Electrodialysis is a membrane separation process driven by the electric potential along ion-exchange membranes, namely anion exchange membranes (AEM) and cation exchange membranes (CEM). The bipolar membrane is a membrane where two kinds of ion exchange membranes (AEM and CEM) are laminated, and water in the membrane gap can be dissociated when an electric potential higher than the one for water molecule dissociation (= 1.23 V) is applied between the two membranes. The water dissociation process releases pairs of proton (H+) and hydroxyl ion (OH-) from the bipolar membrane. This dissociation process can be utilized a variety of applications in the electrodialysis.
In this talk, I am going to introduce some of our recent developments on the applications of the bipolar membrane electrodialysis (BPED) process, especially on the environmental field. The first application is on the carbon capture and sequestration (CCS). CCS has been recognized as a large-measure for reducing the CO2 emission from fossil fuel combustion processes. The biggest challenge of the CCS is the high cost and power consumption for the CO2 capturing process from the flue gas, especially recovery of pure CO2 gas after captured. We have developed a new type of CO2 gas recovery process using BPED. We also have developed a new type of a mineral carbonation process for CCS by using BPED for the recovery of acid/alkali pairs. The second application is the selective recovery of metals or anions from waste stream. A selective recovery process of rare metals from the metal-mixture solutions by using BPED coupled with selective chelating agents. A recovery process of high concentration boric acid solutions from dilute waste water has been also developed based on a multi-stage BPED method. Thus, BPED is a versatile method for environmental applications, with minimal power consumption, chemical wastes, and environmental impacts.
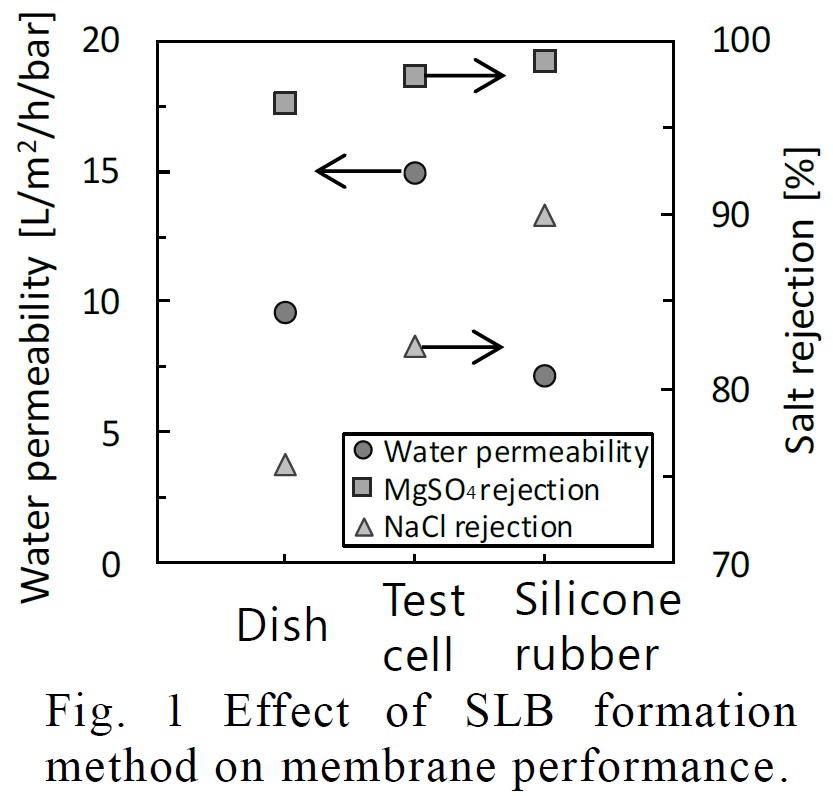
Water purification membranes are expected to a solution for water shortage. However, the significant improvement of conventional polymeric membranes is difficult. While, biological membranes show higher performance than that of polymeric membranes. Biological membranes are consisted of lipid bilayers not to permeate almost all solutes and channel proteins to transport only specific molecules. Therefore, biomimetic membranes have attracted attentions as water purification membranes. Aquaporin Z has been researched for channels of them, however, that structure is so complex that the expression of the water permeability is difficult. In this study, we used Amphotericin B (AmB) as a channel of water purification membranes. We tried to form supported lipid bilayers (SLBs) incorporating AmB. Firstly, AmB:Erg ratio was evaluated to form water permeable channels. AmB:Erg=1:2 was best to form channels because AmB easily binds with Erg under Erg-rich condition. Next, SLBs were formed by three different methods immersing substrate in liposome suspension, (i) in dish (call this method as "Dish"), (ii) fix substrate in permeability test cell ("Test Cell"), and (iii) fix substrate between the silicon rubbers, ("Silicon Rubber"). We quantified the amounts of phospholipids on the surface and calculated the layer numbers of each SLB. Fig. 1 shows the water permeability and salt rejection, and the SLB number is 4.6, 2.3 and 3.1, respectavely. Comparing "Test Cell" to "Dish", due to supress the adsorption of excess lipid on the back side of the support, the water permeability and salt rejection increased and the SLB number decreased. In addition, comparing "Silicon Rubber" to "Test Cell", due to reduce of SLB defects near the boundary between support and O-ring of test cell, the water permeability decreased and salt rejection further increased. Conclusively, we succeeded to form defectless SLB by "Silicon Rubber" and achived high water permeability salt rejection and low SLB number.
