of removing heavy metal ions usually had higher cost and limited effectiveness. Therefore, many studies have used low-cost and reusable biological adsorbent to remove the pollutants in wastewater. The wasabi is a common edible plant. However, when people used wasabi, the part of leaves often was discarded. The main purpose of this study is to use the leaves of wasabi powder for heavy metal chromium, copper, nickel and lead adsorption. The adsorption experiment was to investigate the effect of reaction time, pH, initial concentration, different wasabi powder, the dose of wasabi powder and the particle size of wasabi powder.
According to the results the adsorption capacity of heavy metal ion in the order was : Pb (II) > Cu (II) > Cr (II) > Ni (II). The adsorption data of these metal ions were fitted well to the Pseudo second order rate law. Using NaOH pretreatment the wasabi powder for these metal ions had greater than 90% removal efficiency. The pH value has a significant impact on the adsorption. The adsorption capacity of these metal ions will increase to a certain value with the increase of pH value. Because the higher pH value, metal ions, will be accompanied by precipitation. The adsorption isotherms of these metal ions were all fitted well to the Langmuir isotherm. The adsorption capacity decreased with the dose of wasabi powder increased. The wasabi powder agglomerates, thus the surface area of adsorption decreased. The particle size of wasabi powder have no a significant effect on the adsorption.
The air pollution has become a serious issue in Korea with the quick advancement of urbanization and industrialization. Besides, the concerns about air contamination are increasing more since it influences both human health and economic development. However, there are limits to the temporal and spatial resolution in the air quality forecast, due to the low observation density of the traditional fine dust monitoring network. To solve these problems, the objectives of this research are to construct effective air quality big data based on a high-resolution monitoring system and to develop an improved air quality forecasting methodology using deep learning approach. The mobile sensors which were developed to be attached to the vehicle were applied to construct the big data about air quality. The pollutant considered for this research was Particulate Matter 10 (PM10). The PM10 concentration was monitored at intervals of 1 second by the sensors. Also, its spatial resolution was from tens to hundreds of meters. Recurrent Neural Network (RNN), which is widely used deep learning model, was used for forecasting based on the constructed PM10 time series data. To figure out the trend of data and improve the accuracy of the model, noises of the monitored data with mobile sensors were removed by a filter. The Exponentially Weighted Moving Average Filter (EWMAF) was applied in this research. It is observed from this study that the model performance for predicting PM10 was comparatively well (R2>0.9). Finally, this study may play an important role as a beginning step to improve the air quality monitoring and forecasting system. Furthermore, the suggested methodology in this research can help to develop an air pollutant emission reduction process in response to increased fine dust.