
Chemical oxygen demand (COD) concentration has increased for these decades in the Seto Inland Sea in Japan, though a total pollutant load control system has been employed since 1979 according to the Law Concerning Special Measures for Conservation of the Environment of the Sea. On the other hand, dissolved oxygen (DO) concentration at the bottom layer of Seto inland sea has increased too. As the possible cause for the increase of COD, we focussed on the loading of organic carbon from sediment, because a previous research on the sediment brought to the land by the tsunami in Japan showed the increase of organic carbon content due to the exposure to the atmosphere. This research aimed at investigating whether organic carbon loading occurs via increase of organic carbon content in sediment due to a change of DO at bottom layer. Sediment and overlying water layers were established in the columns and maintained under an anaerobic condition for a week, and then the columns were aerated. In the control experiment, aeration was not carried out. The result of sediment sample analyses confirmed the increase of organic carbon content while changing the sediment surface in the columns from anaerobic to aerobic conditions. In addition, the dissolved organic carbon (DOC) concentration increased too along with this change, though no increase of organic carbon content was observed in the control experiment. An analysis of the microorganisms showed the predominance of sulfur oxidizing bacteria in the sediment samples that demonstrated the increase of organic carbon content. These results collectively showed that the change of sediment environment due to the increase of DO caused the increase of organic matters in the sediment via stimulating the certain autotrophic bacteria such as sulfur oxidizing bacteria, which might contribute to the increase of DOC concentration in the overlying water.
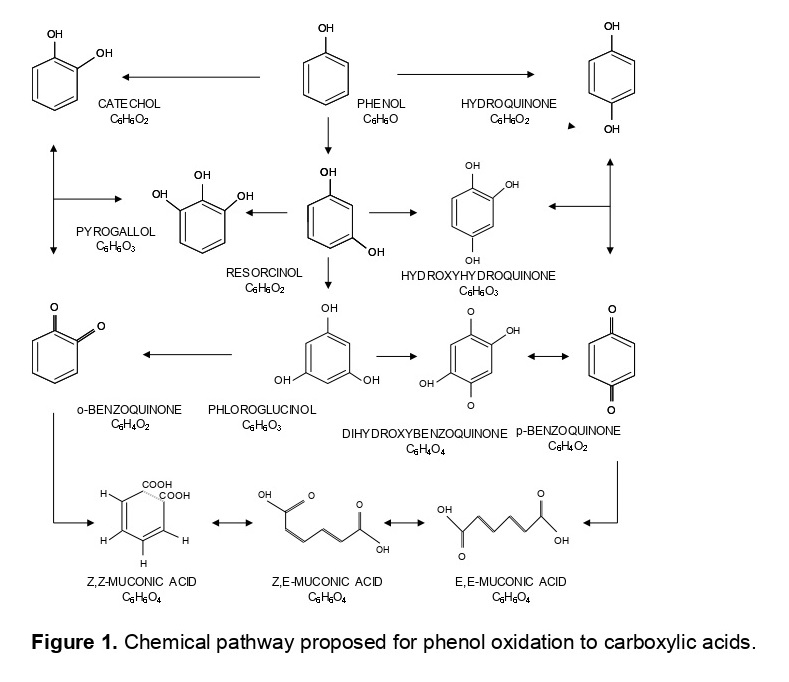
This work studies the kinetics of phenol oxidation, adding new reactions to the current literature and elucidating their intermediates. The mechanism depends on the dosage of oxidant. When utilizing ratios R=1.0 mol H2O2/mol C6H6O, phenol degrades to species with dihydroxylated rings, uncoloured and more toxic than phenol itself. Using R=2.0, ortho and para-substituted intermediates decompose to their corresponding substituted benzoquinones. As a remark, the o-benzoquinone is very unstable and the m-benzoquinone does not exist in nature, due to its low stability. Besides, they cause strong dark coloration to wastewaters, because of chromophore groups. Furthermore, para-substituted benzoquinones can generate complexes of charge transfer (quinhydrone) with hydroquinone. Moreover, ortho-substituted benzoquinones can react with the ferric ions originated from the added catalyst, creating iron compounds.
Applying R=3.0, there is an increase of the reactions involved in the degradation mechanism, occurring parallel reactions of various oxidation levels. Moreover, trihydroxylated rings appear, ortho and para-substituted, like hydroxihydroquinone and pyrogallol. Finally, the oxidation of hydroxylated benzoquinones leads to muconic acid. Performing with R=4.0, the trihydroxylated benzene rings are also decomposed to muconic acid that are not harmful for dumping. Due to the double bond of oxygen in the ring, formation of ferric complexes can happen when reacting ferric ions with carboxylic acids as muconic or oxalic. At higher ratios, decomposition of the toxic and recalcitrant species leads to biodegradable acids. So, with R=6.0 para-substituted quinones evolve to 2,5-dioxo-hexenoic acid. The oxidation of this acid and muconic creates carboxylic acids in the system, such as 1,4-dioxo-2-butane utilising R=6.0; 4-oxo-2-butanoic acid employing R=7.0 and maleic acid operating at R=8.0. When enlarging the stoichiometric ratio to R=14.0, the benzene rings open, generating linear acids, which undergo a shortening of the chain and emit carbon dioxide.