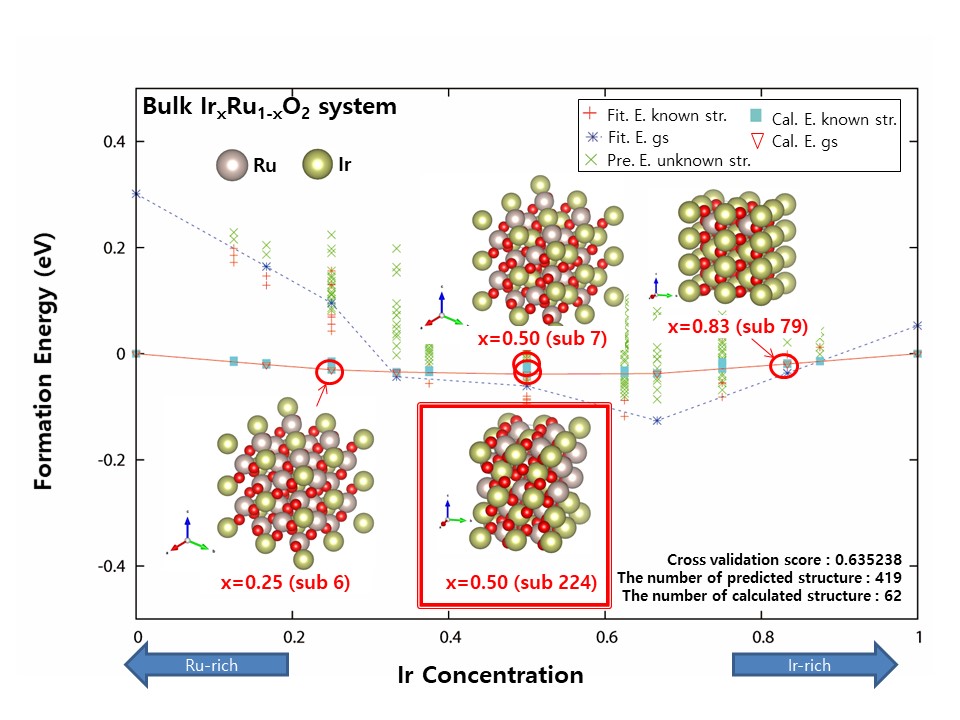
The chlor-alkali process for chlorine evolution reaction (ChER) uses an RuO2-based anode, commonly referred to as Dimensional stable anode (DSA®). Although DSA is regarded as one of the most important inventions in the 21st electrochemistry field, it is recognized as a disadvantage in that the activity of ChER becomes lowered when oxygen is generated, and also Ru is a precious metal. The development of alloy catalysts modified with Ir, Pb, Pt, Ta, Sn, Ti, V, and W has thus been pursued to improve durability and economic feasibility. In this study, the thermodynamic stabilities of MxRu1-xO2 alloys (M = V, W, Ti, Ir) were evaluated by using density function theory (DFT) calculations coupled with the cluster expansion (CE) technique, which can predict the thermodynamic properties of the alloys as the function of element compositions. Once the ground states of alloy structures were determined at different element compositions, the ChER efficiency of the alloys was evaluated by comparing the activation energies of Cl2 desorption on the alloy systems.
