
Geopolymers are inorganic polymers composed by alumino-silicate frameworks containing alkali metal ions. Geopolymers can be used as a building material substituting for concrete because of their low cost, low-curing temperatures, and no CO2 emission for production. Geopolymers have porous structures, and their porosity can be controlled by the preparation conditions including the compositions of raw materials, curing temperatures. In this study, we report the fabrication of self-supporting geopolymer membranes having various porous structures, and the gas permeation characteristics of membranes were examined.
Metakaolin, silica gel, potassium hydroxide, and water were used as the raw materials for the geopolymer membranes. An aqueous solution of potassium hydroxide was mixed with silica gel and metakaolin powder, and stirred for 30 seconds at 100rpm; the slurry mixture was used as a geopolymer paste. The solid-liquid ratio in the paste was set 0.5, and the composition of the raw materials was changed. The geopolymer paste so prepared was cast into a silicone rubber mold, of which the inner diameter was 46 mm, and the thickness was 3mm. Then the rubber mold filled the geopolymer paste was sandwiched by glass plates, and cured under a constant temperature (25 to 100 °C) for a given period (1 to 3 days). The paste was solidified by the curing to form a disk membrane with the 46-mm diameter and 3-mm thickness. The geopolymer membrane was taken out from the mold, and dried for given conditions. The gas permeability of pure gases such as hydrogen, nitrogen, and carbon dioxide was measured under the pressure difference at 0.10MPa and the room temperature.
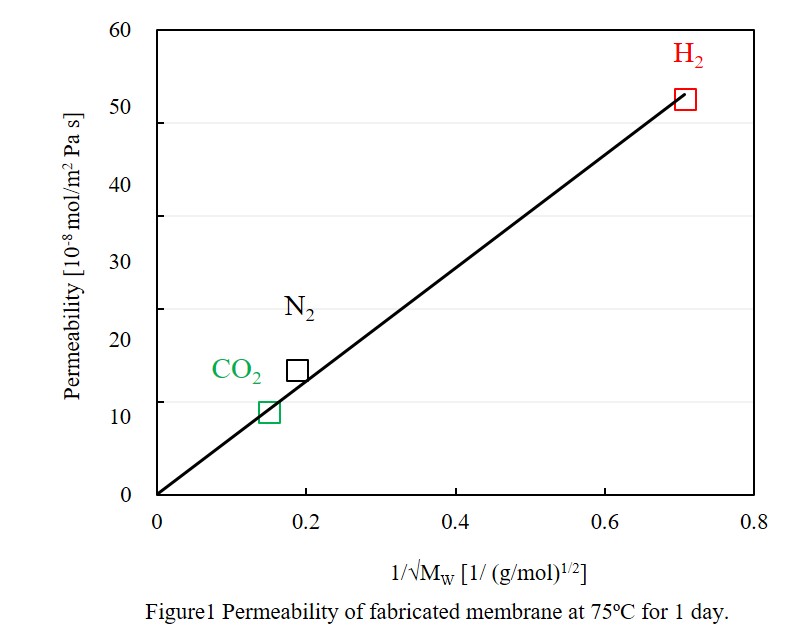
We have successfully fabricated geopolymer membranes without visible cracks only when the molar ratio of SiO2:Al2O3:K2O in casting solution was 65:22.5:12.5. The observed gas permeability was in proportion to the inverse of the square root of the molecular mass of the gas, indicating Knudsen diffusion mechanism.
We investigated the ozonolysis of various VOCs in the presence of nitrogen monoxide (NO) and/or hydroxyl radicals (OH) radicals. A VOC gas was introduced into a fluorine resin bag (50 L) diluted with dry air. The initial concentration of the VOC was set 1200 ppm. Then, ozone and nitrogen monoxide (NO) and/or tetramethylethylene (TME) were added to bag to initiate the ozonolysis reactions. The initial concentration of NO was set 300 ppm. The initial concentration of TME was set 30-60 ppm, which will generate hydroxyl radicals (OH). The bag was shaded with an aluminum bag to avoid the photo-induced reactions.
Under the presence of ozone without NO nor TME, α-pinene, styrene, m-xylene were decomposed, while no decomposition was observed for ethyl acetate and toluene. The decomposition rate α-pinene was largest among the VOCs tested followed by styrene. For α-pinene, the addition of TME decreased the ozonolysis rate, but the addition of TMA and NO increased the ozonolysis rate. For styrene, the addition of TME reduced the ozonolysis rate, and the addition of NO in addition to TME further reduced the ozonolysis rate. For styrene, the addition of TME slightly reduced the ozonolysis rate, but the addition of TMA and NO significantly reduced the ozonolysis of styrene. In the presence of TME (OH radicals), both toluene and ethyl acetate was decomposed. However, the presence of NO in addition to ozone and TME reduced the decomposition rates of these VOCs.
The ozonolysis rates of these VOCs were plotted against the maximum incremental reactivity (MIR), an ozone forming potentials in Fig. 1; no correlations were observed between these values. The present results suggest that MIR is not necessary an appropriate indicator for ozone formation.