The objective of this study is to optimise the wastewater treatment plant of poultry industry. The optimisation of simulation model was done using the SuperPRO designer as simulator. The performance of the wastewater treatment plant for the poultry industry has improved significantly at the minimum cost. The COD value reduced from 133 mg/L to 0.06 mg/L at the discharge stream 1 and 81.1 mg/L for discharge stream 2. In addition, the BOD5 value reduced from 66.7 mg/L to 0.03 mg/L at the discharge stream 1 and 49.8 mg/L at the discharge stream 2. Further to this, the TTS reduced from 33.3 mg/L to 0.0 mg/L at the discharge stream 1 and 21.8 mg/L at the discharge stream 2. Oil and grease also reduced from 3.3 mg/L to 0.06 mg/L at the discharge stream 1 and 1.9 mg/L at the discharge stream 2. The existing model and system can improve the wastewater treatment plant of poultry industry.
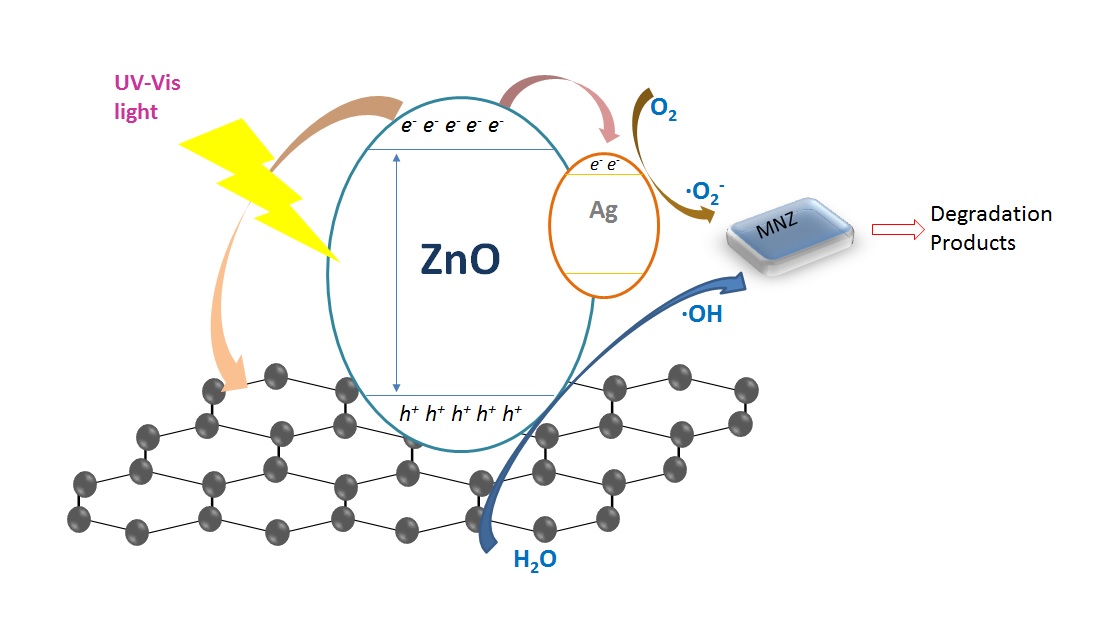
In this study, the ZnO nanoparticles that were doped with Ag and then hybridized on graphite (GP) layer (Ag-ZnO/GP) were prepared by a facile hydrothermal method. The morphologies, structure, and optimal properties of synthesized samples were investigated. It was found that the extent of the enhancement of photocatalytic activity strongly depended on the amounts of graphite and dopant Ag. The optimal weight ratio was observed to be 0.5% GP/ZnO and 1% Ag/ZnO. This fabricated composite was applied as photocatalyst to degrade metronidazole (MNZ) antibiotic in aqueous solution. Experimental results showed that 90% of 30-mg/L MNZ could be removed after 60 min of 100-W UV irradiation over Ag-ZnO/GP composite with a dose of 0.5 g/L. The presence of Ag would cause a reduced recombination rate of electron-hole pairs and therefore an improved photocatalytic activity in the wide range of light wavelengths. After 180 min of solar light irradiation, 71% of 30-mg/L MNZ could be eliminated. In addition, graphitic surface could act as an election sink to successfully inhibit the photocorrosion of ZnO, which revealed the photostability of Ag-ZnO/GP composite after 5 recycles. Moreover, the roles of reactive agents (for example, hole h+, hydroxyl radical ·OH, and superoxide radical ·O2-) during the degradation of MNZ was clarified through the detection of reaction intermediates using an ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTof/MS, Waters). The present results demonstrated the promising application of as-prepared composites for efficient removal of antibiotics from aqueous solutions.
Municipal wastewater reclamation and reuse is one of the most reliable approaches to solve the problem of water scarcity. Compared with wastewater treatment and discharge, water reuse faces more challenges on water safety insurance. The risks caused by pathogens, harmful components of bacteria (such as endotoxin) and microbial regrowth must be controlled in priority during the utilization of reclaimed water.
In order to achieve comprehensive and long-lasting control of biological risks of reclaimed water, the risks of conventional pathogenic (indicator) microorganisms as well as some emerging microbial harmful components, including antibiotic resistant bacteria, endotoxin and so on were investigated.
In order to improve the effectiveness of disinfection, to inhibit the reactivation of microorganisms and to reduce the associated risks during disinfection caused by toxic and harmful disinfection by-products (DBPs), a novel disinfection technology of electroporation was developed.
In order to inhibit microbial regrowth during the distribution and utilization of reclaimed water, the effects of biodegradable organic matters (BOMs) and the microbial community structure on microbial growth were investigated. Furthermore, the BOM removal by advanced treatment was studied, and the transformation mechanism of BOM during advanced treatment was discussed.
Based on the research mentioned above, the systematic control principles of microbial risks during the advanced treatment, disinfection, distribution and utilization process of reclaimed water will be discussed.