CO2 reduction reaction (CRR) offers a promising solution to offset the greenhouse gas emission issues which break the carbon balance in nature. In this work, we introduce a microbial fuel cell (MFC)-driven electrocatalytic CO2 reduction system which harnesses the metabolic activities of microorganism to generate electricity from substrate oxidation. However, the intermittence and periodicity of MFC presents a substantial impediment of maintaining high electrocatalytic efficiency and stability for practical applications. Hence, we developed a redox-medium-assisted system that stores the reduction energy in MFC using a Fe(CN)63+/Fe(CN)64+ redox pair, which can be controllably transfer into CRR to lower power input, then proceeds CO2 reducing into carbon monoxide (CO) with a Zinc oxide/Zinc catalyst cathode electrode. The Fe(CN)63+/Fe(CN)64+ redox pair has the advantages of non-toxicity and good reversibility which is generally used as a cathode electron acceptor for MFC. After being reduced in MFC system, the medium is then transferred to the CRR system to replace the traditional OER reaction in the anode. The experimental results show that the MFC&CRR coupling system can achieve stable energy conversion and reduce the cathode potential from + 1.2 V to + 0.3 V, which greatly improves the energy efficiency. The maximum energy efficiency can reach up to 194%. We also demonstrate a circulating flow system on the basis of intermittent operation inspired by the concept of fluid battery, which increases the capacity of the device and prolongs the reaction time of the device. MFC-driven electrochemical CO2 reduction can more efficiently produce value-added chemicals and offers a potential route to reform carbon in the global environment.
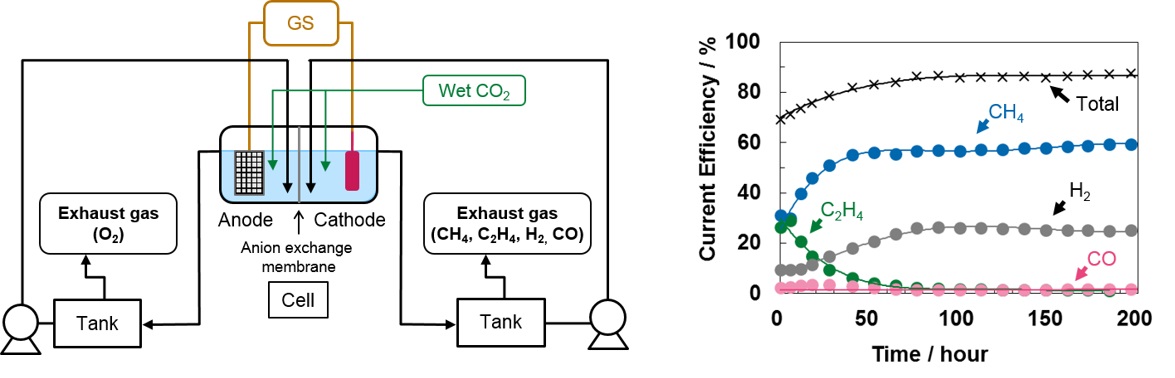
Introduction: Since the Paris Agreement entered into force in 2016, the reduction of greenhouse gas emissions is the task for all countries. In order to achieve the middle- and long-term goal, innovative technology developments and the installation of new system are strongly required. Electrochemical CO2 reduction is a candidate technology and Cu metal electrode is well-known to reduce CO2 to hydrocarbons and alcohols electrochemically. Although long-term stability is essential for practical use, few studies investigated it and the time dependence on reduction product. In this report, we present the time-course of product selectivity and the surface morphology of Cu electrode. The ratio of reduction product was related to the morphological changes.
Methods: Electrochemical CO2 reduction was carried out in 0.1M KHCO3 with two-compartment cell with electrolyte-flow devices (Figure, left) at room temperature. Polycrystalline Cu and Pt mesh were used as cathode and anode, respectively. Electrolysis with constant current was performed at 5 mA cm-2. The gas outlet was connected to gas chromatograph quantified hydrocarbons, H2, and CO. The liquid-phase products were evaluated by gas chromatograph and ion chromatograph.
Results: The generation of C2H4 decreased to 1% and CH4 increased to 60% and became predominant in 50 hours, and the selectivity was almost stabilized from 100 hours, though the main products of CO2 reduction had been CH4 and C2H4 and current efficiencies for them were about 30% each at the beginning of the reaction (Figure, right). That suggests that the morphological change of Cu electrode affects the product selectivity.