The study aimed to investigate the effect of the initial dye concentration, pH, and treatment time on the degradation of Acid red 88 in a batch reactor setup. Raschig rings were dipped and dried in a photocatalyst solution of TiO2 and ethanol for 10 minutes each. It was then calcined at 400 °C for 6 hours with a ramp rate of 3 °C/min. A Box-Behnken design was followed in preparing solutions of 150 mL Acid red 88 dye at 10, 35, and 60 ppm, with a pH of 4,7, and 10. The reactor setup was then filled with 100 mL dye solution. The setup was then run for 30, 60, and 90 minutes according to the experimental design. Samples were collected before and after each run, and its concentrations were analyzed via UV/vis spectroscopy. The immobilized catalyst was also examined under SEM to determine the effectiveness of the immobilization technique. After each run, the Raschig rings are cleaned by running the reactor setup with distilled water. The results indicate that for the most efficient degradation of acid red 88, low initial concentration, low pH must, and high run time must be implemented and that the cleaning procedure was effective in regenerating the catalyst. It can be concluded from the data that the immobilization technique employed in this study is proven to be effective. It is recommended that future studies investigate cationic dyes, optimization of immobilization process, and effect of air sparging on reactor conversion.
Water utilities, commercial and industrial establishments are required to upgrade or install new treatment systems to comply with the revised effluent standards issued by the Department of Environment and Natural Resources – Environment Management Bureau (DENR – EMB) which now includes removal and monitoring of nutrients (nitrogen and phosphorus components). One solution is to utilize a biological nutrient removal technology (BNRT) system capable of removing nutrients from sewage. The on-going study aims to investigate the performance of the pilot-scale system in the removal of nutrients from sewage. The anaerobic-anoxic-oxic (A2O) process was designed and operated in an existing sewage treatment plant (STP) at a flow rate of 1 m3/day. System modification was adapted to ensure continuous operation. Dissolved oxygen (DO) and temperature of each compartment were evaluated after 45 days of system modification. The DO of the anaerobic and oxic compartment remained within the required range, while the internal recycling flowrate and/or aeration must be adjusted to achieve a DO concentration of 0.20 – 0.50 mg/L in the anoxic compartment. The research is financially supported by the Philippine Council for Industry, Energy and Emerging Technology Research and Development of the Department of Science and Technology (PCIEERD Project No. 04176).
Acid mine drainage (AMD) is an environmental problem observed in mine sites rich in pyrites and characterized by low pH, and high concentrations of heavy metals and sulfates. Various water treatment methods are being used to neutralize AMD before being discharged to nearby bodies of water. Passive treatment using a neutralizing agent like limestone is a common method for both operational and abandoned mine sites. The study investigates the potential of locally available serpentinite as an alternative media for AMD neutralization, due to its high magnesium content. Raw material characterization was conducted including XRD and surface area. A batch test was designed to determine the effect of water/rock ratio in treating a synthetic AMD solution. The solution was set at a pH of 2.14 ± 0.06, 1860 ppm Fe, 200 ppm Al, 289 ppm Ni, 51 ppm Cu, 64 ppm Mn, and 20 ppm Mg. The initial and final values of the following parameters: pH, redox potential (ORP), total dissolved solids (TDS), and concentration of heavy metals were monitored. The change in the aforementioned parameters indicate that a low water/rock ratio using serpentinite has the highest neutralizing capacity. Since the available serpentinite is clayey in nature, it is recommended that techniques to increase particle size while maintaining porosity be explored. By doing so, an industrial application of the media may be developed.
Recalcitrant pollutants in wastewater need to be removed to maintain clean, safe and secure environment. This is done often through sequentially integrated physiochemical and biological treatment processes[1-17]. The treatment processes are often multistep, require high energy input and large land area. Until recently, with the development of electrode membrane, bio-electrochemical and photo electrochemical process, we demonstrate the successful treatment of industrial wastewater in a simultaneous bio- / physiochemical reactor with only one step treatment. This is realized by using low cost commercial material in a simple reactor with (1) an electrode / photo-electrochemical cathode (2) bio-anode with microbes and graphite /activated carbon (3) a sand layer separator between the anode and the cathode. The removed COD/TOC from (initial >3700mg/L COD) coking wastewater easily reach 70% to over 90%, 95%, 99%, depending on aeration intensity, acclimation of anodic microbes and total hydraulic residence time, with or without light irradiation. Satisfactory effluent meeting discharge standard can be obtained. The simultaneous electricity generation is realized( the cell voltage 0.3V-0.5V). The Qe for COD to electricity is significantly higher with light. The obtained power density normalized to the cathode area is around 11- 26 W/m2 without light and 29-88W/m2 with light. The progresses achieved in the above signify the high potential of such technology in practical application. The investment cost and energy consumption is estimated at 1000$ and 0.3-0.5KWh/ m3[17].